ABSTRACT
Glycine max L. (soybean) is an important leguminous staple crop. In this study, nutritional value, bioactive compounds, and antioxidant properties of soybean milled fractions (MF) were determined. Protein contents in different milled fractions ranged from 4.6% to 47.2%, and fat contents ranged from 1.9% to 22.1%. MF amino acid contents ranged from 6.8% to 37.9 g/100 g. Glycitein and genistin were the most concentrated isoflavones in the cotyledon and germ fractions. Although MF phenolics of protocatechuic, chlorogenic, hydroxybenzoic, vanillic, caffeic, and sinapic acids were abundant in bound and free phenolic extracts. In addition, MF total phenolic indexes ranged from 65 to 270.1 GAE/100 g and total flavonoid 7.5 to 92.3 mg GAE/100 g, which were found to be the most active of FRAP (80.2–197.1) and ABTS (28.2–125.6 µmol TEAC/100 g), respectively. The overall results provided useful information and encouraged the effective utilization of soybean-milled fractions as functional food ingredients for health requirements.
Introduction
Glycine max L. (soybean) is a vital legume crop of the plant Fabaceae family that has been used as a dietary staple crop in Asian countries for about 5000 years (Jiang et al., Citation2023). According to the US Agriculture reports, global soybean production reached 391.17 million metric tons in 2022–2023 (US Department of Agriculture [USDA], Citation2023). Soybean is widely utilized in human food and animal feed due to its high nutritional value and excellent functional properties. Soybean seeds are also well known for their high content of protein, oils, carbohydrates, vitamins, minerals, fiber, and phytochemicals with antioxidant and anti-carcinogenic potential (Canaan et al., Citation2022; Liu et al., Citation2016; Qin et al., Citation2022; Tyug et al., Citation2010; Xue et al., Citation2016; Yang et al., Citation2015). Literature also highlights soybean nutritional benefits, prevention of chronic and cardiovascular diseases, cholesterol, hypertension, postmenopausal symptoms, and improving cognitive and immune functions (Jiang et al., Citation2023; Kim et al., Citation2014; Maria John et al., Citation2016). Moreover, soybean consumption aids menopause relief, prevents osteoporosis, plays a role in controlling type II diabetes, improves lung function, reduces certain cancers, and prevent several types of diseases (Alu’datt et al., Citation2013; Canaan et al., Citation2022; Liu et al., Citation2016; Tsantili et al., Citation2011).
Soybean seeds are dehulled to the cotyledon fraction before being processed for soybean flour, soy protein isolate, soymilk, soymilk-powder, soy cheese, tempeh, soy sauce, cooked beans, soybean oil, desserts, noodles, snacks, and nutritional supplements (Canaan et al., Citation2022; Tyug et al., Citation2010; Yang et al., Citation2015). Soybean milling and processing result in the production of by-products. Milled results showed that dehulled cotyledon accounts for 90%, whereas germ, seed coat, plumule, and aleurone layers enriched in seed coat fractions collectively accumulate 10%. Hence, 39.11 million metric tons of soybean milled fractions are produced annually and can be utilized as a potential source of nutrition in human food and animal feed. However, during processing, these milled fractions are discarded as an environmental hazard, and some of them are used as animal feed. Due to the nutritional and health benefits of soybean seeds, there is a distinct need to exploit their milled fractions. The world’s population will be 9.8 billion by 2050, which demands a 70% increase in food supply (Zhao et al., Citation2020). The reutilization of agricultural waste and processed food sub-products has gained importance for combating hunger and global food sustainability. Whole-grain foods have been given special consideration for their health benefits. Whereas their milled fractions have attracted attention because of their inexpensiveness, rich source of nutrients, human health promotion, and aggregating economic values (Girish et al., Citation2012; Manco et al., Citation2023; Sreerama et al., Citation2010; Zhang et al., Citation2021).
Soybean is a significant plant-based protein source globally, with a higher crude protein percentage compared to other legumes or pulses. Unlike soybeans, the chemical composition and biological activities of milled fractions of grains like wheat, rice, grams, barley, lentils, and maize have also been investigated by the researchers (Berhow et al., Citation2006; Chaya et al., Citation2022; Das & Singh, Citation2016; Girish et al., Citation2012; Kamani & Meera, Citation2021; Kong & Lee, Citation2010; Manco et al., Citation2023). However, no comparative studies had been carried out on this wide genetic resource of milled fractions. Therefore, this study aimed to elucidate the chemical composition, nutrients, multi-minerals, bioactive components, and their antioxidant activities in different milled fractions of soybean. The results may provide useful information for the utilization of 39.11 million metric tons of milled fractions produced annually as functional food ingredients.
Materials and methods
Yellow Soybean seeds (cv. IA2032) were provided by the Department of Agronomy, Zhejiang University, Hangzhou, China. Isoflavones and phenolic acid standards were purchased from LC- Laboratories (Woburn, MA, USA). Dimethyl sulfoxide, methanol, gallic acid, ascorbic acid, potassium persulfate, and ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] were all provided by Sigma-Aldrich Co. (St. Louis, USA). Folin–Ciocalteu’s phenol reagent was purchased from Merck. All other reagents were of analytical grade.
Milled and separation of soybean fractions
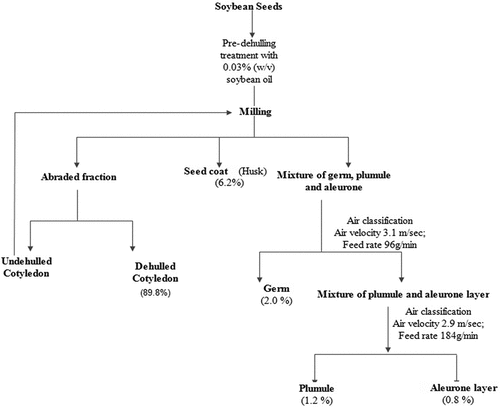
Soybean seeds with a moisture content of ~8.4% were oven-dried at 50°C for 72 h, as this degree yields a good extent of milled fraction separation (Kim et al., Citation2013). Seeds (8 kg) were milled using a mill (Dae-Ryuk Food Machine, Daegu, Korea). The seeds were dehulled at a constant coarse wheel speed, although time varied depending on the degree of dehulling. The flow diagram () shows the process of dehulling and separation of the milled fractions of soybean seeds. The scraped fractions were manually separated into dehulled and un-dehulled cotyledons. To obtain completely dehulled cotyledons, the remaining un-dehulled cotyledons were further passed through another round of dehulling. After dehulling, the aspirated milled fractions were sieved with seed coats (hulls), and mixtures of embryonic axe (germ), plumule, and aleurone layer enriched in seed coat (ALESC) were collected by passing through the sieves and separated by the method of air classification (Ajila et al., Citation2009; Girish et al., Citation2012). All the resultant fractions were weighed and then articulated as a part of the total weight of the seeds. The separated fractions were ground into fine powder using a blender (MX-291 N, National, Osaka, Japan) and stored in airtight containers for further analysis.
Determination of proximate composition
Moisture, fat, ash, and total dietary fiber (TDF) concentrations were determined as described by (Sinkovič et al., Citation2022). The total nitrogen (N) and protein factor (N × 6.25) content was determined through a nitrogen analyzer. The carbohydrates and energy contents calculated are as follows:
Carbohydrates = 100 − (moisture + ash + protein + fat + TDF)
Energy (kcal) = 4 × (protein + total available carbohydrate) + 2 × (fiber) + 9 × (fat)
Mineral analysis
Whole seed and their seed fractions (200 mg) were separately mixed with 6.0 mL nitric acid 65% (v/v) and hydrogen peroxide 2 mL (30% v/v). The microwave-digested solutions were volumed up to 50 mL with double-deionised water. The concentrations of macronutrient (K, P, Mg, Na, Ca) and micronutrient (Fe, Zn, Mn, Cu) in the filtrates were determined using Inductively Coupled Plasma-mass Spectrometry (ICP-MS, Agilent, 7500a, CA, USA) according to the procedure described by Herwig et al. (Citation2011).
Amino acid analysis
The amino acid composition of whole soybean seed and their milled fractions were determined as described by Yang et al. (Citation2015). Briefly, each 0.1 g of dry, finely milled fraction was separately placed in an ampoule and mixed with 5 ml of 6 M HCl. After sealing the ampoule with nitrogen, the sample was hydrolyzed at 110°C for 24 h. After cooling on ice, the hydrolysate evaporated to remove HCl, filtered through a 0.2 μm nylon filter and 400 μl filtrate transferred into a 2-mL eppendorf centrifuge tube containing 200 μL of 200 mg/L norvaline and 400 μl ice-cold 6 M NaOH. The extracts were analyzed using a High-Speed Amino Acid Analyzer (L-8800; Hitachi, Japan). All the samples were analyzed in triplicate and results are expressed in g/100 g of protein.
Analysis of isoflavone contents
Soybean fraction isoflavones were extracted using the method followed by Kim et al. (Citation2014). Briefly, each 1 g of dry, finely milled fraction was mixed with HCl (2 mL of 100 mM), acetonitrile (7 mL), and distilled water (3 mL). The mixture was shaken for 2 h in a shaker and then centrifuged at 2,208 × g for 10 min and filtered through filter paper. The filtrates were dried with nitrogen gas and dissolved in 1 mL of methanol and again filtered through a 0.2 μm syringe filter. Isoflavone analysis of soybean fractions was performed using a HPLC (SIL-20A, Shimadzu, Tokyo, Japan) equipped with a column C18 (4.6 mm × 250 mm, 4 μm). The analysis was performed at a wavelength of 260 nm with a flow rate of 1.0 mL/min. Quantification of isoflavones was conducted based on standard curves of isoflavones. Standards of isoflavones (i.e., daidzein, genistein, glycitein, daidzin, glycitin, and genistin) were dissolved in dimethylsulfoxide at different concentrations of (0.002, 0.005,0.01,0.02,0.05,0.1,0.2,0.5, 1.0 mg ml−1) for the calibration curve, the linearity ranges were daidzein [r2 = 0.98], genistein [r2 = 0.99], glycitein [r2 = 0.99], daidzin [r2 = 0.99], genistin [r2 = 0.99], and glycitin [r2 = 0.99]. Each isoflavone was further identified by its retention time, and the concentrations were calculated by comparing the peak areas of the samples with those of the standards. The results were expressed as (mg/g of the sample).
Extraction of phenolic acids
Free and bound phenolics of soybean fractions were extracted according to the method followed by (Alu’datt et al., Citation2013). For the free phenolics, each fraction (2 g) is blended with 50 mL of 80% (v/v) chilled acetone for 10 min using a homogenizer. The re-extracted collected residues and supernatant were centrifuged for 10 min at 3500 × g. Supernants of different fractions were pooled and concentrated under vacuum at 45°C. Subsequently, each concentrated slurry was diluted with cold methanol (25 mL) to obtained free phenolics. For the bound phenolics, to remove lipid fatty acids, the residues were extracted twice with hexane. Each residue was hydrolyzed with 40 mL of 2 -M NaOH and shaken at room temperature for 2 h under nitrogen gas. The pH was immediately adjusted to 1.0 by adding 6 M HCl. The liberated residues were then extracted five times with 100 mL of ethyl acetate in dark place, and concentrated at 40°C, then re-dissolved with 10 mL methanol to obtain extracts and stored at −20°C until use. The extracts were analyzed by an Agilent 1260 HPLC system. Phenolic acid standards were purchased from Sigma Aldrich (St. Louis, MO, USA), and each phenolic was used at different concentrations for the calibration curve, and linearity ranges, respectively. Results are expressed as (g/100 g of the sample).
Determination of total phenolic and flavonoid contents
The total phenolic content (TPC) and total flavonoid contents (TFC) of free and bound fractions of whole soybean seed and their milled fractions were determined using the colorimetric method (Das & Singh, Citation2016). Briefly, the proper dilution of the extracts was oxidized with Folin–Ciocalteu reagent, and the reaction was neutralized with sodium carbonate (20%). After 30 min, the resulting blue color was measured at 750 nm using spectrophotometer (Shimadzu UV-1601, Japan). Gallic acid was used for a calibration curve and the results were expressed of gallic acid equivalents (GAE) per 100 g of sample. Total flavonoid content (TFC) extracts were mixed with sodium nitrite (5% v/v), aluminum chloride (10% v/v), and sodium hydroxide (1 M) and centrifuged for 10 min at 5,000g. After centrifugation, absorbance of the supernatant was measured at 510 nm. The results were expressed in galic acid equivalents (GAE) per 100 g of sample. Gallic acid was used for a calibration curve, and the results were expressed as gallic acid equivalents (GAE 100 g−1). TP and TF linearity ranges were r2 = 0.98 and 0.99, respectively.
Determination of antioxidant capacity
Determination of ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] scavenging activity was conducted according to the Xue et al. (Citation2016). Briefly, ABTS + was produced by reacting 7 mM ABTS and 2.45 mM potassium persulfate solutions in equal quantities and allow them to react at room temperature for 12 h in the dark. The solution was then diluted by mixing PBS (pH 7.4) and ABTS+ at a ratio as 1:100 (v/v) for 6 min incubation in the dark to obtain an absorbance of 0.70 ± 0.03 at 734 nm by using the spectrophotometer. The whole soybean seed and their milled fraction extracts (50 μL) were allocated to react with 950 μL of the ABTS+ solution for 6 min in a dark condition. The absorbance was then measured at 734 nm through spectrophotometer. Ascorbic acid at 25 μM was used as a positive control. The relative activity was calculated using a trolox calibration curve, and the results were converted to TEAC (trolox equivalent antioxidant capacity) value for further relative contribution of antioxidant analysis.
Ferric reducing antioxidant power assay (FRAP) assay was determined according to Tyug et al. (Citation2010), each sample extract of 1 ml was mixed with 2.5 mL each of 0.2 M phosphate buffer (pH 6.6) and 1% potassium ferricyanide, incubated at 50°C for 20 min and then added with 2.5 mL of 10% trichloroacetic acid (TCA) and centrifuged at 3000 rpm for 10 min. After centrifugation, the supernatant was added with equal part of distilled water and 0.5 mL of 0.1% FeCl3. The absorbance was measured at 700 nm by using spectrophotometer.
FRAP was expressed as the GAE, μmol/100 g−1 DW powder samples.
Statistical analysis
Analysis of variance (ANOVA) and Duncan’s multiple-range tests were used to determine significant differences using SPSS statistical software (v.19.0, SPSS Inc., Chicago, IL, USA). A value of p < .05 was considered statistically significant. All values reported were the means of three independent replicates from samples.
Results and discussion
Milled fraction and their proximate contents
Soybean is a plant protein source with basic nutritive constituents, consumed as the cotyledon fraction in fermented and unfermented products. Soybean seed milled fractions are obtained by separating the hulls from seeds through a milling process. shows the processing flow diagram of the soybean milling process. During the milled process, different byproducts could be distinguished with yield proportions of cotyledon (~90%), husk (~6.1%), embryonic axe (germ) (~2.0%), plumule (~1.1%), and aleurone enriched in seed coat (ALESC) (~0.8%) of the seed content. The nutritional composition of milled fractions is presented in . The cotyledon fraction showed a higher ash content (5.54%) followed by the seed coat (5.72%), germ (4.68%), ALESC (4.15%), and plumule (3.26%) fractions. Ash content is a quality index of poultry and cattle feeding (Berhow et al., Citation2006), so plumule, seed coat, and ALESC fractions with lower ash contents could be suitable feeding materials. Rayaprolu et al. (Citation2015) reported similar moisture profiles in soybean lines. The protein content of soybean fractions varied between for the cotyledon (47.15%), germ (35.37%), ALESC (34.20%), plumule (4.61%), seed coat (4.80%) fractions. A consistent trend was observed for fat and dietary fiber contents, ranging from 1.89% to 22.14% and between 3.10% and 74.53%. The milled process was used to differentiate soybean fractions in terms of ash, moisture, protein, fat, total dietary fiber, carbohydrate, and energy content, as previously demonstrated by Girish et al. (Citation2012) for black grams, Bahr et al. (Citation2014) for lupin cultivars and Sinkovič et al. (Citation2022) for buckwheat fractions. The results of protein content and ash contents were in accordance with the results obtained for defatted soybean reported by Bahr et al. (Citation2014). The protein content in the cotyledon fraction was higher than Yang et al. (Citation2015) report in soybean sprouts, but lower than the progressively increased protein content in other fractions. The germ fraction trend for protein (35%), fat (10%), and fiber 3% was consistent with the results reported by Kim et al. (Citation2014). Similarly, milled fractions of different legumes like chickpea having ash contents ranged 2.6–3.9%, protein 7.3–23.8%, fat 1.6–7.8%, crude fiber 1.8–17.6%, carbohydrate 61.6–83.9, for horse gram ash contents 2.2–3.8%, protein 9.1–22.6%, fat 0.6–2.6%, crude fiber 1.6–21.8%, carbohydrate 66.9–82.6, and black gram ash 3.0–5.3%, protein 12.2–26.8%, fat 0.9–3.4%, crude fiber 1.2–33.7%, and carbohydrate 18.2–58.0 are also comparable to soybean milled fractions (He & Chen, Citation2013; Sreerama et al., Citation2010). Nowadays, whole grains and their fractions are used in the production of various types of food products and prioritized for health benefits. However, utilization of these milled fractions of flour can enhance the ability of flour to absorb and retain oil, improve structure, and enhance the soy flavor of food products. Therefore, soybean milled fractions offer good contents of protein, fat, fiber, carbohydrate, and energy levels, making them valuable ingredients in food formulations for maximum benefits.
Table 1. Proximate composition of whole soybean grain and their milling fractions (% DW).
Mineral contents
Minerals are essential micronutrients and play a role in various metabolic processes. The multi-mineral contents quantified in whole soybean seed and their milled fractions are shown in . Milled fraction multi-minerals can be divided into macroelements and microelements. Micronutrients Fe, Cu, Mn and Zn ranged from 47.49% to 66.27%, 8.53% to 16.49%, 24.01% to 36.72%, and 21.63–51.87%, respectively. The macronutrients in descending order were of K (0.83–2.23%), P (0.48–0.73%), Ca (0.24–0.41%), and Mg (0.02–0.06%). Ca (0.41%) showed higher contents in seed coat, while Na (0.33%) in whole seeds. The cotyledon, germ, and ALESC fractions showed the most abundant levels of minerals, Mg (0.06%), K (2.23%), and P (0.73%), while Na content was the highest in germ and K was in the seed coat fractions. On the other hand, the highest concentrations of microelements Fe, Cu, Zn, and Mn were found in cotyledon, followed by the germ, plumule, and ALESC fractions. The most significant differences were observed for K, Fe, and Zn. Meanwhile, there were no differences in Cu values for ALESC and plumule fractions. Whole soybean seed and its fraction reported comparable contents of Zn and Mn (Bähr et al., Citation2014). Interestingly, Na and K were abundant among all milled fractions and may influence consumption and body electrolytic balance through high K/Na ratios (Liang et al., Citation2013). Begum et al. (Citation2023), Kamani and Meera (Citation2021), and Chaya et al. (Citation2022) repoted comparable values pertaining to K, Fe, Cu, Mg, and Zn contents in black grams, chickpea varieties, cereals, and pulses. Soybean milled fractions are a valuable source of multi-minerals, providing essential minerals for daily consumption and essential defense mechanisms.
Table 2. Mineral elements’ composition of whole-soybean grain and their milling fractions (n = 3).
Amino acid contents
The composition of amino acids is important in determining nutritional quality. In addition to being multi-mineral, soybean-milled fractions are a good source of free amino acid (FAA) reserves. FAA composition of soybean seed milled fractions is shown in . The FAAs’ contents ranged from 6.82 to 37.9 g−1. The highest FAA was observed in the cotyledon fraction (36.46 g−1), while the seed coat had the least (6.79 g−1) compared to other fractions. The total essential amino acids (EAA) ranged from 2.55% to 16.16%, while the non-essential amino acids (NEAA) ranged from 4.24% to 22.63%. The most predominant EAAs were arginine (0.35–3.29 g−1), leucine (0.45–2.94 g−1), and lysine (0.59–2.97 g−1) in all fractions. Phenylalanine (0.31–1.96 g−1), threonine (0.24–1.52 g−1), and histidine (0.24–1.08 g−1) were in trace amounts. Among the NEAAs, glutamic acid (7.47–0.80 g−1) and aspartic acid (0.68–5.02 g−1) were in higher concentrations, while alanine (0.37–1.93 g−1), glycine (0.81–1.90 g−1), proline (1.86–0.44 g−1), serine (1.56–0.36 g−1), and tyrosine (0.32–1.08 g−1) were in low amounts. However, methionine (0.06 g−1) and cysteine (0.03 g−1) were also found in trace quantities in the seed coat fraction. Soybean milled fraction contains a higher percentage of essential amino acids than non-essential ones, suggesting it could be a suitable source for meeting protein needs (Kamani & Meera, Citation2021). However, the husk and ALESC low-protein fractions also exhibited a suitable profile for conditionally essential amino acids. It was worth noting that higher glutamic acid contents were observed in all milled fraction, which aids the gamma-aminobutyric acid (GABA) strength. The glutamate generating system comprises GABA which has functions in reducing an increase in systolic blood pressure. Soybean milled fractions contain GABA stimulants which can potentially be used in GABA-enriched functional foods (Yang et al., Citation2015). Soybean protein contains significantly more amino acids (37.9 g/100 g) than wheat flour (32.20 g/100 g) of protein (Begum et al., Citation2023). The ratio of EAA to NEAA for soybean milled fractions was 0.60–0.80%, which is considerably higher than adults need (0.38) as recommended by the WHO (Citation2007). The chemical scores of EAA with respect to the reference protein (FAO/WHO, Citation1991), presented in . Among EAA isoleucine and lysine branched amino acids were present in higher percentages compared to methionine and cysteine which were in negligible amounts unlike most other legumes. These branched-chain amino acids are important nitrogen outcomes for glutamine and alanine synthesis in muscle (Holecek, Citation2002). Therefore, the amino acid profiles of soybean fractions are appreciable and can be utilized for food fortification and in food dispersion systems to address essential amino acid deficiency.
Table 3. Amino acid composition of whole-soybean grain and their milling fractions (g/100 g DW, n = 3).
Table 4. Essential amino acid composition of milling fractions compared with the FAO/WHO pattern (g/16 N g = grams of amino acid per 100 g protein).
Isoflavones contents
Soybean isoflavones have gained attention for their anti-atherosclerotic, antioxidant, antitumor, and anti-estrogenic properties in recent decades (Kong & Lee, Citation2010). Isoflavones also offer health benefits thus, increasing the daily consumption of soybeans and soy products. The contents of different forms of soybean milled fraction isoflavones are shown in . The aglycone contents of soybean seeds and their milled fractions were 0.012–0.996 mg g−1. Whole seeds had the highest accumulative aglycones (1.111 mg g−1), followed by cotyledon (0.399 mg g−1) and ALESC fractions (0.996 mg g−1), while plumule fractions had the lowest aglycones (0.098 mg g−1). Glycitein was the most abundant aglycone among fractions, followed by daidzein and genistein. The 7-O-β-glucosides contents in whole seeds and their milled fractions were daidzin (0.022–1.102 mg g−1), glycitin (0.014–0.322 mg g−1), and genistin (0.022–1.150 mg g−1). Whole seeds had the highest total 7-O-β-glucosides contents (2.574 mg g−1), followed by cotyledon fractions (1.993 mg g−1) and germ fractions (0.565), while the least was observed in seed coat (0.058 mg g−1) fraction. Kim and Kim (Citation2020) also reported the content of genistin (392.63 μmol/g) and daidzin (240.82 μmol/g) in hybrid soybean seeds which were comparable to the soybean milled fractions. The daidzein in seed coat, plumule, and ALESC, glycitein in plumule and seed coat, whereas genistein in seed coat and germ fraction isoflavones were present in only low concentrations compared to other fractions. The presence of daidzin in seed coat, glycitein in seed coat and ALESC, genistin in germ and seed coat was found to be in low concentrations compared to other fractions. However, in accordance with the results of Žilić et al. (Citation2013) the total isoflavones of soybean seed coats and cotyledon fractions were comparable than those of whole seeds. Žilić et al. (Citation2013) also found that the aglycone constitutes up about 60% of the glucoside. Isoflavone glycosides are generally found to be less biologically active than aglycones. Kim et al. (Citation2014) reported that most isoflavones are found in cotyledon and germ fractions, which aligns with the findings of the study. The impact of genetic and environmental factors on isoflavone contents and variation in milled fractions also revealed different isoflavone biosynthesis preferences. The content of isoflavones can significantly vary depending on the synthesis and accumulation of isoflavones. The variation in profile may indicate varying isoflavone biosynthesis preferences among soybean milled fractions. Soybean isoflavones have antioxidant, anti-inflammatory, and anti-carcinogenic properties in various cancer models, as well as physiological benefits like reducing coronary heart disease and preventing osteoporosis (Kim et al., Citation2013). Southeast Asian diets’ intake has high isoflavones (±100 mg/day), lowering the risks of cancer and cardiovascular diseases. Whereas in the western population, dietary isoflavone intake is much lower (±1–2 mg/day). However, commercially available isoflavones are comparatively expensive, hence their cost-effective supply is essential for healthy foods (Kim et al., Citation2014). Yellow soybean milled fractions have the potential to provide isoflavones for functional foods. The recommendation is for further large-scale isoflavones isolation to be scaled up at a commercial level.
Table 5. Isoflavones content of whole-soybean grains and their milling fractions (mg/g).
Phenolic acid contents
The researchers are actively studying the effects of phenolic acids due to their potential as antioxidative agents. Soybean polyphenol offers therapeutic benefits against reactive oxygen species and free radicals (Multari et al., Citation2016). Phenolic acid impacts food color, sensory attributes, taste perception, nutritional content, and antioxidant properties. The concentrations of phenolic acids in different soybean milled fractions are shown in . Most of the studied phenolic acids were found in the whole soybean seed and the cotyledon fraction, a significant quantity was also found in germ, plumule and ALESC fractions. Protocatechuic acid, vanillic acid, coumaric acid, and chlorogenic acid were the most significant flavanol compounds found in soybean milled fractions. Protocatechuic acid in free form is the predominant phenolic acid (g/100 g) in whole seed (22.9 ± 0.1 g/100 g), tailed by cotyledon (7.5 ± 0.2 g/100 g), germ (11.4 ± 0.1 g/100 g), ALESC (9.7 ± 1.1 g/100 g), whereas bound form was higher in ALESC (26.6 ± 1.8 g/100 g), plumule (11.9 ± 1.8 g/100 g), seed coat (4.4 ± 0.8 g/100 g), germ (1.22 ± 0.02 g/100 g), whole seed (1.1 ± 0.3 g/100 g), and cotyledons (0.8 ± 0.0 g/100 g) (), respectively. Chlorogenic acid is the abundant phenolic acid found in bound form of milled, whereas, in free from was cotyledon 10.1 ± 0.4, germ has 9.6 ± 0.4, seed coat 1.0 ± 0.2, ALESC 1.4 ± 0.2 and plumule has 0.3 ± 0.0 g/100 g. Previous reports have also confirmed that chlorogenic acid is the primary phenolic compound found in soybeans (Alu’datt et al., Citation2013; Tyug et al., Citation2010; Xue et al., Citation2016). Bound hydroxybenzoic acid was the main phenolic acid in the seed coat fraction, which has 22.6 ± 0.0 g/100 g, followed by germ 14.2 ± 1.0, while vanillic acid is the higher phenolic acid in the whole seed 16.1 ± 0.1 g/100 g, germ 11.9 ± 0.3 g/100 g, cotyledon 5.8 ± 0.4 g/100 g, and plumule fraction 1.3 ± 0.2 g/100 g. Free caffeic acid was the highest phenolic acid in germ 15.1 ± 0.1, followed by whole seed 3.9 ± 0.0 and cotyledons 3.8 ± 0.1 g/100 g fractions. Sinapic acid was higher in cotyledon 8.1 ± 0.2, germ 7.4 ± 0.1, plumule 6.2 ± 0.8, ALESC 4.5 ± 1.2, and seed coat 2.5 ± 0.2 g/100 g fractions. Free forms of studied phenolic acids were observed in all fractions except caffeic acid in ALESC, gallic acid in the seed coat, chlorogenic acid in germ, seed coat, ALESC and plumule fractions, hydroxybenzoic acid in cotyledon, germ, seed coat, ALESC and plumule fractions, vanillic acid in whole seed, cotyledon, seed coat and plumule fractions, caffeic acid in the seed coat, ALESC and plumule fractions, coumaric acid in germ and sinapic acid in germ, seed coat, ALESC and plumule fractions. Similarly, protocatechuic was reported as a major phenolic in black gram (Girish et al., Citation2012). Substantial amounts of bound phenolic acids were also observed in soybean milled fractions. Significant differences (p < .05) were perceived in phenolic percentages in whole seeds (79, 21%), cotyledon (47, 21%), germ (74, 2.5%), seed coat (28.6, 7.3%), ALESC (20.8, 33.4%), and plumule (18.7, 12.7%) fractions. Soybean milled fractions are an ideal material for the extraction of polyphenols. Moreover, the bioavailability of milled fraction phenolics is crucial to future studies as their concentrations significantly influence their biological activity.
Table 6. Phenolic acid contents in whole soybean grains and their milling fractions (g/100 g).
Total phenolic and flavonoid contents
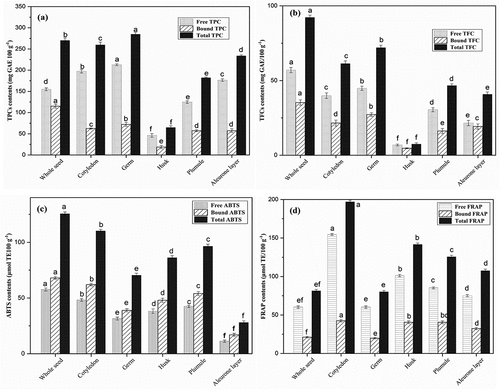
Significant variations in total phenolic (TP) and flavonoid (TF) contents in soybean-milled fractions were observed (). Free and bound TP contents in milled fractions ranged from 46.23 to 212.88 and 18.71 to 115.16 mg GAE/100 g with a coefficient variation of (CV) 7.71% (). The TP results are in good agreement with the previous study (Tyug et al., Citation2010). Germ fraction had maximum TP (free + bound) contents (285.04 mg GAE/100 g DW), followed by cotyledon (259.50 mg GAE/100 g) and plumule fraction (233.92 mg GAE/100 g DW), while seed coat fraction had the lowest TP contents. Flavonoids have the ability to directly scavenge molecules of active oxygen and are highly reactive oxidants (Tsantili et al., Citation2011). Free and bound TF contents of all milled fractions ranged from (6.87–57.01 and 4.7–35.27 mg CE/100 g) (). While total TF (free + bound) values in milled fractions varied from 7.46 to 92.28 mg CE/100 g DW, with a CV of 14.92%. The seed coat fraction showed the lowest TP (64.94 mg GAE/100 g WD) and TF (7.46 mg CE/100 g DW), which are comparable with previous results (Žilić et al., Citation2013). Both TP and TF contents of whole seed, germ, and cotyledon fractions were found to be higher and showed higher values than other fractions.
Antioxidant capacity
Legumes’ antioxidants significantly mitigate cellular and molecular damage by reducing reactive oxygen species. Antioxidant capacity through free scavenging avoids oxidation species and prevents pathologies (Tyug et al., Citation2010; Žilić et al., Citation2013). Soybean consumption is gaining global attention due to its positive impact on human health. FRAP and ABTS antioxidant scavenging capacities varied between soy milled fractions, as shown in (). The free ABTS contents of whole soy seed and their milled fraction ranged from 31.5 to 57.61 mg TE/100 g DW (). Bound fractions ranged from 17.11 to 68.32 mg TE/100 g DW (). Total ABTS (free + bound) of soybean seeds and their milled fractions ranged from 70.5 to 125.61 mg TE/100 g DW with a 4.45% coefficient of variations (CV). Soy germ (102.81 mg TE/100 g DW), plumule (96.55 mg TE/100 g DW), cotyledon (91.48 mg TE/100 g DW), and seed coat (86.26 mg TE/100 g DW) fractions showed the highest ABTS values. The FRAP values of different milled fractions are shown in . Free FRAP values in soybean seed and their milled fractions ranged from 75.27 to 160.26 mg TE/100 g DW (), whereas bound FRAP values ranged from 21.23 to 40.53 mg TE/100 g DW. The total FRAP values varied from 107.67 to 196.59 mg TE/100 g DW, with a 7.72% of CV. The soy germ fraction exhibited higher FRAP scavenging activity, followed by the cotyledon and seed coat fractions, while ALESC fraction had the lowest values. The FRAP reaction consisted of distinct electron transfer mechanism, hence phenolic acid functional group in germ fraction might be represented more active in transferring electrons (Sreerama et al., Citation2010; Xue et al., Citation2016). Our results of cotyledons and germ fractions are in good accordance with the research conducted by Žilić et al. (Citation2013). Soybean seed coat and cotyledon fractions also have a comparable range of antioxidants to the previous reports (Multari et al., Citation2016; Tyug et al., Citation2010; Žilić et al., Citation2013). Antioxidants have plentiful benefits for the human therefore, physically milled fractions should be considered an important feature of soybean seeds.
Correlations among antioxidant capacity (TPC, TFC, ABTS, and FRAP) are statistically analysed in Table S1. A strong and positive correlation (p < .01) existed between TPC and TFC (r = 0.91) as well as TPC and FRAP (r = 0.89). The FRAP and ABTS assays exhibited a positive correlation (r = 0.88, p < .01), suggesting that the two assays are recommendable for evaluating antioxidant capacity in soybean milled fraction extracts. The correlation obtained is in agreement with those reported by Tyug et al. (Citation2010) in soybean byproducts, whereas a significant moderate positive correlation was demonstrated between the TPC and ABTS (r = 0.76, p < .01), TFC and ABTS (r = 0.71, p < .01), TFC and FRAP (r = 0.65, p < .01).
Conclusion
In this study, the nutrients, macro- and microelements, elements, polyphenols, and antioxidant properties of soybean different milled fractions (MF) were investigated. The MF in protein, dietary fiber, energy, and mineral contents showed great variations. Cotyledon, Germ, and ALESC fractions are the main reserves. For adults, the MF-required ratio of essential to non-essential amino acids (0.6–0.8) was comparable to the recommended WHO standards (0.38). Therefore, MF are recognized as low-cost protein sources and could be used as protein replacers, extenders, and products with soy protein importance. Aglycones and 7-O-β-glucosides were the abundant isoflavones in cotyledon, ALESC, plumule, and germ fractions. However, significant quantities of individual chlorogenic, protocatechuic, coumaric, hydroxybenzoic, and canillic phenolics are also present, which are potent inhibitors of digestive enzymes. The soybean fractions also contained abundant free and bound total polyphenols and antioxidant activities. These results determined that the physical separation of different milling fractions has the greatest impact on the nutrient contents of soybeans. The study suggests that soybean MF can be utilized as a functional ingredient in food products for health purposes. Further, our work on MF is in progress for scale-up extraction and its utilization in processed foods, considering its health-promoting capabilities.
Supplemental Material
Download MS Word (11.5 KB)Acknowledgement
Princess Nourah bint Abdulrahman University researchers supporting project (PNURSP2024R155), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia is acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19476337.2023.2290831.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ajila, C. M., & Prasada Rao, U. (2009). Purification and characterization of black gram (Vigna mungo L.) husk peroxides. Journal of Molecular Catalysis B, Enzymatic, 60(1–2), 36–10. https://doi.org/10.1016/j.molcatb.2009.03.014
- Alu’datt, M. H., Rababah, T., Ereifej, K., & Alli, I. (2013). Distribution, antioxidant and characterisation of phenolic compounds in soybeans, flaxseed and olives. Food Chemistry, 139(1–4), 93–99. https://doi.org/10.1016/j.foodchem.2012.12.061
- Bähr, M., Fechner, A., Hasenkopf, K., Mittermaier, S., & Jahreis, G. (2014). Chemical composition of dehulled seeds of selected lupin cultivars in comparison to pea and soya bean. LWT-Food Science and Technology, 59(1), 587–590. https://doi.org/10.1016/j.lwt.2014.05.026
- Begum, N., Xiao, Y., Wang, L., Li, D., Irshad, A., & Zhao, T. (2023). Arbuscular mycorrhizal fungus rhizophagus irregularis alleviates drought stress in soybean with overexpressing the GmSPL9d gene by promoting photosynthetic apparatus and regulating the antioxidant system. Microbiology Research, 273, 127398. https://doi.org/10.1016/j.micres.2023.127398
- Berhow, M. A., Kong, S. B., Vermillion, K. E., & Duval, S. M. (2006). Complete quantification of group a and group B soyasaponins in soybeans. Journal of Agricultural and Food Chemistry, 54(6), 2035–2044. https://doi.org/10.1021/jf053072o
- Canaan, J. M. M., Brasil, G. S. A. P., de Barros, N. R., Mussagy, C. U., Guerra, N. B., & Herculano, R. D. (2022). Soybean processing wastes and their potential in the generation of high value added products. Food Chemistry, 373, 131476. https://doi.org/10.1016/j.foodchem.2021.131476
- Chaya, H. C., Kumar, S. S., Jayarama, S., & Mahadevappa, P. (2022). Comprehensive nutritional analysis, antioxidant activities, and bioactive compound characterization from seven selected cereals and pulses by UHPLC-HRMS/MS. ACS Omega, 7(35), 31377–31387. https://doi.org/10.1021/acsomega.2c03767
- Das, K. S., & Singh, V. (2016). Antioxidative free and bound phenolic constituents in botanical fractions of Indian specialty maize (Zea Mays L.) genotypes. Food Chemistry, 201, 298–306. https://doi.org/10.1016/j.foodchem.2016.01.099
- FAO/WHO. (1991). Protein quality evaluation report of joint FAO/WHO expert consultation, food and agriculture organization of the United Nations. FAO Food and Nutrition Paper, 51, 247.
- Girish, T. K., Pratape, V. M., & Prasada Rao, U. J. S. (2012). Nutrient distribution, phenolic acid composition, antioxidant and alpha-glucosidase inhibitory potentials of black gram (Vigna mungo L.) and its milled by-products. Food Research International, 46(1), 370–377. https://doi.org/10.1016/j.foodres.2011.12.026
- He, F., & Chen, J. (2013). Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence, differences between Chinese women and women in Western countries and possible mechanisms. Food Science and Human Wellness, 2(3–4), 146–161. https://doi.org/10.1016/j.fshw.2013.08.002
- Herwig, N., Stephan, K., Panne, U., Pritzkow, W., & Vogl, J. (2011). Multi-element screening in milk and feed by SF-ICP-MS. Food Chemistry, 124(3), 1223–1230. https://doi.org/10.1016/j.foodchem.2010.07.050
- Holecek, M. (2002). Relation between glutamine, branched chain amino acids and protein metabolism. Nutrition, 18(2), 130–133. https://doi.org/10.1016/S0899-9007(01)00767-5
- Jiang, G.-L., Rajcan, I., Zhang, Y.-M., Han, T., & Mian, R. (2023). Editorial: Soybean molecular breeding and genetics. Frontiers in Plant Science, 14, 1157632. https://doi.org/10.3389/fpls.2023.1157632
- Kamani, M. H., & Meera, M. S. (2021). Assessment of black gram milling by-product as a potential source of nutrients. Journal of Food Science and Technology, 58(10), 3844–3852. https://doi.org/10.1007/s13197-020-04845-0
- Kim, M. A., & Kim, M. J. (2020). Isoflavone profiles and antioxidant properties in different parts of soybean sprout. Journal of Food Science, 85(3), 689–695. https://doi.org/10.1111/1750-3841.15058
- Kim, J. K., Kim, E. H., Park, I., Yu, B. R., Lim, J. D., Lee, Y. S., Lee, J. H., Kim, S. H., & Chung, I. M. (2014). Isoflavones profiling of soybean [Glycine max (L.) Merrill] germplasms and their correlations with metabolic pathways. Food Chemistry, 153, 258–264. https://doi.org/10.1016/j.foodchem.2013.12.066
- Kim, S. L., Lee, J. E., Kwon, Y. U., Kim, W. H., Jung, G. H., Kim, D. W., Lee, C. K., Lee, Y. Y., Kim, M. J., Kim, Y. H., Hwang, T. Y., & Chung, I. M. (2013). Introduction and nutritional evaluation of germinated soy germ. Food Chemistry, 136(2), 491–500. https://doi.org/10.1016/j.foodchem.2012.08.022
- Kong, S., & Lee, J. (2010). Antioxidants in milled fractions of black rice cultivars. Food Chemistry, 120(1), 278–281. https://doi.org/10.1016/j.foodchem.2009.09.089
- Liang, Q., Cui, J., Li, H., Liu, J., & Zhao, G. (2013). Florets of sunflower (Helianthus Annuus L.), potential new sources of dietary fiber and phenolic acids. Journal of Agricultural and Food Chemistry, 61(14), 3435–3442. https://doi.org/10.1021/jf400569a
- Liu, J., Yang, C., Zhang, Q., Lou, Y., Wu, H., Deng, J., Yang, F., & Yang, W. (2016). Partial improvements in the flavor quality of soybean seeds using intercropping systems with appropriate shading. Food Chemistry, 207, 107–114. https://doi.org/10.1016/j.foodchem.2016.03.059
- Manco, A., Gerardi, C., Romano, G., D’Amico, L., Blanco, A., Milano, F., DiSansebastiano, G. P., Balech, R., & Laddomada, B. (2023). Phenolic profile of whole seeds and seed fractions of lentils and its impact on antioxidant activity. Food Bioscience, 102887. https://doi.org/10.1016/j.fbio.2023.102887
- Maria John, K. M., Natarajan, S., & Luthria, D. L. (2016). Metabolite changes in nine different soybean varieties grown under field and greenhouse conditions. Food Chemistry, 211, 347–355. https://doi.org/10.1016/j.foodchem.2016.05.055
- Multari, S., Neacsu, M., Scobbie, L., Cantlay, L., Duncan, G., Vaughan, N., Stewart, D., & Russell, W. R. (2016). Nutritional and phytochemical content of high-protein crops. Journal of Agricultural and Food Chemistry, 64(41), 7800–7811. https://doi.org/10.1021/acs.jafc.6b00926
- Qin, P., Wang, T., & Luo, Y. (2022). (2022). A review on plant-based proteins from soybean, health benefits and soy product development. Journal of Agriculture and Food Research, 7, 100265. https://doi.org/10.1016/j.jafr.2021.100265
- Rayaprolu, S., Hettiarachchy, N., Horax, R., Satchithanandam, E., Chen, P., & Mauromoustakos, A. (2015). Amino acid profiles of 44 soybean lines and ACE‐I inhibitory activities of peptide fractions from selected lines. Journal of the American Oil Chemists’ Society, 92(7), 1023–1033. https://doi.org/10.1007/s11746-015-2655-y
- Sinkovič, L., Deželak, M., Kopinˇ, R., & Meglič, V. (2022). Macro/Microelements, nutrients and bioactive components in common and Tartary buckwheat (Fagopyrum spp.) grain and stone-milling fractions. LWT - Food Science & Technology, 161, 113422. https://doi.org/10.1016/j.lwt.2022.113422
- Sreerama, Y. N., Neelam, D. A., Sashikala, V. B., & Pratape, V. M. (2010). Distribution of nutrients and antinutrients in milled fractions of chickpea and horse gram, seed coat phenolics and their distinct modes of enzyme inhibition. Journal of Agricultural and Food Chemistry, 58(7), 4322–4330. https://doi.org/10.1021/jf903101k
- Tsantili, E., Konstantinidis, K., Christopoulos, M. V., & Roussos, P. A. (2011). Total phenolics and flavonoids and total antioxidant capacity in pistachio (Pistachia vera L.) nuts in relation to cultivars and storage conditions. Scientia Horticulturae, 129(4), 694–701. https://doi.org/10.1016/j.scienta.2011.05.020
- Tyug, T. S., Prasad, K. N., & Ismail, A. (2010). Antioxidant capacity, phenolics and isoflavones in soybean by-products. Food Chemistry, 123(3), 583–589. https://doi.org/10.1016/j.foodchem.2010.04.074
- U.S. Department of Agriculture. (2023). World soybean production 2022/2023. https://www.fas.usda.gov/data/commodities/soybeans
- WHO. (2007). Protein and amino acid requirements in human nutrition. World Health Organization Technical Report Series, 935, 1–265. https://apps.who.int/iris/handle/10665/43411
- Xue, Z., Wang, C., Zhai, L., Yu, W., Chang, H., Kou, X., & Zhou, F. (2016). Bioactive compounds and antioxidant activity of mung bean (Vigna radiata L.), soybean (Glycine max L.) and black bean (Phaseolus vulgaris L.) during the germination process. Czech Journal of Food Sciences, 34(1), 68–78. https://doi.org/10.17221/434/2015-CJFS
- Yang, H., Gaob, J., Yang, A., & Chen, H. (2015). The ultrasound-treated soybean seeds improve edibility and nutritional quality of soybean sprouts. Food Research International, 77(4), 704–710. https://doi.org/10.1016/j.foodres.2015.01.011
- Zhang, M., Xu, Y., Xiang, J., Zheng, B., Yuan, Y., Luo, D., & Fan, J. (2021). Comparative evaluation on phenolic profiles, antioxidant properties and α-glucosidase inhibitory effects of different milling fractions of foxtail millet. Journal of Cereal Science, 99, 103217. https://doi.org/10.1016/j.jcs.2021.103217
- Zhao, H., Zhu, M., Wang, K., Yang, E., Su, J., Wang, Q., Cheng, N., Xue, C., Wuc, L., & Cao, W. (2020). Identification and quantitation of bioactive components from honeycomb (Nidus Vespae). Food Chemistry, 314, 126052. https://doi.org/10.1016/j.foodchem.2019.126052
- Žilić, S., Serpen, A., Serpen, A., Perić, V., & Gökmen, V. (2013). Comparisons of phenolic compounds, isoflavones, antioxidant capacity and oxidative enzymes in yellow and black soybeans seed coat and dehulled bean. European Food Research and Technology, 237(3), 409–418. 10.1007/s00217-013-2005-y