ABSTRACT
Antibiotics are commonly used to address animal health issues and enhance overall productivity in animal agriculture. However, overuse and misuse of antibiotics has become a concern in the field of agriculture and pose potential food safety risks. Efficiently controlling antibiotic resistance in animal agriculture is of vital importance. Various technologies have been developed and employed to prevent and control antibiotic-resistant bacteria in this field, such as ozone sterilization technology, among others. This review provides a comprehensive overview of current sterilization technologies in animal agriculture, including chemical sanitizers, plasma oxidation, bacteriophage, ozone, ultraviolet, ultrasonic irradiation, and photodynamic inactivation. Furthermore, we present novel insights and future perspectives on the prevention and control of antibiotic resistance in animal agriculture.
1. Introduction
Antibiotics have been critical in treating diseases caused by pathogens (Bennett & Chung, Citation2001). However, overuse or abuse of antibiotics has led to an increase in antibiotics remaining in the environment and food, accelerating the emergence and spread of antibiotic-resistant bacteria (ARB) or antibiotic-resistant genes (ARGs), which pose serious health risks to humans. In 2017, World Health Organization (WHO) released the list of antibiotic-resistant bacteria that cause the greatest threat to human health for the first time, including Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species, collectively known as ESKAPE pathogens (Tacconelli et al., Citation2018).
ESKAPE pathogens have been discovered as multidrug-resistant (MDR), extensively drug-resistant (XDR), and pan-drug-resistant (PDR) (Patil et al., Citation2021). The WHO has estimated that antibiotic resistance caused more than 700,000 deaths annually. A report commissioned by the British Government showed that antibiotic resistance may cause 10 million deaths by 2050, resulting in economic losses of $100 trillion and the WHO named antimicrobial resistance (AMR) as one of the top ten global health hazards due to its effect on human health in 2019 (Mancuso et al., Citation2021). It is reported that 4.95 million deaths were associated with antibiotic-resistant bacterial infections in 2019, with 1.27 million directly attributable to antibiotic resistance, making it the third leading cause of death in the world (Murray et al., Citation2022).
Antibiotics such as sulfonamides, tetracyclines, quinolones, macrolides, and other antibiotics are critical for the treatment of infectious diseases in animal agriculture, and there are residues of antibiotics present (Wei et al., Citation2022). Now, antibiotics are gradually used as feed additives, preventives, and growth promoters (Shao et al., Citation2021). It has been estimated that antibiotics used in animal agriculture accounted for over 50% of all antibiotic use worldwide, amounting to 131,109 tons in 2013, and predicted to exceed 200,000 tons by 2030 (Van Boeckel et al., Citation2015, Citation2017). In 2017, the total sales of antibiotics for food-producing animals in Europe reached over 6,000 tons (Zalewska et al., Citation2021). In the United States, it is estimated that the antibacterial treatment of food-producing animals accounted for about 80% of the total annual use (Van Boeckel et al., Citation2015).
The misuse or overuse of antibiotics has led to the detection of antibiotic residues in the ecological environment and agricultural products (Shao et al., Citation2021). Research has indicated that about 75% of antibiotics are not absorbed by animals and are excreted into environment through animal waste; antibiotics facilitate the development of antibiotic resistance in gastrointestinal bacteria, which might persist in the manure (Chee‐Sanford et al., Citation2009). Animal agriculture has become a significant source for the transmission of antibiotics, ARB or ARGs in the ecosystem, potentially posing risks to the environment and human health. Therefore, it is essential to develop accessible and efficient technologies to combat ARB and ARGs in animal agriculture.
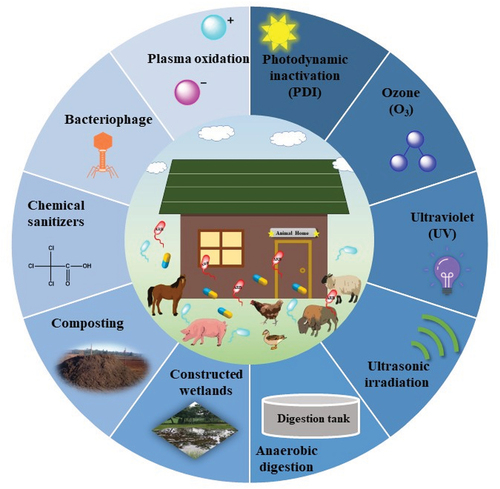
In this review, we first introduce the use of antibiotics and the spread of antibiotic resistance in animal agriculture. We systematically review existing sterilization technologies, including chemical sanitizers, anaerobic digestion, composting, constructed wetlands, plasma oxidation, bacteriophages, ozone, ultraviolet light, ultrasonic irradiation, and photodynamic inactivation, to combat antibiotic resistance in animal agriculture (). Additionally, we propose prospects for novel strategies to control antibiotic resistance. By providing an overview of the current state of antibiotic use and resistance in animal agriculture, this review aims to raise awareness of the urgent need for innovative solutions. It highlights the importance of developing and implementing effective sterilization technologies to reduce the spread of ARB and ARGs in the ecosystem. With a comprehensive understanding of existing technologies and potential future strategies, we can work together to protect the environment and human health from the growing threat of antibiotic resistance.
2. The use of antibiotics and the prevalence of antibiotic-resistance in animal agriculture
Antibiotics are commonly used in animal agriculture to promote weight gain, prevent diseases as feed antibiotics and treat infections as veterinary antibiotics. Consequently, antibiotics may persist in the livestock’s digestive system and increase the survival chances of gastrointestinal bacteria with ARGs. ARB and ARGs can then be excreted and dispersed in the soil, water, and air; ARGs might be further disseminated into other human pathogens through horizontal gene transfer mechanisms (e.g. conjugation, transformation, transduction) and lead to the development of ARB. The digestion of contaminated water and animal source food or the inhalation of contaminated air result in the transfer of ARB into and colonize the human gut, potentially causing bacterial infectious diseases (Fang et al., Citation2015; Zhang et al., Citation2013).
Moreover, antibiotics at sub-inhibitory concentrations, which are concentrations below the level required to completely inhibit bacterial growth, can still have significant effects on bacteria. These effects include changes in gene expression, horizontal gene transfer, and mutation. Antibiotic-induced gene expression can have an impact on virulence, but boosted mutagenesis and horizontal gene transfer can promote resistance to antibiotics and spread (Viswanathan, Citation2014).
Research has identified ARGs, including tetA, tetX and strB, in the treated wastewater of broiler farms (T. Chen et al., Citation2022). Additionally, studies have found that most ARGs collected from farms, feedlots, and dairy farms were associated with tetracycline resistance, with tetQ and tetW being the most abundant among ARGs (Noyes et al., Citation2016). Other antibiotic-resistant genes, such as tetB, mecA, ermF and other antibiotic resistance genes were also discovered to appear in swine-sow, -nursery, and -finisher farm manure lagoon wastewater (Brooks et al., Citation2014). These studies also revealed that the abundance of ARGs in the soil with livestock manure was strongly higher than that in farmland without manure, with the order of abundance of ARGs in all samples was as follows: pig farm > chicken farm > beef farm (the dominant ARGs in farm soil: tetX, sul1, sul2 and tetG).
Given that animal agriculture already serves as the major reservoir of ARB and ARGs and may play a role in the dissemination of antibiotic resistance from agricultural animals to the environment and eventually to humans, it is crucial to monitor and efficiently control ARB and ARGs in animal agriculture.
3. Existing strategies to combat the antibiotic resistance in animal agriculture
3.1. Chemical sanitizers
Chemical sanitizers, including ethanol, chlorine dioxide, hydrogen peroxide, phenolic compounds, peracetic acid, sodium hypochlorite and quaternary ammonium compounds, are commonly employed to control the microbial contamination in animal agriculture (Sengun et al., Citation2021). Miklasińska-Majdanik et al. (Citation2018) reviewed the latest report on the antibacterial effects of polyphenols on antibiotic-resistant S. aureus strains, further demonstrating that chemical fungicides can effectively inhibit antibiotic-resistant bacteria.
Despite their effectiveness, chemical sanitizers also have some drawbacks. Chlorine- and bromine-based sanitizers produce harmful byproducts (e.g. trichloroacetic acid, halo nitriles, bromide precursors, trihalomethane), which probably results in a serious impact on human health (Pandian et al., Citation2022). Some studies indicated that several chemical sanitizers might have limited capacity to remove ARGs. In the study by Q. B. Yuan et al. (Citation2015), it is found that about 40% erythromycin-resistant genes and 80% tetracycline-resistant genes still remained after chlorination (15 mg Cl2 min/L). The above indicates that chemical sanitizers have limitations in treating antibiotic-resistant bacteria and antibiotic-resistance genes.
In light of these concerns, researchers have explored the use of specific chemical sanitizers to target antibiotic-resistant bacteria. Campo et al. (Citation2020) observed that the inactivation rate of AmpR E. coli was faster than that of total E. coli when examine the inactivation mechanisms of peroxyacetic acid (PAA) and performic acid (PFA) on antibiotic-resistant E. coli in secondary effluent. The initial concentrations of total E. coli and AmpR E. coli were 6.0 and 5.6 log units, respectively; when PAA ICT(the integral estimate of the time-dependent residual disinfectant concentration) = 15.8 mg min/L, it was observed that AmpR E. coli removed half log, while when PAA ICT was ~30 mg min/L, almost one log inactivation was obtained (Two initial concentrations in waste water: PAA: 3–4 mg/L; PFA: 1–2 mg/L); in the case of PFA, a reduction of 1 and 2 log was achieved when the ICT was 4.7 and 6.8 mg min/L for AmpR E. coli, respectively, and a reduction of less than 100 CFU/100 mL was observed when the PFA ICT was 10 mg min/L.
Similarly, Wu and Xu (Citation2019) demonstrated that ClO2 could effectively inactivate ARB in the soil around a hennery, with S. aureus exhibiting the strongest resistance. The killing log values of all antibiotic-resistant bacteria increased with the concentrations of ClO2 2, 4, 6, 8 and 10 mg/L.
Stange et al. (Citation2019) investigated the removal of ARB and ARGs through three disinfection processes (chlorination, ozone and UV treatment) on a laboratory scale using E. coli (tet(A), ampC) and E. faecium (ermB, vanA) and determined bacterial inactivation through plate counting method and quantify ARGs damage using real-time PCR. They found that using 0.5 mg/L free chlorine (with 30 min of contact time) resulted in a reduction of 3.8–5.6 log of bacteria and 0.8–2.8 log of ARGs.
These studies suggest that while chemical sanitizers can be effective against ARB and ARGs, their limitations and potential drawbacks must be considered when evaluating their use in animal agriculture. Using it alone may not completely kill bacteria, or it may not be comprehensive enough. If it can be combined with other sterilization strategies in the future, it may have better sterilization effects.
3.2. Anaerobic digestion
Anaerobic digestion is an alternative method for treating animal manure, and solid anaerobic digestion (SAD) has proven to be an efficient approach for handling animal feces. Sun et al. (Citation2019) investigated the resistance of SAD to ARGs (tetracycline-resistant genes -tetA, tetB/P, tetC, teteE, tetG, tetM, tetO, tetQ, tetT, tetW and tetX; sulfonamide-resistant genes-sul1 and sul2; fluoroquinolone-resistant genes-aac (6’)-Ib-cr, qnrA, parC, and qnrC; macrolide-resistant genes-ermB, ermQ and ermX), mobile genetic elements and microbial communities with cattle manure using high temperature SAD 55°C, medium temperature SAD 35°C, and liquid anaerobic digestion 35°C. Their findings demonstrated that SAD significantly reduced at least 7 out of 10 ARGs and all considered mobile genetic elements compared to liquid anaerobic digestion.
Building on these results, Sun et al. (Citation2019) also observed a rapid and long-lasting reduction in tetC abundance by SAD. By the fifth day, the tetC abundance in moderate and high temperature SAD treatments was significantly lower than that in LAD treatment. By the end of digestion (Day 60), the abundance of tetC in moderate temperature SAD and high temperature SAD was 20.9% and 17.7% of that on day 0, and this study showed that SAD was an effective method to reduce tetC (Sun et al., Citation2019); furthermore, the abundance of sul2, ermQ, ermX, qnrA and aac (6’)-Ib-cr in SAD products decreased significantly (0.31–0.76 log) compared with LAD, the abundance of tetW in moderate temperature SAD products decreased significantly (0.43 log), the abundance of tetG in moderate temperature SAD products decreased significantly by 46.5%, and the abundance of tetX was removed by high temperature SAD.
In another study, Sun et al. (Citation2016) investigated the impact of temperature on anaerobic digestion by conducting experiments with cow feces at moderate (20°C), mesophilic (35°C), and thermophilic (55°C); and analysed the changes of tetracycline-resistant gene, sulfonamide-resistant gene, fluoroquinolone-resistant gene and integrase gene, and analysed the dynamics of ARGs and bacterial communities by quantitative PCR and 16S rRNA gene sequencing. They found that in the process of high temperature digestion, 8/10 ARGs (tetracycline-resistant genes-tetC, tetM, tetQ, tetW, tetX; sulfonamide-resistant genes-sul1 and sul2; fluoroquinolone-resistant genes-gyrA; integrase genes-intI1 and intI2) were decreased, and 5/10 ARGs were decreased more than 1.0 log, while only 4 and 5 ARGs were decreased in the process of moderate and mesophilic digestion respectively.
Moreover, Sun et al. (Citation2016) noted that moderate and mesophilic treatments enrich tetC, tetM, tetQ and tetX; in contrast, only tetC was enriched by thermophilic treatment, while tetQ did not change significantly, and tetM, tetW, and tetX decreased by 0.1–1.5 log under thermophilic treatment; while all treatments decreased the absolute abundance of sul1 and sul2, moderate treatment enriched sul1, and thermophilic treatment had the highest reduction rate of these two genes (both >1.0 log); after digestion, the absolute abundance of gyrA decreased by 47.3%, and there was no significant difference between the three treatments. All treatments greatly declined intI1 and intI2 1.2–1.5 log. These findings suggest that thermophilic anaerobic digestion, specifically thermophilic treatment, is more effective at reducing ARGs than moderate and mesophilic temperature digestion (Sun et al., Citation2016).
In summary, anaerobic digestion has been shown to effectively eliminate antibiotic resistance genes and is related to their temperature and state. Finding an appropriate temperature is crucial for anaerobic digestion. These study highlights the potential of anaerobic digestion as a sterilization strategy to mitigate the risks associated with the spread of antibiotic resistance from farm animals to the environment and humans.
3.3. Composting
Composting is recognized as an effective and economical method for the harmless treatment of animal waste, with the resulting compost being nutrient-rich for crop growth; superabsorbent polymers (SAP) are considered to be suitable modifiers for reducing the selection pressure caused by the abundance of heavy metals and ARGs during composting (Guo et al., Citation2017).
This promising development led Guo et al. (Citation2017) to conduct a study applying three levels of SAP (0, 5, and 15 mg/kg compost) to examine their effects on tetracycline resistance genes (tetC, tetG, tetQ, tetW and tetX) of ARGs, sulfonamide resistance genes (sul1, sul2 and dfrA7), macrolide resistance genes (ermF, ermQ and ermX), and quinolone-resistant genes (qnrS, qnrD and aac(6”)-ib-cr) during swine manure composting. After composting, the abundance of ARGs decreased to varying degrees, and the removal efficiency of tetW, dfrA7, ermX, arc (6”)-ib-cr exceeded 90%; the high concentration of SAP significantly reduced ARGs, and showed that the application of 15 mg/kg SAP was suitable for reducing ARGs in compost (Guo et al., Citation2017).
Meanwhile, another study conducted by Peng et al. (Citation2018) investigated the effect of factory-scale chicken manure compost with zeolite (F), superphosphate (G), or zeolite and ferrous sulfate (FL) simultaneously on ARGs (tetG, tetL, tetM, tetO, tetB(P), sul1 and sul2), and found that the ARGs in the feces of the control group decreased by 67.3% after composting, while the ARGs in F, G and FL decreased by 86.5%, 68.6% and 72.2%, respectively. Moreover, the study noted that the maximum removal rates of several ARGs reached impressively high percentages. The maximum removal rates of tetM, tetO and tetB (P) were 99.9%, 99.6% and 98.5%, respectively, while the maximum removal rates of sul1, sul2, tetG and tetL were 82.1%, 92.8%, 69.2% and 87.6%, respectively.
However, the study also identified challenges. Notably, sul1 was the main ARGs gene in the compost, and intI1 was the main integrase gene, while the removal rates of sul1 and intI1 in the control were 52.6% and 28.5%, respectively, which are difficult to be removed by composting; and the removal rate of sul1 and intI1 increased to 82.1% and 63%, respectively, with the addition of F (Peng et al., Citation2018).
In brief, composting is considered an effective sterilization strategy for removing antibiotic-resistant genes. In addition, adding substances such as zeolite (F), superphosphate (G), zeolite and ferrous sulfate (FL) can improve the removal rate of antibiotic-resistant genes. Therefore, some supplementary strategies are needed to remove this stubborn ARGs during composting.
3.4. Constructed wetland
Constructed wetlands (CWs) have been established with the intention of manipulating natural wastewater treatment processes. Compared with traditional wastewater treatment technologies, CWs offer economic and environmental advantages owing to low cost, ease of operation, and low maintenance costs. A variety of wastewater types, as well as any potential antibiotic-resistant bacteria and genes present, have been treated using constructed wetland systems (J. Chen et al., Citation2016). The fate of antibiotics and ARGs in constructed wetlands has recently received more attention.
Using 12 scales of CWs with three hydraulic loading rates (HLR; 10, 20, and 30 cm/day) and four substrates (oyster shell, zeolite, medical stone, and ceramic), J. Chen et al. (Citation2016) studied the removal of chemical oxygen demand, total nitrogen ammonia nitrogen, antibiotics, and ARGs in domestic sewage in raw domestic wastewater, and results showed that the removal rate of total antibiotics (7 target antibiotic: erythromycin-H2O, lincomycin, monensin, ofloxacin, sulfamerazine, sulfamethazine and novobiocin) was between 17.9% and 98.5%, the removal rate of total ARGs (quinolone-resistant genes-qnrB, qnrD and qnrS; chloramphenicol-resistant genes-cmlA, fexA, fexB and floR and integrase genes-int1 and int2) ranged from 50.0% to 85.8%; and after considering their water removal rate and their mass removal rate, CW with zeolite as matrix and HLR of 20 cm/day was selected as the best choice.
In another study, Huang et al. (Citation2017) evaluated the impact of hydraulic flow direction and substrate type (brick rubble or oyster shell) on the removal of antibiotics and ARGs from swine wastewater. All treatments removed more than 84% of oxytetracycline and difloxacin. Due to the properties of brick, including large porosity and micropore size and 32% Fe2O3, the brick column has stronger antibacterial agent removal ability for the removal of ARGs. The removal efficiency of tetracycline-resistant gene (tet) and the integrase gene(intI1) is between 33.2% and 99.1%. The study showed that the antibiotic removal of constructed wetlands is greatly affected by the type of substrate, and the relative abundance of ARGs in sewage and soil depends on the direction of hydraulic flow (Huang et al., Citation2017).
Additionally, Huang et al. (Citation2015) evaluated the removal efficiency of vertical up-flow constructed wetlands (VUF-CWs) on tetracycline compounds (TCs) and tet gene in swine wastewater, the residual TCs and tet gene in soil and plants, and the impact of TCs accumulation on nutrient removal and tet gene development. The removal efficiency of oxytetracycline (OTC), tetracycline (TC) and chlortetracycline (CTC) was higher (69.0–99.9%) with or without OTC in the influent. The concentration of TCs in surface soil decreased after plant harvest. The absolute abundances of all target genes (tetO, tetM, tetW, tetA, tetX and intI1) were significantly reduced, with the units ranging from 0.26 to 3.3 log. VUF-CWs are an effective alternative for remove TC and tet gene (Huang et al., Citation2015).
3.5. Plasma oxidation
The fourth state of matter is known to as plasma, which is composed of positive and negative ions, electrons, excited and neutral atoms, free radicals, molecules in their ground and excited states, and UV photons. Plasma oxidation has received increasing attention for microbial decontamination owing to its broad spectrum of antimicrobial activity, easy operation, and no use of chemical reagents.
In order to understand the sterilizing effect of nanosecond pulsed plasma (NPP) as a liquid discharge plasma and Argon gas-feeding barrier discharge (Ar-DBD) as a surface plasma, Hoon Park et al. (Citation2015) used wild-type S. aureus and multidrug-resistant bacteria (Penicillium-resistant S. aureus (PRSA), Methicillin-resistant S. aureus (MRSA) and Gentamicin-resistant S. aureus (GRSA)) for experiments. They took 107 CFU/mL for all strains; after the fourth NPP discharge, 102 CFU/mL for wild S. aureus and 103 CFU/mL for PRSA, MRSA and GRSA, which showed that after the discharge of NPP plasma, log 4–5 bacteria were inactivated; and after the action of Ar-DBD plasma, log 3 bacteria were inactivated, which showed that 104 CFU/ml bacteria survive (Hoon Park et al., Citation2015).
Liao et al. (Citation2021) used the plasma discharge on the wastewater system to degrade E. coli, which contains a plasmid (pBR322) encoding ampicillin (blaTEM) and tetracycline (tet) resistance, and the results showed that low intensity (0.71 kJ/cm2) plasma treatment would lead to a reduction of CFU/mL in E. coli higher than 3 log. However, at the plasma intensity of 0.18 kJ/cm2, the degradation kinetics of e-blaTEM and e-tet did not decrease at all; when the plasma dose exceeded 0.18 kJ/cm2, the concentrations of e-blaTEM and e-tet began to decline rapidly. In the case of i-blaTEM and i-tet degradation, the results showed that the plasma dose of 1.41 kJ/cm2 (8 min treatment) was sufficient to cause a reduction of more than 1 log copies/mL (Liao et al., Citation2021).
A surface plasma was developed using AR E. coli as a model to inactivate the donor ARB, remove ARGs, and stop the conjugative transfer of ARGs in water, emphasizing the need of accompanying inorganic ions; surface plasma oxidation and the presence of NO3-, Cu2+and Fe2+ significantly inactivated AR E. coli (harbored a plasmid (a transferable plasma of IncI1) containing the tetC, tetW, blaTEM-1 and aac (3) – II) (Li et al., Citation2021a). Within 10 min of treatment, about 4.5 log of AR E. coli was inactivated, and it increased to 7.4 log of AR E. coli after adding Fe2+ (Li et al., Citation2021a). In addition, the study revealed the potential mechanism of inhibiting ARGs transfer: decreased levels of reactive oxygen species, decreased DNA damage induction response, decreased intercellular contact and down-regulated expression of plasmid transfer genes (Li et al., Citation2021a).
Li et al. (Citation2021b) reported that the presence of inorganic ions NO3-, Cu2+ and Fe2+ in the aqueous solution enhanced the elimination of ARB by promoting the generation of •OH in the process of mesoporous plasma oxidation, and ARGs (tetC, tetW, blaTEM-1, aac (3)-I and integration (intI1)) were also removed. The highest ARGs removal efficiency reached 3.34-log within 10 min of the mesoporous plasma treatment in the presence of Fe2+, and more than 95% of the selected genes (tetC, tetW, blaTEM-1, aac (3)-I and integration (intI1)) were eliminated.
In addition, glow discharge plasma has also been proved to be able to inactivate antibiotic-resistant E. coli with resistance genes (tetA, tetR, aphA) and transposase genes (tnpA), remove ARGs and reduce the risk of gene transfer; the level of E. coli measured by 16S rRNA decreased by about 4.7 log after 15 min of discharge treatment; the test of propidium monoazide-quantitative polymerase chain reaction (PMA-qPCR) showed that the cell structure of 4.8 log E. coli was destroyed within 15 min; after 30 min of discharge treatment, the decrease of tetA, tetR, aphA and tnpA genes increased to 5.8, 5.4, 5.3 and 5.5 log, respectively; it was also found that the total abundance of ARGs decreased by 3.9 log in 30 min after removal of ARGs from high-salinity wastewater (Yang et al., Citation2020).
3.6. Bacteriophage
Bacteriophages, natural viruses with specificity for bacterial hosts, exist in large quantities in nature (Rotman et al., Citation2020). After infection, bacteriophages insert their own genetic materials into bacterial host cells to change the metabolism of the host and then promote the bacteriophage genetic replication. Finally, the bacteriophage-encoded endolysin decomposes the bacterial cell wall from inside, which leads to the release of phage from offspring and death of bacterial host (Rotman et al., Citation2020).
In recent years, the application of bacteriophages as an antibiotic growth promoter, preventative measures, and alternatives for decolonizing animal-derived pathogens at the farm level has gained widespread attention in edible animal production (Svircev et al., Citation2018). Miller et al. (Citation2010) found that the mixture of five Clostridium perfringens phases (CPAS-7, CPAS-12, CPAS-15, CPAS-16 and CPLV-42) can effectively control necrotic enteritis in broiler chickens, and improve feed conversion rate and weight gain.
Studies have also confirmed the efficacy of bacteriophages in treating bacterial diseases in animal models using E. coli, S. typhimurium and P. aeruginosa. Atterbury et al. (Citation2003) showed that Campylobacter specific bacteriophages with high-titer are applied to the surface of chicken skin inoculated with Campylobacter, which significantly reduces the number of recoverable cells. In addition, within 30 min of contact with Listeria bacteriophage Listex P100 on the flat stainless-steel surface, Listeria cells rapidly inactivated. Phage treatment was carried out on the L. monocytogenes biofilm that have been growing for a week, and the content of biofilm would also be reduced (Chaitiemwong et al., Citation2014).
To determine the feasibility, dynamics and limitations of bacteriophage therapy, the mouse model of Vibrio vulnificus infection was used and the bacteriophage of the infected strain was injected into the mice, and it was found that it can prevent local and systemic diseases caused by V. vulnificus. Moreover, the bacteriophage CK-2 has an inhibitory effect on V. vulnificus MLT403, and the bacteriophage 153A–5 has inhibitory effect on V. vulnificus MO6/24–0 and VV1009 (Cerveny et al., Citation2002). Petsong et al. (Citation2018) found that combining Salmonella P22 (ATCC 97,541) with ciprofloxacin effectively inhibited the growth of S. Typhimurium, increased its sensitivity to ciprofloxacin and erythromycin, and reduced the minimum inhibitory concentration (MIC) by more than twofold.
Despite their many advantages, bacterial pathogens may develop resistance to bacteriophages after years of usage (Örmälä & Jalasvuori, Citation2013). However, the combination of bacteriophages has achieved good sterilizing efficacy with combined use of A. baumannii phase vB_AbaS_D0 and vB_AbaP_D2 treat multidrug-resistant A. baumannii showed that bacterial cells were killed at time points 12 and 24 h; besides, the survival rate of mice treated with bacteriophage mixture was 100% (Y. Yuan et al., Citation2019).
In conclusion, bacteriophage mixtures appear to be more effective than single bacteriophage in inhibiting the growth of host cells, indicating that bacteriophages hold great potential for widespread application in animal agriculture.
3.7. Ozone
Ozone (O3), a powerful oxidant, has been utilized for disinfection purposes due to its sterilizing effect on both gram-positive and gram-negative bacteria (Epelle et al., Citation2022; Restaino et al., Citation1995). Ozone kills bacteria in the following ways: Ozone kills bacteria through several mechanisms: by gradually oxidizing essential cell components, interacting with the cell membrane to cause cell rupture and leakage, and increasing cell permeability, ultimately leading to cell dissolution (Daş et al., Citation2006).
Antibiotics in animals are typically excreted through urine and stools, and discharged into the anaerobic lagoon for biological treatment and temporary storage. Over time, they accumulate in the lagoons, leading to the emergence of ARB. Macauley et al. (Citation2006) showed that ozone at dose of 100 mg/L resulted in 3.3-log reduction of antibiotic-resistant bacteria in lagoon A sample which was mainly detected four antibiotics: LIN (1.47 mg/L), oxytetracycline (0.11 mg/L), isochromotetracycline (0.4 mg/L), and SMN (1.24 mg/L). Michael-Kordatou et al. (Citation2017) showed that a low ozone dose of 0.3 mg/L efficiently degraded the erythromycin (ERY) and ethylparaben (EtP) within 2 min, and completely inactivated ERY- and EtP-resistant E. coli after 15 min.
Combining ozone and other technologies has been proposed to improve the removal efficiency of ARB or ARGs. Oh et al. (Citation2014) evaluated the disinfection effect by checking the survival rate of ARB and ARGs, and they found that ozone can reduce ARB (E. coli DH5α, containing a multi-resistance gene (pB10)) and ARGs (pB10) decreased by more than 90% with ozone concentration of 3 mg/L. Stange et al. (Citation2019) used E. coli (tetA, ampC) and E. faecium (ermB, vanA) with ARG studied the removal of ARB and ARGs through chlorination, ozone and ultraviolet treatment in drinking water with laboratory scale. They found that in ozone treatment, when 1 mg/L ozone was used and the exposure time was 5 min, it was observed that the inactivation rate of bacterial cells and ARGs was similar, which led to a decrease of 5.0 log (more than 99.999%) of bacterial cells and ARB, and a decrease of 4.3–4.6 log (more than 99.5%) of ARGs (Stange et al., Citation2019).
Lee et al. (Citation2011) found that combining O3 and UV improved the inactivation effect on the chlortetracycline-resistant bacteria in piggery wastewater, reducing ARB by more than 5 log within 20 min. These findings suggest that the combined use of ozone and other technologies holds great promise for the efficient removal of antibiotic-resistant bacteria and genes in various environments.
3.8. Ultraviolet (UV)
Ultraviolet (UV) is the technology utilizes light within an electromagnetic spectrum, ranging from 100 to 400 nm, to inactivate bacteria (Turtoi & Borda, Citation2014). Solar electromagnetic radiation consists of UV-rays that lie between visible light and X-ray. UV light could be divided into four spectral regions: UV-A (315–400 nm), UV-B (280–315 nm), UV-C (200–280 nm), and vacuum-UV (10–195 nm); UV-A contains long wavelength ultraviolet wave, which usually leads to the change of melanin in human skin, known as tanning; UV-B contains medium-length ultraviolet wave; UV-C is a short-wave ultraviolet wave, which can effectively inactivate microorganisms. UV radiation induces cross-linking between pyrimidine nucleoside bases in DNA, inhibiting its synthesis, transcription, and replication, ultimately leading to bacterial cell death (Block, Citation1977).
Wells et al. (Citation2010), the combination of UV-C (intensity is about 11 mW/cm2) and hydrogen peroxide (1.5%) was applied for the decontamination of eggshells; the combined treatment for 8 min achieved a maximum reduction of 3 log CFU/eggs total bacteria count, while individual UV-C or hydrogen peroxide exposure resulted in a reduction 2 log CFU/egg total bacteria count. Macauley et al. (Citation2006) applied UV-C with a dose of 220 mJ/cm2 and 770 mJ/cm2 to treat two samples of pig wastewater and it is found that the bacteria inactivation efficiency of 3.4–4.2 log can be reached, thus reducing the number of bacteria in all samples to less than 1, 000 CFU/mL. And when UV-C treatment time was extended to 30 min, almost all the bacteria were completely removed from the swine wastewater.
Traditionally, mercury lamps have been the primary UV source. However, their toxic mercury content and high energy consumption limit widespread application. UV light-emitting diodes (UV-LEDs) have emerged as an alternative UV source for disinfection, offering advantages such as high efficiency, low cost, and less pollution. LED is a semiconductor device that uses semiconductor materials to form p-n junctions (holes and electrons), and electrons and holes recombine at the junction to emit radiation, and different semiconductor materials determine the wavelength of radiation. The combination of UV-LED and other technologies has been developed for removing ARB or ARGs. For example, Kim et al. (Citation2020) found that the combination of UV LED (275 nm) and chlorine (free available chlorine (FAC): 15 mg/L) treatment achieved a completed inactivation of ciprofloxacin-resistant E. faecium within 5 min, while the individual chlorination (15 mg/L) or UV LED (275 nm) photolysis required 60 min to result in 6 log reduction.
In the study of Shin et al. (Citation2023), the combination of peracetic acid (50 ppm) and UVC-LED efficiently degraded the ampicillin resistance (ampR) gene on the surface of tomato and cabbage with a rate of 2.69 × 10−2 cm2/mJ and 2.48 × 10−2 cm2/mJ, respectively (Shin et al., Citation2023). Additionally, Liu and Hu (Citation2020) studied the effects of ultraviolet irradiation, chlorination, and four combinations of ultraviolet and chlorination on the degradation of sulfanilamide-resistant gene-sul1. The results showed that the simultaneous UV (288 mJ/cm2)/Cl2 (20 mg Cl2/L) combined process and UV-Cl2 process (followed by 288 mJ/cm2 UV irradiation, 20 mg/L free chlorine and 60 min exposure time) provided the highest inactivation rate, and the observed synergistic effects reached 0.61 log and 0.49 log, respectively; finally, 1.46 and 1.41 log of sul1 gene can be removed respectively (Liu & Hu, Citation2020). Through the elimination of carbapenem-resistant K. pneumoniae in water by UV-C, UV-C/persulfate and UV-C/H2O2, and the evaluation of the reaction of antibiotics, the residual effect of the process and the removal of antibiotic-resistant genes, Serna-Galvis et al. (Citation2020) found that considering the different treatment time (60 s, 300 s and 600 s), under three conditions (namely, UV-C, UV-C/H2O2 and UV-C/persulfate), after 60 s of treatment, carbapenem-resistant K. pneumoniae was completely inactivated, but the bla-KPC3 resistance-gene still exists (Serna-Galvis et al., Citation2020). However, UV-C/H2O2 (1 mM) and UV-C/persulfate (1 mM) processes can eliminate more than 98% of bla-KPC3 resistance-gene at 5 min. At the same time, UV-C light (8W, 254 nm, 2.3 µW/cm2) only removed a similar percentage of resistance-gene after 10 min, and it can also be noted that the percentage of genes removed by UV/persulfate (about 80%) is higher than that obtained by using UV-C/H2O2 process (about 67%) after 60 s of treatment, and the effect of using UV-C together is better than using UV-C alone (Serna-Galvis et al., Citation2020).
3.9. Ultrasonic irradiation
Ultrasound (US) refers to sound waves with frequencies of more than 20 kHz (J. Chen et al., Citation2012). This technology has the ability to destroy cell membrane structures, disrupt cell membrane permeability, and cause leakage of organic substances from cells, ultimately leading to bacterial cell death (Dai et al., Citation2020). However, individual US treatment generally show limited microbial inactivation. To improve removal efficiency, other technologies have been combined with US treatment.
In the study of Rebecca Annisha et al. (Citation2020), the combination of UV at dose of 600 mW s/cm2 and US at dose of 130 kHz for 240 min was used. This approach reduced the sulfonamide-resistant E. coli from 3.8 log units to 0.005 log units and tetracycline resistant E. coli from 4.4 log units to 0.002 log units.
3.10. Photodynamic inactivation (PDI)
Photodynamic inactivation (PDI) is an effective method for controlling microorganisms, consisting of three major components: a photosensitizer (PS), light, and molecular oxygen (3O2) (Sheng et al., Citation2022). PS is excited by the resonance wavelength of light, leading to a series of reactions that generate generates reactive oxygen species (ROS). ROS oxidize the cellular components such as DNA, enzymes, membranes and lipids to inactivate microbial cells (Sheng et al., Citation2022).
In a study by GulíGulíAs et al. (Citation2020), assessed a range of antibiotic-resistant E. coli (It is resistant to Ampicillin, Amoxicillin, Clavulanic acid, Cephalotin, Cefuroxime, Aztreonam, Nalidixic acid, Norfloxacin, Azithromycin, Tetracycline, Gentamycin, Fosfomycin, Nitrofurantoin, Trimethoprim, and Sulfamethoxazole) strains’ susceptibility to Methylene Blue (MB; as PS)-mediated antimicrobial photodynamic therapy, and found that photodynamic therapy induced a decrease of more than 3 log10 (MB: 31 µM) in the colony forming units of all strains in planktonic state, and half of them decreased by more than 6 log10. Furthermore, under biofilm conditions, a 3 log10 sterilizing effect was achieved by increasing the MB dose by two and a half times (GulíGulíAs et al., Citation2020).
Another study by Sarker and Ahn (Citation2022) used two green phytochemicals (pyrogallol (PGL) and terpinolene (TPN) as PS, and white and blue light emitting diode (LED) lamps to treat multidrug-resistant bacteria (MDRB) and total coliform in the secondary treatment wastewater, the two MDRB (E. coli and S. aureus; 6.55 log CFU/mL, 3.5 × 106 cells/mL; resistant to ampicillin, cefotaxime, gentamicin, tetracycline, and vancomycin) and total coliform (TC); in the susceptibility test, it was found that PGL inhibited 50% of E. coli and S. aureus cells at 0.186 and 0.103 mg/mL, respectively; in contrast, TPN required 0.812 and 0.758 mg/mL for the same efficacy, respectively (Sarker & Ahn, Citation2022). They found that after 80 min of exposure with a white LED lamp (17 mW/cm2), MDRB bacteria were completely eradicated at the MIC of PGL (0.156 mg/mL for E. coli and 0.078 mg/mL for S. aureus) (Sarker & Ahn, Citation2022).
Tan et al. (Citation2022) conducted a PDI of blue light LED (455–460 nm) and photosensitizer sodium copper chlorophyll combined with slightly alkaline electrolyzed water (SAlEW) and found that this PDI efficiently eradicated V. parahaemolyticus and Shewanella putrefaciens mixed biofilm, which has higher resistance towards external stress (e.g., low pH, antibiotics, etc.). The additional application of SAlEW contributed to a higher production of singlet oxygen and assisted the penetration of the photosensitizer through the exopolysaccharides (EPS) of the biofilm, ultimately killing the bacterial cells (Tan et al., Citation2022). The joint use of PDI and other sterilization technologies should be further explored and promoted in the future.
4. Summary and prospect
This review primarily focuses on the advancements in strategies for removing antibiotic-resistant bacteria and antibiotic-resistance genes in the field of animal agriculture (). Over the past decades, significant efforts have been made to develop efficient and environmentally friendly techniques to combat antibiotic resistance in animal agriculture. Future research should emphasize the following issues:
Table 1. Summary of existing sterilization technologies in animal agriculture.
First, it is critical to explore novel combat strategies. For instance, environment-friendly plant-derived antimicrobials (PDAs), such as trans-cinnamaldehyde, carvacrol, and eugenol, have been proposed to counter ARB (Upadhyaya et al., Citation2013).
Second, the synergistic effects of various antibacterial techniques should be investigated to enhance the removal efficacy of ARB and ARGs in animal agriculture. For example, nanobubbles (NBs), also known as ultra-fine bubbles or nano gases, combined with NEW have been found to improve microbial inactivation (Shiroodi et al., Citation2021). Combining the various sterilization technologies mentioned in this review with other new technologies such as NBs for animal agriculture sterilization is of great significance for the animal agriculture environment and food safety.
Third, the study of antibiotic-resistant bacteria and antibiotic-resistant genes should be deepened in conjunction with other sterilization technologies, such as electrolyzed water. This technology has potential for use in animal agriculture (Ni et al., Citation2016); however, no relevant research on antibiotic-resistant bacteria and genes has been conducted.
Lastly, most experiments have been conducted in laboratory settings, so the efficiency of these technologies in actual animal agriculture settings should be carefully evaluated and validated.
Author contributions
Tianming Xu: Writing-original draft, Investigation, Visualization Jing Liu: Investigation, Validation, Data curation. Qian Wu: Investigation, Validation Xiaoran Hui: Supervision, Validation Weidan Duan: Supervision, Validation Zhaohuan Zhang: Data curation Xinyu Liao: Conceptualization, Supervision, Writing-review & editing Yong Zhao: Supervision, Conceptualization, Visualization.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Atterbury, R. J., Connerton, P. L., Dodd, C. E., Rees, C. E. D., & Connerton, I. F. (2003). Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Applied and Environmental Microbiology, 69(10), 6302–12. https://doi.org/10.1128/AEM.69.10.6302-6306.2003
- Bennett, J. W., & Chung, K. T. (2001). Alexander fleming and the discovery of penicillin. Advances in Applied Microbiology, 49, 163–184. https://doi.org/10.1016/s0065-2164(01)49013-7
- Block, S. S. (1977). Disinfection, sterilization, and preservation. Soil Science, 124(6), 378. https://doi.org/10.1097/00010694-197712000-00013
- Brooks, J. P., Adeli, A., & McLaughlin, M. R. (2014). Microbial ecology, bacterial pathogens, and antibiotic resistant genes in swine manure wastewater as influenced by three swine management systems. Water Research, 57, 96–103. https://doi.org/10.1016/j.watres.2014.03.017
- Campo, N., De Flora, C., Maffettone, R., Manoli, K., Sarathy, S., Santoro, D., Gonzalez-Olmos, R., & Auset, M. (2020). Inactivation kinetics of antibiotic resistant Escherichia coli in secondary wastewater effluents by peracetic and performic acids. Water Research, 169, 115227. https://doi.org/10.1016/j.watres.2019.115227
- Cerveny, K. E., DePaola, A., Duckworth, D. H., & Gulig, P. A. (2002). Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infection and Immunity, 70(11), 6251–6262. https://doi.org/10.1128/IAI.70.11.6251-6262.2002
- Chaitiemwong, N., Hazeleger, W. C., & Beumer, R. R. (2014). Inactivation of Listeria monocytogenes by disinfectants and bacteriophages in suspension and stainless steel carrier tests. Journal of Food Protection, 77(12), 2012–2020. https://doi.org/10.4315/0362-028X.JFP-14-151
- Chee‐Sanford, J. C., Mackie, R. I., Koike, S., Krapac, I. G., Lin, Y.-F., Yannarell, A. C., Maxwell, S., & Aminov, R. I. (2009). Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. Journal of Environmental Quality, 38(3), 1086–1108. https://doi.org/10.2134/jeq2008.0128
- Chen, J., Ren, Y., Seow, J., Liu, T., Bang, W., & Yuk, H. (2012). Intervention technologies for ensuring microbiological safety of meat: Current and future trends. Comprehensive Reviews in Food Science and Food Safety, 11(2), 119–132. https://doi.org/10.1111/j.1541-4337.2011.00177.x
- Chen, J., Wei, X. D., Liu, Y. S., Ying, G.-G., Liu, S.-S., He, L.-Y., Su, H.-C., Hu, L.-X., Chen, F.-R., & Yang, Y.-Q. (2016). Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Optimization of wetland substrates and hydraulic loading. Science of the Total Environment, 565, 240–248. https://doi.org/10.1016/j.scitotenv.2016.04.176
- Chen, T., Zhang, S., Zhu, R., Zhao, M., Zhang, Y., Wang, Y., Liao, X., Wu, Y., & Mi, J. (2022). Distribution and driving factors of antibiotic resistance genes in treated wastewater from different types of livestock farms. Science of the Total Environment, 849, 157837. https://doi.org/10.1016/j.scitotenv.2022.157837
- Dai, J., Bai, M., Li, C., Cui, H., & Lin, L. (2020). Advances in the mechanism of different antibacterial strategies based on ultrasound technique for controlling bacterial contamination in food industry. Trends in Food Science & Technology, 105, 211–222. https://doi.org/10.1016/j.tifs.2020.09.016
- Daş, E., Gürakan, G. C., & Bayındırlı, A. (2006). Effect of controlled atmosphere storage, modified atmosphere packaging and gaseous ozone treatment on the survival of Salmonella enteritidis on cherry tomatoes. Food Microbiology, 23(5), 430–438. https://doi.org/10.1016/j.fm.2005.08.002
- Epelle, E. I., Macfarlane, A., Cusack, M., Burns, A., Amaeze, N., Mackay, W., & Yaseen, M. (2022). The impact of gaseous ozone penetration on the disinfection efficiency of textile materials. Ozone: Science & Engineering, 45(3), 1–15. https://doi.org/10.1080/01919512.2022.2066503
- Fang, H., Wang, H., Cai, L., & Yu, Y. (2015). Prevalence of antibiotic resistance genes and bacterial pathogens in long-term manured greenhouse soils as revealed by metagenomic survey. Environmental Science & Technology, 49(2), 1095–1104. https://doi.org/10.1021/es504157v
- GulíGulíAs, Ò., McKenzie, G., Bayó, M., Agut, M., & Nonell, S. (2020). Effective photodynamic inactivation of 26 Escherichia coli strains with different antibiotic susceptibility profiles: A planktonic and biofilm study. Antibiotics, 9(3), 98. https://doi.org/10.3390/antibiotics9030098
- Guo, A., Gu, J., Wang, X., Zhang, R., Yin, Y., Sun, W., Tuo, X., & Zhang, L. (2017). Effects of superabsorbent polymers on the abundances of antibiotic resistance genes, mobile genetic elements, and the bacterial community during swine manure composting. Bioresource Technology, 244, 658–663. https://doi.org/10.1016/j.biortech.2017.08.016
- Hoon Park, J., Kumar, N., Hoon Park, D., Yusupov, M., Neyts, E. C., Verlackt, C. C. W., Bogaerts, A., Ho Kang, M., Sup Uhm, H., Ha Choi, E., & Attri, P. (2015). A comparative study for the inactivation of multidrug resistance bacteria using dielectric barrier discharge and nano-second pulsed plasma. Scientific Reports, 5(1), 13849. https://doi.org/10.1038/srep13849
- Huang, X., Liu, C., Li, K., Su, J., Zhu, G., & Liu, L. (2015). Performance of vertical up-flow constructed wetlands on swine wastewater containing tetracyclines and tet genes. Water Research, 70, 109–117. https://doi.org/10.1016/j.watres.2014.11.048
- Huang, X., Zheng, J., Liu, C., Liu, L., Liu, Y., & Fan, H. (2017). Removal of antibiotics and resistance genes from swine wastewater using vertical flow constructed wetlands: Effect of hydraulic flow direction and substrate type. Chemical Engineering Journal, 308, 692–699. https://doi.org/10.1016/j.cej.2016.09.110
- Kim, T. K., Kim, T., Park, H., Lee, I., Jo, A., Choi, K., & Zoh, K.-D. (2020). Degradation of ciprofloxacin and inactivation of ciprofloxacin resistant E. faecium during UV-LED (275 nm)/chlorine process. Chemical Engineering Journal, 394, 124803. https://doi.org/10.1016/j.cej.2020.124803
- Lee, H., Lee, E., Lee, C. H., & Lee, K. (2011). Degradation of chlorotetracycline and bacterial disinfection in livestock wastewater by ozone-based advanced oxidation. Journal of Industrial and Engineering Chemistry, 17(3), 468–473. https://doi.org/10.1016/j.jiec.2011.05.006
- Liao, X., Liu, D., Chen, S., Ye, X., & Ding, T. (2021). Degradation of antibiotic resistance contaminants in wastewater by atmospheric cold plasma: Kinetics and mechanisms. Environmental Technology, 42(1), 58–71. https://doi.org/10.1080/09593330.2019.1620866
- Li, H., Song, R., Wang, Y., Zhong, R., Zhang, Y., Zhou, J., Wang, T., Jia, H., & Zhu, L. (2021a). Inhibited conjugative transfer of antibiotic resistance genes in antibiotic resistant bacteria by surface plasma. Water Research, 204, 117630. https://doi.org/10.1016/j.watres.2021.117630
- Li, H., Song, R., Wang, Y., Zhong, R., Zhang, Y., Zhou, J., Wang, T., & Zhu, L. (2021b). Simultaneous removal of antibiotic-resistant bacteria and its resistance genes in water by plasma oxidation: Highlights the effects of inorganic ions. Separation and Purification Technology, 278, 119672. https://doi.org/10.1016/j.seppur.2021.119672
- Liu, X., & Hu, J. Y. (2020). Effect of DNA sizes and reactive oxygen species on degradation of sulphonamide resistance sul1 genes by combined UV/free chlorine processes. Journal of Hazardous Materials, 392, 122283. https://doi.org/10.1016/j.jhazmat.2020.122283
- Macauley, J. J., Qiang, Z., Adams, C. D., Surampalli, R., & Mormile, M. R. (2006). Disinfection of swine wastewater using chlorine, ultraviolet light and ozone. Water Research, 40(10), 2017–2026. https://doi.org/10.1016/j.watres.2006.03.021
- Mancuso, G., Midiri, A., Gerace, E., & Biondo, C. (2021). Bacterial antibiotic resistance: The most critical pathogens. Pathogens, 10(10), 1310. https://doi.org/10.3390/pathogens10101310
- Michael-Kordatou, I., Andreou, R., Iacovou, M., Frontistis, Z., Hapeshi, E., Michael, C., & Fatta-Kassinos, D. (2017). On the capacity of ozonation to remove antimicrobial compounds, resistant bacteria and toxicity from urban wastewater effluents. Journal of Hazardous Materials, 323, 414–425. https://doi.org/10.1016/j.jhazmat.2016.02.023
- Miklasińska-Majdanik, M., Kępa, M., Wojtyczka, R. D., Idzik, D., & Wąsik, T. (2018). Phenolic compounds diminish antibiotic resistance of staphylococcus aureus clinical strains. International Journal of Environmental Research and Public Health, 15(10), 2321. https://doi.org/10.3390/ijerph15102321
- Miller, R. W., Skinner, J., Sulakvelidze, A., Mathis, G. F., & Hofacre, C. L. (2010). Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with Clostridium perfringens. Avian Diseases, 54(1), 33–40. https://doi.org/10.1637/8953-060509-Reg.1
- Murray, C. J. L., Ikuta, K. S., Sharara, F., Swetschinski, L., Robles Aguilar, G., Gray, A., Han, C., Bisignano, C., Rao, P., Wool, E., Johnson, S. C., Browne, A. J., Chipeta, M. G., Fell, F., Hackett, S., Haines-Woodhouse, G., Kashef Hamadani, B. H., Kumaran, E. A. P., …, Dolecek, C. (2022). Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. The Lancet, 399(10325), 629–655. https://doi.org/10.1016/S0140-6736(21)02724-0
- Ni, L., Zheng, W., Zhang, Q., Cao, W., & Li, B. (2016). Application of slightly acidic electrolyzed water for decontamination of stainless steel surfaces in animal transport vehicles. Preventive Veterinary Medicine, 133, 42–51. https://doi.org/10.1016/j.prevetmed.2016.09.010
- Noyes, N. R., Yang, X., Linke, L. M., Magnuson, R. J., Cook, S. R., Zaheer, R., Yang, H., Woerner, D. R., Geornaras, I., McArt, J. A., Gow, S. P., Ruiz, J., Jones, K. L., Boucher, C. A., McAllister, T. A., Belk, K. E., & Morley, P. S. (2016). Characterization of the resistome in manure, soil and wastewater from dairy and beef production systems. Scientific Reports, 6(1), 1–12. https://doi.org/10.1038/srep24645
- Oh, J., Salcedo, D. E., Medriano, C. A., & Kim, S. (2014). Comparison of different disinfection processes in the effective removal of antibiotic-resistant bacteria and genes. Journal of Environmental Sciences, 26(6), 1238–1242. https://doi.org/10.1016/S1001-0742(13)60594-X
- Örmälä, A. M., & Jalasvuori, M. (2013). Phage therapy: Should bacterial resistance to phages be a concern, even in the long run? Bacteriophage, 3(1), e24219. https://doi.org/10.4161/bact.24219
- Pandian, A. M. K., Rajamehala, M., Singh, M. V. P., Sarojini, G., & Rajamohan, N. (2022). Potential risks and approaches to reduce the toxicity of disinfection by-product–A review. Science of the Total Environment, 822, 153323. https://doi.org/10.1016/j.scitotenv.2022.153323
- Patil, A., Banerji, R., Kanojiya, P., & Saroj, S. D. (2021). Foodborne ESKAPE biofilms and antimicrobial resistance: Lessons learned from clinical isolates. Pathogens and Global Health, 115(6), 339–356. https://doi.org/10.1080/20477724.2021.1916158
- Peng, S., Li, H., Song, D., Lin, X., & Wang, Y. (2018). Influence of zeolite and superphosphate as additives on antibiotic resistance genes and bacterial communities during factory-scale chicken manure composting. Bioresource Technology, 263, 393–401. https://doi.org/10.1016/j.biortech.2018.04.107
- Petsong, K., Uddin, M. J., Vongkamjan, K., & Ahn, J. (2018). Combined effect of bacteriophage and antibiotic on the inhibition of the development of antibiotic resistance in Salmonella typhimurium. Food Science and Biotechnology, 27(4), 1239–1244. https://doi.org/10.1007/s10068-018-0351-z
- Rebecca Annisha, O. D., Li, Z., Zhou, X., Madgil Don Stenay, N., & Donde, O. O. (2020). Performance evaluation of combined ultraviolet-ultrasonic technologies in removal of sulfonamide and tetracycline resistant Escherichia coli from domestic effluents. Journal of Water, Sanitation and Hygiene for Development, 10(2), 276–285. https://doi.org/10.2166/washdev.2020.144
- Restaino, L., Frampton, E. W., Hemphill, J. B., & Palnikar, P. (1995). Efficacy of ozonated water against various food-related microorganisms. Applied and Environmental Microbiology, 61(9), 3471–3475. https://doi.org/10.1128/aem.61.9.3471-3475.1995
- Rotman, S. G., Sumrall, E., Ziadlou, R., Grijpma, D. W., Richards, R. G., Eglin, D., & Moriarty, T. F. (2020). Local bacteriophage delivery for treatment and prevention of bacterial infections. Frontiers in Microbiology, 11, 538060. https://doi.org/10.3389/fmicb.2020.538060
- Sarker, M. A. R., & Ahn, Y. H. (2022). Photodynamic inactivation of multidrug-resistant bacteria in wastewater effluent using green phytochemicals as a natural photosensitizer. Environmental Pollution, 311, 120015. https://doi.org/10.1016/j.envpol.2022.120015
- Sengun, I. Y., Senturk, S., Gul, S., & Kilic, G. (2021). Potential of essential oil combinations for surface and air disinfection. Letters in Applied Microbiology, 72(5), 526–534. https://doi.org/10.1111/lam.13445
- Serna-Galvis, E. A., Salazar-Ospina, L., Jiménez, J. N., Pino, N. J., & Torres-Palma, R. A. (2020). Elimination of carbapenem resistant Klebsiella pneumoniae in water by UV-C, UV-C/persulfate and UV-C/H2O2. Evaluation of response to antibiotic, residual effect of the processes and removal of resistance gene. Journal of Environmental Chemical Engineering, 8(1), 102196. https://doi.org/10.1016/j.jece.2018.02.004
- Shao, Y., Wang, Y., Yuan, Y., & Xie, Y. (2021). A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Science of the Total Environment, 798, 149205. https://doi.org/10.1016/j.scitotenv.2021.149205
- Sheng, L., Li, X., & Wang, L. (2022). Photodynamic inactivation in food systems: A review of its application, mechanisms, and future perspective. Trends in Food Science & Technology, 124, 167–181. https://doi.org/10.1016/j.tifs.2022.04.001
- Shin, M., Kang, J. W., & Kang, D. H. (2023). A study on antibiotic resistance gene degradation in fresh produce using peracetic acid combined with an ultraviolet-C light-emitting-diode. Food Control, 145, 109478. https://doi.org/10.1016/j.foodcont.2022.109478
- Shiroodi, S., Schwarz, H. M., Nitin, N., & Ovissipour, R. (2021). Efficacy of Nanobubbles Alone or in combination with neutral electrolyzed water in removing Escherichia coli O157:H7, Vibrio parahaemolyticus, and listeria innocua biofilms. Food and Bioprocess Technology, 14(2), 1–11. https://doi.org/10.1007/s11947-020-02572-0
- Song, X., Su, R., Wang, Y., Zhang, Y., Gao, B., Wang, Y., Ma, D., & Li, Q. (2023). Visible light-driven chlorite activation process for enhanced sulfamethoxazole antibiotics degradation, antimicrobial resistance reduction and biotoxicity elimination. Chemical Engineering Journal, 452, 139103. https://doi.org/10.1016/j.cej.2022.139103
- Stange, C., Sidhu, J. P. S., Toze, Toze, S., & Tiehm, A. (2019). Comparative removal of antibiotic resistance genes during chlorination, ozonation, and UV treatment. International Journal of Hygiene & Environmental Health, 222(3), 541–548. https://doi.org/10.1016/j.ijheh.2019.02.002
- Sun, W., Gu, J., Wang, X., Qian, X., & Peng, H. (2019). Solid-state anaerobic digestion facilitates the removal of antibiotic resistance genes and mobile genetic elements from cattle manure. Bioresource Technology, 274, 287–295. https://doi.org/10.1016/j.biortech.2018.09.013
- Sun, W., Qian, X., Gu, J., Wang, X.-J., & Duan, M.-L. (2016). Mechanism and effect of temperature on variations in antibiotic resistance genes during anaerobic digestion of dairy manure. Scientific Reports, 6(1), 30237. https://doi.org/10.1038/srep30237
- Svircev, A., Roach, D., & Castle, A. (2018). Framing the future with bacteriophages in agriculture. Viruses, 10(5), 218. https://doi.org/10.3390/v10050218
- Tacconelli, E., Carrara, E., Savoldi, A., Harbarth, S., Mendelson, M., Monnet, D. L., Pulcini, C., Kahlmeter, G., Kluytmans, J., Carmeli, Y., Ouellette, M., Outterson, K., Patel, J., Cavaleri, M., Cox, E. M., Houchens, C. R., Grayson, M. L., Hansen, P., … Yilmaz, F. O. (2018). Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. The Lancet Infectious Diseases, 18(3), 318–327. https://doi.org/10.1016/S1473-3099(17)30753-3
- Tan, L., Zhao, Y., Li, Y., Peng, Z., He, T., Liu, Y., Zeng, Q., & Wang, J. J. (2022). Potent eradication of mixed-species biofilms using photodynamic inactivation coupled with slightly alkaline electrolyzed water. LWT, 155, 112958. https://doi.org/10.1016/j.lwt.2021.112958
- Turtoi, M., & Borda, D. (2014). Decontamination of egg shells using ultraviolet light treatment. World’s Poultry Science Journal, 70(2), 265–278. https://doi.org/10.1017/S0043933914000282
- Upadhyay, A., Upadhyaya, I., Kollanoor-Johny, A., & Venkitanarayanan, K. (2013). Antibiofilm effect of plant derived antimicrobials on listeria monocytogenes. Food Microbiology, 36(1), 79–89. https://doi.org/10.1016/j.fm.2013.04.010
- Van Boeckel, T. P., Brower, C., Gilbert, M., Grenfell, B. T., Levin, S. A., Robinson, T. P., Teillant, A., & Laxminarayan, R. (2015). Global trends in antimicrobial use in food animals. Proceedings of the National Academy of Sciences, 112(18), 5649–5654. https://doi.org/10.1073/pnas.1503141112
- Van Boeckel, T. P., Glennon, E. E., Chen, D., Gilbert, M., Robinson, T. P., Grenfell, B. T., Levin, S. A., Bonhoeffer, S., & Laxminarayan, R. (2017). Reducing antimicrobial use in food animals consider user fees and regulatory caps on veterinary use. Science, 357(6358), 1350–1352. https://doi.org/10.1126/science.aao1495
- Viswanathan, V. K. (2014). Off-label abuse of antibiotics by bacteria. Gut microbes, 5(1), 3–4. https://doi.org/10.4161/gmic.28027
- Wei, Z., Decheng, S., Xia, F., Xiao, Z., Zhang, H., Zhou, Z., Huo, X., & Chong, Y. (2022). Occurrence and risk assessment of five kinds of antimicrobial in mattress on swine farm use ectopic fermentation systems in Zhejiang Province. Environmental Science and Pollution Research International, 29(46), 70591–70607. https://doi.org/10.1007/S11356-022-22891-3
- Wells, J. B., Coufal, C. D., Parker, H. M., & McDaniel, C. D. (2010). Disinfection of eggshells using ultraviolet light and hydrogen peroxide independently and in combination. Poultry Science, 89(11), 2499–2505. https://doi.org/10.3382/ps.2009-00604
- Wu, M. S., & Xu, X. (2019). Inactivation of antibiotic-resistant bacteria by chlorine dioxide in soil and shifts in community composition. RSC Advances, 9(12), 6526–6532. https://doi.org/10.1039/c8ra07997h
- Yang, Y., Wan, K., Yang, Z., Li, D., Li, G., Zhang, S., Wang, L., & Yu, X. (2020). Inactivation of antibiotic resistant Escherichia coli and degradation of its resistance genes by glow discharge plasma in an aqueous solution. Chemosphere, 252, 126476. https://doi.org/10.1016/j.chemosphere.2020.126476
- Yuan, Q. B., Guo, M. T., Yang, J., & Zhou, Z. (2015). Fate of antibiotic resistant bacteria and genes during wastewater chlorination: Implication for antibiotic resistance control. PLoS One, 10(3), e0119403. https://doi.org/10.1371/journal.pone.0119403
- Yuan, Y., Wang, L., Li, X., Tan, D., Cong, C., & Xu, Y. (2019). Efficacy of a phage cocktail in controlling phage resistance development in multidrug resistant Acinetobacter baumannii. Virus Research, 272, 197734. https://doi.org/10.1016/j.virusres.2019.197734
- Zalewska, M., Błażejewska, A., Czapko, A., & Popowska, M. (2021). Antibiotics and antibiotic resistance genes in animal manure – consequences of its application in agriculture. Frontiers in Microbiology, 12, 610656. https://doi.org/10.3389/fmicb.2021.610656
- Zhang, Y., Snow, D. D., Parker, D., Zhou, Z., & Li, X. (2013). Intracellular and extracellular antimicrobial resistance genes in the sludge of livestock waste management structures. Environmental Science & Technology, 47(18), 10206–10213. https://doi.org/10.1021/es401964s