ABSTRACT
Molasses is a valuable byproduct in the sugar and citrus fruit processing industries. This versatile substance used in various sectors, including beverage production, acetic acid synthesis, baker’s yeast cultivation, formulation of animal feed components, and even as a fertilizer. Beet molasses is used for creation of diverse bakery and confectionery products. In agriculture, beet sugar molasses is employed to enhance nutrient uptake efficiency and stimulate soil biological activity. Molasses also contributes to human health. Sugarcane molasses serves as a source of iron, containing approximately 0.7% iron by weight. Studies have indicated that molasses can have positive effects on glucose regulation, reducing both the peak and total levels of glucose, enhancing the secretion of insulin, amylin, and gastric inhibitory polypeptide after oral intake. Despite its widespread usage, molasses had its own drawbacks like it causes diseases (molasses toxicity, urea toxicity, and bloat) and increased greenhouse gas emissions.
1. Introduction
Molasses is a significant byproduct of the beet sugar (Beta vulgaris var. saccharifera), sugar cane (Saccharum L.), and citrus fruit processing industries, adding value to their operations. It is generated as a byproduct of sugar manufacturing, emerging as the residual substance once the primary crystallization process is complete. When the solution no longer allows for further sugar crystallization, the resulting liquid, known as molasses, is extracted. This molasses contains approximately 50% sucrose. On average, it is possible to obtain 3.5 to 4.5 kg of molasses for every 100 kg of processed plant material (Ahmed, Citation2017). Molasses, distinguished as either cane molasses or beet molasses depending on its origin, stands out as one of the most economical sources of carbohydrates (Göksungur & Zorlu, Citation2001). Sucrose, glucose, fructose, raffinose, melibiose, and galactose collectively constitute 45–55% of the fermentable components (Atiyeh & Duvnjak, Citation2003; Bekatorou et al., Citation2006). Molasses can be extracted from at least two types of plants found in both tropical and temperate climates, making it accessible in a variety of regions. Elevated temperatures, especially exceeding 60°C, trigger thermal degradation in molasses through the Maillard reaction, leading to the loss of sugars. According to Lea and Piggott (Citation2012), Molasses remains stable up to 45°C, making it an ideal temperature for storage. Due to its ease of storage and cost-effectiveness, molasses serves as an excellent untreated material for crafting spirits (Medeiros et al., Citation2017).
In the year 2014, molasses production achieved a total of 64 million tonnes (OECD & FAO, Citation2016). In descending order, Brazil, India, Thailand, China, Pakistan, and the United States stand out as the top producers of molasses. Similarly, in the realm of exports, the leading countries are Thailand, India, Pakistan, Indonesia, and Australia.
The global consumption of molasses extends across various industries, notably in the feed and biofuel sectors. There is considerable variation in molasses usage among countries. For instance, in 2014, Brazil allocated 84% of its molasses for biofuel, while India dedicated 58% for the same purpose. The global trend has shown an increase in the application of molasses for bioethanol, rising from 15% in 1996 to 45% in 2014. Conversely, the use of molasses in nutrition has remained relatively consistent in total volume, hovering between 13 million tonnes and 16 million tonnes (OECD & FAO, Citation2016).
Molasses serves as a cost-effective feedstock primarily designated for ethanol production, making it readily available in any sugar-producing region and facilitating international trade. Its versatile applications encompass the production of beverages, glycerol, acetic acid, baker’s yeast, and lysine, as well as serving as a crucial component in animal feed and compost. The composition of molasses exhibits significant variability, influenced by factors such as the non-sucrose content of raw juice and processing technology. For sugarcane molasses, a general approximation includes 75–85% total solids, 30–36% sucrose, 10–17% (fructose + glucose), 10–16% ash, along with smaller amounts of polysaccharides, oligosaccharides, organic acids, proteins, and nitrogen compounds (Rein, Citation2007). It serves as an excellent raw material for fermentation processes, boasting an approximate 50% content of fermentable sugars. Sugar beet molasses shares a composition akin to sucrose but features a lower concentration of reducing sugars and a higher sucrose concentration. Traditionally, molasses has been employed as an alternative sugar source and a common ingredient in various food products (Rein, Citation2007). Globally, molasses finds its primary application as livestock feed, as it fosters the digestion of fiber and non-protein nitrogen by augmenting microbial growth in the animal’s rumen (Rein, Citation2007). Furthermore, molasses has been extensively advertised for its therapeutic properties, due to it has high mineral content (Bor-Sen et al., Citation2011). Molasses has found diverse applications in both food and non-food processes, owing to its elevated content of nitrogenous compounds, carbohydrates, and sweet flavor. In the realm of road maintenance, molasses serves the purpose of (i) mitigating dust on footpaths near sugar factories (Ndegwa, Citation2011) and (ii) the production of molasses-based materials for road de-icing (Sarka et al., Citation2012). The significance of examining this paper lies in elucidating the applications, health implications, and adverse effects of molasses on end-users and the environment, both when utilized as a raw material and as a resource for various products.
2. Application of molasses
2.1. Osmotic dehydration of fruits and vegetables
In the food industry segment, the main objectives revolve around preserving food products to extend their shelf life and guaranteeing the safety and quality of the food. According to (Yadav & Singh, Citation2014), Sugar beet molasses plays a role in the microbiological aspects of both raw and processed food by controlling moisture content and water activity values. Osmotic dehydration proves to be an effective technique for prolonging the shelf life of fruits and vegetables through the reduction of moisture content (Lazarides, Citation2019). In the process of osmotic dehydration for fruits and vegetables using sugar beet molasses as a hypertonic solution, concentrated solutions of sucrose, NaCl, or their combinations are the prevalent choices for achieving the desired osmotic effect (Mišljenović et al., Citation2011). Due to its elevated solid content (80%, w/w), sugar beet molasses serves as an exceptional medium for osmotic dehydration, facilitating the diffusion of liquid from the cells into the osmotic solution (Mišljenović et al., Citation2011).
The primary technological benefit of molasses stems from its high solid content and minimal liquid state. For instance, the utilization of high-concentration sucrose solutions has been associated with certain challenges, such as sluggish sucrose dissolution and persistent recrystallization throughout the process (Šarić et al., Citation2016). The osmotic dehydration process is mainly impacted by temperature, duration of immersion, and the concentration of the hypertonic solution. According to Filipović et al. (Citation2012), The osmotic dehydration of carrot and apple juice was most significantly influenced by the solid concentration in the solution of sugar beet molasses and the immersion time, with the least impact observed from the solution temperature. The kinetics of osmotic dehydration for carrot and apple in both beet sugar molasses and pure sucrose solution were compared. According to Koprivica (Citation2013), the impact of immersion time on the kinetics of molasses is more pronounced than the concentration of the hypertonic solution, while, in the case of sucrose, the concentration of the hypertonic solution takes precedence over immersion time. The efficiency of the osmotic dehydration kinetics for sugar beet molasses increases with an extended immersion time, attributed to the viscous nature of the molasses solution. The assessment of osmotic dehydration efficiency is gauged by the ratio of water loss to solids gain. When employed as a hypertonic solution in apple osmotic dehydration, sugar beet molasses yields higher ratios compared to the use of a 70% sucrose solution. As per the findings of Koprivica et al. (Citation2010) and Gordana et al. (Citation2014), the optimal conditions for osmotic dehydration of plum and apple in sugar beet molasses involve utilizing pure molasses heated to 45°C with an immersion time of 3 hours. The most favorable outcomes in terms of final dry matter content were achieved by employing undiluted sugar beet molasses in the osmotic treatment of red cabbage, with a 5-hour immersion time (Nevena et al., Citation2009; Miljenovi et al., Citation2011). The most significant elevation in dry matter content during the osmotic dehydration of carrots occurred when using a water-based solution, a temperature of 45°C, and an immersion time of 5 hours (Miljenovi et al., 2011). Following osmotic dehydration with molasses, the dry matter content of treated apples reached 63.4% (w/w), which was five times higher than that of fresh apples (Nevena et al., Citation2010).
2.2. Bakery products
Comprehensive research indicates that the incorporation of beet molasses in the production of diverse bakery items, confections, and meat analogs does not compromise their palatability or acceptance. Beet molasses can serve as an additive in wheat-based bread and cookies at levels ranging from 5% to 10% on a flour basis in bread (Filipčev et al., Citation2010); 25% flour basis in semi-sweet cookies (Simurina et al., Citation2006); and half the quantity of honey in ginger nut biscuit formation (Filipčev et al., Citation2012). The use of filtered molasses concentrate derived from sugar cane has proven effective in reducing the glycemic index of sugar-rich foods, including refined bread, energy-rich bars, and cereal bricks. Additionally, it has been observed to lower the insulin response in consumers (Wright et al., Citation2014). Molasses can be explored as a valuable additive with the potential to enhance the nutritional quality of food, given its concentrated form containing various naturally occurring beneficial compounds. Beyond its nutritional advantages, studies have shown that different varieties of beet molasses can exert varying effects on the texture and color of gluten-free cookies. Furthermore, they contribute to diversifying the range of gluten-free cookies available in the market (Filipčev et al., Citation2016). The incorporation of viscous molasses led to the production of gluten-free cookies with a spongy texture, increased spread, and more pronounced color properties. On the other hand, the addition of dry molasses resulted in improved dough characteristics but yielded cookies with a tougher, more brittle texture.
2.3. Compounds fertilizer
In agriculture, the primary role of sugar beet molasses is to enhance nutrient uptake efficiency and promote soil organic activity (Samavat, Citation2014). Molasses is utilized as a fertilizer, particularly on clay and sandy soils characterized by poor structure (Pyakurel et al., Citation2019). Molasses alters the C: N ratio, exerting a positive impact on the soil microbial ecosystem, and contributes to reducing plant parasitic nematodes, among other advantages for plant growth (Schenck, Citation2001). In soils prone to hard settings, molasses aids in restoring soil aggregation and reducing surface crusting (Wynne & Meyer, Citation2002). Likewise, molasses is employed to cleanse soil and enhance nitrogen fixation (Singh et al., Citation2021). Synthetic fertilizers decrease soil productivity over time and pose a substantial threat to agroecology (Zhang et al., Citation2018), hence, molasses could serve as a potential alternative. Condensed molasses soluble (CMS) exhibited a superior nutrient composition compared to conventional sugar mill molasses (Wynne & Meyer, Citation2002). CMS is utilized as a composite fertilizer for economic crops (Kang et al., Citation2004). Studies have demonstrated that CMS enhances the availability of nitrogen (N), phosphorus (P), potassium (K), and other organic matter in the soil in comparison to chemical fertilizers, leading to an overall improvement in crop yield. In the case of sugarcane, the modification with CMS resulted in increased surfacing rate, tillering rate, chlorophyll content, and yield compared to artificial fertilization. Additionally, it contributed to enhancing the physical and chemical properties of the soil (Jiang et al., Citation2012). Applying CMS directly resulted in enhanced growth of sugarcane seedlings and an increased net photosynthetic rate without any adverse effects. Moreover, irrigation with CMS elevated nitrogen (N) uptake, along with increased activities of polyphenol oxidase (PPO), peroxidase (POD), and catalase (CAT), in addition to higher levels of chlorophyll, total nitrogen, and water-soluble protein content (Wang et al., Citation2006). Substantial research indicates that the application of CMS benefits crop production enhances the physical structure of the soil, and promotes an increase in the biological function of beneficial microorganisms (Suganya & Rajannan, Citation2009).
2.4. Culture media component
According to Strop (Citation2014), molasses derived from sugar cane, primarily consisting of non-crystallized sugars, is generated as a byproduct of cane sugar production. The disposal of this waste into the environment poses a significant environmental concern. Therefore, there is a need for innovative applications for this byproduct. One effective solution is utilizing sugar cane molasses as a growth/culture medium for the production of microbial transglutaminase (MTGase). MTGase is an enzyme that catalyzes the conversion of glutamine residues to lysine residues. It serves as a food additive, altering the functional properties of proteins, including gelation, emulsification, bubble formation, gumminess, and water-holding capacity in foods with high protein content (Gaspar & de Góes-Favoni, Citation2015).
In the production of baked goods, yeast undergoes an aerobic fermentation process, heavily dependent on the efficient transfer of oxygen and nutrients to the microorganisms. The commercial use of yeast commenced in the late 19th century following Pasteur’s identification and isolation of yeast for industrial purposes. Yeast cultivation takes place in carefully controlled industrial environments, using media composed of both beet and cane molasses. Under optimal growth conditions, a yeast cell reproduces approximately every two to three hours (Bekatorou et al., Citation2006). The fermentation process of raw materials significantly contributes to the production costs of low-value items, such as baker’s yeast. Baker’s yeast, available in pressed or dry forms (active dry yeast), serves as a leavening agent in bakeries and is derived from the organism Saccharomyces cerevisiae. Before yeast fermentation, molasses undergoes a clarification process involving heat treatment and the removal of sediments. Following this, the clarified solution is introduced into fermenters. To fulfill the nutritional requirements of the organisms, molasses is enriched with salts like ammonium phosphate, ammonium sulfate, ammonia, or magnesium sulphate (Sherif, Citation2018). Based on the original composition of molasses, the fermentation mixture typically undergoes pH adjustment to a range between 4.5 to 5.0. This adjustment is further enhanced by the addition of supplementary nutrients, including minerals and trace amounts of vitamins, commonly incorporating biotin (Bekatorou et al., Citation2006).
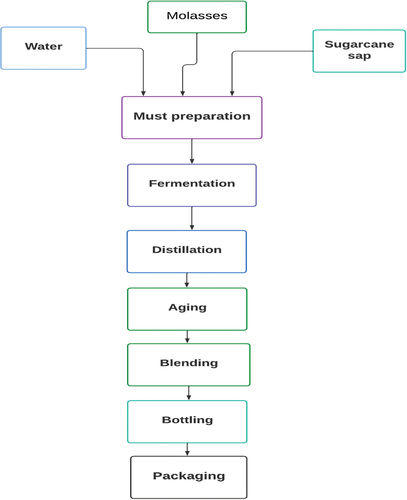
2.5. Rum production
Rum is an alcoholic beverage produced through the fermentation of molasses (Mangwanda et al., Citation2021). The precise origin of the term “rum” remains uncertain, though it might stem from the Latin word “Saccharum,” signifying sugar, or “rumbullion,” denoting a “kill-devil spirit” due to the beverage’s perceived harshness (Medeiros et al., Citation2017). The production of rum follows the fundamental steps common to other distilled beverages. While the overall process of rum production aligns with that of other distilled beverages (), the primary distinction lies in the preparation of the “must” (Medeiros et al., Citation2017). The physicochemical and nutritional analysis of molasses holds significant importance for fermentation in the rum manufacturing industry (). It provides crucial information on parameters such as Brix, pH, and total dissolved solids (TDS), allowing for the adjustment and optimization of fermentation conditions (Tinashe et al., Citation2023). Following the dilution and fermentation of molasses with the addition of water, the distillation process yields a distilled spirit known as rum (Mulye, Citation2019). The production of top-quality rum hinges on a precisely controlled fermentation process, shaped by the physicochemical and nutritional characteristics of the molasses serving as a substrate. The flavor, aroma, and alcohol concentration of the end product are profoundly affected by the composition of the molasses (Tinashe et al., Citation2023).
Table 1. The physicochemical properties of sugar cane molasses used for rum production.
2.6. Lactic acid production
Lactic acid, a powerful chemical, finds applications as a preservative, acidulant, and flavor enhancer in the food industry, while also being utilized in the textile and pharmaceutical industries (Åkerberg & Zacchi, Citation2000). Numerous studies have been previously documented regarding the utilization of molasses in the production of lactic acid (LA). For instance, lactic acid was generated from sugar beet molasses at concentrations ranging from 17 to 90 g/l (Kotzamanidis et al., Citation2002). Moreover, additional pre-treatments of molasses with sulfuric acid may be necessary to enhance fermentation efficiency. This is attributed to molasses containing heavy metals that could potentially impede the growth of microorganisms responsible for lactic acid production, leading to the dormancy of enzymes involved in the formation of the final product (Roukas, Citation1998).
Oliveira et al. (Citation2016) identified molasses as a carbon source that resulted in the highest lactic acid production, reaching 88%. The combination of molasses with yeast products was found to provide the microorganism with all the necessary nutrients for rapid growth and lactic acid production (). Despite molasses yielding less than carbohydrates (100%), its productivity was significantly higher, exerting a positive influence on lactic acid production. de Oliveira et al. (Citation2018) observed a reduction in both productivity and yield in the lactic acid production process when combining molasses with liqueurs derived from hydrolyzed bagasse. The lactic acid productivity of C5 and C6 liqueurs, when combined with molasses, was 1.20 g L−1 h−1 and 1.00 g L−1 h−1, respectively. The average lactic acid yield in these combinations was 77%, which marked an 11% decrease compared to using pure molasses. This decline in yield and efficiency is strongly associated with inhibitory factors present in the fluid from hydrolyzed bagasse. The findings suggest that components in the hydrolysate, particularly furfural and hydroxyl methyl furfural, exert a negative impact (Gutiérrez-Rivera et al., Citation2015). Numerous compounds, such as phenolic compounds, are recognized for their challenging removal from the hydrolysate. The principal phenolic components in the hydrolysate include 4-hydroxybenzoic acid, vanillin, and catechol (Palmqvist & Hahn-Hägerdal, Citation2000). These compounds also pose a substantial obstacle to the complete and efficient utilization of hydrolyzed liquors in biotechnological processes and the production of second-generation products.
Table 2. Utilizing molasses as a raw material for the production of lactic acid (LA) with specific microorganisms.
2.7. Ethanol production
The global concerns about oil shortages and environmental issues have brought significant attention to the production and utilization of biofuels. Despite numerous studies on bio-ethanol production, the majority have predominantly concentrated on ethanol derived from cereals, such as maize and wheat (Kim & Dale, Citation2002; Shapouri et al., Citation2004). Ethanol has emerged as a prominent alternative candidate due to its environmental friendliness and compatibility with gasoline in existing combustion engine systems (Hansen et al., Citation2005). Utilizing agricultural wastes, such as molasses, can help diminish dependence on fossil fuels (Ghorbani et al., Citation2011). Due to its retained sugar content ranging from 50–60%, this substance holds promise as a raw material for bioethanol production (Basso et al., Citation2011). Ethanol producers find molasses appealing due to its cost-effectiveness, elevated sucrose content, and the fact that it serves as a substrate not requiring pre-treatment before fermentation (Bouallagui et al., Citation2013). Various microorganisms, including bacteria, yeast, and fungi, possess the ability to ferment sugar-rich substances for the production of bioethanol. Saccharomyces cerevisiae stands out as the most suitable microorganism for ethanol production through alcoholic fermentation from a variety of sugar-rich raw materials (Gasmalla et al., Citation2012). The fermentation process must take into account the mineral content of molasses, including Mg, Ca, and K, along with the sugar concentration (Morais et al., Citation2007). In the fermentation process, yeast utilizes the enzyme invertase to transform sucrose into reducing sugar. In the production of ethanol from sugarcane molasses, the elevated calcium content in molasses hinders yeast activity by inhibiting the activity of invertase enzyme (Chotineeranat et al., Citation2010).
2.8. Diets for dairy cows
According to Hackmann and Firkins (Citation2015), molasses can be regarded as a suitable feed for lactating cows owing to its optimal conditions for growth and its efficacy for rumen microorganisms. In the first stomach, protozoa convert molasses sugar into glycogen, serving as a reserve energy source within the cells of lactating cows. Hall (Citation2011) found that an elevated sugar content leads to increased total microbial glycogen accumulation, with protozoal glycogen accounting for 51% of the total rumen microbial glycogen accumulation. Soybean molasses is recognized as a beneficial feed ingredient for dairy cows due to its high carbohydrate content. Carbohydrates are crucial as they serve as the primary source of energy in dairy cow diets, comprising approximately 60–70% of the total dry matter intake (). Incorporating soy molasses into dairy cow diets enhances the availability of carbohydrates, which, upon degradation, yield energy for both ruminal microorganism growth and the host animal (Rakita et al., Citation2021). Consequently, this amplifies microbial protein synthesis utilizing non-protein nitrogen sources. Soybean molasses is highly regarded as a valuable dietary component for dairy cows due to its rich carbohydrate content, which serves as a primary energy source, comprising 60–70% of the total dry matter in their diets. The inclusion of soy molasses in dairy cow diets amplifies the availability of carbohydrates, which, upon degradation, furnish energy for both ruminal microorganism proliferation and the host animal (Rakita et al., Citation2021). Consequently, this fosters heightened microbial protein synthesis using non-protein nitrogen sources. Additionally, sugars derived from soybean molasses have been shown to enhance rumen nitrogen utilization efficiency (Patidar et al., Citation2022), diminish ammonia nitrogen concentrations in rumen fluid, and augment milk protein yield (P. Chen et al., Citation2022).
Table 3. Chemical composition of soy molasses for a nutritional regimen for cows.
Employing soybean molasses at appropriate levels (<15% of dry matter) in dairy cow feed can also influence increased butyrate production in the rumen, consequently promoting enhanced blood flow through the rumen epithelium. This may facilitate the rapid transfer of volatile fatty acids from epithelial cells to the bloodstream and elevate ruminal pH levels (Martel et al., Citation2011). Studies have indicated that soybean molasses can be effectively integrated into total mixed rations for lactating dairy cows as a source of readily fermentable carbohydrates in the rumen, with no adverse effects on rumen pH (Martel et al., Citation2011).
The concentration of a readily degradable portion of carbohydrates stimulates the synthesis of microbial protein in the first stomach when utilizing soybean molasses in the diet of dairy cows. According to Vallimont et al. (Citation2004), elevated rates of readily degradable portions of carbohydrates in the first stomach of cows positively influenced microbial protein synthesis and milk yield. As per Broderick and Radloff (Citation2004), the favorable impact on milk yield in lactating cow diets was observed with an increase of up to 3% dry mass of molasses. Augmenting the molasses content in the lactating cow diet (ranging from 125 to 370 g/day) results in an elevation in milk production, increasing from 15.5 to 17.6 kg/day (Gasmalla et al., Citation2012). The cows provided with diets featuring a higher proportion of soybean molasses yielded more milk compared to cows fed standard rations (Gao & Oba, Citation2016).
2.9. Biohydrogen production
According to the report by Wu and Lin (Citation2004) hydrogen serves as a primary energy source. Utilizing anaerobic microorganisms for the conversion of organic waste into hydrogen gas offers an energy-generating process. The microbial fermentation of molasses results in the production of soluble condensed molasses (SCM). As per Chang et al. (Citation2008), soluble condensed molasses (SCM) comprises a varied array of microorganisms. The application of thermal pre-treatment to certain feeding substrates enhances hydrogen production by suppressing specific microorganisms. According to Noike et al. (Citation2002), the inhibitory impact of lactic acid bacteria (LAB) on hydrogen production is attributed to the bacteriocins generated by LAB. The application of heat treatment at temperatures ranging from 50°C to 90°C for 30 minutes alleviated the inhibition of hydrogen production. As per the findings of Lin et al. (Citation2007) enhancements in hydrogen production were observed through heat treatment at temperatures of 70°C for 10–30 minutes for a continuously stirred tank reactor (CSTR) and up-flow anaerobic fermentor systems.
2.10. Substrate for production of sophorolipids
Sophorolipids (SLs) are a category of glycolipids generated extracellularly by specific Candida species (Daverey & Pakshirajan, Citation2009). Candida bombicola is extensively researched due to its significant production of a substantial quantity of Sophorolipids (Guilmanov et al., Citation2002). Numerous investigations have been carried out on Sophorolipids (SLs) owing to their diverse potential applications, including serving as wetting agents, emulsifiers, antimicrobials, and a source of specialty chemicals such as sophorose and hydroxylated fatty acids (Rau et al., Citation2001). Due to its elevated nitrogen-containing molecule content, soy molasses has the potential to be utilized as a nitrogen source in microbial fermentation, contributing to the production of proteins and peptides. As per the findings of Solaiman et al. (Citation2007), the synthesis of Sophorolipids (SLs) by C. bombicola achieved a volumetric yield of 53 g/l culture when soy molasses was solely employed as a combined nitrogen and carbon source, along with oleic acid serving as a lipid co-substrate ().
Table 4. Sophorolipids production yields from molasses.
2.11. Production of flavonoid glycosides and limonoids
According to Kuroyanagi et al. (Citation2008), Tangerine orange molasses(Citrus unshiu Markovich) is a by-product derived from the production of tangerine orange juice. It serves as a rich source of organic compounds, including flavonoids and limonoids. Citrus molasses is obtained from the pressed liquor generated during the pressing of orange peel. The significant utilization of tangerine oranges for juice extraction leads to the generation of byproducts, such as citrus molasses. Numerous natural compounds have been identified in citrus plants, including aglycones of flavones (Nogata et al., Citation2006, flavone glycosides (Horie et al., Citation1986), and limonoids (Tan & Luo, Citation2011). Flavonoids with high methoxylation levels identified in citrus peel have been recognized for their role as cell differentiators (L.-X. Chen et al., Citation2011). As per the findings of Kuroyanagi et al. (Citation2008), flavonoids such as hesperidin, narirutin, eriodictyol, 3“,4”,5“,6”,7“,8”-hexamethoxy-3-O-b-D-glucopyranoxyloxy flavone, and limonin were extracted from tangerine orange molasses.
3. Health usage of molasses
3.1. Iron deficiency anemia (IDA)
Sugarcane serves as a notable iron source, with clarified sugarcane juice containing approximately .7% iron by weight (Jain & Venkatasubramanian, Citation2017). The iron concentration in blackstrap cane molasses ranged from 11.3 to 20 mg/100 g (Jain & Venkatasubramanian, Citation2017). The iron content in molasses varies depending on the type, with A, B, and C molasses exhibiting iron contents of 3.2, 6.0, and 11.3 mg/100 g, respectively (Noureldin et al., Citation2020).
The iron content results of molasses with ascorbic acid were comparable to those of ferrous sulfate and ascorbic acid (Soltan, Citation2013). According to the research conducted by Soltan (Citation2013) on Sprague Dawley rats, the combination of ascorbic acid with molasses led to elevated iron levels. Jain and Venkatasubramanian (Citation2017) reported similar findings in in vitro studies with C-molasses, attributing it to the capability of ascorbic acid to convert ferric to ferrous ions and prevent the formation of insoluble iron compounds. Given that grape molasses and other sugarcane products are rich in iron, research on their impact on iron deficiency anemia (IDA) may offer valuable insights. The sole human clinical trial involving infants that utilized grape molasses observed statistically significant improvements in iron levels for both normal and IDA infants (Soltan, Citation2013).
3.2. Antioxidant and polyphenol activity
Preclinical investigations on a sugarcane extract rich in polyphenols have indicated its potential to modulate carbohydrate metabolism and provide protection against metabolic disorders (Ji et al., Citation2019). Sugarcane molasses demonstrated inhibition of nitric oxide production in lipopolysaccharide-stimulated macrophages and exhibited anti-mutagenic properties in a bacterial model, suggesting anti-inflammatory activity (Bor-Sen et al., Citation2011). In vitro studies showed that sugarcane molasses effectively protected DNA from oxidative stress in human HepG2 cells, demonstrating an effect comparable to the positive control tocopherol. Tocopherol is a potent antioxidant known for its peroxyl radical scavenging properties (Valli et al., Citation2012). The antioxidant activity of the tested molasses fractions was directly linked to the reduction in deoxyribose degradation and DNA scission observed in sugarcane molasses (Guimaraes et al., Citation2007). Numerous studies have been undertaken to investigate the antioxidant activity of sugarcane molasses (Asikin et al., Citation2013; Bor-Sen et al., Citation2011; Guan et al., Citation2014; Yu et al., Citation2017).
Polyphenols exhibit antioxidant properties that extend beyond merely scavenging free radicals. These compounds play a role in regulating carbohydrate metabolism, inhibiting enzymes, and preventing various diseases, including cardiovascular and neurodegenerative conditions (Ji et al., Citation2019; Scalbert et al., Citation2005). Fractionation enhanced the antioxidant efficacy of sugar cane molasses extract (ME), with a further boost observed in the antioxidant potential of the molasses extract-resin bound fraction (ME-RBF). Sugarcane molasses, as an alternative sweetener to refined sugar, boasts the highest ferric-reducing antioxidant power (FRAP) value among sweeteners (Phillips et al., Citation2009). Sugar cane molasses exhibited superior total antioxidant capacity (TAC), as assessed through the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, in comparison to sugar beet molasses. Additionally, it demonstrated increased effectiveness in preventing induced oxidative stress in HepG2 cells compared to its sugar beet counterpart (Valli et al., Citation2012). Food fortified with beet molasses showcased an elevated mineral and antioxidant composition (Filipčev et al., Citation2016). It is suitable for substitution in wheat bread at levels ranging from 5–10% (on a flour basis), semi-sweet biscuits at up to 25%, and gingerbread-type biscuit formulations at up to 50% when used as a replacement for honey (Filipčev et al., Citation2012).
3.3. Lowering the glycemic index and insulin response
In a metabolic study involving rats, molasses was found to reduce both the peak and overall response of glucose while enhancing insulin, amylin, and gastric inhibitory polypeptide levels following oral ingestion (St-Pierre et al., Citation2014). In a clinical investigation, it was noted that the substance led to decreased plasma glucose and insulin reactions to carbohydrate intake (Wright et al., Citation2014). To diminish the glycemic response, a functional ingredient known as filtered molasses concentrate (FMC), extracted from sugar cane, was employed in various carbohydrate-rich foods (Jamir et al., Citation2021). In comparison to untreated controls and accredited glycemic index (GI) assessments, the postprandial glucose reactions in the tested products were reduced by 5–20% (Wright et al., Citation2014). The decrease in glucose response exhibited a dose-dependent pattern, correlating linearly with the ratio of added filtered molasses concentrate (FMC) to the available carbohydrates in the test products. Additionally, the inclusion of FMC led to a reduction in insulin response compared to untreated controls. It’s noteworthy that FMC was incorporated into test foods as an additive to the existing formulations without replacing any of the original ingredients (Wright et al., Citation2014). Molasses are enriched with essential minerals such as magnesium, calcium, and potassium, potentially contributing to improved carbohydrate metabolism. Additionally, magnesium deficiency has been associated with insulin resistance (Barbagallo & Dominguez, Citation2007), calcium supplementation has been linked to increased insulin sensitivity (Pikilidou et al., Citation2009), and low potassium levels have been linked to an increased risk of developing diabetes, especially in young men (Chatterjee et al., Citation2012). Supplementing filtered molasses concentrate (FMS) with organic acids results in a reduction of postprandial glucose and insulin responses. Despite a substantial decrease in the quantity of organic acid in filtered molasses concentrate (FMC), carbohydrate metabolism remains unaltered (Liljeberg et al., Citation1995). According to Wright et al. (Citation2014), FMC appears to inhibit intestinal glucose transport and absorption because it does not appear to inhibit digestive enzymes or increase insulin responses. If this is the case, it may explain why the magnitude of the reduction in both glucose and insulin responses was found to be comparable. As indicated by various studies, aqueous extracts containing polyphenols and flavonoids have been shown to decrease sugar absorption (Macarulla et al., Citation1989) or impede intestinal glucose transport by competing with receptor sites (Kobayashi et al., Citation2000; Kwon et al., Citation2007).
4. Negative impact of using molasses
4.1. Cause of disease
Despite the asserted health advantages attributed to molasses, numerous studies have indicated its potential to adversely affect livestock (Masgoret Cuellar, Citation2007). The inclusion of molasses in livestock diets has been linked to various ailments, including molasses toxicity, urea toxicity, and bloat (Azizi-Shotorkhoft et al., Citation2013). Nevertheless, there is some debate surrounding the potential endothelial dysfunction associated with molasses. As per Masgoret et al. (Citation2009), molasses has been reported to induce endothelial dysfunction in vitro. However, in a subsequent in vitro study, these effects could not be reproduced in Holstein bull calves (Masgoret et al., Citation2009). However, these reports suggest that molasses might pose a potential risk in the onset of diseases in both animals and humans, highlighting the need for toxicity testing (Rahiman & Pool, Citation2016).
4.2. Increasing greenhouse gas emission
As reported by Whittaker et al. (Citation2011), the emissions of direct ethanol from molasses in Pakistan and South Africa are recorded at 77 and 87 g CO2e/MJ, respectively. Given the assumption that converter facilities in these regions are exclusively powered by coal combustion, the conversion phase emerges as the primary contributor to ethanol’s lifecycle emissions. However, the study highlights that molasses conversion emissions are comparatively lower in the United Kingdom, registering a carbon intensity of 39 gCO2e/MJ when utilizing coal and natural gas for power (RFA, Citation2009). In this report, the assumption of zero carbon intensity for feedstock and fuel transport emissions in the UK is acknowledged as unrealistic. The California Air Resources Board (ARB) evaluates direct carbon intensities for biofuel producers participating in the Low Carbon Fuel Standard program. Detailed information on these calculations is accessible for certain historical biofuel pathways (Yeh & Witcover, Citation2016), yet, the agency has reevaluated these pathways, and the updated carbon intensities are not provided in a disaggregated manner (Yeh et al., Citation2009). For instance, in the legacy pathway application for the Copersucar facility Usina Barra Grande (ARB, Citation2015), The estimated direct emissions from the production and transportation of molasses ethanol are 8 gCO2e/MJ. Other studies reviewed here primarily focused on computing emissions related to feedstock production. Among these studies, those estimating feedstock production emissions by allocating a share of upstream emissions from sugar production reported estimates ranging from 15 to 29 gCO2e/MJ (El Takriti et al., Citation2017). In the legacy pathway application for the Copersucar Usina Barra Grande facility, the California Air Resources Board (ARB) calculated the total upstream emissions to be 21 gCO2e/MJ (ARB, Citation2015).
Three studies discovered that the assigned upstream emissions from sugar production did not encompass emissions related to indirect land use change (ILUC) from sugarcane (Gopal & Kammen, Citation2009; Khatiwada et al., Citation2016; Tsiropoulos et al., Citation2014). The California Air Resources Board (ARB) incorporates sugarcane indirect land use change (ILUC) emissions in every assessment of molasses pathways, with the present value standing at 12 gCO2e/MJ. The cumulative emissions for all evaluated molasses ethanol pathways vary from 38 to 54 gCO2e/MJ, encompassing direct emissions from fuel production and transport, allocated upstream emissions from sugar production, and ILUC (El Takriti et al., Citation2017).
5. Conclusion
Molasses, a by-product of the sugar industry, is rich in sugars and minerals, offering various benefits often overlooked by many as mere waste. Its versatility extends to applications in beverages, acetic acid production, baker’s yeast cultivation, fermentation processes, and utilization as a component in animal feed and fertilizer. Moreover, owing to its high solid content, molasses proves advantageous for enhancing the microbiological quality of fruits and vegetables and serves as an effective medium for osmotic dehydration. Extensive research has highlighted its potential in diverse bakery and confectionery product formulations. From a health perspective, sugarcane molasses emerges as a valuable source of iron, crucial for preventing iron deficiency. Consumption of molasses has been associated with reduced peak and overall glucose responses, while also enhancing insulin, amylin, and gastric inhibitory polypeptide levels post oral ingestion. However, despite its widespread use in both edible and non-edible products, molasses may pose certain drawbacks, including disease risks and contributing to increased greenhouse gas emissions.
Author contributors
The data were collected by the MG and developed and guided by AM. Technical comments were given by AM, MT, NS, and BD. Revising the manuscript critically for important intellectual content: AM, NS, MT, BD, and DA. Approval of the version of the manuscript to be published (the names of all authors must be listed): Messenbet Geremew, Aynadis Molla, Mikru Tesfa, Neela Satheesh, Biresaw Demelash, Desye Alemu.
Ethical statement
All authors verify that the study in the manuscript does not involve any human or animal trial experiments. However, in the case of the sensory evaluation was conducted according to all regulations, and informed written consent was obtained from all the assessors.
Acknowledgments
We express our gratitude to the Food Science and Nutrition Laboratory, Addis Ababa University for its support and provision of access to all necessary materials needed for the successful completion of this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmed, S. M. H. (2017). Impact of added graded levels of sugar cane molasses to drink water on performance of broiler chicks. Sudan University of Science & Technology.
- Åkerberg, C., & Zacchi, G. (2000). An economic evaluation of the fermentative production of lactic acid from wheat flour. Bioresource Technology, 75(2), 119–11. https://doi.org/10.1016/S0960-8524(00)00057-2
- Al Loman, A., & Ju, L.-K. (2016). Soybean carbohydrate as a fermentation feedstock for the production of biofuels and value-added chemicals. Process Biochemistry, 51(8), 1046–1057. https://doi.org/10.1016/j.procbio.2016.04.011
- ARB. (2015). Air resources board final (issue June).
- Asikin, Y., Takahashi, M., Mishima, T., Mizu, M., Takara, K., & Wada, K. (2013). Antioxidant activity of sugarcane molasses against 2, 2′-azobis (2-amidinopropane) dihydrochloride-induced peroxyl radicals. Food Chemistry, 141(1), 466–472. https://doi.org/10.1016/j.foodchem.2013.03.045
- Atiyeh, H., & Duvnjak, Z. (2003). Production of fructose and ethanol from cane molasses using Saccharomyces cerevisiae ATCC 36858. Acta Biotechnologica, 23(1), 37–48. https://doi.org/10.1002/abio.200390005
- Azizi-Shotorkhoft, A., Rezaei, J., & Fazaeli, H. (2013). The effect of different levels of molasses on the digestibility, rumen parameters, and blood metabolites in sheep-fed processed broiler litter. Animal Feed Science and Technology, 179(1–4), 69–76. https://doi.org/10.1016/j.anifeedsci.2012.12.001
- Barbagallo, M., & Dominguez, L. J. (2007). Magnesium metabolism in type 2 diabetes mellitus, metabolic syndrome, and insulin resistance. Archives of Biochemistry and Biophysics, 458(1), 40–47. https://doi.org/10.1016/j.abb.2006.05.007
- Basso, L. C., Basso, T. O., & Rocha, S. N. (2011). Ethanol production in Brazil: The industrial process and its impact on yeast fermentation. Biofuel Production-Recent Developments and Prospects, 1530(3), 85–100.
- Bekatorou, A., Psarianos, C., & Koutinas, A. A. (2006). Production of food-grade yeasts. Food Technology and Biotechnology, 44(3), 407–415.
- Bor-Sen, W., Chang, L.-W., Kang, Z.-C., Chu, H.-L., Tai, H.-M., & Huang, M.-H. (2011). Inhibitory effects of molasses on mutation and nitric oxide production. Food Chemistry, 126(3), 1102–1107. https://doi.org/10.1016/j.foodchem.2010.11.139
- Bouallagui, H., Touhami, Y., Hanafi, N., Ghariani, A., & Hamdi, M. (2013). Performances comparison between three technologies for continuous ethanol production from molasses. Biomass and Bioenergy, 48, 25–32. https://doi.org/10.1016/j.biombioe.2012.10.018
- Broderick, G. A., & Radloff, W. J. (2004). Effect of molasses supplementation on the production of lactating dairy cows fed diets based on alfalfa and corn silage. Journal of Dairy Science, 87(9), 2997–3009. https://doi.org/10.3168/jds.S0022-0302(04)73431-1
- Celligoi, M. A. P. C., Silveira, V. A. I., Hipólito, A., Caretta, T. O., & Baldo, C. (2020). Sophorolipids: A review on production and perspectives of application in agriculture. Spanish Journal of Agricultural Research, 18(3), e0301–e0301. https://doi.org/10.5424/sjar/2020183-15225
- Chajuss, D. (2004). Soy molasses: Processing and utilization as a functional food. Soybeans as Functional Foods and Ingredients, AOCS Press, Champaign. Ill., USA, 201–208.
- Chang, J.-J., Wu, J.-H., Wen, F.-S., Hung, K.-Y., Chen, Y.-T., Hsiao, C.-L., Lin, C.-Y., & Huang, C.-C. (2008). Molecular monitoring of microbes in a continuous hydrogen-producing system with different hydraulic retention times. International Journal of Hydrogen Energy, 33(5), 1579–1585. https://doi.org/10.1016/j.ijhydene.2007.09.045
- Chatterjee, R., Colangelo, L. A., Yeh, H. C., Anderson, C. A., Daviglus, M. L., Liu, K., & Brancati, F. L. (2012). Potassium intake and risk of incident type 2 diabetes mellitus: The coronary artery risk development in young adults (CARDIA) study. Diabetologia, 55(5), 1295–1303. https://doi.org/10.1007/s00125-012-2487-3
- Chen, L.-X., He, H., & Qiu, F. (2011). Natural withanolides: An overview. Natural Product Reports, 28(4), 705–740. https://doi.org/10.1039/c0np00045k
- Chen, P., Li, Y., Shen, Y., Cao, Y., Li, Q., Wang, M., Liu, M., Wang, Z., Huo, Z., Ren, S., Gao, Y., & Li, J. (2022). Effect of dietary rumen-degradable starch to rumen-degradable protein ratio on in vitro rumen fermentation characteristics and microbial protein synthesis. Animals, 12(19), 2633. https://doi.org/10.3390/ani12192633
- Chotineeranat, S., Wansuksri, R., Piyachomkwan, K., Chatakanonda, P., Weerathaworn, P., & Sriroth, K. (2010). Effect of calcium ions on ethanol production from molasses by Saccharomyces cerevisiae. Sugar Technology, 12(2), 120–124. https://doi.org/10.1007/s12355-010-0024-6
- Daverey, A., & Pakshirajan, K. (2009). Production, characterization, and properties of sophorolipids from the yeast Candida bombicola using a low-cost fermentative medium. Applied Biochemistry and Biotechnology, 158(3), 663–674. https://doi.org/10.1007/s12010-008-8449-z
- de Oliveira, R. A., Komesu, A., Rossell, C. E. V., & Maciel Filho, R. (2018). Challenges and opportunities in lactic acid bioprocess design—from economic to production aspects. Biochemical Engineering Journal, 133, 219–239. https://doi.org/10.1016/j.bej.2018.03.003
- Dumbrepatil, A., Adsul, M., Chaudhari, S., Khire, J., & Gokhale, D. (2008). Utilization of molasses sugar for lactic acid production by Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 in batch fermentation. Applied and Environmental Microbiology, 74(1), 333–335. https://doi.org/10.1128/AEM.01595-07
- El Takriti, S., Searle, S., & Pavlenko, N. (2017). Indirect greenhouse gas emissions of molasses ethanol in the European Union. The International Council on Clean Transportation. https://theicct.org/sites/default/files/publications/EUmolassesethanolemissionsICCTzworkingpaper27092017%20vF.pdf
- Filipčev, B., Bodroža-Solarov, M., Šimurina, O., & Cvetković, B. (2012). Use of sugar beet molasses in processing of gingerbread type biscuits: Effect on quality characteristics, nutritional profile, and bioavailability of calcium and iron. Acta Alimentaria, 41(4), 494–505. https://doi.org/10.1556/AAlim.41.2012.4.11
- Filipčev, B., Lević, L., Bodroža-Solarov, M., Mišljenović, N., & Koprivica, G. (2010). Quality characteristics and antioxidant properties of breads supplemented with sugar beet molasses-based ingredients. International Journal of Food Properties, 13(5), 1035–1053. https://doi.org/10.1080/10942910902950526
- Filipčev, B., Mišan, A., Šarić, B., & Šimurina, O. (2016). Sugar beet molasses as an ingredient to enhance the nutritional and functional properties of gluten-free cookies. International Journal of Food Sciences and Nutrition, 67(3), 249–256. https://doi.org/10.3109/09637486.2016.1157140
- Filipović, V. S., Ćurčić, B. L., Nićetin, M. R., Plavšić, D. V., Koprivica, G. B., & Mišljenović, N. M. (2012). Mass transfer and microbiological profile of pork meat dehydrated in two different osmotic solutions. Hemijska Industrija, 66(5), 743–748. https://doi.org/10.2298/HEMIND120130033F
- Gao, X., & Oba, M. (2016). Effect of increasing dietary non-fiber carbohydrate with starch, sucrose, or lactose on rumen fermentation and productivity of lactating dairy cows. Journal of Dairy Science, 99(1), 291–300. https://doi.org/10.3168/jds.2015-9871
- Gasmalla, M. A. A., Yang, R., Nikoo, M., & Man, S. (2012). Production of ethanol from Sudanese sugar cane molasses and evaluation of its quality. Journal of Food Process Technology, 3(7), 163–165.
- Gaspar, A. L. C., & de Góes-Favoni, S. P. (2015). Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chemistry, 171(2015), 315–322. https://doi.org/10.1016/j.foodchem.2014.09.019
- Ghorbani, F., Younesi, H., Sari, A. E., & Najafpour, G. (2011). Cane molasses fermentation for continuous ethanol production in an immobilized cells reactor by Saccharomyces cerevisiae. Renewable Energy, 36(2), 503–509. https://doi.org/10.1016/j.renene.2010.07.016
- Göksungur, Y., & Güvenç, U. (1999). Production of lactic acid from beet molasses by calcium alginate immobilized Lactobacillus delbrueckii IFO 3202. Journal of Chemical Technology & Biotechnology, 74(2), 131–136. https://doi.org/10.1002/(SICI)1097-4660(199902)74:2<131:AID-JCTB996>3.0.CO;2-Q
- Göksungur, Y., & Zorlu, N. (2001). Production of ethanol from beet molasses by Ca-alginate immobilized yeast cells in a packed-bed bioreactor. Turkish Journal of Biology, 25(3), 265–275.
- Gopal, A. R., & Kammen, D. M. (2009). Molasses for ethanol: The economic and environmental impacts of a new pathway for the lifecycle greenhouse gas analysis of sugarcane ethanol. Environmental Research Letters, 4(4), 44005. https://doi.org/10.1088/1748-9326/4/4/044005
- Gordana, B. K., Pezo, L. L., Ćurčić, B. L., Lević, L. B., & Šuput, D. Z. (2014). Optimization of osmotic dehydration of apples in sugar beet molasses. Journal of Food Processing and Preservation, 38(4), 1705–1715. https://doi.org/10.1111/jfpp.12133
- Guan, Y., Tang, Q., Fu, X., Yu, S., Wu, S., & Chen, M. (2014). Preparation of antioxidants from sugarcane molasses. Food Chemistry, 152, 552–557. https://doi.org/10.1016/j.foodchem.2013.12.016
- Guilmanov, V., Ballistreri, A., Impallomeni, G., & Gross, R. A. (2002). Oxygen transfer rate and sophorose lipid production by Candida bombicola. Biotechnology and Bioengineering, 77(5), 489–494. https://doi.org/10.1002/bit.10177
- Guimaraes, C. M., GIao, M. S., Martinez, S. S., Pintado, A. I., Pintado, M. E., Bento, L. S., & Malcata, F. X. (2007). Antioxidant activity of sugar molasses, including protective effect against DNA oxidative damage. Journal of Food Science, 72(1), C039–C043. https://doi.org/10.1111/j.1750-3841.2006.00231.x
- Gutiérrez-Rivera, B., Ortiz-Muñiz, B., Gómez-Rodríguez, J., Cárdenas-Cágal, A., González, J. M. D., & Aguilar-Uscanga, M. G. (2015). Bioethanol production from hydrolyzed sugarcane bagasse supplemented with molasses “B” in a mixed yeast culture. Renewable Energy, 74, 399–405. https://doi.org/10.1016/j.renene.2014.08.030
- Hackmann, T. J., & Firkins, J. L. (2015). Maximizing efficiency of rumen microbial protein production. Frontiers in Microbiology, 6, 465. https://doi.org/10.3389/fmicb.2015.00465
- Hall, M. B. (2011). Isotrichid protozoa influence the conversion of glucose to glycogen and other microbial products. Journal of Dairy Science, 94(9), 4589–4602. https://doi.org/10.3168/jds.2010-3878
- Hansen, A. C., Zhang, Q., & Lyne, P. W. L. (2005). Ethanol–diesel fuel blends––a review. Bioresource Technology, 96(3), 277–285. https://doi.org/10.1016/j.biortech.2004.04.007
- Horie, T., Tsukayama, M., Yamada, T., Miura, I., & Nakayama, M. (1986). Three flavone glycosides from citrus sudachi. Phytochemistry, 25(11), 2621–2624. https://doi.org/10.1016/S0031-9422(00)84522-7
- Jain, R., & Venkatasubramanian, P. (2017). Sugarcane molasses–a potential dietary supplement in the management of iron deficiency anemia. Journal of Dietary Supplements, 14(5), 589–598. https://doi.org/10.1080/19390211.2016.1269145
- Jamir, L., Kumar, V., Kaur, J., Kumar, S., & Singh, H. (2021). Composition, valorization and therapeutical potential of molasses: A critical review. Environmental Technology Reviews, 10(1), 131–142. https://doi.org/10.1080/21622515.2021.1892203
- Jiang, Z.-P., Li, Y.-R., Wei, G.-P., Liao, Q., Su, T.-M., Meng, Y.-C., Zhang, H.-Y., & Lu, C.-Y. (2012). Effect of long-term vinasse application on physico-chemical properties of sugarcane field soils. Sugar Technology, 14(4), 412–417. https://doi.org/10.1007/s12355-012-0174-9
- Ji, J., Yang, X., Flavel, M., Shields, Z. P.-I., & Kitchen, B. (2019). Antioxidant and anti-diabetic functions of a polyphenol-rich sugarcane extract. Journal of the American College of Nutrition, 38(8), 670–680. https://doi.org/10.1080/07315724.2019.1587323
- Kang, G.-H., Kang, B.-H., Park, K.-D., Chung, K.-Y., Sohn, B.-K., Ha, H.-S., Heo, J.-S., & Cho, J.-S. (2004). Effects of condensed molasses soluble on chemical and biological properties of soil, and nitrogen mineralization. Korean Journal of Soil Science and Fertilizer, 37(2), 124–130.
- Khatiwada, D., Venkata, B. K., Silveira, S., & Johnson, F. X. (2016). Energy and GHG balances of ethanol production from cane molasses in Indonesia. Applied Energy, 164, 756–768. https://doi.org/10.1016/j.apenergy.2015.11.032
- Kim, S., & Dale, B. E. (2002). Allocation procedure in ethanol production system from corn grain I. system expansion. The International Journal of Life Cycle Assessment, 7(4), 237–243. https://doi.org/10.1007/BF02978879
- Kobayashi, Y., Suzuki, M., Satsu, H., Arai, S., Hara, Y., Suzuki, K., Miyamoto, Y., & Shimizu, M. (2000). Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. Journal of Agricultural and Food Chemistry, 48(11), 5618–5623. https://doi.org/10.1021/jf0006832
- Koprivica, G. (2013). Nutritive profile and sensorial quality of osmotically dehydrated fruits and vegetables in sugar beet molasses and sucrose solutions.
- Koprivica, G., Mišljenović, N., Lević, L., & Jevrić, L. (2010). Mass transfer kinetics during osmotic dehydration of plum in sugar beet molasses. Journal on Processing and Energy in Agriculture, 14(1), 27–31.
- Kotzamanidis, C. H., Roukas, T., & Skaracis, G. (2002). Optimization of lactic acid production from beet molasses by Lactobacillus delbrueckii NCIMB 8130. World Journal of Microbiology and Biotechnology, 18(5), 441–448. https://doi.org/10.1023/A:1015523126741
- Kuroyanagi, M., Ishii, H., Kawahara, N., Sugimoto, H., Yamada, H., Okihara, K., & Shirota, O. (2008). Flavonoid glycosides and limonoids from Citrus molasses. Journal of Natural Medicines, 62(1), 107–111. https://doi.org/10.1007/s11418-007-0198-8
- Kwon, O., Eck, P., Chen, S., Corpe, C. P., Lee, J., Kruhlak, M., & Levine, M. (2007). Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. The FASEB Journal, 21(2), 366–377. https://doi.org/10.1096/fj.06-6620com
- Lazarides, H. N. (2019). Reasons and possibilities to control solids uptake during osmotic treatment of fruits and vegetables. In CRC press (Ed.), Osmotic dehydration & vacuum impregnation (pp. 33–42). CRC Press.
- Lea, A. G., & Piggott, J. R. (2012). Fermented beverage production. Springer Science & Business Media.
- Liljeberg, H. G. M., Lönner, C. H., & Björck, I. M. E. (1995). Sourdough fermentation or addition of organic acids or corresponding salts to bread improves nutritional properties of starch in healthy humans. The Journal of Nutrition, 125(6), 1503–1511. https://doi.org/10.1093/jn/125.6.1503
- Lin, C.-Y., Lin, C.-Y., Wu, J.-H., & Chen, C.-C. (2007). Effect of thermal pretreatment of influent on the fermentative hydrogen production from molasses. Journal of Environmental Engineering and Management, 17(2), 117.
- Long, C. C., & Gibbons, W. R. (2013). Conversion of soy molasses, soy solubles, and dried soybean carbohydrates into ethanol. International Journal of Agricultural and Biological Engineering, 6(1), 62–68.
- Macarulla, M. T., Martinez, J. A., Barcina, Y., & Larralde, J. (1989). Intestinal absorption of D-galactose in the presence of extracts from Phaseolus vulgaris hulls. Plant Foods for Human Nutrition, 39(4), 359–367. https://doi.org/10.1007/BF01092073
- Mangwanda, T., Johnson, J. B., Mani, J. S., Jackson, S., Chandra, S., McKeown, T., White, S., & Naiker, M. (2021). Processes, challenges, and optimization of rum production from molasses—a contemporary review. Fermentation, 7(1), 21. https://doi.org/10.3390/fermentation7010021
- Martel, C. A., Titgemeyer, E. C., Mamedova, L. K., & Bradford, B. J. (2011). Dietary molasses increases ruminal pH and enhances ruminal biohydrogenation during milk fat depression. Journal of Dairy Science, 94(8), 3995–4004. https://doi.org/10.3168/jds.2011-4178
- Masgoret, M. S., Botha, C. J., Myburgh, J. G., Naudé, T. W., Prozesky, L., Naidoo, V., Van Wyk, J. H., Pool, E. J., & Swan, G. E. (2009). Molasses as a possible cause of an“endocrine disruptive syndrome” in calves. Onderstepoort Journal of Veterinary Research, 76(2), 209–225. https://doi.org/10.4102/ojvr.v76i2.46
- Masgoret Cuellar, M. S. (2007). Molasses as a possible cause of“endocrine disruptive syndrome” in cattle. University of Pretoria.
- Medeiros, A. B. P., de Matos, M. E., de Pinho Monteiro, A., de Carvalho, J. C., & Soccol, C. R. (2017). Cachaça and rum. In Current developments in biotechnology and bioengineering (pp. 451–468). Elsevier.
- Mišljenović, N. M., Koprivica, G. B., Jevrić, L. R., & Lević, L. J. B. (2011). Mass transfer kinetics during osmotic dehydration of carrot cubes in sugar beet molasses. Romanian Biotechnological Letters, 16(6), 6790–6799.
- Morais, P. B., Rosa, C. A., Linardi, V. R., Carazza, F., & Nonato, E. A. (2007). Production of fuel alcohol by saccharomyces strains from tropical habitats. Biotechnology Letters, 18(11), 1351–1356. https://doi.org/10.1007/BF00129969
- Mordenti, A. L., Giaretta, E., Campidonico, L., Parazza, P., & Formigoni, A. (2021). A review regarding the use of molasses in animal nutrition. Animals, 11(1), 115. https://doi.org/10.3390/ani11010115
- Mulye, S. (2019). The effect of distillation conditions and molasses concentration on the volatile compounds of unaged rum. Auckland University of Technology.
- Ndegwa, J. K. (2011). The effect of cane molasses on the strength of expansive clay soil. Journal of Emerging Trends in Engineering and Applied Sciences, 2(6), 1034–1041.
- Nevena, M., Koprivica, G., & Lević, L. (2010). Comparison of the kinetics of osmotic drying apples in sugar beet molasses and sucrose solutions. Journal on Processing and Energy in Agriculture, 14(1), 32–35.
- Nevena, M., Koprivica, G. B., Lević, L. B., Filipčev, B. V., & Kuljanin, T. A. (2009). Osmotic dehydration of red cabbage in sugar beet molasses: Mass transfer kinetics. Acta Periodica Technologica, 40(40), 145–154. https://doi.org/10.2298/APT0940145M
- Nogata, Y., Sakamoto, K., Shiratsuchi, H., Ishii, T., Yano, M., & Ohta, H. (2006). Flavonoid composition of fruit tissues of citrus species. Bioscience, Biotechnology, and Biochemistry, 70(1), 178–192. https://doi.org/10.1271/bbb.70.178
- Noike, T., Takabatake, H., Mizuno, O., & Ohba, M. (2002). Inhibition of hydrogen fermentation of organic wastes by lactic acid bacteria. International Journal of Hydrogen Energy, 27(11–12), 1367–1371. https://doi.org/10.1016/S0360-3199(02)00120-9
- Noureldin, H. A., Salman, K. H., Ali, H. M., & Mansour, A. I. A. (2020). Using of sugarcane molasses on novel yoghurt making. Archives of Agriculture Sciences Journal, 3(2), 156–167. https://doi.org/10.21608/aasj.2020.37510.1027
- OECD & FAO. (2016). Agriculture in sub-saharan Africa: Prospects and challenges for the next decade. OECD-FAO Agricultural Outlook, 2025(181), 1–39.
- Oliveira, R. A. D., Maciel Filho, R., & Rossel, C. E. V. (2016). High lactic acid production from molasses and hydrolyzed sugarcane bagasse. Chemical Engineering Transactions, 50, 307–312.
- Palmonari, A., Cavallini, D., Sniffen, C. J., Fernandes, L., Holder, P., Fagioli, L., Fusaro, I., Biagi, G., Formigoni, A., & Mammi, L. (2020). Short communication: Characterization of molasses chemical composition. Journal of Dairy Science, 103(7), 6244–6249. https://doi.org/10.3168/jds.2019-17644
- Palmqvist, E., & Hahn-Hägerdal, B. (2000). Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresource Technology, 74(1), 25–33. https://doi.org/10.1016/S0960-8524(99)00161-3
- Patidar, V., Dixit, S., Ghandour, M. M. A., Keshri, A., Singh, M., & Kundu, S. S. (2022). Carbohydrate and protein fractionations of commonly used forages and agro-industrial byproducts as per Cornell Net carbohydrate and protein system (CNCPS). Journal of Livestock Science, 13(3), 182–187. https://doi.org/10.33259/JLivestSci.2022.182-187
- Phillips, K. M., Carlsen, M. H., & Blomhoff, R. (2009). Total antioxidant content of alternatives to refined sugar. Journal of the American Dietetic Association, 109(1), 64–71. https://doi.org/10.1016/j.jada.2008.10.014
- Pikilidou, M. I., Lasaridis, A. N., Sarafidis, P. A., Befani, C. D., Koliakos, G. G., Tziolas, I. M., Kazakos, K. A., Yovos, J. G., & Nilsson, P. M. (2009). Insulin sensitivity increases after calcium supplementation and changes in intraplatelet calcium and sodium–hydrogen exchange in hypertensive patients with type 2 diabetes 1. Diabetic Medicine, 26(3), 211–219. https://doi.org/10.1111/j.1464-5491.2009.02673.x
- Pyakurel, A., Dahal, B. R., & Rijal, S. (2019). Effect of molasses and organic fertilizer in soil fertility and yield of spinach in Khotang, Nepal. International Journal of Applied Sciences and Biotechnology, 7(1), 49–53. https://doi.org/10.3126/ijasbt.v7i1.23301
- Rahiman, F., & Pool, E. J. (2016). The effect of sugar cane molasses on the immune and male reproductive systems using in vitro and in vivo methods. Iranian Journal of Basic Medical Sciences, 19(10), 1125.
- Rakita, S., Banjac, V., Djuragic, O., Cheli, F., & Pinotti, L. (2021). Soybean molasses in animal nutrition. Animals, 11(2), 514. https://doi.org/10.3390/ani11020514
- Rau, U., Hammen, S., Heckmann, R., Wray, V., & Lang, S. (2001). Sophorolipids: A source for novel compounds. Industrial Crops and Products, 13(2), 85–92. https://doi.org/10.1016/S0926-6690(00)00055-8
- Rein, P. W. (2007). Developments in sugarcane processing over the last 25 years. Sugar Industry/zuckerindustrie, 132(6), 435–444.
- RFA. (2009). Carbon and sustainability reporting within the renewable transport fuel obligation - technical guidance part one (issue January).
- Rodríguez, A., Gea, T., & Font, X. (2021). Sophorolipids production from oil cake by solid-state fermentation. Inventory for economic and environmental assessment. Frontiers in Chemical Engineering, 3, 632752. https://doi.org/10.3389/fceng.2021.632752
- Roukas, T. (1998). Pretreatment of beet molasses to increase pullulan production. Process Biochemistry, 33(8), 805–810. https://doi.org/10.1016/S0032-9592(98)00048-X
- Samavat, S. (2014). The effects of fulvic acid and sugar cane molasses on yield and qualities of tomato. International Research Journal of Applied and Basic Sciences, 8(3), 266–268.
- Šarić, L. Ć., Filipčev, B. V., Šimurina, O. D., Plavšić, D. V., Šarić, B. M., Lazarević, J. M., & Milovanović, I. L. (2016). Sugar beet molasses: Properties and applications in osmotic dehydration of fruits and vegetables. Food and Feed Research, 43(2), 135–144. https://doi.org/10.5937/FFR1602135S
- Sarka, E., Bubnik, Z., Hinkova, A., Gebler, J., & Kadlec, P. (2012). Molasses as a by-product of sugar crystallization and a perspective raw material. Procedia Engineering, 42, 1219–1228. https://doi.org/10.1016/j.proeng.2012.07.514
- Scalbert, A., Manach, C., Morand, C., Rémésy, C., & Jiménez, L. (2005). Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition, 45(4), 287–306. https://doi.org/10.1080/1040869059096
- Schenck, S. (2001). Molasses soil amendment for crop improvement and nematode management. Hawaii Agricultural Research Center, 3, 1–7.
- Shapouri, H., Duffield, J., Mcaloon, A. J., & Wang, M. (2004, June 24–25). The 2001 net energy balance of corn-ethanol. Proceedings of the Conference on Agriculture as a Producer and Consumer of Energy, Arlington, VA. www.usda.gov/oce/reports/energy/net_energy_balance.pdf
- Sherif, N. J. (2018). Characterization and optimization of lactic acid produced from sugar cane molasses by using lactobacillus plantarium bacteria isolated from “Kocho”. Addis Ababa University Addis Ababa.
- Simurina, O., Filipcev, B., Levic, L., Pribis, V., & Pajin, B. (2006). Sugar beet molasses as an ingredient in tea-cookie formulations. PTEP (Serbia and Montenegro), 10(3–4), 93–96.
- Singh, R., Yadav, M., Kumar, V., Sharma, I., Singh, M., & Upadhyay, S. K. (2021). Effect of molasses on the growth of okra, abelmoschus esculentus (L.) Moench (Dicotyledonae: Malvaceae). BioScience Research Bulletin-Biological Sciences, 37(1), 1–14. https://doi.org/10.5958/2320-3161.2021.00002.X
- Solaiman, D. K. Y., Ashby, R. D., Zerkowski, J. A., & Foglia, T. A. (2007). Simplified soy molasses-based medium for reduced-cost production of sophorolipids by Candida bombicola. Biotechnology Letters, 29(9), 1341–1347. https://doi.org/10.1007/s10529-007-9407-5
- Soltan, S. S. A. (2013). The protective effect of soybean, sesame, lentils, pumpkin seeds, and molasses on iron deficiency anemia in rats. World Applied Sciences Journal, 23(6), 795–807.
- St-Pierre, P., Pilon, G., Dumais, V., Dion, C., Dubois, M.-J., Dubé, P., Desjardins, Y., & Marette, A. (2014). Comparative analysis of maple syrup to other natural sweeteners and evaluation of their metabolic responses in healthy rats. Journal of Functional Foods, 11, 460–471. https://doi.org/10.1016/j.jff.2014.10.001
- Strop, P. (2014). Versatility of microbial transglutaminase. Bioconjugate Chemistry, 25(5), 855–862. https://doi.org/10.1021/bc500099v
- Suganya, K., & Rajannan, G. (2009). Effect of onetime post-sown and pre-sown application of distillery spent wash on the growth and yield of maize crop. Botany Research International, 2(4), 288–294.
- Tan, Q.-G., & Luo, X.-D. (2011). Meliaceous limonoids: chemistry and biological activities. Chemical Reviews, 111(11), 7437–7522. https://doi.org/10.1021/cr9004023
- Tinashe, M., Mani, J. S., Johnson, J. B., Jackson, S., McKeown, T., & Naiker, M. (2023). Physicochemical and nutritional analysis of molasses for rum fermentation. Biology and Life Sciences Forum, 26(1), 105.
- Tsiropoulos, I., Faaij, A. P. C., Seabra, J. E. A., Lundquist, L., Schenker, U., Briois, J.-F., & Patel, M. K. (2014). Life cycle assessment of sugarcane ethanol production in India in comparison to Brazil. The International Journal of Life Cycle Assessment, 19(5), 1049–1067. https://doi.org/10.1007/s11367-014-0714-5
- Valli, V., Gómez-Caravaca, A. M., DiNunzio, M., Danesi, F., Caboni, M. F., & Bordoni, A. (2012). Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. Journal of Agricultural and Food Chemistry, 60(51), 12508–12515. https://doi.org/10.1021/jf304416d
- Vallimont, J. E., Bargo, F., Cassidy, T. W., Luchini, N. D., Broderick, G. A., & Varga, G. A. (2004). Effects of replacing dietary starch with sucrose on ruminal fermentation and nitrogen metabolism in continuous culture. Journal of Dairy Science, 87(12), 4221–4229. https://doi.org/10.3168/jds.S0022-0302(04)73567-5
- Wang, B. S., Chang, L. W., Kang, Z. C., Chu, H. L., Tai, H. M. & Huang, M. H. (2011). Inhibitory effects of molasses on mutation and nitric oxide production. Food Chemistry, 126(3), 1102–1107.
- Wang, Y., Wei, M., Bi, L., Li, Y., Wang, W., & Ye, Y. (2006). Effect of irrigating vinasse waste liquor on the activity of three kinds of enzymes and agronomic characters at seedling stage in sugarcane. Southwest China Journal of Agricultural Sciences, 19(3), 482–485.
- Wang, L., Zhao, B., Liu, B., Yu, B., Ma, C., Su, F. & Xu, P. (2010). Efficient production of L-lactic acid from corncob molasses, a waste by-product in xylitol production, by a newly isolated xylose utilizing Bacillus sp. strain. Bioresource Technology, 101(20), 7908–7915.
- Wee, Y.-J., Kim, J.-N., Yun, J.-S., & Ryu, H.-W. (2004). Utilization of sugar molasses for economical L (+)-lactic acid production by batch fermentation of Enterococcus faecalis. Enzyme and Microbial Technology, 35(6–7), 568–573. https://doi.org/10.1016/j.enzmictec.2004.08.008
- Whittaker, C., McManus, M. C. & Hammond, G. P. (2011). Greenhouse gas reporting for biofuels: A comparison between the RED, RTFO and PAS2050 methodologies. Energy Policy, 39(10), 5950–5960.
- Wright, A. G., Ellis, T. P., & Ilag, L. L. (2014). Filtered molasses concentrate from sugar cane: Natural functional ingredient effective in lowering the glycaemic index and insulin response of high carbohydrate foods. Plant Foods for Human Nutrition, 69(4), 310–316. https://doi.org/10.1007/s11130-014-0446-5
- Wu, J.-H., & Lin, C.-Y. (2004). Biohydrogen production by mesophilic fermentation of food wastewater. Water Science and Technology, 49(5–6), 223–228. https://doi.org/10.2166/wst.2004.0757
- Wynne, A. T., & Meyer, J. H. (2002). An economic assessment of using molasses and condensed molasses solids as a fertilizer in the South African sugar industry. Proceedings South African Sugar Technologists Association, 76(200), 71–78.
- Yadav, A. K., & Singh, S. V. (2014). Osmotic dehydration of fruits and vegetables: A review. Journal of Food Science and Technology, 51(9), 1654–1673. https://doi.org/10.1007/s13197-012-0659-2
- Yeh, S., Sumner, D. A., Kaffka, S. R., Ogden, J. M., Jenkins, B. M., Lee, H., Parker, N. C., Tittmann, P. W., & Mishra, G. (2009). Developing a sustainability framework for the California low carbon fuel standard. Institute of Transportation Studies, University of California.
- Yeh, S., & Witcover, J. (2016). Status review of California’s low carbon fuel standard, 2011–2015.
- Yu, P., Xu, X.-B., & Yu, S.-J. (2017). Inhibitory effect of sugarcane molasses extracts on the formation of Nε-(carboxymethyl) lysine and Nε-(carboxyethyl) lysine. Food Chemistry, 221, 1145–1150. https://doi.org/10.1016/j.foodchem.2016.11.045
- Zhang, L., Yan, C., Guo, Q., Zhang, J., & Ruiz-Menjivar, J. (2018). The impact of agricultural chemical inputs on environment: Global evidence from informetrics analysis and visualization. International Journal of Low-Carbon Technologies, 13(4), 338–352. https://doi.org/10.1093/ijlct/cty039
- Zhong, Y., & Zhao, Y. (2015). Chemical composition and functional properties of three soy processing by-products (soy hull, okara, and molasses). Quality Assurance and Safety of Crops & Foods, 7(5), 651–660. https://doi.org/10.3920/QAS2014.0481