?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Many plants are well-known for their use in traditional medicine to treat diseases associated with inflammations and oxidative stress. This study aimed to investigate a medicinal plant from the Saharan region of Algeria, Pituranthos chloranthus. Phytochemical analysis showed that it is rich in polyphenols (8.93 ± 0.736 mg GAE/g) and flavonoids (0.78 ± 0.023 mg EQ/g). The treatment of hypoglycemic individuals with a P. chloranthus extract showed effectiveness when compared to control diabetic rats not treated with this extract. The latter showed a high average glucose at 3.11 ± 0.769 g/L when compared to the two treated rat groups, 0.8 ± 023 g/L and 0.54 ± 0.08 g/L when fed 500 and 250 mg/Kg body weight of P. chloranthus extract, respectively. The in vitro antioxidant evaluation, using the DPPH (IC50 = 0.156 ± 0.23 mg/mL) and FRAP (IC50 = 0.718 ± 0.09 mg/mL) tests and ratio to ascorbic acid 0.0037 ± 0.505 mg/mL showed an important antioxidant potential.
Introduction
Historically, plants have served not only as nutritional sources but also as integral components of traditional remedies for various health issues, a fact documented in ancient Arabic, Chinese, Egyptian, Hindu, Greek, and Roman literature (Kamatou et al., Citation2017). 70 to 95% of the population living in developing countries uses medicinal plants for primary treatments due to the lack of access to prescribed medications. The biological activities of various chemical compounds, particularly secondary metabolites like alkaloids, flavonoids, polyphenols, polyterpenes, saponifies, sterols, and tannins, which are used in treatments as enzyme inhibitors, antioxidants, antidiabetics, antibacterials, anticancer, antifungals, diuretics, etc., are responsible for these therapeutic effects (Khacheba et al., Citation2014; N’Guessan et al., Citation2009). Traditional medicine is based on the use of medicinal plants for the treatment of many diseases, including diabetes which is a metabolic disease characterized by a disorder in the regulation of carbohydrate metabolism leading to hyperglycemia. Statistical results from 2017 showed that 451 million people were living with diabetes worldwide which will increase to 693 million by 2045 . Whereas, 7 million diabetics have been estimated in Africa (Bouchenak et al., Citation2018). Furthermore, diabetes is also characterized by hyperglycemia and insufficient secretion or action of endogenous insulin (Derouiche et al., Citation2017). Diabetes is generally accompanied by an increase in the production of free radicals or impaired antioxidant defenses (Ceriello, Citation2000) which induces a state of oxidative stress (Tiwari et al., Citation2013). Secondary metabolites are also known to function as anti-inflammatories, facilitating the body’s defensive reactions to diverse assaults, including physical, chemical, or biological origins (immune response) or infectious agents (Dièye et al., Citation2008). Inflammation can sometimes be detrimental. The detrimental effect stems from the interplay of various factors, including the virulence of the pathogen, its prolonged presence, and the specific location of the inflammatory response. Pituranthos chloranthus, locally known as “Guezzah,” belongs to the Apiaceae family and is among the most utilized medicinal plants with significant antidiabetic and hypoglycemic properties in Algeria, as well as neighboring countries such as Tunisia and Morocco. It is recommended for the treatment of several diseases (Dahia et al., Citation2007; Yangui et al., Citation2009). The primary objective of our study is to evaluate the antidiabetic, antioxidant, and anti-inflammatory properties of the aqueous extract of Pituranthos chloranthus through in vitro and in vivo experiments.
Material and methods
Plant material
The plant species examined in this study is frequently employed in traditional medicine for its anti-diabetic properties (Hammiche & Maiza, Citation2006), antioxidant effects, and anti-inflammatory characteristics. Pituranthos chloranthus (Coss. and Dur.) () was harvested in March 2022 from the province of El Menia (Algerian Sahara). The samples were dried at room temperature and protected from light. After drying, the specimens were cut and partially crushed using an electric mill, specifically the IKA/MF 10 basic Microfine grinder (Germany/Deutschland). They were then stored in tightly closed jars in a dry location until needed (Gao et al., Citation2015).
Preparation of the aqueous extract by maceration
Traditionally, water extraction commonly employed in traditional medicine was carried out as described by Majhenic et al. (Citation2007), and Bougandoura and Bendimerad (Citation2012), with some modifications. P. chloranthus powder (10 g) was added to 100 mL of distilled water, then shaken for 21 h, and finally allowed to macerate for an additional 3 hours at 25°C. Subsequently, it was filtered using Whatman No. 1 filter paper. After filtration, the extract was dried in an oven at a temperature not exceeding 50°C. The resulting extract obtained in the form of a thin solid film was scraped off using a flat spatula, stored in closed glass vials covered with aluminum foil, and kept in a 4°C refrigerator until ready for use within 30 days. Qualitative phytochemical tests were conducted on the aqueous P. chloranthus extract to detect polyphenols (flavonoids, tannins), reducing sugars, alkaloids, and saponins.
High-performance liquid chromatography (HPLC)
In the current work, we used a High-Performance Liquid Chromatography (HPLC) system, type Shimadzu LC 20 AL equipped with a universal injector (Hamilton 25 µL). The analytical column used was a Shim-pack VP-ODS C18 (4.6 mm × 250 mm, 5 µm), type Shimadzu. A UV-VIS detector SPD 20A (Shimadzu) was used. The mobile phase was a mixture of acetonitrile and acetic acid 0.1%. The contents of the mobile phase were filtered before use through a 0.45 μm membrane filter, sonicated, and pumped from the solvent reservoir to the column at a flow rate of 1 mL/min. According to Chouikh et al. (Citation2018), and 20 µL of plant extract solution was injected into the flow of the mobile phase. We adjusted the high pressure that drives the mobile phase by using a pump. The separated compounds shall be determined using the column for 50 min with the mobile phase in the effluent detected at λ = 268 nm and to the computer which records the results as chromatographic curves. In this study, the quantification of some peaks was compared by calibration of standards as mentioned below.
Ultra-performance liquid chromatography-mass spectrometry (UPLC/MS-MS)
For polyphenol standards optimization, we employed direct injection of 5 µL without a column (Restek Ultra C18 3 µm 150 × 4.6 mm) on a SHIMADZU 8040 Ultra-High Sensitivity system with UFMS technology, equipped with a binary pump Nexera XR LC-20AD. Gradient elution was performed using a mobile phase composed of solvent A (water with 0.1% formic acid) and solvent B (methanol), with a total flow rate of 0.4 ml/min. The ESI conditions were as follows: CID gas, 230 KPs; conversion dynode, −6.00 Kv; DL temperature, 250 ◦C; nebulizing gas flow, 3.00 L/min; heat block, 400 ◦C; drying gas flow, 15.00 L/min. A summary of MS/MS detection parameters is presented in .
Table 1. LC/MS detection parameters for analysed compounds in P. chloranthus (negative and positive ionization mode) (n = 3).
Animal material
Our study was conducted on female Wistar Albino rats aged between 9 and 11 weeks, with weights ranging from 150 g to 180 g. These animals were brought from the Pasteur Institute in Algiers, and raised in an animal facility at the Faculty of Natural and Life Sciences at the University of El-Oued. The rats were acclimatized and housed for two weeks prior to the commencement of the experiment under controlled conditions of light and temperature (12-hour light cycle, temperature maintained at 24°C). They were kept in plastic cages filled with sawdust, which was changed three times a week throughout the duration of the experiment. All procedures involving the treatment and handling of rats were conducted in accordance with the guidelines outlined in the manuals for the care and use of experimental animals, as per the standards established by the Canadian Council on Animal Care (CCAC) in 1984. In addition, these experiments received approval from the ethical committee, as evidenced by the report under code 35 EC/DCMB/FNSL/EU2024. The female rats weight change was monitored twice a week using an electronic scale.
Study of the toxicity of P. chloranthus extract on rats
The acute toxicity of P. chloranthus extract was assessed using 15 female Wistar rats, randomly allocated into three groups, each comprising 5 rats. The groups were designated as follows: G1 (untreated), G2 (treated with 500 mg/kg of body weight), and G3 (treated with 1000 mg/kg of body weight). Prior to administration, the rats underwent a 16-hour fasting period, with access to water provided ad libitum. Each group received a single daily dose of the aqueous extract orally using a gavage syringe, with all rats being maintained under consistent conditions throughout the study. The signs of toxicity and the weight of the rats were recorded during the duration of the experiment (7 days) according to the method of Pissang et al. (Citation2022) with some modifications.
All individuals were monitored at regular intervals during the initial 24 hours post-administration, with particular attention given to the first 4 hours. Subsequently, they were monitored daily for the duration of the experiment following the administration of the solution. Weight variations were calculated and recorded (El Kabbaoui, Citation2019).
Evaluation of the antidiabetic activity of P. chloranthus in vivo
After the toxicity test, another group of 15 adult Wistar Albino rats (between 150 g and 190 g) were injected with alloxan (Sigma, UK) after fasting for 12 h, intraperitoneal injection, prepared freshly in a volume of 5 mL of physiological water solution at a dose of 150 mg/kg b.w., was induced (Sabu et al., Citation2002). After the injection, the water bottles were replaced with those containing 5% glucose solution for 24 h to overcome any normal or fatal hypoglycemia that could occur. Alloxan-induced destruction of pancreatic β cells and the massive release of insulin (Chahlia, Citation2009).
Induced diabetes was confirmed in each of the rats to be studied after 48 h by measuring fasting blood sugar (Owoyele et al., Citation2005). Treatment with P. chloranthus extract was initiated 48 h after diabetes induction (El Kabbaoui, Citation2019), they were administered orally (gastric gavage) daily for a total treatment duration (21 days). All rats were divided into four groups, 5 rats for each. Normal group on a standard diet (G1); diabetic group subjected to a standard diet (G2); diabetic group subjected to a standard diet and treated daily with 500 mg/Kg b.w. of P. chloranthus extract (G3); diabetic group subjected to a standard diet and treated daily with 250 mg/Kg b.w. of P. chloranthus extract. During the treatment period, two parameters were monitored at regular intervals, namely: weight and blood sugar. In addition, biochemical parameters were taken into consideration i.e. glucose, triglycerides, total cholesterol, serum urea, creatinine, aspartate aminotransferase (ASAT) and alanine aminotransferase (ALT). Moreover, histological sections of the liver, heart, kidneys and spleen were made according to the technique described by Houlot (Citation1984).
Evaluation of the in vitro antioxidant activity of P. chloranthus
The capacity of an extract or a plant compound to eliminate free radicals was estimated in several ways.
Free radical scavenging test (DPPH)
This method involves completely dissolving 4 mg of DPPH in methanol. 12 mg of the extract was dissolved in 2 mL of methanol. 200 μL of the extract dissolved in methanol was added to 800 μL of the DPPH solution (3 repetitions), after which the solution was homogenized and incubated in the dark for 30 minutes. Finally, the absorbance of the prepared solutions were measured at 517 nm by a spectrophotometer (Azouaou et al., Citation2020; Dziri et al., Citation2012). Ascorbic acid was used as a standard.
Ferric reducing-antioxidant power (FRAP) iron reduction test
The activity of the reducing power was determined according to the method of Oyaizu (Citation1986), Benzie and Strain (Citation1996). 0.25 mL of the aqueous extract of the plant, at different concentrations (between 0.0625 and 1 mg/mL) was added to 0.625 mL of a buffer solution (sodium phosphate: 0.2 M, pH = 6.6). The mixture was added to 0.625 mL of a 1% solution of potassium ferricyanide [K3Fe (CN)6]. Test tubes containing the reaction mixture were incubated in a water bath at 50°C for 20 min. To stop the reaction, 2.5 mL of 10% trichloroacetic acid (TCA) was added. All tubes were centrifuged at 3000 rpm for 10 min. 0.625 mL of the supernatants were transferred to separate test tubes, into which 0.625 mL of distilled water and 0.125 mL of a freshly prepared solution of ferric chloride FeCl3 0.1% were already present. The absorbance reading of the reaction mixture was measured at 700 nm against a similarly prepared blank. The positive control was represented by ascorbic acid.
Evaluation of anti-inflammatory activity in vitro
To evaluate the anti-inflammatory activity, we used egg whites, according to the protein denaturation method described by Chandra et al. (Citation2012) with some modifications. We mixed 200 µL of fresh egg albumin with 2.8 mL of phosphate buffer saline (PBS, pH 6.4) and 2 mL of aqueous extract at different concentrations (0.0625 mg/mL). For the control, it consists of 2 mL of distilled water instead of the aqueous extract. Acetylsalicylic acid was used as the reference drug. Then, the mixtures were left at room temperature for 15 min, and then heated in a water bath at 70°C for 5 min. After cooling, the absorbance was measured at 660 nm. The percentage inhibition (PI%) of protein denaturation was calculated according to the formula of Bouhlali et al. (Citation2018):
Evaluation of anti-inflammatory activity in vivo
This activity was evaluated according to the method of Amezouar et al. (Citation2013) and Harchaoui (Citation2019) with some modifications. The rats were starved for 16 h before administering the product intragastrically. The rats were divided into four groups with five individuals for each. The first group was a negative control, the second was a positive control (reference), the third was treated with 500 mg/Kg b.w. and the last was treated with 250 mg/Kg b.w. The percentage of inhibition of edema was calculated according to the following formula:
- Dn: the diameter of the leg at time t after the injection of carrageenan.
- D0: the initial diameter of the paw before causing edema.
Statistical analysis
Statistical analysis was carried out using the statistical software R 4.2.3. The analysis included Principal Component Analysis (PCA) and ANOVA.
Results
In the present study, we obtained a dry extract weighing 1.012 g, corresponding to a yield of 10.12% but displayed low levels of alkaloids, terpenes, and tannins. Saponins and reducing sugars were found to be absent ().
Table 2. Phytochemical test of aqueous extract of P. chloranthus.
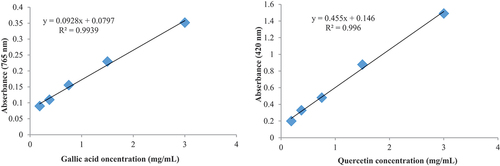
Based on the qualitative results obtained showing the significant presence of polyphenols and flavonoids, we selected these last two metabolites to carry out quantitative tests. According to the results, we saw that our extract contains polyphenols at a rate of 8.93 ± 0.74 mg GAE/g and also flavonoids estimated at 0.78 ± 0.02 mg EQ/g ().
The present experiment revealed the presence of polyphenols and flavonoids in the aqueous extract of P. chloranthus. According to Chabane et al. (Citation2013), flavonoids and tannins play a crucial role in the hypoglycemic effect of this plant. Additionally, they contribute to the body’s defense against free radical damage through their antioxidant and anti-enzymatic properties, as well as in limiting inflammatory reactions in various tissues (Hertel, Citation2003; Stoclet & Schini-Kerth, Citation2011). In addition, they are responsible for hepato-protective activity (Sangare et al., Citation2012). It also contains biological properties such as anti-allergic, antiviral, antibacterial and anti-tumor (Middleton, Citation1998). The antioxidant property of polyphenols is likely to prevent molecular and cellular oxidative damage inducing various pathologies (Amiot et al., Citation2009). In our study, the quantification of polyphenols revealed higher concentrations compared to previous research. Seladji (Citation2013) reported 4.089 ± 0.38 mg EAG/g in the aqueous extract of P. chloranthus, while Ben Nasr et al. (Citation2020) found 1.36 ± 0.02 mg EAG/g. In contrast, reported a higher quantity of polyphenols in this plant, amounting to 11.22 ± 0.98 mg EAG/g. The variation in phenol values across different extracts can be attributed to the presence of diverse phenolic compounds and their respective proportions. Their characteristics varies depending on their chemical structure and the environment in which they are situated, including factors such as acidity and basicity (Hayouni et al., Citation2007; Mellouk, Citation2013). Regarding the quantitative results of flavonoids, our results were close to those found by Khacheba et al. (Citation2014).
High-performance liquid chromatography (HPLC)
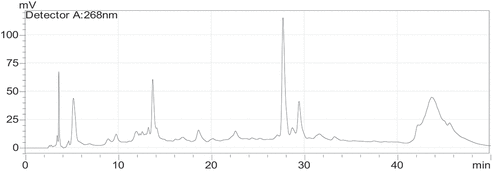
Phenolic compounds detected in the P. chloranthus fraction by HPLC are listed in .
Table 3. The concentration (µg/mg extract) of major phenolic compounds identified by HPLC in extracts of P. chloranthus (n = 3).
The HPLC analyzes have identified seven phenolic compounds in the aqueous extract of P. chloranthus, e.g. gallic acid, chlorogenic acid, vanillic acid, caffeic acid, p-coumaric acid, rutin, and quercetin (, ).
Ultra-performance liquid chromatography-mass spectrometry (UPLC/MS-MS)
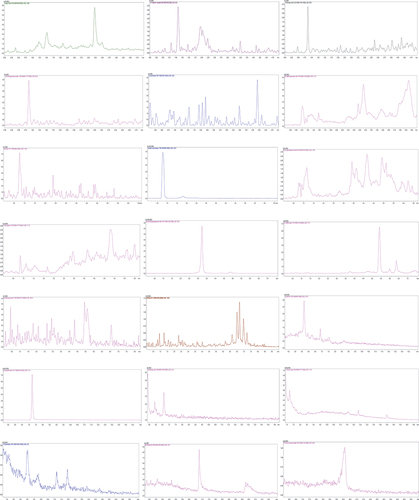
UPLC/MS chromatography profiles detailing the detection and quantification of the 21 separated phenols are shown in . The detected compounds in the aqueous extract of P. chloranthus are kojic acid, catechin hydrate, synaptic acid, chlorogenic acid, kaempferol, 2-methoxybenzoic acid, 4-methoxybenzoic acid, thymol, salicylic acid, coumaric acid, vanillin, naringenin, syringic acid, ferulic acid, 3,5 dihydroxybenzoic acid, p-coumaric acid, caffeic acid, quercetin, hespetin, hydroxyquenolin, and rutin.
Toxicity of P. chloranthus
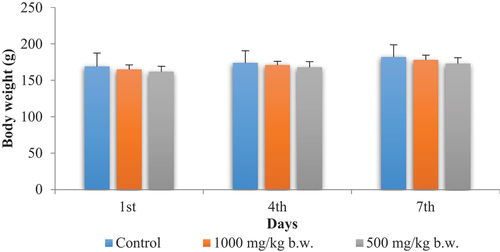
The oral administration of the aqueous extract of P. chloranthus at doses of 500 and 1000 mg/kg body weight to rats did not elicit any signs of acute toxicity throughout the 14-day observation period. There were no observable effects on the skin, hair, eyes, behavior, somato-motor activity, sleep, or mortality. A normal course of body growth was observed ().
The oral administration of P. chloranthus extract via gavage resulted in a natural increase in the growth rate for the two treated batches compared to the control group. Importantly, no signs of toxicity were observed during the study period. This observation indicates the non-toxic nature of these doses. Consequently, we proceeded to investigate its antidiabetic and anti-inflammatory activity at these doses in vivo.
Effect of diabetes induction in rats
In this study, rats made diabetic by intraperitoneal injection with 150 mg/kg of Alloxan (Sabu et al., Citation2002) showed hyperglycemia after 48 hours after the injection compared to control rats (). This was consistent with the results of other previous studies. This effect was explained by the cytotoxicity of Alloxan to pancreatic Langerhans cells, which can cause severe necrosis of the latter and thus causes a significant drop in insulinemia, which induces type 1 diabetes (Lenzen, Citation2008; Szkudelski, Citation2001).
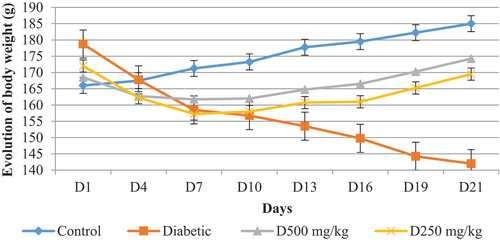
After 48 h of diabetes induction, the hypoglycemic activity of the aqueous extract of P. chloranthus was studied by following the evolution of body weight (). During the first days of treatment, severe weight loss was recorded in the three groups of diabetic rats (control and treated), while the healthy control exhibited normal growth development. Auroba (Citation2010) indicated that the loss of body weight in diabetic rats was caused by alloxan. From the second week, an increase in body weight was observed in all treated and control groups.
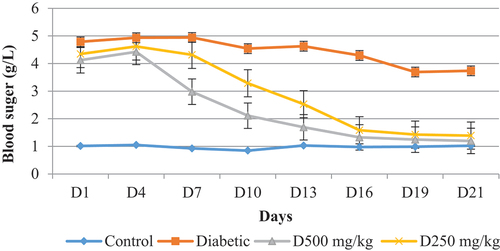
On the 16th day of the experiment, the treatments administered with P. chloranthus extract resulted in a reduction in blood sugar levels compared to the untreated diabetic group. This explains the biological potential of the extract to reduce hyperglycemia induced by alloxan.
The decrease in body weight was generally attributed to the stimulation of gluconeogenesis. The acceleration of protein and fat catabolism, due to the carbohydrate deficit. This deficit was due to continuous lipolysis by a lack of insulin because of the onset of diabetes leading to a significant loss of body weight after an increase in muscle atrophy and loss of tissue proteins (Daisy et al., Citation2012; Sathishsekar & Subramanian, Citation2005). The increase and improvement in body weight was perhaps due to the protective effect of our plant against weight loss associated with diabetes disease and ensuring normal growth through the activation of structural protein synthesis (Gandhi et al., Citation2012). In addition, this plant can stimulate pancreatic secretion of insulin, which promotes the storage of lipids and triglycerides (Babu et al., Citation2007; Kim et al., Citation2006) to control hyperglycemia.
After the injection of alloxan, the results showed an increase in blood sugar levels in control diabetic rats and rats treated with the extract, this effect is due to the administration of alloxan leading to the selective destruction of β cells of the islets of Langerhans, which leads to permanent hyperglycemia (Etuk, Citation2010). Alloxan is a glucose analog that preferentially accumulates in pancreatic β cells via the GLUT2 transporter and thus acts to destroy pancreatic cells (Pinheiro et al., Citation2011).
The effect of P. chloranthus extract represented a reduction in blood sugar levels, which was evident from the 16th day of the experiment in the group treated with this extract. Thus, the molecules included in the composition of this plant can influence the stimulation of insulin secretion at the level of pancreatic β cells by activating the SIRTUIN 1 enzyme, which stimulates the signaling chain of insulin secretion (Jang et al., Citation2000; Kilic et al., Citation1998). In addition, they can influence the absorption of glucose and its use by different tissues.
Variations in organ weight
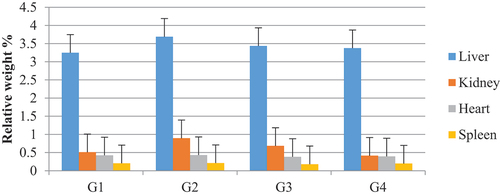
In the present study, a significant increase in the weight of organs including the liver, kidneys, and heart was observed in untreated diabetic rats compared to the other groups (). While the weight of the spleen did not present a difference between the treated (G3, G4) and control (G1) groups, except the treated group (G2).
According to studies carried out on the diabetogenic effect of alloxan, a loading of the liver is marked by the increase in the hepatic concentration of free TG and AG, which is due to their mobilization of adipose tissues towards the liver and the reduction of their degradation. This increase is caused by the absence of the hormone insulin, which leads to the breakdown of fats and glycogen under the influence of the hormone glucagon (Mihaela et al., Citation2006). Concerning the relative weight of the kidneys, its increase in diabetic rats is due to the proliferation of glomerular cells (Kumar et al., Citation2007).
In our study, we found that the treatment of diabetic rats with P. chloranthus extract attenuated the diabetogenic effect of alloxan and reduced the degree of diabetes as well as improving the lipo-distribution mechanisms controlling overloads lipids in non-adipose tissues such as the liver and heart (Unger, Citation2002). In addition, it contains chemical compounds that eliminate free radicals from pancreatic β cells, which leads to improving their efficiency in secreting insulin, which contributes to the conversion of glucose into glycogen (Ghorbani et al., Citation2019). It is attributed to the fact that diabetes causes a deficiency of hemoglobin (accompanied by high creatine levels (Babu et al., Citation2007), which leads to anemia, that causes the spleen to absorb iron and maintain a constant blood flow in the capillaries (Ghorbani et al., Citation2019). This decrease in hemoglobin levels can be explained by the increase in antibodies released during the onset of diabetes, resulting from the destruction of β cells in islets of Langerhans under the effect of autoimmunity (Beam, Citation2001).
Analysis of biochemical parameters
The results illustrated in the below showed the antidiabetic potential of the aqueous extract of P. chloranthus on rats treated with it (250-500 mg/Kg b.w.).
Table 4. Biochemical parameters.
The application of P. chloranthus extract on hypoglycemic individuals showed the existence of a very significant effectiveness compared to controls whose diabetic rats not treated with this extract recorded a very high average glucose (3.11 ± 0.77 g/L) compared to treated individuals (D500 = 0.8 ± 023; D250 = 0.54 ± 0.08) (). Idem, all the other biochemical parameters tested were very high in untreated diabetic individuals whose other diabetic subjects treated with the P. chloranthus extract recorded values similar to or close to the control individuals ().
Analysis of the correlation of doses and biochemical parameters
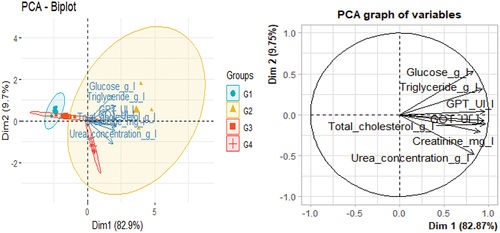
The analysis of the matrix revealed high correlation coefficients, 65.31% of which their variables were significantly correlated (). The eigenvalues representing the dosage of biochemical parameters of the groups studied on the axes are average, 82.9% for the first axis and 9.7% for axis 2, thus giving a good contribution to the total variance. The total information explained by the first three axes from the PCA was 92.6%.
The positive part of axis 1 was explained by triglyceride, GTP and GOT, while the positive part of axis 2 was explained by glucose. On the other hand, the negative part was explained by urea, creatinine and total cholesterol. The projection of the groups indicated the presence of a negative correlation between G1 and G3 with a disturbance of biochemical parameters. While G2 and G4 are positively correlated with these ().
Figure 10. Combination analysis between PCA and ANOVA of the biochemical parameters of the studied groups.
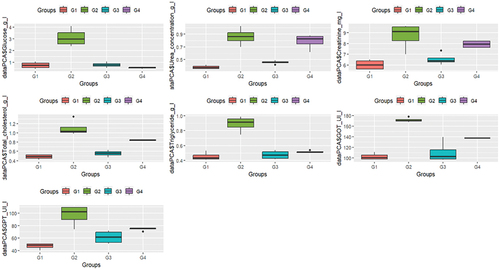
According to the analysis of ANOVA and PCA, the results indicated a significant relationship between groups 1 and 2 in stabilizing biochemical parameters, while the other groups exhibited a notable association with the disruption of these parameters ().
In our study, a very highly significant increase in the serum concentration of total cholesterol was recorded in rats rendered diabetic by alloxan because insulin inhibited the 3-hydroxy-3-methyl-glutaryl coenzyme. Insulin deficiency results in the inability to activate lipoprotein lipase, thereby causing hypertriglyceridemia and hypercholesterolemia (Shirwaikar et al., Citation2005). The differences in glucose levels between treated and non-treated rats may be explained by decreasing the expression of genes that control gluconeogenesis, increasing the storage of glucose in the liver and reducing the degradation of glycogen, inhibiting glucose transporters in the intestines (Sarkhail et al., Citation2007), and the conversion into fatty acids in the liver (Palsamy & Subramanian, Citation2008). Additionally, the reduction in uric acid levels may be due to the reduction in lipid peroxidation of triglycerides and cholesterol, while the elevation of these metabolites may increase uric acid synthesis (Derouiche, Citation2020). As for the increase in creatinine being explained by the effect of diabetes on the kidneys, it has been found that high doses of alloxan cause necrosis of the renal tubules (Nicolas, Citation2010). This result was explained by the accumulation of amino acids (alanine) in the serum coming from the degradation of protein compounds in the body (Bouiddouh, Citation2012). As a result, these amino acids can be transformed under the action of serum transaminases into carboxylic compounds such as α ketoglutarate and pyruvate. Which then implies a strong enzymatic activity of TGO and TGP. This can also be explained by the hepatotoxic effect of alloxan (Bouiddouh, Citation2012).
Histological sections
Histological sections made from treated and untreated individuals showed congestion of blood and vacuolation of cytoplasm in the liver of untreated diabetic rats; unlike the diabetic individuals treated with P. chloranthus extract presented just one small congestion of the blood (). Regarding the kidneys, the untreated batches showed some histopathological changes represented by a clear dilation of Bowman’s capsule, congestion of the blood vessels, congestion within the glomeruli and dilation of the tubules. Furthermore, the observation of an improvement in renal tissue following treatment with P. chloranthus extract was noted. This improvement was characterized by the restoration of glomerular size with reduced inflammation. Additionally, slight blood congestion was observed in certain areas with the administration of a higher dose (500 mg/kg) (), while examination of the heart revealed congestion of cardiac muscles in untreated diabetic individuals. However, both treated groups exhibited slight histological changes, manifested by the divergence of cardiac muscle fibers, particularly at lower doses. These changes could potentially be attributed to the effects of Alloxan (). Concerning the spleen, normal morphology was observed in the control batches and the treated diabetic batches, as for the untreated diabetic batch, the white pulp was densely compared to the red pulp, because it contains cells, which participate in the immune response resulting an inflammation in the spleen ().
Figure 11. Histological section of the different organs of treated and untreated individuals (G: 40x10) (BC: Blood congestion; VC: Vacuolization of the cytoplasm).
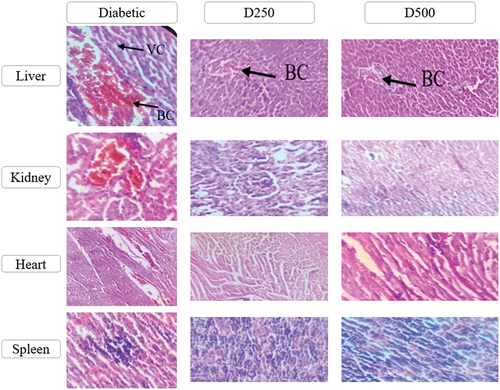
In this study, we noted a complete absence of fatty agglomerations in the liver of treated groups, whilst the presence of blood congestion was observed in the diabetic group. According to Mir et al. (Citation2008), all these signs are caused by poor blood drainage following hepatic venous obstruction, causing cessation or disruption of blood flow through hepatic visceral cells. On the other hand, a vacuolation of cytoplasm in diabetic and untreated rats was observed. It was caused by damage to liver cells resulting from immunological causes or from the toxic effect of alloxan (oxidative stress) resulting from the accumulation of radicals which cause the destruction of liver cells by lipid peroxidation of cell membrane or mitochondrial membranes, causing the emergence of an inflammatory and immune response, leading to severe blood congestion, hepatic hemorrhage, and the destruction of hepatocytes, as described by Majumdar et al. (Citation2008).
The untreated group exhibited some histopathological changes represented by a clear dilation of Bowman’s capsule, congestion of the blood vessels, congestion within the glomeruli and dilation of the tubules. It has been observed that kidney necrosis is among the changes induced by diabetes, as highlighted by Teoh et al. (Citation2010). Oxidative stress plays a significant role in the development of diabetic nephropathy, as emphasized by Abo-Salem et al. (Citation2009).
The notable improvement observed can be attributed to the active terpenoid and flavonoid components present in the tested plant. These compounds contribute to a reduction in lipid peroxidation and an increase in antioxidants in the rats. This leads to a decrease in accumulated fat and hypoglycemia, ultimately resulting in the enhancement of renal tissues (Abd-Alla et al., Citation2014). In the untreated diabetic group, the white pulp exhibited denser characteristics compared to the red pulp. This denser appearance may be attributed to the presence of cells involved in the immune response, leading to inflammation. It is plausible that alloxan toxicity contributed to the occurrence of this reaction (Tennenbaum et al., Citation2023).
Evaluation of the antioxidant activity of P. Chloranthus
In this study, the utilization of free radical trapping test (DPPH), revealed an antioxidant potential of our extract, demonstrating an inhibitory concentration of 0.156 ± 0.23 mg/mL. Comparatively, ascorbic acid exhibited exceptionally strong anti-radical activity, with an IC50 value of 0.0037 ± 0.505 mg/mL. The lower the IC50 value, the more the extract is considered a powerful antioxidant. On the other hand, the iron reduction test (FRAP) revealed that our extract has a lower reducing activity (0.718 ± 0.17 mg/mL) when compared with the standard ascorbic acid (0.064 ± 0.08 mg/mL). It was clear that our extract has considerable modest reductive power. This discrepancy could be explained by the variation in the phenolic compounds present in our extract.
Our extract showed lower antioxidant activity than the aqueous extract of Kadouri and Tabaa (2019) as well as Ben Nasr et al. (Citation2020) who found an inhibitory concentration equal to 0.54 ± 0.011 mg/mL, and 0.44 ± 0.01 mg/mL, respectively. On the other hand, Seladji (Citation2013) recorded a higher antioxidant activity with an IC50 = 501.7 mg/mL. In another study, the methanolic extract of P. chloranthus was 2.01 ± 0.34 µg/ml (Yangui et al., Citation2009). The presence of inhibitory capacity in the extract was attributed to the presence of active substances capable of scavenging or capturing the free radicals. Mohammedi (Citation2013) confirmed the existence of a direct correlation between flavonoid content and antioxidant capacity.
Regarding the effectiveness of iron reduction in our plant, it exceeded the values reported by Seladji (Citation2013), who found 7.32 ± 0.69 mg/mL. Additionally, Dridi (Citation2018) reported values of 0.0389 mg/mL and 0.0659 mg/mL for extracts obtained before and after the flowering period, respectively, using a methanolic extract of the same species. The functional groups present in phenolic compounds play an important role in the adsorption and neutralization of free radicals, quenching of singular and triplet oxygen or decomposition of peroxides (Amarowicz et al., Citation2004; Zheng & Wang, Citation2001). In addition, the antioxidant activity is linked to the structure and nature of the compounds contained in the extract (Baghiani et al., Citation2010). Phenolic compounds can be free radical acceptors or hydrogen donors or by readily donating an electron or proton (Yordi et al., Citation2012).
Anti-inflammatory activity in vitro and in vivo
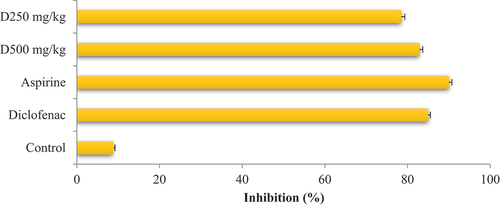
The results of the anti-inflammatory activity in vitro indicated an inhibition percentage equal to 82.32% with concentrations of 1 mg/mL of the extract, while acetylsalicylic acid presented an inhibition rate of 89.64% for the same concentration. On the other hand, the inhibitory concentration of the extract and acetylsalicylic acid was almost identical, i.e. 0.305 mg/mL and 0.203 mg/mL, respectively.
Regarding the in vivo test, we observed that the inflammation caused by carrageenan increased as a function of time, reaching a maximum of 6.96 ± 0.02 mm at the third hour. In addition, we have noted that acetylsalicylic acid (reference) gradually reduced the edema.
Furthermore, the P. chloranthus extract exhibited a highly significant inhibition against inflammation, with the diameter reduced to 5.74 ± 0.01 mm for the extract dose of 250 mg/kg body weight and 4.81 ± 0.02 mm for the extract dose of 500 mg/kg body weight. The progression of the kinetics of edema inhibition showed that the two extracts have a considerable anti-inflammatory effect, at 5 hours, with a very similar effect of Diclofinac. We can deduce the order of this inhibition as follows: Aspirin ˃ Diclofenac ˃ Extract (500 mg/Kg b.w.) ˃ Extract (250 mg/Kg b.w.) ().
Given the limited research on our species as an anti-inflammatory substance, we compared our results to those reported by Bouhlali et al. (Citation2018) on seeds of Cuminum cyminum, which belongs to the same family. They reported an IC50 value of 234.87 µg/mL. In addition, Derrouiche et al. (Citation2020) found that the IC50 equal to 247.12 µg/mL. The anti-inflammatory potential increased with increasing concentrations of P. chloranthus extract. These results, when compared to those of acetylsalicylic acid, the reference anti-inflammatory, indicated that the inhibitory effect of the tested extract varied depending on the dose. Consequently, the extract demonstrated potent anti-inflammatory action, particularly in preventing the thermal denaturation of egg white proteins. This effect can be attributed to the presence of the B vitamins group, notably vitamin B6, which possesses anti-inflammatory properties, along with vitamin B12 (Hosseinzadeh et al., Citation2012; Paez-Hurtado et al., Citation2023). Several studies report the anti-inflammatory potential of plants belonging to the Apiaceae family. After 3 h of carrageenan injection, the methanolic extract of Thapsia garganica at a dose of 500 mg/Kg caused an inhibition (48.7%) lower than that of our plant.
The inhibition of plantar edema in rats suggests the presence of bioactive molecules in the plant extract, particularly phenolic compounds such as flavonoids and tannins. These compounds potentially function as non-steroidal anti-inflammatories by inhibiting the enzymatic activity of cyclooxygenase (COX) and lipoxygenase (LOX). Consequently, they help reduce the production of pro-inflammatory mediators during the immune process. Additionally, flavonoids, as a subgroup of polyphenols, are known to modulate prostaglandin biosynthesis (Adebayo et al., Citation2015).
Conclusion
With the use of in vitro and in vivo tests, the current study enabled us to determine the biological characteristics of the Algerian Saharan medicinal plant. Its chemical composition, which is particularly rich in polyphenols, confers both therapeutic and commercial significance. According to the findings of the current study, the compounds present in P. chloranthus exhibit promising pharmacological properties that could be valuable in traditional medicine. These include anti-diabetic, antioxidant, and anti-inflammatory properties.
Ethical approval and consent to participate
The experimental procedures adhered to ethical guidelines and followed an approved protocol. In addition, these experiments were approved according to the Algerian ethical committee report under a code 35 EC/DCMB/FNSL/EU2024.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abd-Alla, H. I., Aly, H. F., Shalaby, N. M., Albalawy, M. A., & Aboutabl, E. A. (2014). Hunting for renal protective phytoconstituents in Artemisia judaica L. and Chrysanthemum coronarium L. (Asteraceae). Egyptian Pharmaceutical Journal, 13(1), 46–57. https://doi.org/10.4103/1687-4315.135597
- Abo-Salem, O. M., El-Edel, R. H., Harisa, G. E., El-Halawany, N., & Ghonaim, M. M. (2009). Experimental diabetic nephropathy can be prevented by propolis: Effect on metabolic disturbances and renal oxidative parameters. Pakistan Journal of Pharmaceutical Sciences, 22(2), 205–210.
- Adebayo, S. A., Dzoyem, J. P., Shai, L. J., & Eloff, J. N. (2015). The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complementary and Alternative Medicine, 15(1), 1–14. https://doi.org/10.1186/s12906-015-0669-5
- Amarowicz, R., Pegg, R. B., Rahimi-Moghaddam, P., Barl, B., & Weil, J. A. (2004). Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chemistry, 84(4), 551–562. https://doi.org/10.1016/S0308-8146(03)00278-4
- Amezouar, F., Badri, W., Hsaine, M., Bourhim, N., & Fougrach, H. (2013). Evaluation des activités antioxydante et anti-inflammatoire d’Erica arborea L. du Maroc. Pathologie Biologie, 61(6), 254–258. https://doi.org/10.1016/j.patbio.2013.03.005
- Amiot, M. J., Riollet, C., & Landrier, J. F. (2009). Polyphénols et syndrome métabolique: Polyphenols and metabolic syndrome. Médecine des maladies métaboliques, 3(5), 476–482. https://doi.org/10.1016/S1957-2557(09)73293-6
- Auroba, M. (2010). Study antidiabetic effect of Momordica Charantia (bitter gourd) seeds on Alloxan induced diabetic rats. The Iraqi Journal of Veterinary Medicine, 34(1), 165–170. https://doi.org/10.30539/iraqijvm.v34i1.675
- Azouaou, K., Touazi, K., Ayadi, B., & Seddaoui, I. (2020). Contribution à l’étude de la phytotherapie traditionnelle dans la région de Tizi-ouzou et à l’étude d’Asphodelus tenuifolius Cav [ Ph.D thesis]. University of Mouloud Mammeri Tizi-Ouzou,
- Babu, P. S., Prabuseenivasan, S., & Ignacimuthu, S. (2007). Cinnamaldehyde-a potential antidiabetic agent. Phytomedicine, 14(1), 15–22. https://doi.org/10.1016/j.phymed.2006.11.005
- Baghiani, A., Boumerfeg, S., Belkhiri, F., Khennouf, S., Charef, N., Harzallah, D., & Mosaad Attia, A. W. (2010). Antioxidant and radical scavenging properties of Carthamus caeruleus L extracts grow wild in Algeria flora. Comunicata Scientiae, 1, 28–136. https://doi.org/10.14295/cs.v1i2.50
- Beam, H. A. (2001). The effects of diabetes mellitus on fracture healing in BB Wistar rats. Rutgers the State University of New Jersey-New Brunswick.
- Ben Nasr, S., Aazza, S., Mnif, W., & Miguel, M. (2020). In-vitro antioxidant and anti-inflamatory activities of Pituranthos chloranthus and Artemisia vulgaris from Tunisia. International Journal of Applied Pharmaceutical Sciences and Research, 11(2), 605–614. https://doi.org/10.13040/IJPSR.0975-8232.11(2).605-14
- Benzie, I. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measure “Antioxidant power”: The FRAP assay. Analytical Biochemistry, 239(1), 70–76. https://doi.org/10.1006/abio.1996.0292
- Bouchenak, O., Yahiaoui, K., Benhabyles, N., Laoufim, R., & Arab, K. (2018). Phyco-chemical and chromatographic characterization of the essential oil of Nigella Sativa L. and evaluation of its antioxidant, hypoglycemic and anti-diabetic activities. Journal of Fundamental & Applied Sciences, 10(55), 82–99. https://doi.org/10.4314/jfas.v10i5s.5
- Bougandoura, N., & Bendimerad, N. (2012). Effet antifongique des extraits aqueux et méthanolique de Satureja calamintha ssp. (Nepeta) briq. Revue des Bio Ressources, 2(1), 1–7. http://dspace.univ-ouargla.dz/jspui/handle/123456789/6701
- Bouhlali, E. T., El-Hilali, J., Ennassir, J., Benlyas, M., Alem, C., Amarouch, M. Y., & Zegwouit, M. (2018). Anti-inflammatory properties and phenolic profile of six Moroccan date fruit (Phoenix dactylifera L.) varieties. Journal of King Saud University – Science, 30(4), 519–526. https://doi.org/10.1016/j.jksus.2017.08.011
- Bouiddouh, F. Z. (2012). Evolution des paramètres biochimiques sériques chez les rats wistar traités par l’extrait ethanolique des graines de la coloquinte (Citrullus colocynthis) [ MSc thesis]. University of Abu Baker Belkaid.
- Ceriello, A. (2000). Oxidative stress and glycemic regulation. Metabolism: clinical and experimental, 49(2), 27–29. https://doi.org/10.1016/s0026-0495(00)80082-7
- Chabane, D., Saidi, F., Rouibi, A., & Azine, K. (2013). Activité hypoglycémique de l’extrait aqueux d’Ajuga iva L. schreber chez les rats diabétiques induite par l’alloxane. Afrique Science Revue Internationale Des Sciences et Technologie, 9(1), 120–127..
- Chahlia, N. (2009). Effect of Capparis decidua on hypolipidemic activity in rats. Journal of Medicinal Plants Research, 3(6), 481–4.
- Chandra, S., Priyanka, C., Protapaditya, D., & Sanjib, B. (2012). Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pacific Journal of Tropical Biomedicine, 2(1), S178–S180. https://doi.org/10.1016/S2221-1691(12)60154-3
- Chouikh, A., Alia, F., Neffar, S., Rebiai, A., Adjal, E. H., & Chefrour, A. (2018). Evaluation of phenolic contents (quantitative and qualitative) and antioxidant activities in different physiological phases of Genista saharae coss. & dur. growing in the Sahara of Algeria. Analele Universitatii Din Oradea, Fascicula Biologie, 25(2), 83–89.
- Dahia, M., Laouer, H., Chaker, A. N., Prado, S., Meierhenrich, U. J., & Baldovini, N. (2007). Chemical composition and antibacterial activity of Pituranthos chloranthus volatile oil. Natural Product Communications, 2(11), 1159–1162. 1934578X0700201123. https://doi.org/10.1177/1934578X0700201123
- Daisy, P., Feril, G., & KANI, J. (2012). Evaluation of anti-diabetic activity of various extracts of Cassia auriculata L. Bark on streptozotocin induced diabetic wistar rats. International Journal of Pharmacy and Pharmaceutical Sciences, 4(4), 312–318.
- Derouiche, S. (2020). Oxidative stress associated with SARS-Cov-2 (COVID-19) increases the severity of the lung disease - A systematic review. Journal of Infectious Diseases and Epidemiology, 6(3), 121. https://doi.org/10.23937/2474-3658/1510121
- Derouiche, S., Seghieri, I., & Zebidi, M. (2017). Etude de l’activité antidiabétique et antioxydante de l’extrait aqueux d’Oudneya africana R. Br. de la région d’El Oued chez des rattes rendues diabétiques par l’alloxane [ MSc thesis]. University of EL Oued.
- Dièye, A. M., Sarr, A., Diop, S. N., Ndiaye, M., Sy, G. Y., Diarra, M., Gaffary, I. R., Sy, A. N., & Faye, B. (2008). Medicinal plants and the treatment of diabetes in Senegal: Survey with patients. Fundamental & Clinical Pharmacology, 22(2), 211–216. https://doi.org/10.1111/j.1472-8206.2007.00563.x
- Dridi, A. (2018). Etude phytochimique et activité biologique des deux espèces: Teucrium polium L. et Pituranthos chloranthus Coss et Dur [ PhD. Thesis]. University of Badji Mokhtar.
- Dziri, S., Hassen, I., Fatnassi, S., Mrabet, Y., Casabianca, H., Hanchi, B., & Hosni, K. (2012). Phenolic constituents, antioxidant and antimicrobial activities of rosy garlic (Allium roseum var. odoratissimum). Journal of Functional Foods, 4(2), 423–432. https://doi.org/10.1016/j.jff.2012.01.010
- El Kabbaoui, M. (2019). Etude de l’activité antidiabétique et du profil toxicologique de Cistus ladaniferus et Thymus satureioides [ PhD Thesis]. University of Sidi Mohamed Ben Abdellah.
- Etuk, E. U. (2010). Animals models for studying diabetes mellitus. Agriculture and Biology Journal of North America, 1(2), 130–134.
- Gandhi, G. R., Ignacimuthu, S., & Paulraj, M. G. (2012). Hypoglycemic and β-cells regenerative effects of aegle marmelos (L.) Corr. bark extract in streptozotocin-induced diabetic rats. Food and Chemical Toxicology, 50(5), 1667–1674.. https://doi.org/10.1016/j.fct.2012.01.030
- Gao, J., Zhang, T., Jin, Z. Y., Xu, X. M., Wang, J. H., Zha, X. Q., & Chen, H. Q. (2015). Structural characterisation, physicochemical properties and antioxidant activity of polysaccharide from lilium lancifolium thunb. Food Chemistry, 169, 430–438.. https://doi.org/10.1016/j.foodchem.2014.08.016
- Ghorbani, A., Rashidi, R., & Shafiee-Nick, R. (2019). Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomedicine & Pharmacotherapy, 111, 947–957. https://doi.org/10.1016/j.biopha.2018.12.127
- Hammiche, V., & Maiza, K. (2006). Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. Journal of Ethnopharmacology, 105(3), 358–367. https://doi.org/10.1016/j.jep.2005.11.028
- Harchaoui, L. (2019). Etude biotechnologique,biochimique de Deverra scoparia, plante endémique de Tamanrasset : Recherche de quelques activités biologiques [ PhD. Thesis]. University of Houari Boumediene USTHB.
- Hayouni, E. A., Abedrabba, M., Bouix, M., & Hamdi, M. (2007). The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chemistry, 105(3), 1126–1134. https://doi.org/10.1016/j.foodchem.2007.02.010
- Hertel, J. M. (2003). Plantes médicinales et diabète. Nouveau Magazine de phytomania.
- Hosseinzadeh, H., Sadeghnia, H. R., Ghaeni, F. A., Motamedshariaty, V. S., & Mohajeri, S. A. (2012). Effects of saffron (crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytotherapy Research, 26(3), 381–386. https://doi.org/10.1002/ptr.3566
- Houlot, R. (1984). Techniques d’histopathologie et de cytopathologie. Ed Maloine, 19, 225–227.
- Jang, Y. Y., Song, J. H., Shin, Y. K., Han, E. S., & Lee, C. S. (2000). Protective effect of boldine on oxidative mitochondrial damage in STZ-induced diabetic rats. Pharmacological Research: The Official Journal of the Italian Pharmacological Society, 42(4), 361–371. https://doi.org/10.1006/phrs.2000.0705
- Kamatou, G. P. P., Van Zyl, R. L., Van Vuuren, S. F., Figueiredo, A. C., Hubert, A., & Dorota, K. R. (2017). Food preservatives from plants. Chapter 3. In D. N. Karunaratne and G. Pamunuwa (Eds.), Food Additives. IntechOpen. https://www.intechopen.com/chapters/56355
- Khacheba, I., Djeridane, A., & Yousfi, M. (2014). Twenty traditional Algerian plants used in diabetes therapy as strong inhibitors of α-amylase activity. International Journal of Carbohydrate Chemistry, 12, 1–12. https://doi.org/10.1155/2014/287281
- Kilic, N., Malhatun, E., Elmali, E., & Altan, N. (1998). An investigation into the effect of sulfonylurea glyburide on glutathione Peroxidase activity in streptozotocin-induced diabetic rat muscle tissue. General Pharmacology: The Vascular System, 30(3), 399–401. https://doi.org/10.1016/S0306-3623(97)00277-2
- Kim, S. H., Hyun, S. H., & Choung, S. Y. (2006). Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. Journal of Ethnopharmacology, 104(1–2), 119–123. https://doi.org/10.1016/j.jep.2005.08.059
- Kumar, G., Banu, G. S., Murugesan, A. G., & Pandian, M. R. (2007). Effect of Helicteres isora bark extract on protein metabolism and marker enzymes in Streptozotocin induced diabetic rats. Iranian Journal of Pharmaceutical Research, 6(2), 123–129.
- Lenzen, S. (2008). The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia, 51(2), 216–226. https://doi.org/10.1007/s00125-007-0886-7
- Majhenic, L., Kerget, M. S., & Knez, Z. (2007). Antioxidant and antimicrobial activity of guarana seed extracts. Food Chemistry, 104(3), 1258–1268. https://doi.org/10.1016/J.FOODCHEM.2007.01.074
- Majumdar, A., Saraf, M., Andrades, N., & Kamble, R. (2008). Preliminary studies on the antioxidant activity of Tribulus terrestris and Eclipta alba. Pharmacognosy Magazine, 4(13), 102–107..
- Mellouk, K. (2013). Étude des activités antioxydante et antimicrobienne des flavonoïdes et des fractions flavoniques de la partie aérienne de Pituranthos chloranthus (Guezzeh) de la région de Biskra [ MSc. Thesis]. University of Abou Bekr Belkaid.
- Middleton, J. R. (1998). Effect of plant flavonoids on immune and inflammatory cell function. Advances in Experimental Medicine and Biology, 439, 175–182.. https://doi.org/10.1007/978-1-4615-5335-9_13
- Mihaela, H., Anca-Mihaela, P. S., Malina, C. E., & Mehedinti, T. (2006). L’effet de l’alloxane sur l’histologie du tissu pancréatique. Analele universităti Dunărea De Jos’’ Galati Medicină: Fascicula, 17(7), 29–34.
- Mir, S. H., Baqui, A., Bhagat, R. C., Darzi, M. M., & Shah, A. W. (2008). Biochemical and histomorphological study of streptozotocin-induced diabetes mellitus in rabbits. Pakistan Journal of Nutrition, 7(2), 359–64. https://doi.org/10.3923/pjn.2008.359.364
- Mohammedi, Z. (2013). Etude phytochimique et activités biologiques de quelques plantes médicinales de la région nord et sud-ouest de l’Algérie [ PhD. Thesis]. University of Abou Bekr Belkaid.
- N’Guessan, K., Kadja, B., Zirihi, G. N., Traoré, D., & Aké-Assi, L. (2009). Screening phytochimique de quelques plantes médicinales ivoiriennes utilisées en pays Krobou (Agboville, Côte-d‘Ivoire). Sciences & Nature, 6(1), 1–15. https://doi.org/10.4314/scinat.v6i1.48575
- Nicolas, G. (2010). Identification et caractérisation de gènes différemment exprimés dans les tubules proximaux de reins diabétiques et impliqués dans le développement de la néphropathie diabétique [ PhD. Thesis]. University of Montréal.
- Owoyele, V. B., Adeyemi, F. M., & Soladoye, A. O. (2005). Effect of aqueous leaves extract of Ocimum gratissimum (sweet basil) on alloxan induced diabetic rats. Pharmacognosy Magazine, 1(2), 62–64.
- Oyaizu, M. (1986). Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Japan Journal of Nutrition, 44(6), 307–315. https://doi.org/10.5264/eiyogakuzashi.44.307
- Paez-Hurtado, A. M., Calderon-Ospina, C. A., & Nava-Mesa, M. O. (2023). Mechanisms of action of vitamin B1 (thiamine), B6 (pyridoxine), and B12 (cobalamin) in pain: A narrative review. Nutritional Neuroscience, 26(3), 235–253. https://doi.org/10.1080/1028415X.2022.2034242
- Palsamy, P., & Subramanian, S. (2008). Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomedicine and Pharmacotherapy, 62(9), 598–605. https://doi.org/10.1016/j.biopha.2008.06.037
- Pinheiro, L. S., Dutra de Melo, A., Andreazzi, A. E., de Caires Júnior, L. C., Costa, M. B., & González Garcia, R. M. (2011). Protocol of insulin therapy for streptozotocin ¬Diabetic rats based on a study of food ingestion and glycemic variation. Scandinavian Journal of Laboratory Animal Science, 38(2), 117–127.
- Pissang, P., Koukoura, K. K., Gbekley, E. H., Hoekou, Y., Effoe, S., & Tchacondo, T. (2022). Toxicité aigüe de Pteleopsis suberosa Engl. & Diels: effet sur les paramètres sanguins Pastre. Afrique Science, 21(4), 82–87.
- Sabu, M. C., Smitha, K., & Kuttan, R. (2002). Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. Journal of Ethnopharmacology, 83(1–2), 109–116.. https://doi.org/10.1016/S0378-8741(02)00217-9
- Sangare, M. M., Bayala, B., Ategbo, J. M., Loko, F., & Dramane, K. L. (2012). Effets de l’extrait aqueux de Gomphrena celosioides (amaranthaceae) sur les enzymes hépatiques. Afrique Science, 8(3), 107–115.
- Sarkhail, P., Rahmanipour, S., Fadyevatan, S., Mohammad, I. A., Dehghan, G., Amin, G., Shafiee, A., & Abdollahi, M. (2007). Antidiabetic effect of phlomis anisodonta: Effects on hepatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Pharmacological Research, 56(3), 261–266. https://doi.org/10.1016/j.phrs.2007.07.003
- Sathishsekar, D., & Subramanian, S. (2005). Antioxidant properties of Momordica Charantia (bitter gourd) seeds on Streptozotocin induced diabetic rats. Asia Pacific journal of clinical nutrition, 14(2), 153–158.
- Seladji, S. M. C. E. (2013). Étude phytochimique et activités biologiques (antioxydante et antimicrobienne) des composés phénoliques des extraits de la partie aérienne de Pituranthos chloranthus (Guezzeh) de la région de Biskra [ MSc. Thesis]. University of Abou Bekr Belkaid.
- Shirwaikar, A., Rajendran, K., & Punitha, I. S. (2005). Antidiabetic activity of alcoholic stem extract of coscinium fenestratum in streptozotocin-nicotinamide induced type 2 diabetic rats. Journal of Ethnopharmacology, 97(2), 369–374. https://doi.org/10.1016/j.jep.2004.11.034
- Stoclet, J. C., & Schini-Kerth, V. (2011). Flavonoïdes alimentaires et santé humaine. Annales Pharmaceutiques Françaises, 69(2), 78–90. https://doi.org/10.1016/j.pharma.2010.11.004
- Szkudelski, T. (2001). The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiological Research, 50(6), 537–546.
- Tennenbaum, J., Volle, G., Buffet, P., Ranque, B., Pouchot, J., & Arlet, J. B. (2023). Dysfonction splénique au cours de la drépanocytose: mise au point. La Revue de Médecine Interne, 44(7), 335–343. https://doi.org/10.1016/j.revmed.2023.01.005
- Teoh, S. L., Latiff, A. A., & Das, S. (2010). Histological changes in the kidneys of experimental diabetic rats fed with Momordica charantia (bitter gourd) extract. Romanian Journal of Morphology and Embryology, 51(1), 91–5.
- Tiwari, B. K., Pandey, K. B., Abidi, A. B., & Rizvi, S. I. (2013). Markers of oxidative stress during diabetes mellitus. Journal of Biomarkers, 2013, 1–8. https://doi.org/10.1155/2013/378790
- Unger, R. H. (2002). Lipotoxic diseases. Annual Review of Medicine, 53(1), 319–336. https://doi.org/10.1146/annurev.med.53.082901.104057
- Yangui, T., Bouaziz, M., Dhouib, A., & Sayadi, S. (2009). Potential use of Tunisian Pituranthos chloranthus Essential Oils as a natural disinfectant. Letters in Applied Microbiology, 48(1), 112–117. https://doi.org/10.1111/j.1472-765X.2008.02499.x
- Yordi, E. G., Pérez, E. M., Matos, M. J., & Villares, E. U. (2012). Antioxidant and pro-oxidant effects of polyphenolic compounds and structure-activity relationship evidence. Nutrition, Well-Being and Health, 2, 23–48. https://doi.org/10.5772/29471
- Zheng, W., & Wang, S. Y. (2001). Antioxidant activity and phenolic compounds in selected herbs. Journal of Agricultural and Food Chemistry, 49(11), 5165–5170. https://doi.org/10.1021/jf010697n