 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Recovering biocompounds from industrial by-products is crucial to avoid food waste. Microwave-assisted extraction (MAE) has been employed to recover some biocompounds from vegetal matrices using ethanol and his solutions as solvent. Optimization of MAE conditions over total soluble polyphenols (TSP) and antioxidant capacity (DPPH and FRAP) from avocado residual paste (ARP) from oil industry was carried out. Optimal extract was used to elaborate an artisanal ham. Using response surface methodology (RSM), optimal conditions for MAE were 480 W of power, absolute ethanol as solvent, and extraction time of 180 s. The extract contained TSP of 31.24 mg GAE/g DW, DPPH inhibition of 24.51 mg TE/g DW and FRAP inhibition of 21.58 mg TE/g DW. The extract was added in the ham formulation at 0, 0.5, 1, and 1.5% w/w. Ham with 1% extract had the highest scores for acceptance. Extracts from ARP may be used in ham with adequate sensory attributes.
1. Introduction
Avocado (Persea americana) cv. Hass is a tropical fruit, originally from South Central Mexico, with a creamy texture, peculiar flavor, and high nutritional value (Cervantes-Paz & Yahia, Citation2021). The worldwide avocado production experienced a compound annual growth rate of 7% over the last decade, reaching around 8.4 million metric tons in 2022 (RaboResearch, Citation2023). Due to its high oil content, a significant quantity of avocado fruit is used to produce oil using different methods (Cervantes-Paz & Yahia, Citation2021). Early reports point out that most of the oil content is obtained from ripe avocado fruit due to the increased activity of polygalacturonase and cellulase enzymes that degrade the parenchyma cell walls, resulting in the release of oil bodies in emulsion form (Platt-Aloia et al., Citation1980). The worldwide avocado oil market is projected to grow 5.5%, being USD$556 million in 2022 and projected to USD$950 million for 2032 (Eyres, Citation2023). Generally, avocado oil is extracted from dried fruit tissue by organic solvents. New technologies for avocado oil extraction have increased considerably, giving rise to different available methods as extraction with solvent, mechanical extraction by cold pressing and aqueous extraction with subsequent centrifugation (Permal et al., Citation2020). After avocado oil extraction, peel, seed, and organic paste are the main residues generated. This last one is characterized by a hydrated and defatted pulp, with great potential as raw material for the food industry (Cervantes-Paz & Yahia, Citation2021).
Organic paste is deemed a significant agricultural byproduct, as out of the total 0.525 million tons used annually for avocado oil extraction, approximately 47% is discarded as residue (Qin & Zhong, Citation2016). The usage of by-products may create functional products and contribute to reduce environmental pollution (Jiménez-Velázquez et al., Citation2020). Other alternative is to employ them as a source of bioactive compounds, such as phenolic compounds (PCs). Salazar-López et al. (Citation2020) reported the presence of diverse PCs as hydroxycinnamic and hydroxybenzoic acids, phenolic-alcohol derivatives, proanthocyanins and flavonoids in flavonoids in avocado seeds and husks. PCs have caught the attention in recent years because of their antioxidant characteristics, for example, studies have found that PCs possess promising effects in chronic non-communicable diseases like diabetes, cancer, and hypertension (Nishikito et al., Citation2023).
PCs can be recovered from organic paste and used as an ingredient in cosmetics, pharmaceuticals, and the food industry. Some methodologies for recovering PCs from vegetal by-products involve novel technologies such as pulsed electric field, ultrasound-assisted extraction, and microwave-assisted extraction (MAE); MAE uses green solvents (water and ethanol), friendlier to the environment with short extraction times (Jimenez-Champi et al., Citation2023; Salazar et al., Citation2023). The variables with more effect in the extraction processes of bioactive compounds are microwave power and treatment time (which means the intensity of the treatment), as well as the solvent type (because of the nature and affinity of the biocompounds to be extracted). Agregán et al. (Citation2021) extracted PCs from eggplant peels via optimized microwave conditions: solvent ratio, microwave power and extraction time. Furthermore, Quiles-Carrillo et al. (Citation2019) optimized MAE parameters to maximize the PCs recovery from carob fruit peels (Ceratonia siliqua L.) to develop functional packages.
The development of optimization models allows us to describe a process and predict the yield of a target response by the effect of the involved variables. Response surface methodology (RSM) is a tool that combines statistics with mathematics for the optimization of processes that can involve a series of response variables with the independent variables. This correlation occurs through second-order equation, which provides acceptable results and reduces the number of experimental runs (Sai-Ut et al., Citation2023).
The extract containing PCs may be used as an ingredient in food matrices, to provide new benefits to the original product (Salem et al., Citation2023). The use of plant extracts as a source of bioactive compounds is becoming a strategy to increase quality and health-related characteristics in meat products (Munekata et al., Citation2020). In this context, utilizing optimized extracts derived from avocado residual pulp and integrating them into meat matrices can enhance the value and confer functional attributes to diverse formulated products. However, the addition of vegetable ingredients to meat products may affect quality attributes and affect the sensory acceptance; then, the sensory assessment is important to search for an adequate amount of the new ingredient in the final product (Ruiz-Hernández et al., Citation2023). Cooked pork ham is one of the most popular meat products in Latin America and Europe (Lorenzo et al., Citation2020). Thus, this work aims to optimize by RSM the MAE conditions over the PCs from avocado agri-industrial by-products to add the best extract in an artisanal ham formulation, which be sensorily acceptable. The novelty of the study is the proposal to recover biocompounds from avocado industrial by-products to avoid food waste, without to affect the sensory attributes of ham, but improving its phenolics content.
2. Materials and methods
2.1. Materials
ARP from the avocado “Hass” variety was donated by an avocado oil industry in El Salto, Jalisco, Mexico. The ARP was transported in sealed 20 L cuvettes to the lab in Irapuato, Guanajuato at room temperature (around 25ºC). The moisture content of the ARP was 95%, wet basis. The ARP was packed and frozen in 1-kg resealable plastic bags until experimentation. Thawed ARP was submitted to MAE as described below.
2.2. Microwave extraction of PCs from ARP
PCs from ARP were extracted by MAE using a microwave oven (io Mabe, model IO160MDI, China, nominal power = 1100 W, frequency = 2450 MHz). PCs were extracted using water and ethanolic solution as solvents (0%, 70% or 100%) at a solid-to-solvent ratio of 1:1 (w/v). The solvent type (XS1: 0, 70 or 100% ethanol/water solution), microwave power (XP2, at 100, 300 and 480 W), and the extraction time (Xt3, 1.3, 2.3 and 4 min) were varied. Thawed ARP without any MAE treatment or addition of solvents, served as control. After the extraction, the flasks were cooled in an ice bath for 15 min and the samples were transferred to falcon tubes (15 mL). Immediately, the samples were centrifuged at 18,400g for 15 min at 4°C (Hermle, model Z 326 K, Germany). The supernatants were filtered through Whatman No.1 paper and the volumes were volumetric to 50 mL. The extracts were preserved at −35°C for further determinations.
2.3. Experimental design
A 3k-p factorial design was applied. Solvent type, microwave power and extraction time were considered as the factors, while PC, and antioxidant capacity measured by DPPH and FRAP were the responses. The design was executed in three levels, with 9 treatments in triplicate, for a total of 27 experiments, and analyzed in STATISTICA software, version 10.0 (Stat Soft. Inc. 1984–2007, Tulsa, OK, U.S.A.). The experimental data were fitted to the regression model:
where Y is the response variable, β0 is the model constant, βi, βii and βij are the regression coefficients of the linear intercept, quadratic Xi2 and XiXj are the effects of the interactions and ε corresponds to the residual error. Subsequently, an optimization of the process was carried out to determine the optimal conditions that guarantee the best extraction of PC and antioxidant capacity measured by DPPH and FRAP. In addition, experimental validation tests of the mathematical model obtained were carried out using a T-student test to establish if there are differences between the predicted and experimental values for each response variable.
2.4. Analysis of total soluble polyphenols (TSP)
From the extracts obtained by MAE containing PCs, total soluble polyphenols (TSP) were quantified. The extracts were centrifuged at 8000g for 15 min at 4°C (Hermle, model Z 326 K, Germany) and TSP were measured with the methodology established by Singleton and Rossi (Citation1965). The method is based on the reduction reaction of the Folin-Ciocalteu reagent in the presence of a reducing agent. Briefly, 0.25 mL of the extract obtained was taken and 0.25 mL of the Folin-Ciocalteu reagent was added, previously diluted 1:4 v/v with distilled water and 2 mL of 1% sodium carbonate. The mixture was left standing in the dark for 1 h and absorbance was measured at 765 nm using a spectrophotometer (model Lambda XLS, Perkin Elmer, Waltham, MA, U.S.A.). TSP was calculated using a gallic acid calibration curve and the results were expressed as mg of gallic acid equivalents (GAE)/g of ARP, dry weight basis (DW).
2.5. Determination of the antioxidant capacity of the extracts
There is a large variety of methods to determine this parameter, and the variability of experimental conditions found in the literature for each of the methods hinders such selection and the possibility of easily comparing the obtained results with those of other authors. Antioxidants can neutralize the formation of free radical by two mechanisms. The first mechanism involves hydrogen atom transfer (HAT), for which DPPH is commonly used to assess the ability of compounds to act as free radical scavengers or hydrogen donors. The second mechanism operates through single electron transfer (SET), where the antioxidant capacity is assessed using the ferric reducing ability (FRAP assay), which capitalizes on electron-transfer reactions. According to the literature, it is recommended to employ at least one quantification method for each of the antioxidant mechanisms. The commonly utilized methods for assessing antioxidant capacity daily include the DPPH and FRAP assays (Chaves et al., Citation2020).
2.5.1. Antioxidant capacity by antiradical scavenging capacity (DPPH)
Methodology proposed by Brand-Williams et al. (Citation1995), with some modifications suggested by Ozuna et al. (Citation2020) was followed. An amount of 2.95 mg of the DPPH radical (2,2-diphenyl-1-picrylhidazil) was solved in 50 ml of absolute methanol and the absorbance (515 nm) was adjusted to 0.70 ± 0.02. Then, 2 mL of the extract was taken and mixed with 3.2 mL of the DPPH radical solution. The mixture was incubated in the dark for 15 min and the absorbance was read at 515 nm. The capacity of the antioxidants to reduce the radical, after the incubation period, was expressed as mg of Trolox equivalents (TE)/g in dry weight basis (DW).
2.5.2. Antioxidant capacity by reducing power (FRAP)
The FRAP methodology was applied according to Benzie and Strain (Citation1999). FRAP reagent was prepared by mixing 25 mL of sodium acetate buffer (0.03 M, pH 3.6), 2.5 mL 10 mM TPTZ (in 40 mM HCl), and 2.5 mL of ferric chloride (FeCl3, 20 mM). Then, 65 μL of the extract was mixed with 1950 μL of the FRAP reagent, 195 μL of distilled water and incubated for 30 min in the dark at room temperature. The absorbance was read at 595 nm, and the Fe 2+ concentration was calculated by linear regression using a Trolox calibration curve. Results were expressed as mg of Trolox equivalents (TE)/g of dry weight basis (DW).
2.6. Preparation of ham added with the optimized extract
displays the formulations for artisanal hams elaboration. Four formulations were prepared as follows: Control treatment was formulated without extract content, T-0.5%, T-1.0% and T-1.5% were formulated replacing the amount of water used in the formulation by 0.5, 1 and 1.5% (w/w) respectively by the optimized extract from ARP. Meat from pork legs was obtained from a local market in the city of Irapuato, Guanajuato, Mexico. The additives for making the brine were purchased from Bekarem® company, Mexico City.
Table 1. Ham formulations, control and added with extracts obtained by MAE optimized conditions from avocado residual pulp (ARP) at 0.5, 1 and 1.5% (w/w).
For the ham production, first, the subcutaneous fat and visible connective tissue were removed. Second, the meat was portioned in two parts: 2/3 was ground in a mill with a size of 10–14 mm (meat miller model 63245, Hamilton Beach, U.S.A.) and 1/3 was sliced manually in the form of thin slices of steak. Third, the brine was prepared with the curing salts (mixture of sodium nitrite, ascorbate, and polyphosphate), cornstarch, NaCl, maltodextrin, carrageenan, and spices. Extracts were incorporated as components of the brine solution. Subsequently, the brine was added uniformly to all the meat as proposed by Dutra et al. (Citation2021). Then, the meat mixture was submitted to a maceration process for 24 h at 4°C to allow the incorporation of all the ingredients. Finally, the emulsion was transferred to a filling machine (ET-25, Torrey, Querétaro, QRO, Mexico) and embedded in collagen ham casings of 50 mm (Salvigar, Monterrey, Nuevo León, Mexico). After, the hams were cooked in a convection electrical oven (model CE3F, Shel lab, Cornelius, OR, U.S.A.) at 75°C for 45 min (Oliveira et al., Citation2018).
2.7. Color parameters
Ham was coated in slices (around 2 mm of thickness) and placed into the measuring container. Color was determined using a colorimeter (ColorFlex model, Hunterlab, Reston, VIR, U.S.A.), calibrated using the black and white tiles. Values were measured employing a CIELab coordinates, with brightness D65, observation angle of 10° and aperture diameter of 8 mm. The equipment provided the following colorimetric parameters: lightness (L*), red/green component (a*), and yellow/blue component (b*). Three replicates were measured.
2.8. Total soluble polyphenols content in hams
Determination of TSP were carried out as described in section 2.4. In this case, the analysis was conducted on the ham samples added with the optimized extract, using 1 g from each ham. The results were expressed as mg GAE/g of ham, dry weight basis (DW).
2.9. Sensory assay
Sensory evaluation was performed by a semi-trained panel to assess the color, aroma, taste, and general acceptance of the four cooked hams. The panel was composed by 30 students and professors from the University of Guanajuato, from them, 19 were women and 11 men, between 21 and 45 years old. The sensory protocol was approved by the University Ethical Committee under project CEPIUG-P62-2023. Analysis was carried out in the Sensory Lab with individual booths under white light, and the samples were coded with 3-digit random numbers. Purified water at room temperature was served at the beginning of the session and between samples to clean the paladar and eliminate residual flavors. Cooked ham samples were evaluated for each parameter throughout a 9-point hedonic scale, from 1 “dislike extremely”, to 5 “not like neither dislike” to 9 “like extremely” (Kroehnke et al., Citation2018).
2.10. Statistical analysis
Statistical analysis was performed using the STATISTICA software, version 10.0 (Stat Soft. Inc. 1984–2007, Tulsa, OK, U.S.A.). Data were analyzed by analysis of variance (ANOVA) and Tukey´s pairwise comparison; the adequacy of the response surface model was determined by evaluating the lack of fit and coefficient of determination (R2). The statistical significance of the model and its variables was determined at 95% of probability level. The optimal conditions for MAE of ARP were obtained based on modeling and desirability functions that could be visually explained in terms of three-dimensional response surface plots and contour plots. All experiments were conducted in triplicate.
3. Results and discussion
3.1. Effect of microwave-assisted extraction on total soluble polyphenols and antioxidant capacity in the extracts
shows the influence of the assessed factors on the extraction of TSP and the antioxidant capacity measured by DPPH and FRAP. Treatments in which water was used as solvent resulted in lower extraction of TSP than the control treatment (ARP without MAE treatment). Thus, water is not working as a suitable solvent for TSP extraction. This is related to an oxidation process during the MAE. The collision induced by ionic polarization and dipole rotation of water molecules lead to the degradation of free or non-linked phenolics compounds. In this sense, Kroehnke et al. (Citation2018) reported that applying microwave treatments in aqueous media generates the oxidation of phenolic compounds and flavonoids. Moreover, regarding to linked-phenolic compounds the utilization of water appears to have limited effectiveness in disrupting the bonds to which these compounds are linked, as it is established that phenolics compounds naturally are in the cell wall of vegetal forms bonds with polysaccharides such as hemicellulose, lignin, and cellulose through different weak interactions, such as van der Waals, hydrophobic interactions, hydrogen bonds, or electrostatic forces (Siemińska-Kuczer et al., Citation2022). The response generated in treatments 7, 8 and 9 also indicate that PC are not solubilizing in water, and inefficient extraction is occurring.
Table 2. 3k-p factorial design with responses for total soluble polyphenols (TSP) and antioxidant capacity indices (FRAP and DPPH) of extracts obtained by microwave-assisted extraction (MAE) from avocado residual paste (ARP).
The results indicate best extraction of TSP is obtained when using a higher concentration of ethanol in the extracts. Further, an increase in microwave power generates a greater extraction of phenolics independently of the solvent type. On the other hand, an increasing extraction time leads to a negative effect on the TSP content. Treatments 1 and 5 were those that guaranteed a greater release of TSP with values of 31.70 ± 0.45 and 31.17 ± 0.54 mg GAE/g DW, respectively (p ≤ .05). The use of ethanol and the hydroalcoholic mixture (30:70) favors the release. In the case of treatment 1, a higher value is obtained when the sample is exposed to low extraction times and powers. Increasing both parameters generated a reduction of TSP values. The general behavior indicates that the use of ethanol favors the extraction of PC compared to the use of water as the main solvent. Evidence indicates that a high concentration of ethanol accelerates the transfer of substances, increases solubility, and consequently improves the extraction rate of phenolic compounds (Çiftci et al., Citation2023; Gil-Martín et al., Citation2022).
Antioxidant capacity for both assays DPPH and FRAP varied significantly with the solvent employed. All treatment under MAE shows higher values compared to the control which imply a higher antioxidant capacity (p ≤ .05). The antioxidant capacity results demonstrate the existing direct correlation with the TSP content. Higher antioxidant capacity values by DPPH and FRAP correspond to the treatment with the highest extraction of TSP (p ≤ .05). This behavior demonstrated that the application of MAE on ARP led the increase of the antioxidant capacity (AOC). However, there is a difference between both antioxidant assays, and this can be explained by the different mechanisms involved to neutralize the formation of oxidant spices. DPPH assays principally measured the ability of PC to donate a hydrogen atom (H+) to the DPPH● free radical. On the other side, FRAP methodology is based on the capability of PC to reduce the complex ferric ion-TPTZ by electron transfer. Both mechanisms help to elucidate how efficient are the chemical compounds present in the extracts when acting as antioxidants (Fernández-Marín et al., Citation2021).
ANOVA analysis of the experiment is presented in , outlining the factors and significant interactions (p ≤ .05) to elucidate the behavior of the dependent variable (TSP). The correlation observed between the factors and the response variable yielded an R2 value of 0.996, adequate for predicting the behavior of TSP. Additionally, the adjusted R2 value suggests a strong fit of the mathematical model (Equation 2) in accurately predicting the extraction of TSP. The coefficient of variation (CV) was 2.13% and the adjusted precision were 72.483. Values indicate that exists a minimum of variation in the model due to factors outside the mathematical model. In addition, adjusted precision measures the signal-to-noise ratio, in this case the ratio was greater than 4, indicating an adequate signal, so the model can be used to navigate the design space.
Table 3. Analysis of variance of the regression model for the content of phenolic compounds, antioxidant activity measured by DPPH and FRAP in avocado residual paste (ARP).
The predictive, second-order, polynomial model for the TSP of ARP extracts obtained by MAE, represented in terms of coded factor levels is:
The relationship between the factors and TSP content can be illustrated through three-dimensional response surfaces (). shows microwave power and solvent type interaction on the TSP content. When each factor is examined separately, it becomes evident that an increase in ethanol content leads to an increase in the TSP values for all the concentrations studied. Conversely, as microwave power increased, there is an augmentation in the content up to 400 W, but beyond that point, higher power levels result in a decrease in TSP. Within the response surface analysis, the combined influence of solvent type and microwave power improves the extraction of TSP, with the highest extraction occurring at high ethanol concentrations and low microwave power. The observed behavior suggests that the composition of the solvent plays a dominant role in governing the extraction process of TSP. In contrast, the power level appears to have a less pronounced impact on the extraction of these compounds compared to the solvent ratio.
Figure 1. Response surface plot for total soluble polyphenols (TSP), as phenolic compounds, of extracts obtained by microwave-assisted extraction (MAE) of avocado residual paste (ARP). (a) Effect of solvent ratio (XS1), and microwave power (XP2); (b) Effect of extraction time (Xt3), and microwave power (XP2).
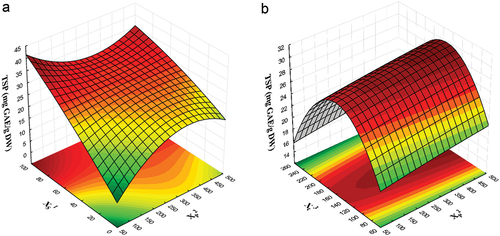
The type of solvent significantly influences the quantity of compounds extracted from various food matrices (Kaderides et al., Citation2019). As an example, water is typically classified as a moderate microwave absorber, whereas ethanol is regarded as a strong microwave absorber. This unique property allows microwaves to be absorbed with greater efficiency and converted into heat, thus promoting the swift breakdown of plant material, and facilitating the diffusion of phenolic compounds (Valenzuela-González et al., Citation2023).
presents the behavior of extraction time and solvent ratio, and their impact on the content of TSP. Contour plots illustrate the presence of a maximum value and two minimum values across the response surface, indicating that the extraction of the compounds rises to a point between 150 and 180 s, after which it starts to decrease. This behavior may be attributed to when the plant material undergoes a brief treatment, it may not ensure the complete release of the compounds of interest. However, when the material is subjected to treatments that extend beyond 180 s, there is a reduction in the TSP. This decrease could be associated with the occurrence of degradation and/or oxidation reactions. Quintero-Quiroz et al. (Citation2019) suggested that when plant material is exposed to microwave process above 300 s, oxidation reaction in phenolic compounds may occur. Other factors might influence the transfer of the desired compounds into the solvent, including the particle size of the plant material, the solvent employed, and the ratio of solute/solvent (Rodsamran & Sothornvit, Citation2019). In our specific research, the material under study is an agri-industrial residue already being processed. Consequently, it can be expected that extraction times will be reduced compared to samples that were only conditioned for the MAE process.
presents the ANOVA results considering both methods of expression of antioxidant capacity. Models indicate that the factors significantly influence the behavior of the dependent variables. Additionally, the combined effect of solvent type with microwave power, quadratic effects of the factors, and the interaction of XS1x (XP2)2 were also found to be significant. The R2 and adjusted R2 values exhibit consistency for both dependent variables. In terms of DPPH, the adjusted R2 indicates that 99.99% of the variance in the DPPH parameters can be accounted by the design employed. Similarly, for FRAP, 99.98% of the variance can be elucidated by the mathematical model. In essence, the ANOVA findings suggest that the model could serve as a reliable tool for forecasting the performance of antioxidant capacity using DPPH and FRAP. The values of CV were very low with values close to 0: for DPPH CV = 0.198% and for FRAP = .613%, which is desirable. The adjusted precision for DPPH was 1109.363 and for FRAP 456.015. The values indicate that for both variables the mathematical model can predict the behavior of the response variables with a minimum of variation.
The predictive, second-order, polynomial model for the antioxidant capacity by DPPH and FRAP of ARP extracts obtained by MAE, represented in terms of coded factor levels, is expressed as:
Overall, increased ethanol content in the solvent resulted in increased antioxidant activity in the extracts for both DPPH and FRAP assays (). Furthermore, it was observed that prolonging the extraction time led to higher antioxidant capacity values in both methodologies (). Specifically, illustrates the trends in antioxidant capacity measured by DPPH. Values surpassing 20 mg TE/g DW were achieved with ethanol concentrations exceeding 80%. Regarding extraction time, elevated values were also obtained with its increase, albeit to a lesser degree than the impact of the solvent used. Interestingly, the microwave power applied does not seem to markedly affect the rise in antioxidant capacity, as there is no discernible increase on the response surface when the microwave power is elevated (p ≤ .05). Consequently, the results suggest a significant enhancement in antioxidant capacity with higher ethanol concentrations, a relatively minor impact with increased exposure time, and a lower influence of the microwave power utilized on these values.
Figure 2. Response surface plot for antioxidant capacity indices of extracts obtained by microwave-assisted extraction (MAE) of avocado residual paste (ARP). (a) Effect of solvent ratio (XS1), and microwave power (XP2) over DPPH; (b) Effect of extraction time (Xt3), and microwave power (XP2) over DPPH; (c) Effect of solvent ratio (XS1), and microwave power (XP2) over FRAP; (d) Effect of extraction time (Xt3), and microwave power (XP2) over FRAP.
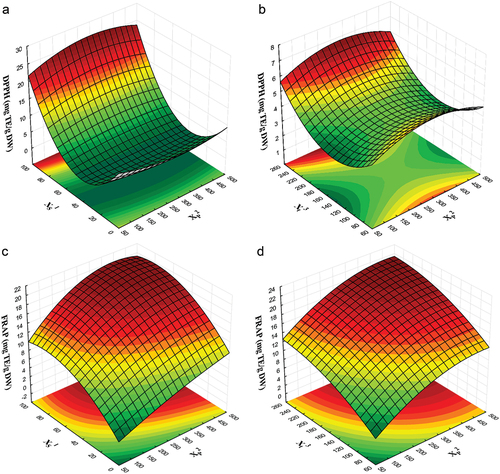
Similar results were found by Almusallam et al. (Citation2021) who investigated the ultrasound-assisted extraction of bioactive compounds from date palm spikelets, finding that the DPPH radical scavenging was enhanced as ethanol concentration increased over 50%. In another report, this time using MAE to recover polyphenols from Thymus serpyllum industrial waste, authors reported that lineal and quadratics terms of ethanol concentration and extraction time exhibited a highly significant influence on DPPH values. The highest scavenging capacity (6.107 mg TE/g) was observed using 75% ethanol for 720 s (Mrkonjic et al., Citation2022). While direct comparisons of the results are not feasible due to the distinct conditions and equipment requirements, the discernible trend of increased DPPH values with higher alcohol concentration and prolonged exposure time remains undeniable. The trend can be explained by the fact that the increase in ethanol concentration promotes the dissolution of PC during the MAE process (Fadimu et al., Citation2020). The mixtures of water and ethanol play a vital role in TSP extraction. Water acts as an agent that causes swelling, whereas ethanol disrupts the bonds between the phenolic compounds and cellular structures. Thus, an increase in ethanol concentration results in the extraction of more phenolic compounds with a high antioxidant capacity. On the contrary, reducing ethanol concentration results in the extraction of more contaminating materials that interferer with the quantification leading to a low antioxidant capacity (Mathews et al., Citation2024).
The values obtained in FRAP exhibit a distinct behavior when compared to the antioxidant capacity measured by the DPPH method. illustrates that the combined effect of the solvent ratio with the microwave power doubles the antioxidant capacity values (22 mg TE/g DW), contrasting with independent values that only reach up to 10 mg TE/g DW. Additionally, demonstrates that the interaction between extraction time and microwave power enhances the antioxidant capacity in the extracts compared to the independent effects of these factors. Exposure time, microwave power, and solvent ratio positively contribute to the increase the antioxidant capacity in the microwave-treated ARP (p ≤ .05). Increased ethanol concentration in the solvent aided in extracting certain components, thereby enhancing the antioxidant capacity measured by FRAP. Similarly, longer extraction times contribute to breaking down cell walls and releasing bioactive compounds into the extraction solvent and increase the antioxidant capacity of the extracts. Another important aspect is the fact that the methodologies employed to assess the antioxidant capacity of ARP extract might analyze different groups of antioxidant compounds, which could partially coincide. On the one hand, the FRAP method evaluates the content of electron-donating species with a certain redox potential, whereas the DPPH method assesses the free radical scavenging capacity of a sample (Solaberrieta et al., Citation2022).
Given the close connection between antioxidant capacity and TSP content, a similar pattern regarding ethanol concentration is evident. The phenomenon could be explained by the increase of the dipole moment of solvent due to more excellent microwave absorption. Furthermore, solvent type exhibits a stronger attraction toward moderately lipophilic polyphenols, resulting in an improvement of solvent penetration into plant cells, thereby enhancing heating and facilitating mass transfer from solid to liquid phases (Martić et al., Citation2022).
The optimal conditions were identified through the maximization of response desirability. Multiple responses required simultaneous consideration and seeking the optimal response among the collective responses becomes essential. Derringer function or desirability function (d) stands out as a crucial and widely employed multicriteria methodology in optimization procedures (Bezerra et al., Citation2008). The optimal conditions were to submit the ARP to a microwave power of 480 W, with a concentration of 100% ethanol for 180 s. The global desirability value was 0.960. The experimental value was determined for all the responses and contrasted with the predicted values (). The experimental results demonstrate that the model is reliable and predicts the behavior of the response variables. Furthermore, the adjusted R2 value indicates a good agreement between the experimental values and those predicted by the statistical method.
Table 4. Experimental validation of predicted values at optimal conditions for microwave-assisted extraction (MAE) of total soluble polyphenols (TSP) and antioxidant capacity indices (DPPH and FRAP) from avocado residual paste (ARP).
3.2. Characteristics of hams added with the optimum extract of ARP
shows the color parameters of the hams added with the optimized extract from ARP and control (without extract). Addition of 1.5% of ARP extract decreased the lightness (L* parameter) of the ham. In addition, an increasing in the a* value suggests that adding 1.5% extract intensifies the red component of the hams. In relation to the b* values, it is observed that including extracts at levels of 1% and 1.5% contributes to raising this parameter. This increment might lead to amplified yellow tones in the resulting products.
Figure 3. Color parameters evaluated in the formulated hams. Control: ham without added extract; T-0.5%: ham with the addition of 0.5% extract; T-1.0%: ham with the addition of 1% extract; T-1.5%: ham with the addition of 1.5% extract. Different literals in each parameter indicate significant differences (p ≤ .05).
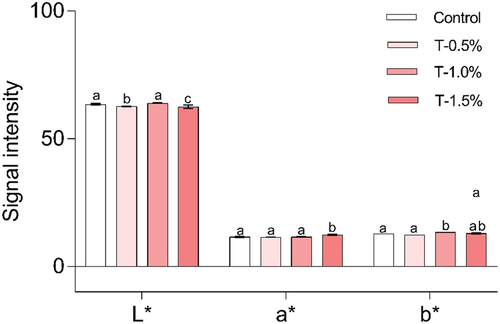
Some authors refers that the inclusion of naturals extracts to meat and meat products can modified in different ways the color parameters in the resulting products. For instance, Aksu and Turan (Citation2022) reported that the addition of black carrot extract to fresh meat products decreased L* and improved red color (a*) in vacuum packed samples. In other report, the effect of albedo of grapefruit on color parameters of turkey patties was investigated demonstrated that the extracts showed no impact on the color values for a* and b* parameters (Babaoğlu et al., Citation2022). Results in both investigations suggested the variability of the results can be attributed to the nature of the phenolics extracts and to the specific food matrix selected to add the extracts (Pereira et al., Citation2022). Moreover, our study revealed that adding ARP extract changed the color parameters as increasing the concentration of extract in the formulated hams. Adding phenolics compounds has the potential to elevate the a* parameters and reduce L * . This effect is attributable to the antioxidant properties of the phenolics compounds within the extract, neutralizing the formation of free radicals. Their presence promotes the conversion of nitrites into nitric oxide, which subsequently interacts with the primary proteins in the meat matrix (myoglobin), resulting in the formation of nitrosomyoglobin. Notably, this compound is characterized by its stable red hue, a quality preserved through heat treatment during cooking process of the hams (Deng et al., Citation2022).
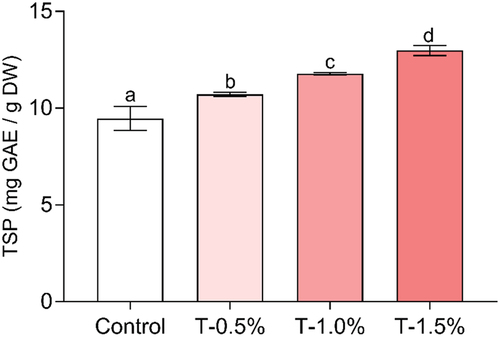
Total soluble polyphenols were determined in the elaborated hams and lead that the incorporation of optimized ARP extract increase the parameter evaluated as concentration rise (). The highest content was observed when 1.5% of the extract was added, resulting in a 37% increase compared to the control. Similarly, the inclusion of 0.5% and 1.0% led to increases of 13% and 24%, respectively. The results illustrate that integrating the optimized extract leads to a product that gains increased value due to its higher phenolic content. Comparable findings were achieved when the inclusion of roselle extract in beef patty formulation increased phenolic content of the product (Pérez‐Báez et al., Citation2020). Other study shows how the addition of buckwheat by-product (1 to 3% w/w) is an excellent option to obtain Frankfurter sausages with a high content of phenolic compounds (Salejda et al., Citation2022). Consequently, addition of optimized extracts from ARP is an alternative to enhance the phenolics content, highlights appreciated by its antioxidant characteristic and create a new type of meat product with a substantial value augmentation.
Figure 4. Total soluble polyphenols (TSP) content in the formulated hams. Control: ham without added extract; T-0.5%: ham with the addition of 0.5% extract; T-1.0%: ham with the addition of 1% extract; T-1.5%: ham with the addition of 1.5% extract. Different literals indicate differences (p ≤ .05).
provides the web graphics for the sensory scores obtained from the panelists. The color attribute was not influenced by the concentration of the extracts (p > .05). While the instrumental analysis indicates variations in color due to the addition of extracts, this suggests that the panelists were unable to discern the potential impact of extract additions on the color of hams. Regarding aroma, including 1% of the extract promotes a favorable perception, while higher concentrations result in a moderate liking of the product by consumers. The concentration of ARP extracts caused differences in the taste, which followed the trend T1.5<T0.5<Control<T1.0. The formulation T1.0 achieved the highest general acceptability, averaging 8 points, indicating a strong preference (“like very much”) according to the employed scale. Conversely, T1.5 was the least accepted formulation, receiving scores around 6, indicating that consumers only slightly favored this ham. The findings suggest that the inclusion of 1.0% of the extract resulted in the highest level of acceptance among consumers for the formulated ham.
Figure 5. Sensory scores for cooked ham (control) and formulations added with extracts (0.5, 1 and 1.5% w/w) obtained by microwave-assisted extraction (MAE) of avocado residual paste (ARP).
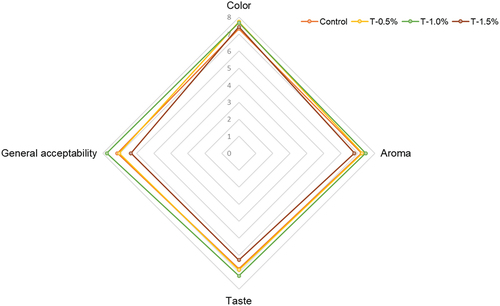
Oliveira et al. (Citation2018) added lactulose to a cooked ham formulation and did not find changes in the sensory characteristics in comparison with the control when added at 30 g lactulose/kg. The use of antioxidant sources, for example, essential oils increase the evaluations obtained for aroma and general acceptability up to concentrations of 1.5 mg/g. This suggests that panelists can identify the element added at higher concentrations. Hence, it is advisable that the development of meat product incorporating antioxidant extracts should be paired with sensory studies. This is because, typically, the highest TSP values obtained in the formulations may not align with the optimal acceptability from consumers (Ruiz-Hernández et al., Citation2023). For the commented issues, the adequate amount of new ingredients is crucial to do not affect the sensory perception of the meat products. More studies are required to evaluate the bioaccesibility of the compounds after consumption.
4. Conclusions
The mathematical model to describe the MAE of PC with high TSP and antioxidant capacity adequately predicted the values. The optimal extraction parameters for the evaluated responses were 180 s of extraction time, 100% ethanol concentration and 480 W of microwave power. ARP, which is considered an oil production waste, can be used as a high-value raw material to obtain extracts with the potential to be added as ingredient in cooked ham, at concentration of 1% (w/w). This agro-industrial by-product can be revalorized and transformed into a functional ingredient, enhancing the phenolic compounds content through the application of emerging technologies like microwave-assisted extraction. Moreover, the presence of industrial-scale microwave ovens offers the potential to integrate this process into the avocado processing chain. This represents an opportunity to enhance the economic value of the ARP and contribute to the development of a circular economy in the avocado sector.
Acknowledgements
The voluntary and kind participation of the panelists in the sensory analyses is highly appreciated.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agregán, R., Munekata, P. E., Feng, X., Astray, G., Gullón, B., & Lorenzo, J. M. (2021). Recent advances in the extraction of polyphenols from eggplant and their application in foods. LWT, 146, 111381. https://doi.org/10.1016/j.lwt.2021.111381
- Aksu, M. I., & Turan, E. (2022). Properties of black carrot extract and its efficacy for improving the storage quality of vacuum packaged fresh meat products. Packaging Technology and Science, 35(4), 339–11. https://doi.org/10.1002/pts.2631
- Almusallam, I. A., Mohamed Ahmed, I. A., Babiker, E. E., Al Juhaimi, F. Y., Fadimu, G. J., Osman, M. A., Al Maiman, S. A., Ghafoor, K., & Alqah, H. A. S. (2021). Optimization of ultrasound-assisted extraction of bioactive properties from date palm (Phoenix dactylifera L.) spikelets using response surface methodology. LWT, 140, 110816. https://doi.org/10.1016/j.lwt.2020.110816
- Babaoğlu, A. S., Ainiwaer, T., Özkan, H., & Karakaya, M. (2022). Grapefruit and pomelo peel extracts as natural antioxidants for improved storage stability of Turkey patties during refrigerated storage. Journal of Food Science and Technology, 59(10), 4067–4074. https://doi.org/10.1007/s13197-022-05458-5
- Benzie, I. F., & Strain, J. (1999). Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In L. Packer (Ed.), Methods in enzymology (Vol. 299, pp. 15–27). Elsevier.
- Bezerra, M. A., Santelli, R. E., Oliveira, E. P., Villar, L. S., & Escaleira, L. A. (2008). Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta, 76(5), 965–977. https://doi.org/10.1016/j.talanta.2008.05.019
- Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1), 25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
- Cervantes-Paz, B., & Yahia, E. M. (2021). Avocado oil: Production and market demand, bioactive components, implications in health, and tendencies and potential uses. Comprehensive Reviews in Food Science and Food Safety, 20(4), 4120–4158. https://doi.org/10.1111/1541-4337.12784
- Chaves, N., Santiago, A., & AlíAlíAs, J. C. (2020). Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants, 9(1), 76. https://doi.org/10.3390/antiox9010076
- Çiftci, B., Karaman, K., & Kaplan, M. (2023). Comparison of antioxidant, Antiradical and antibacterial activities of mistletoe (viscum album L.) fruits and leaves growing on different Host tree genus. Waste and Biomass Valorization, 1–14. https://doi.org/10.1007/s12649-023-02307-0
- Deng, S., Shi, S., & Xia, X. (2022). Effect of plant polyphenols on the physicochemical properties, residual nitrites, and N-nitrosamine formation in dry-fried bacon. Meat Science, 191, 108872. https://doi.org/10.1016/j.meatsci.2022.108872
- Dutra, M. P., Cardoso, G. P., Ramos, E. M., Ramos, A. D. L. S., Pinheiro, A. C. M., & Fontes, P. R. (2021). Technological and sensory quality of restructured low-fat cooked ham containing liquid whey. Ciência e Agrotecnologia, 36(1), 86–92. https://doi.org/10.1590/S1413-70542012000100011
- Eyres, L. (2023). 10th world avocado congress. Food New Zealand, 23(3), 20–23.
- Fadimu, G. J., Ghafoor, K., Babiker, E. E., Al-Juhaimi, F., Abdulraheem, R. A., & Adenekan, M. K. (2020). Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. Journal of Food Measurement and Characterization, 14(3), 1784–1793. https://doi.org/10.1007/s11694-020-00426-z
- Fernández-Marín, R., Fernandes, S. C., Andrés, M. A., & Labidi, J. (2021). Microwave-assisted extraction of curcuma longa l. Oil: Optimization, chemical structure and composition, antioxidant activity and comparison with conventional soxhlet extraction. Molecules, 26(6), 1516. https://doi.org/10.3390/molecules26061516
- Gil-Martín, E., Forbes-Hernández, T., Romero, A., Cianciosi, D., Giampieri, F., & Battino, M. (2022). Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chemistry, 378, 131918. https://doi.org/10.1016/j.foodchem.2021.131918
- Jimenez-Champi, D., Romero-Orejon, F. L., Moran-Reyes, A., Muñoz, A. M., & Ramos-Escudero, F. (2023). Bioactive compounds in potato peels, extraction methods, and their applications in the food industry: A review. CyTA - Journal of Food, 21(1), 418–432. https://doi.org/10.1080/19476337.2023.2213746
- Jiménez-Velázquez, P., Valle-Guadarrama, S., Alia-Tejacal, I., Salinas-Moreno, Y., García-Cruz, L., Pérez-López, A., & Guerra-Ramírez, D. (2020). Separation of bioactive compounds from epicarp of ‘Hass’ avocado fruit through aqueous two-phase systems. Food and Bioproducts Processing, 123, 238–250. https://doi.org/10.1016/j.fbp.2020.07.004
- Kaderides, K., Papaoikonomou, L., Serafim, M., & Goula, A. M. (2019). Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chemical Engineering & Processing - Process Intensification, 137, 1–11. https://doi.org/10.1016/j.cep.2019.01.006
- Kroehnke, J., Szadzińska, J., Stasiak, M., Radziejewska-Kubzdela, E., Biegańska-Marecik, R., & Musielak, G. (2018). Ultrasound-and microwave-assisted convective drying of carrots–process kinetics and product’s quality analysis. Ultrasononic Sonochemistry, 48, 249–258. https://doi.org/10.1016/j.ultsonch.2018.05.040
- Lorenzo, J., Dominguez, R., & Pateiro, M. (2020). Catálogo de productos cárnicos Iberoamericanos, red healthy meat (CYTED 119RT0568). Ourense Spain.
- Martić, N., Zahorec, J., Stilinović, N., Andrejić-Višnjić, B., Pavlić, B., Kladar, N., Šoronja-Simović, D., Šereš, Z., Vujčić, M., Horvat, O., & Rašković, A. (2022). Hepatoprotective effect of carob pulp flour (Ceratonia siliqua L.) extract obtained by optimized microwave-assisted extraction. Pharmaceutics, 14(3), 657. https://doi.org/10.3390/pharmaceutics14030657
- Mathews, A., Arbal, A. V., Kaarunya, A., Jha, P. K., Le-Bail, A., & Rawson, A. (2024). Conventional vs modern extraction techniques in the food industry. In S. M. Jafari & S. Akhavan-Mahdavi (Eds.), Extraction processes in the food industry (pp. 97–146). Elsevier.
- Mrkonjic, Ž., Rakic, D., Takaci, A., Kaplan, M., Teslic, N., Zekovic, Z., Lazarevic, I., & Pavlic, B. (2022). Polyphenols recovery from Thymus serpyllum industrial waste using microwave-assisted extraction–Comparative RSM and ANN approach for process optimization. Foods, 11(9), 1184. https://doi.org/10.3390/foods11091184
- Munekata, P. E. S., Rocchetti, G., Pateiro, M., Lucini, L., Domínguez, R., & Lorenzo, J. M. (2020). Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Current Opinion in Food Science, 31, 81–87. https://doi.org/10.1016/j.cofs.2020.03.003
- Nishikito, D. F., Borges, A. C. A., Laurindo, L. F., Otoboni, A. M. B., Direito, R., Goulart, R. D. A., Nicolau, C. C., Fiorini, A. M., Sinatora, R. V., & Barbalho, S. M. (2023). Anti-inflammatory, antioxidant, and other health effects of dragon fruit and potential delivery systems for its bioactive compounds. Pharmaceutics, 15(1), 159. https://doi.org/10.3390/pharmaceutics15010159
- Oliveira, C. A., Massingue, A. A., Moura, A. P. R., Fontes, P. R., Ramos, A. L., & Ramos, E. M. (2018). Restructured low‐fat cooked ham containing liquid whey fortified with lactulose. Journal of the Science of Food and Agriculture, 98(2), 807–816. https://doi.org/10.1002/jsfa.8529
- Ozuna, C., Mulík, S., Valdez-Rodríguez, B., Abraham-Juarez, M. D. R., & Fernández-López, C. L. (2020). The effect of organic farming on total phenols, total flavonoids, brown compounds and antioxidant activity of spent coffee grounds from Mexico. Biological Agriculture & Horticulture, 36(2), 107–118. https://doi.org/10.1080/01448765.2019.1704876
- Pereira, A., Lee, H. C., Lammert, R., Jr., Wolberg, C., Jr., Ma, D., Immoos, C., Casassa, F., & Kang, I. (2022). Effects of red‐wine grape pomace on the quality and sensory attributes of beef hamburger patty. International Journal of Food Science & Technology, 57(3), 1814–1823. https://doi.org/10.1111/ijfs.15559
- Pérez‐Báez, A. J., Camou, J. P., Valenzuela‐Melendres, M., González‐Aguilar, G., Viuda‐Martos, M., Sebranek, J. G., & Tortoledo‐Ortiz, O. (2020). Effects and interactions of roselle (Hibiscus sabdariffa L.), potato peel flour, and beef fat on quality characteristics of beef patties studied by response surface methodology. Journal of Food Processing and Preservation, 44(9), e14659. https://doi.org/10.1111/jfpp.14659
- Permal, R., Chang, W. L., Seale, B., Hamid, N., & Kam, R. (2020). Converting industrial organic waste from the cold-pressed avocado oil production line into a potential food preservative. Food Chemistry, 306, 125635. https://doi.org/10.1016/j.foodchem.2019.125635
- Platt-Aloia, K. A., Thomson, W. W., & Young, R. E. (1980). Ultrastructural changes in the walls of ripening avocados: Transmission, scanning, and freeze fracture microscopy. Botanical Gazette, 141(4), 366–373. https://doi.org/10.1086/337169
- Qin, X., & Zhong, J. (2016). A review of extraction techniques for avocado oil. Journal of Oleo Science, 65, 881–888. https://doi.org/10.5650/jos.ess16063
- Quiles-Carrillo, L., Mellinas, C., Garrigos, M. D. C., Balart, R., & Torres-Giner, S. (2019). Optimization of microwave-assisted extraction of phenolic compounds with antioxidant activity from carob pods. Food Analytical Methods, 12, 2480–2490. https://doi.org/10.1007/s12161-019-01596-3
- Quintero-Quiroz, J., Celis-Torres, A., Muñoz-Ramirez, L., Silva-Garcia, M., Ciro-Gomez, G., & Rojas-Camargo, J. (2019). Optimization of the microwave-assisted extraction process of bioactive compounds from annatto seeds (Bixa orellana L.). Antioxidants, 8(2), 37. https://doi.org/10.3390/antiox8020037
- RaboResearch. (2023). World avocado map 2023: Global growth far from over. Retrieved January 23, 2024, from https://research.rabobank.com/far/en/sectors/fresh-produce/world-avocado-map-2023-global-growth-far-from-over.html
- Rodsamran, P., & Sothornvit, R. (2019). Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Bioscience, 28, 66–73. https://doi.org/10.1016/j.fbio.2019.01.017
- Ruiz-Hernández, K., Sosa-Morales, M. E., Cerón-García, A., & Gómez-Salazar, J. A. (2023). Physical, chemical, and sensory changes in meat and meat products induced by the addition of essential oils: A concise review. Food Reviews International, 39, 2027–2056. https://doi.org/10.1080/87559129.2021.1939369
- Sai-Ut, S., Kingwascharapong, P., Mazumder, M. A. R., & Rawdkuen, S. (2023). Optimization of extraction of phenolic compounds and antioxidants from passion fruit and rambutan seeds using response surface methodology. Journal of Agriculture and Food Research, 100888. https://doi.org/10.1016/j.jafr.2023.100888
- Salazar-López, N. J., Domínguez-Avila, J. A., Yahia, E. M., Belmonte-Herrera, B. H., Wall-Medrano, A., Montalvo-González, E., & González-Aguilar, G. (2020). Avocado fruit and by-products as potential sources of bioactive compounds. Food Research International, 138, 109774. https://doi.org/10.1016/j.foodres.2020.109774
- Salazar, M. D. Á. R., Urbina, G. R. O., Bezerra, P. D. N., Cunha, V. M., da Silva, M. P., Pires, F. C., E Silva, A. P. D. S., Ferreira, M. C., Barbosa, J. R., & de Sousa, S. H. (2023). Antioxidants extraction from vegetable matrices with green solvents. In I. A. Altalhi (Ed.), Green sustainable process for chemical and environmental engineering and science (pp. 289–308). Elsevier. https://doi.org/10.1016/B978-0-323-95156-2.00010-6
- Salejda, A. M., Olender, K., Zielińska-Dawidziak, M., Mazur, M., Szperlik, J., Miedzianka, J., Zawiślak, I., Kolniak-Ostek, J., & Szmaja, A. (2022). Frankfurter-type sausage enriched with buckwheat by-product as a source of bioactive compounds. Foods, 11(5), 674. https://doi.org/10.3390/foods11050674
- Salem, M. A., Mansour, H. E. A., Mosalam, E. M., El-Shiekh, R. A., Ezzat, S. M., & Zayed, A. (2023). Valorization of by-products derived from onions and potato: Extraction optimization, metabolic profile, outstanding bioactivities, and industrial applications. Waste and Biomass Valorization, 1–36. https://doi.org/10.1007/s12649-022-02027-x
- Siemińska-Kuczer, A., Szymańska-Chargot, M., & Zdunek, A. (2022). Recent advances in interactions between polyphenols and plant cell wall polysaccharides as studied using an adsorption technique. Food Chemistry, 373, 131487. https://doi.org/10.1016/j.foodchem.2021.131487
- Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144–158. https://doi.org/10.5344/ajev.1965.16.3.144
- Solaberrieta, I., Jiménez, A., & Garrigós, M. C. (2022). Valorization of Aloe vera skin by-products to obtain bioactive compounds by microwave-assisted extraction: Antioxidant activity and chemical composition. Antioxidants, 11(6), 1058. https://doi.org/10.3390/antiox11061058
- Valenzuela-González, M., Cárdenas-López, J. L., Burgos-Hernández, A., Salazar-López, N. J., Viuda-Martos, M., Ruiz-Hernández, A. A., & Robles-Sánchez, R. M. (2023). Quinoa treated by an optimized method of microwave heating and their effect on antioxidant activity and phenolic compounds after in vitro gastrointestinal digestion. CyTA - Journal of Food, 21(1), 751–759. https://doi.org/10.1080/19476337.2023.2279186
