ABSTRACT
β-sitosterol is waxy substance, white in color distributed in a variety of natural plant sources cereals, pulses, legumes, vegetables, fruits, and herbs. Several clinical and preclinical investigations indicate that β-sitosterol has a wide range of important health advantages. It prevents many types of malignancies, lowers levels of low density lipoprotein (LDL), lowers the risk of coronary artery disease, heart attacks, and atherosclerosis, and supports the body’s natural healing process. It is found to have cancer-protective properties and has the potency to be beneficial against breast, prostate, colon, lung, stomach, and leukemia. The present review provides a complete analysis and examines the literature on β-sitosterol from different dietary sources, with a focus on its toxicological characteristics and nutraceutical qualities. It lays down the theoretical basis and justification for directing the future planning and execution of clinical studies intended to confirm the importance and possible toxicity of β-sitosterol.
1. Introduction
Numerous compounds that are not only nutrients but can also prevent disease are found in plants. “Phytochemicals” refers to the principal compounds that plants make through their primary and secondary metabolism (Ferahtia, Citation2021). Its name, phyton, is a Greek word that means “plant.” More than a thousand different phytochemicals are produced by plants for a variety of reasons, including growth and defense against diseases and rival plants. The word “Phytochemicals” was first used in Citation1994, and soon after, scientists and researchers started to take an interest in it. Phytosterols, a subtype of steroids, are one of the significant types of bioorganic compounds (Ferahtia, Citation2021). The form of phytosterol β-sitosterol (plant sterol) that is most prevalent can be found in a variety of plant-based foods, including natural oils, soy products, including nuts. Numerous studies have shown that sitosterol has therapeutic and clinical uses that include decreasing cholesterol and low-density lipoprotein levels, neutralizing free radicals in the body, and, most intriguing, curing and preventing cancer (Afzal et al., Citation2022). Alginate/Chitosan nanoparticles with encapsulated -sitosterol (β-sitosterol Alg/Ch/NPs) are being tested for their efficacy in treating breast cancer as well as their in vivo pharmacokinetic characteristics (Afzal et al., Citation2022). Compared to other cancers, breast cancer affects women at a higher rate than the others (Zaheed et al., Citation2020). According to current projections, in situ diagnosis of ductal carcinoma as well as melanoma in the female breast were 49,290 and 101,280, respectively, in Citation2021 (Siegel et al., Citation2021). Sterols, which are regarded as membrane reinforcers because they preserve the domain structure of cell membranes along with control crucial biological processes, is the third most significant class of lipids (Yin et al., Citation2018). The primary sterol in vertebrates is cholesterol, which is mostly located in animal cell membranes. It functions as a secondary messenger in developmental signaling and has an impact on the fluidity of the cell membrane (Akihisa et al., Citation1991). Through a chick embryo chorioallantoic membrane assay, the angiogenic component (β-sitosterol) was isolated from the Aloe-Vera gel, and its impact on damaged blood vessels of the Mongolian gerbil was examined. It was discovered that intraperitoneal administration of β-sitosterol significantly improves the formation or motion recovery of new vessels in gerbil brains damaged (Choi et al., Citation2003). One of the most prevalent phytosterols found in human diets, β-sitosterol is present in high concentrations in the nonpolar portions of plants as well as marine organisms (200–400 mg daily). Consuming β-sitosterol alongside vitamins or plants that may drop blood sugar levels should be done with caution, as well as doses may need to be modified (Ferahtia, Citation2021).
2. Methodology
In our methodology, we gathered literature on β-Sitosterol from various sources, including Science Direct, Google Scholar, Web of Science, and PubMed. For access to paid articles, we collaborated with the The University of Lahore, Lahore, Pakistan, which graciously provided free access. We initially created an outline to establish a coherent structure for the review and then conceptualized the content. Adhering to this framework, we structured the review into sections, delving into the overall sources of β-Sitosterol, its bioactive potential in functional foods, and its capacity to combat chronic diseases.
3. Different sources of β-sitosterol
Among 200 plant sterols distributed in plants (Lagarda et al., Citation2006), β-sitosterol is major (comprising 60% of phytosterols) followed by campesterol (24%), stigmasterol (7%), and minor contents of 5-avenasterol, brassicasterol, beta-sitostanol, and campestanol (Chung et al., Citation2013). Due to significant health benefits associated with β-sitosterol, these biomolecules need to be provided through diet as the human body is incapable of generating these biomolecules (Chanioti et al., Citation2021). According to EFSA, 2009, the daily consumption of 1.5 to 2.4 g/kg of phytosterols can reduce the level of low-density lipoproteins in blood on average by 7 to 10.5%.
β-sitosterol is a waxy substance, white in color (Kakade & Magdum, Citation2012), distributed in a variety of natural plant sources cereals, pulses, legumes, vegetables, fruits (Piironen et al., Citation2002) and herbs (Gahlaut et al., Citation2013). Phytoecdysteroid is a crucial chemical for plant well-being and is derived from β-sitosterol which is distributed in various plant tissues like leaves, fruits, and rhizomes (Bin Sayeed et al., Citation2016). β-sitosterol along with γ-oryzanol acts as a structuring agent in plant oils and their amount influences the different mechanical properties of these oils (Calligaris et al., Citation2020). Moreover, β-sitosterol influences the metabolism of major bio-molecules and acts as a growth regulator when exposed to strident environmental conditions (Z. Li et al., Citation2019).
β-sitosterol is been extracted by using technologies like supercritical fluid extraction (SFE) (Sajfrtova et al., Citation2010), and also by conventional methods of solvent extraction and various chromatographic techniques (J. C. Ye et al., Citation2010).
3.1. Cereals, legumes, pulses and nuts
Cereals are valuable naturally available plant sterol sources and their contents remain the same for cultivars grown in the same area at the same time, but growing conditions affect sterol content, and various milling products of these cereals also give varied yields (Piironen et al., Citation2002). This is supported by a study that states, there is an inverse relation between β-sitosterol and total plant sterols in soya beans at elevated temperatures; there is an increase in plant sterol contents by 2.5-fold at the expense of decreasing β-sitosterol (Vlahakis & Hazebroek, Citation2000). Plant sterol is present either freely or linked through an ester or glycosidic linkage in plants (Hakala et al., Citation2002). Linked state sterols called “conjugates” could be either esterified to fatty acid or hydroxycinnamic acid or glycosylated with any hexose moiety or 6-fatty acyl-hexose (Moreau et al., Citation2002).
β-sitosterol constitute a major portion of plant sterols, and these are non saponifiable lipids (Abidi, Citation2001). Plant sterol contents of individual cereal varieties have been determined by researchers, but there is slight variation in data collected which may be attributed to sample differences, growing conditions, or different analytical methods (Piironen et al., Citation2002). Total sterol contents in cereals like barley, rye, wheat, rice, and oats are in the range of 0.35–1.20 g/kg, while in crude soya beans, they are 3.0–4.4 g/kg (Chanioti et al., Citation2021). Specific vegetable oils such as wheat germ and corn germ oil possess good phytosterol contents (Chanioti et al., Citation2021). Legumes and some seed oils (peanuts, soybean, olives & flaxseeds) contain the highest β-sitosterol contents among plants, which gives these oils stability due to their antioxidant nature; especially in olive oil (Lima & Block, Citation2019).
Due to stability and appealing flavor, vegetable oil; soybean, wheat germ oil, and rice bran oil (RBO) is employed as salad dressings and frying medium (De Deckere & Korver, Citation1996). Rice bran, a milling by-product consisting of pericarp, aleurone, and sub aleurone, is rich in bioactive compounds (Friedman, Citation2013). Certain vegetable oils, especially soya bean are employed for suppression of carcinogenesis (Kakade & Magdum, Citation2012).
Among nuts highest β-sitosterol contents are found in pistachio (Vecka et al., Citation2019). They are synthesized from acetyl co-A, and stabilize the phospholipid bilayer in nuts due to similarity in structure with cholesterol (Hartmann, Citation1998). Extraction and purifying methods could be the reason for reduced phytosterol contents in end products (Gecgel & Demirci, Citation2006). The following elaborates the different sources of β-sitosterol.
Table 1. Different sources of β-sitosterol.
3.2. Fruits
Fruits are a valuable phytosterol source. The yield of β-sitosterol in fruits relative to total phytosterols is 72–92% (Piironen et al., Citation2002). Avocado (Persea americana) is a pebbly dark-skinned fruit grown as a major crop in America and is the highest source of β-sitosterol among fruits (Duester, Citation2001). Individual fruit parts (peel, leaves & seeds) are rich sources of β-sitosterol; seeds of various fruits yield almost 11.8–28.5% plant sterols (Gornas et al., Citation2016).
Malus domestica Borkh. (Apple) is a widely cultivated fruit in the world. According to FAOSTAT globally 86 million tons of apples are produced. Apple leaves were recently discovered to be a great source of β-sitosterol. 1 gram of dried apple leaves contains 5 mg of β-sitosterol, similarly, pear (Pyrus communis) has about 9.4 mg of β-sitosterol per gram of dried leaves (Hammam et al., Citation2023).
β-sitosterol extracted from apple & pear leaves, through high-performance liquid chromatography, has shown good wound healing properties when applied in the form of rich emulgels (Hammam et al., Citation2023). Moreover, phenolic compounds in apple leaves are renowned for their antioxidative properties (Liaudanskas et al., Citation2014).
3.3. Herbs/Shrubs
Berberis is a genus of plants, belonging to the Berberidaceae family which is characterized by spiny, woody, perennial herbs and shrubs, its Species like jaeschkeana, aristate, lyceum Royle and vulgaris of the genus Berberis are well known as a rich source of vital medicinal compounds (Perveen & Qaiser, Citation2010). The stem, bark, root, root bark, leaves, and flowers of these plants are rich sources of phytochemicals and phytosterols (I. Khan et al., Citation2016).
The Fractional composition of sterols in barberry’s (Berberis vulgaris) fruit powder showed the highest β-sitosterol contents alongside campesterol and delta 5-avenasterol (Dubtsova et al., Citation2021). Berberis aristata is used to extract phytochemicals (caffeic acid, quercetin & chlorogenic acid) & root contents of this shrub have the potential to reduce oxidative stress (Singh & Kakkar, Citation2009). β-sitosterol, a secondary metabolite of B.aristata provides effective protection from lipid peroxidation triggered by FeCl2 (C. R. Zhang et al., Citation2013).
The use of Berberis lycium Royle to treat rheumatism, ENT, gastric and skin problem is well known, but recently its root bark has been used to extract β-sitosterol, which possess a considerable cytotoxic potential (Anwar et al., Citation2020).
Alamzeb et al. (Citation2013) reported that some alkaloid and non-alkaloid fractions obtained from Berberis jaeschkeana also had β-sitosterol which is used as an effective antimicrobial agent.
4. Clinical importance of β-sitosterol
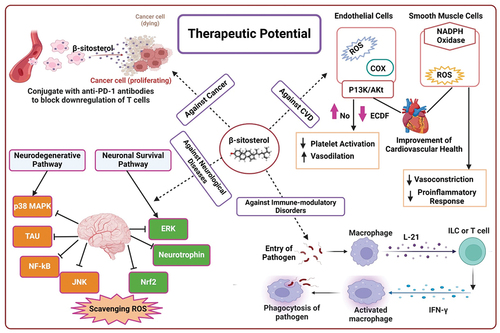
Phytochemicals have long been attracting the interest of researchers due to the countless benefits they offer to human health. Steroids have many subclasses, one such subclass is phytosteroids, which have a structure similar to cholesterol. One of the phytosterols is β-sitosterol. β-sitosterol, which is a plant-derived nutrient, is known to have many health benefits. Much research has been done on this nutrient to study the therapeutic effects β-sitosterol has on the human body. It is found to have cancer-protective properties and has the potency to be beneficial against breast, prostate, colon, lung, stomach, and leukemia. Several studies have been conducted showing the interference of β-sitosterol on numerous cell signaling pathways suggesting its anticancer, anti-inflammatory, hepato-protective, anti-tumor, antioxidant, anti-diabetic, and cardio-protective role, without causing toxicity. β-sitosterol is found in lipid-rich food products such as nuts, legumes, seeds, and extra-virgin olive oil. Owing to the advantages offered by this nutrient, many products that contain β-sitosterol have been commercialized.
Studies have found that consuming low amounts of β-sitosterol may help in cancer by killing cancer cells, therefore decreasing the chances of cancer progression. This cholesterol-like compound is used by humans as a nutrient in amounts ranging from 200 to 400 mg per day. According to the research conducted, this nutrient stops the absorption of cholesterol, hence being beneficial in controlling morbid diseases such as strokes, diabetes, cardiovascular disorders, and obesity. Apart from being a natural chemical that stops the proliferation, angiogenesis, invasion, and metastasis in cancer cells, this nutrient is also known to possess trypanocide and mosquito larvicidal properties (Z. Khan et al., Citation2022). β-sitosterol helps combat diseases such as coronary artery disease, heart attack, and atherosclerosis by reducing the levels of low density lipoprotein LDL in the body, by helping the body’s recovery process in fighting against morbid diseases. Heavily incorporated in oils such as olive oil, sesame seed oil, flaxseed, canola, and corn oil, this phytosterol has pharmacological and therapeutic benefits (E. Gupta, Citation2020).
β-sitosterol is known to possess anti-nociceptive activity, a study that was done on mice, in which the mice were given different concentrations of β-sitosterol, suggested that β-sitosterol in concentrations of 5, 10, and 20 mg/kg has anti-nociceptive properties in mice, which was confirmed by tail flick and hot-plate test in mice (Sakul & Okur, Citation2021). β-sitosterol has positive effects on the colon. In a study that was conducted on mice colon, β-sitosterol was responsible for reducing the colon length and inflammation by reducing the survival chances of Salmonella typhimurium which is known to produce inflammatory responses in the colon (Ding et al., Citation2019). β-sitosterol was linked to balancing sex hormones in PCOS-like mice and had a positive impact on the gut microbiome at the same time. Therefore, leading to the conclusion that β-sitosterol has beneficial applications in the treatment of PCOS (Yu et al., Citation2021). In another study conducted on a mice model, it was observed that β-sitosterol had a positive effect on aged mice suffering from Alzheimer’s disease, not only was it found to be beneficial for reducing the progression of Alzheimer’s disease but also had beneficial effects in later stages of Alzheimer’s disease (J. Y. Ye et al., Citation2020). illustrate the sources, disease prevention and importance of β-sitosterol.
Table 2. The sources, study level, disease prevention and importance of β-sitosterol.
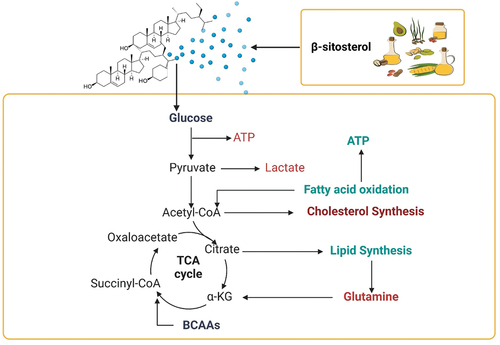
Not only does β-sitosterol have neuro-protective but it also has anti-diabetic properties. In a study conducted on diabetic rats, it was seen that β-sitosterol balanced the levels of blood glucose, serum insulin, testosterone, oxidative stress markers, and lipid profile all the while improving glycemic control suggesting its positive impact as an anti-diabetic agent (Ponnulakshmi et al., Citation2019). According to a study conducted on rats in Citation2020, which focused on the anti-oxidative properties of β-sitosterol with chronic alcohol intake. It was observed that β-sitosterol is linked with reducing oxidative stress caused by excessive alcohol intake. Alcohol-induced liver damage was reduced with the consumption of β-sitosterol in rats. Different concentrations of β-sitosterol had beneficial effects on biochemical indicators that are produced due to the intake of alcohol. The oxidative stress that is produced after the intake of alcohol was significantly reduced in rats such as erythrocyte membrane fluidity was improved, glutathione depletion was reduced and antioxidant enzyme activity was restored (Z. Chen et al., Citation2020). Due to the inflammation-reducing response created by β-sitosterol, it has promising effects on renal tissues as well. Reducing inflammation, and oxidative stress is the main mechanism by which β-sitosterol reduces renal and cardiac necrosis (Koc et al., Citation2021). β-sitosterol, which has a structural similarity with cholesterol, is such a nutrient that can be incorporated into food products and can be used in supplements to get the numerous health benefits it offers to humans. illustrates the effect of β-sitosterol on regulation of lipid metabolism.
4.1. Anticancer
Nowadays, there is a great deal of attention on using everyday lifestyle behaviors to avoid primary cancer. It is well established that β-sitosterol and cholesterol cause apoptosis by inducing caspase-3 activation in cells. Although they are not as readily absorbed as cholesterol, plant sterols nevertheless have an effect on a number of physiological functions. These substances effectively lower blood levels of low-density lipoproteins and minimize the risk of atherosclerosis. Furthermore, they reduce the viability of both cancer and normal cells in a way that depends on exposure time and dosage (Rubis et al., Citation2008). Regular consumption of liposomal β-sitosterol on a daily basis may prevent tumor metastasis by potentially boosting the effectiveness of gut immune surveillance mechanisms (Imanaka et al., Citation2008).
4.1.1. Colon cancer
The proliferation of COLO 320 DM cells is inhibited dose-dependently by β-sitosterol (IC50 266.2 microM), which also scavenges reactive oxygen species to cause apoptosis and suppresses the expression of β-catenin and proliferating cell nuclear antigen in human colon cancer cells (Baskar et al., Citation2010).
Adding β-sitosterol to colon cancer cell lines prevents them from growing without changing the overall amount of phospholipids in the membrane. It is most likely the effect on signal transduction pathways connected to membrane phospholipids that is responsible for this growth inhibition (Gu et al., Citation2023).
4.1.2. Breast cancer
β-sitosterol inhibits the invasion of tumor cells, such as MDA-MB-231, through Matrigel and their adhesion to plates coated with collagen I, collagen IV, fibronectin, and laminin, consequently restricting the adhesive interactions between tumor cells and the basement membrane. Exposure to β-sitosterol leads to its accumulation in the membranes of transformed cells (Awad et al., Citation2007). Apoptosis induction, which is accomplished by inhibiting the extracellular signal-regulated kinase 1 and 2 (ERK1/2) signaling pathway, causes cell death. Increases in proapoptotic Bcl-2 protein levels and decreases in procaspase-9 and procaspase-3 levels are involved in this process. Notably, after treatment, these impacts on normal cell controls are negligible, keeping cell viability levels at ≥ 80% (Tasyriq et al., Citation2012).
4.1.3. Prostate cancer
When human prostate cancer (PC-3) cells are supplemented with β-sitosterol, their proliferation is suppressed, resulting in cell cycle arrest in the G2/M phase. In addition, it promotes prostaglandin secretion, raises ROS levels, and triggers apoptosis. Furthermore, it prevents PC-3 cells from encroaching on Matrigel-coated membranes and from attaching to fibronectin and laminin, which may indicate that growth and metastasis are inhibited (Reddy et al., Citation2022). In vitro primary prostate stromal cell cultures supplemented with β-sitosterol express and secrete transforming growth factor β1 (TGF-β1) and Protein Kinase C-α (PKC-α), which translocates to the cytosol from its membrane-associated active form (Dedić et al., Citation2023).
4.2. Antidiabetic
When diabetic rats induced with streptozotocin received oral doses of β-sitosterol at 10, 15, or 20 mg/kg for 21 days, there was a reduction in glycated hemoglobin, nitric oxide, and serum glucose levels. Simultaneously, there was an increase in antioxidants and serum insulin within pancreatic cells, alongside a decrease in thiobarbituric acid-reactive substances (R. Gupta et al., Citation2011). In a study, oral SIT treatment reduced fasting blood glucose and enhanced oral glucose tolerance in hyperglycemic rats by raising fasting plasma insulin levels; the outcomes were contrasted with those of the conventional medication Glibenclamide (Babu et al., Citation2020).
4.3. Antihyperlipidemic activity
β-sitosterol disrupts the absorption of micellar cholesterol, resulting in reduced influx of plasma membrane cholesterol and secretion of cholesteryl ester (Mel’nikov et al., Citation2004). β-sitosterol reduces the synthesis of cholesterol by affecting the expression of the HMG-CoA reductase gene (Liang et al., Citation2015).
4.4. Immunomodulatory disorders
By inhibiting eosinophil infiltration and goblet hyperplasia-induced mucus hypersecretion, β-sitosterol reduces the generation of reactive oxygen species. In lung tissue and bronchoalveolar lavage fluid, respectively, lactose-β-sitosterol and β-sitosterol block elevated mRNA and protein production of IL-4 and IL-5. One of lactose-β-sitosterol’s (L-BS) anti-asthmatic properties is demonstrated by its ability to obstruct IgE in bronchoalveolar lavage fluid and to reduce mouse splenocyte survival rates (Yuk et al., Citation2007). When activated via the conventional pathway, glucoside-3′-O-β-sitosterol has been demonstrated to exhibit a strong inhibitory effect on the complement system (Ambavade et al., Citation2014).
4.5. Neurological disorders
Dietary β-sitosterol is able to enter the brain and build up in the brain cells’ plasma membrane. β-sitosterol in the mitochondrial membrane enhances the fluidity of the inner mitochondrial membrane while leaving the fluidity of the outer mitochondrial membrane unaffected. As a result, this increase in mitochondrial membrane potential (∆Ψm) and mitochondrial ATP content could potentially offer benefits for neurodegenerative disorders such as Alzheimer’s disease (AD) (Shi et al., Citation2013). This incorporation effectively shields against oxidative stress and lipid peroxidation induced by glucose oxidase. Moreover, it enhances PI3K activity, facilitates the recruitment of PI3K to lipid rafts, and promotes the expression of p-GSK3B. Notably, these effects can be counteracted by estrogen receptor antagonists. Hence, nutrients enriched with β-sitosterol hold promise for ameliorating neurodegenerative disorders such as Alzheimer’s disease (AD) (Sharma et al., Citation2021).
4.6. Reproductive issues
Brown American mink (Neovison vison) were given 50 mg/kg/day of β-sitosterol for 10 months, and this improved reproductive success suggests that β-sitosterol might be employed to improve these mammals’ reproductive capabilities (Balkrishna et al., Citation2021). Plant sterols, predominantly β-sitosterol, elevate testosterone levels in the testes of male pups in the F2 generation, raise plasma testosterone levels, and reduce relative uterine weights in F2 and F4 generation pups. Additionally, they boost plasma estradiol concentrations in female pups of the F3 generation. Despite these temporary alterations, there are no lasting detrimental effects on mouse reproduction (Kopylov et al., Citation2021). elaborates the therapeutic potential of β-sitosterol in detail.
5. Toxicity
The phytosterol, β-sitosterol is known for its benign uses, but there is a limit to its consumption for humans. Even a beneficial compound can have detrimental effects if it is misused. β-sitosterol may have many health benefits, but a limit is set up for its usage daily to avoid toxic buildup in the body. β-sitosterol has many pharmacological uses such as antibacterial, antinociceptive, stabilizing activity, thrombolytic activity, anthelmintic activity, antibiotic activity, anti-diarrheal, and hepato-protective activity but only within a certain range, it is thought to provide these benefits (Kudumula et al., Citation2021). However, the control for supplements is not as strict as it is for drugs. Anyhow, The US Food and Drug Administration has set up daily limits for supplements and drugs to avoid the overconsumption of these substances. β-sitosterol is consumed as a supplement in many forms; it is either incorporated into food items or is taken as a dietary supplement. Various companies have mixed β-sitosterol in margarine, yogurt, and other foods. The daily consumption of β-sitosterol incorporated into food 1.5 to 3 grams per day. Whereas, to reduce the levels of low density lipoprotein LDL in the body by 6% to 15% it is estimated that the body requires almost 2 grams of β-sitosterol per day. For the treatment of a swollen prostate, the limit ranges from about 130 mg to 180 mg per day. It is noteworthy that the intake of β-sitosterol can lead to reduced levels of vitamin E and carotene so, supplementation of this vitamin should be considered along with using β-sitosterol. This is the limit for the consumption of β-sitosterol daily if the limit is exceeded, there can be dire consequences. People who have already had a heart attack were observed to have increased chances of other heart-related diseases. Phytosterols are generally regarded as safe GRAS, but these sterols should be consumed within safe limits as they can trigger an underlying condition. It has been observed that β-sitosterol has been linked to causing many gastrointestinal disturbances such as flatulence, stool discoloration, changes in appetite, dyspepsia, leg cramps, skin rashes, leucopenia, and in a few cases can also cause impotency. In a study that was conducted on rats, it was seen that at higher doses of 5 mg/kg, the rats became infertile. With increased therapy, a decrease in the testicular size and sperm count was observed whereas, a one-year trial in which patients were given 1.6 mg of dosage daily, showed an overall reduction in the cholesterol (Matsuoka, Citation2022).
6. Limitations and enhancements of β-sitosterol
When exposed to 180°C for two hours, 75% of cholesterol and β-sitosterol oxidized, demonstrating their thermal instability. β-sitosterol’s oxidative behavior was similar to that of cholesterol in terms of both the rate of oxidation and the compounds that were produced as a result (Xu et al., Citation2009). The application of β-sitosterol is related to its poor targeted efficacy and absorption (Wang et al., Citation2023). β-Sitosterol (SIT) has a low intestinal absorption capacity and a high rate of biliary excretion, which reduces its bioavailability concentration (Gil-Ramirez et al., Citation2014). Large dosages of β-sitosterol (up to 25 to 50 g/day) are required to achieve a desired drop in serum cholesterol levels (Marangoni & Poli, Citation2010).
The butylated hydroxyl toluene BHT, green tea catechins, alpha-tocopherol, and quercetin were more effective at preventing the oxidation of beta-sitosterol and can improve the thermal instability (Arivarasu, Citation2023). By adding SIT to PLGA nanoparticles, its concentration-dependent antiproliferative effects on MCF-7 and MDA-MB-231 cells were enhanced compared to conventional carrier vehicles (Andima et al., Citation2018). β-sitosterol (SIT) has been created into nanoparticle formulations to overcome its problems relating to low targeting efficacy, poor aqueous solubility, and systemic bioavailability. The usefulness of these SIT-based nanoformulations against cancer has been studied, and the results show a much more promising efficacy (Karim et al., Citation2022).
7. Future prospects of β-sitosterol
β-sitosterol has great potential considering the anti-cancer effect it has on the body. But, currently, the research has slowed down a bit on β-sitosterol. Whereas, if efforts are made in the research industry this plant sterol can do wonders. The only limiting factors for the usage of β-sitosterol include its bioavailability and low targeting effect. As a dietary chemo-preventive and chemotherapeutic agent, β-sitosterol holds good potential. With modern drug delivery systems, the problem of bioavailability can be solved (Wang et al., Citation2023). There is a great deal of data that supports the fact that β-sitosterol has hundreds of benefits, that are not only being used now but will be used in the future.
8. Conclusion
Most typically found in plants, β-sitosterol is also referred to as “plant sterol ester” and has a variety of uses, including in the medical area and the global food industry. Knowing the structure, biosynthesis, as well as behavior of β-sitosterol requires knowledge of chemistry, biochemistry, and biotechnology. Due to significant health benefits associated with β-sitosterol, these biomolecules need to be provided through diet as the human body is incapable of generating these biomolecules. This substance has a significant impact on human physiology and shares structural similarities with cholesterol. β-sitosterol is been extracted by using technologies like supercritical fluid extraction (SFE), and also by conventional methods of solvent extraction and various chromatographic techniques. Studies have shown that β-sitosterol interferes with a number of cellular signaling pathways, which include cell cycle control, apoptosis, proliferation, survival, invasion, angiogenesis, metastasis, anti-inflammatory responses, anticancer characteristics, hepatoprotective effects, antioxidant activity, cardioprotection, and antidiabetic effects. Despite this, β-sitosterol exhibits minimal toxicity in pharmacological evaluations. It is regarded as the astonishing, more effective drug of the future. Understanding the possible advantages offered by this exceptional phytosterol requires consideration of a wide range of scientific applications.
Consent for publication
All authors agree to publish.
Authors contributions
Azka Khan Durrani contribute for the conception of the paper.
Momina Khalid layout the design of paper.
Awais Raza critically analysis the data.
Izza Faiz ul Rasool done the interpretation of the data.
Waseem Khalid drafting the paper.
Muhammad Nadeem Akhtar revising the paper critically for intellectual content.
Ammar Ahmad Khan also revising the paper critically for intellectual content.
Zunair Abdullah also revising the paper critically for intellectual content.
Babirye Khadijah prepare the final draft of paper and after analysis done the final approval before submission.
Acknowledgments
The authors are gratefully thanking the University of Lahore for their support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Abidi, S. L. (2001). Chromatographic analysis of plant sterols in foods and vegetable oils. Journal of Chromatography A, 935(1–2), 173–10. https://doi.org/10.1016/S0021-9673(01)00946-3
- Afzal, O., Akhter, M. H., Ahmad, I., Muzammil, K., Dawria, A., Zeyaullah, M., Altamimi, A. S. A., Khalilullah, H., Mir Najib Ullah, S. N., Rahman, M. A., Ali, A., Shahzad, N., Jaremko, M., Emwas, A.-H., & Abdel Aziz Ibrahim, I. (2022). A β–sitosterol encapsulated biocompatible alginate/Chitosan polymer nanocomposite for the treatment of breast cancer. Pharmaceutics, 14(8), 1711. https://doi.org/10.3390/pharmaceutics14081711
- Akihisa, T., Kokke, W., & Tamura, T. (1991). Naturally occurring sterols and related compounds from plants. In G. W. Patterson & W. D. Nes (Eds.), Physiology and biochemistry of sterols (pp. 172–228). American Oil Chemists’ Society.
- Alamzeb, M., Khan, M. R., Ali, S., Shah, S. Q., & Rashid, M. U. (2013). Antimicrobial properties of extracts and compounds isolated from Berberis jaeschkeana. Bangladesh Journal of Pharmacology, 8(2), 107–109. https://doi.org/10.3329/bjp.v8i2.13551
- Alvarez-Sala, A., Attanzio, A., Tesoriere, L., Garcia-Llatas, G., Barberá, R., & Cilla, A. (2019). Apoptotic effect of a phytosterol-ingredient and its main phytosterol (β-sitosterol) in human cancer cell lines. International Journal of Food Sciences and Nutrition, 70(3), 323–334. https://doi.org/10.1080/09637486.2018.1511689
- Ambavade, S. D., Misar, A. V., & Ambavade, P. D. (2014). Pharmacological, nutritional, and analytical aspects of β-sitosterol: A review. Oriental Pharmacy and Experimental Medicine, 14(3), 193–211. https://doi.org/10.1007/s13596-014-0151-9
- Andima, M., Costabile, G., Isert, L., Ndakala, A. J., Derese, S., & Merkel, O. M. (2018). Evaluation of β-sitosterol loaded PLGA and PEG-PLA nanoparticles for effective treatment of breast cancer: Preparation, physicochemical characterization, and antitumor activity. Pharmaceutics, 10(4), 232. https://doi.org/10.3390/pharmaceutics10040232
- Anwar, M. A., Tabassam, S., Gulfraz, M., Sheeraz Ahmad, M., Raja, G. K., & Arshad, M. (2020). Isolation of oxyberberine and β-sitosterol from Berberis lycium Royle root bark extract and in vitro cytotoxicity against liver and lung cancer cell lines. Evidence-Based Complementary and Alternative Medicine, 2020, 1–9. https://doi.org/10.1155/2020/2596082
- Arivarasu, L. (2023). In-vitro antioxidant potential of beta-sitosterol: A preface. Cureus, 15(9). https://doi.org/10.7759/cureus.45617
- Awad, A. B., Chinnam, M. F. C. S., Fink, C. S., & Bradford, P. G. (2007). β-sitosterol activates fas signaling in human breast cancer cells. Phytomedicine, 14(11), 747–754. https://doi.org/10.1016/j.phymed.2007.01.003
- Babu, S., & Jayaraman, S. (2020). An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomedicine & Pharmacotherapy, 131, 110702. https://doi.org/10.1016/j.biopha.2020.110702
- Babu, S., Krishnan, M., Rajagopal, P., Periyasamy, V., Veeraraghavan, V., Govindan, R., & Jayaraman, S. (2020). Beta-sitosterol attenuates insulin resistance in adipose tissue via IRS-1/Akt mediated insulin signaling in high fat diet and sucrose induced type-2 diabetic rats. European Journal of Pharmacology, 873, 173004. https://doi.org/10.1016/j.ejphar.2020.173004
- Balkrishna, A., Nain, P., Joshi, M., Khandrika, L., & Varshney, A. (2021). Supercritical fluid extract of putranjiva roxburghii wall. Seeds mitigates fertility impairment in a zebrafish model. Molecules, 26(4), 1020. https://doi.org/10.3390/molecules26041020
- Baskar, A. A., Ignacimuthu, S., Paulraj, G. M., & Al Numair, K. S. (2010). Chemopreventive potential of β-sitosterol in experimental colon cancer model-an in vitro and in vivo study. BMC Complementary and Alternative Medicine, 10(1), 1–10. https://doi.org/10.1186/1472-6882-10-24
- Bin Sayeed, M. S., Karim, S. M. R., Sharmin, T., & Morshed, M. M. (2016). Critical analysis on characterization, systemic effect, and therapeutic potential of β-sitosterol: A plant-derived orphan phytosterol. Medicines, 3(4), 29. https://doi.org/10.3390/medicines3040029
- Calligaris, S., Alongi, M., Lucci, P., & Anese, M. (2020). Effect of different oleogelators on lipolysis and curcuminoid bioaccessibility upon in vitro digestion of sunflower oil oleogels. Food Chemistry, 314, 126146. https://doi.org/10.1016/j.foodchem.2019.126146
- Chanioti, S., Katsouli, M., & Tzia, C. (2021). β-sitosterol as a functional bioactive. In M. Mushtaq & F. Anwar (Eds.), A centum of valuable plant bioactives (pp. 193–212). Academic Press.
- Chen, Z., Wu, A., Jin, H., & Liu, F. (2020). β-sitosterol attenuates liver injury in a rat model of chronic alcohol intake. Archives of Pharmacal Research, 43(11), 1197–1206. https://doi.org/10.1007/s12272-020-01271-w
- Chen, Y., Yang, Y., Wang, N., Liu, R., Wu, Q., Pei, H., & Li, W. (2024). β‐sitosterol suppresses hepatocellular carcinoma growth and metastasis via FOXM1‐regulated Wnt/β‐catenin pathway. Journal of Cellular and Molecular Medicine, 28(3), e18072.
- Choi, Y. H., Kong, K. R., Kim, Y., Jung, K. O., Kil, J. H., Rhee, S. H., & Park, K. Y. (2003). Induction of bax and activation of caspases during β-sitosterol-mediated apoptosis in human colon cancer cells. International Journal of Oncology, 23(6), 1657–1662. https://doi.org/10.3892/ijo.23.6.1657
- Chung, I. M., Yong, S. J., Lee, J., & Kim, S. H. (2013). Effect of genotype and cultivation location on β-sitosterol and α-, β-, γ-, and δ-tocopherols in sorghum. Food Research International, 51(2), 971–976. https://doi.org/10.1016/j.foodres.2013.02.027
- De Deckere, E. A. M., & Korver, O. (1996). Minor constituents of rice bran oil as functional foods. Nutrition Reviews, 54(11), S120. https://doi.org/10.1111/j.1753-4887.1996.tb03831.x
- Dedić, A., Džudžević-Čančar, H., Stanojković, T., Roje, M., Damjanović, A., Alispahić, A., & Jerković-Mujkić, A. (2023). HPLC analysis of phytosterols in Prunus spinosa L. Extracts and their antiproliferative activity on prostate cancer cell lines. Kemija u industriji: Časopis kemičara i kemijskih inženjera Hrvatske, 72(5–6), 323–330. https://doi.org/10.15255/KUI.2022.077
- Ding, K., Tan, Y. Y., Ding, Y., Fang, Y., Yang, X., Fang, J., Zhang, S. M., Zhang, H., Lu, W., Li, M., Huang, S.-C., Cai, M.-L., Song, Y., Ding, Y.-J., & Zhang, S.-M. (2019). β‐sitosterol improves experimental colitis in mice with a target against pathogenic bacteria. Journal of Cellular Biochemistry, 120(4), 5687–5694. https://doi.org/10.1002/jcb.27853
- Dubtsova, G. N., Lomakin, A. A., Azimkova, E. M., Kosareva, K. V., Dubtsov, G. G., & Kusova, I. U. (2021, February). Lipid composition of viburnum and barberry fruits. IOP conference series: Earth and environmental science (Vol. 640, No. 4. pp. 042002). IOP Publishing.
- Duester, K. C. (2001). Avocado fruit is a rich source of β-sitosterol. Journal of the Academy of Nutrition and Dietetics, 101(4), 404. https://doi.org/10.1016/S0002-8223(01)00102-X
- Ferahtia, A. (2021). See discussions, stats, and author profiles for this publication. Net/publication/350567414 surface water quality assessment in semi-arid region (el hodna watershed, algeria) based on water quality index (WQI).
- Friedman, M. (2013). Rice brans, rice bran oils, and rice hulls: Composition, food and industrial uses, and bioactivities in humans, animals, and cells. Journal of Agricultural and Food Chemistry, 61(45), 10626–10641. https://doi.org/10.1021/jf403635v
- Gahlaut, A., Shirolkar, A., Hooda, V., & Dabur, R. (2013). β-sitosterol in different parts of Saraca asoca and herbal drug ashokarista: Quali-quantitative analysis by liquid chromatography-mass spectrometry. Journal of Advanced Pharmaceutical Technology & Research, 4(3), 146. https://doi.org/10.4103/2231-4040.116783
- Gecgel, M. T. B. B. Ü., & Demirci, A. Ş. (2006). Phytosterols as functional food ingredients. Tekirdağ Ziraat Fakültesi Dergisi, 3(2), 153–159.
- Gil-Ramirez, A., Ruiz-Rodriguez, A., Marin, F. R., Reglero, G., & Soler-Rivas, C. (2014). Effect of ergosterol-enriched extracts obtained from Agaricus bisporus on cholesterol absorption using an in vitro digestion model. Journal of Functional Foods, 11, 589–597. https://doi.org/10.1016/j.jff.2014.08.025
- Gornas, P., Rudzińska, M., Raczyk, M., & Soliven, A. (2016). Lipophilic bioactive compounds in the oils recovered from cereal by‐products. Journal of the Science of Food and Agriculture, 96(9), 3256–3265. https://doi.org/10.1002/jsfa.7511
- Gu, S., Liu, F., Xie, X., Ding, M., Wang, Z., Xing, X., Tianbao, & Sun, X. (2023). β-sitosterol blocks the LEF-1-mediated Wnt/β-catenin pathway to inhibit proliferation of human colon cancer cells. Cellular signalling, 104, 110585. https://doi.org/10.1016/j.cellsig.2022.110585
- Gupta, E. (2020). β-sitosterol: Predominant phytosterol of therapeutic potential. In P. Mishra, R. R. Mishra & C. O. Adetunji (Eds.), Innovations in Food Technology: Current Perspectives and Future Goals (pp. 465–477). Springer.
- Gupta, R., Sharma, A. K., Dobhal, M. P., Sharma, M. C., & Gupta, R. S. (2011). Antidiabetic and antioxidant potential of β‐sitosterol in streptozotocin‐induced experimental hyperglycemia. Journal of Diabetes, 3(1), 29–37. https://doi.org/10.1111/j.1753-0407.2010.00107.x
- Hakala, P., Lampi, A. M., Ollilainen, V., Werner, U., Murkovic, M., Wähälä, K., Karkola, S., & Piironen, V. (2002). Steryl phenolic acid esters in cereals and their milling fractions. Journal of Agricultural and Food Chemistry, 50(19), 5300–5307. https://doi.org/10.1021/jf025637b
- Hammam, W. E., Gad, A. M., Gad, M. K., Kirollos, F. N., Yassin, N. A., Tantawi, M. E. E., & El Hawary, S. S. E. (2023). Pyrus communis L. (Pear) and Malus domestica Borkh.(apple) leaves lipoidal extracts as sources for β-sitosterol rich formulae and their wound healing evaluation. Natural Product Research, 37(15), 2613–2617. https://doi.org/10.1080/14786419.2022.2056181
- Hartmann, M. A. (1998). Plant sterols and the membrane environment. Trends in plant science, 3(5), 170–175. https://doi.org/10.1016/S1360-1385(98)01233-3
- Imanaka, H., Koide, H., Shimizu, K., Asai, T., Shimizu, N. K., Ishikado, A., Makino, T., & Oku, N. (2008). Chemoprevention of tumor metastasis by liposomal β-sitosterol intake. Biological and Pharmaceutical Bulletin, 31(3), 400–404. https://doi.org/10.1248/bpb.31.400
- Jun-Hua, H. A. N., Yue-Xin, Y. A. N. G., & Mei-Yuan, F. E. N. G. (2008). Contents of phytosterols in vegetables and fruits commonly consumed in China. Biomedical and Environmental Sciences, 21(6), 449–453. https://doi.org/10.1016/S0895-3988(09)60001-5
- Kakade, A. N., & Magdum, C. S. (2012). HPLC analysis of β-sitosterol in herbal medicine and vegetable oils. International Journal of Pharmacy & Life Sciences, 3(5), 1666–1669.
- Karim, S., Akhter, M. H., Burzangi, A. S., Alkreathy, H., Alharthy, B., Kotta, S., Md, S., Rashid, M. A., Afzal, O., Altamimim, A. S. A., & Khalilullah, H. (2022). Phytosterol-loaded surface-tailored bioactive-polymer nanoparticles for cancer treatment: Optimization, in vitro cell Viability, antioxidant activity, and stability studies. Gels, 8(4), 219. https://doi.org/10.3390/gels8040219
- Khan, I., Najeebullah, S., Ali, M., & Shinwari, Z. K. (2016). Phytopharmacological and ethnomedicinal uses of the genus berberis (Berberidaceae): A review. Tropical Journal of Pharmaceutical Research, 15(9), 2047–2057. https://doi.org/10.4314/tjpr.v15i9.33
- Khan, Z., Nath, N., Rauf, A., Emran, T. B., Mitra, S., Islam, F., Chandran, D., Barua, J., Khandaker, M. U., Idris, A. M., Wilairatana, P., & Thiruvengadam, M. (2022). Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chemico-Biological Interactions, 365, 110117. https://doi.org/10.1016/j.cbi.2022.110117
- Koc, K., Geyikoglu, F., Cakmak, O., Koca, A., Kutlu, Z., Aysin, F., Yilmaz, A., & Aşkın, H. (2021). The targets of β-sitosterol as a novel therapeutic against cardio-renal complications in acute renal ischemia/reperfusion damage. Naunyn-Schmiedeberg’s Archives of Pharmacology, 394(3), 469–479. https://doi.org/10.1007/s00210-020-01984-1
- Kopylov, A. T., Malsagova, K. A., Stepanov, A. A., & Kaysheva, A. L. (2021). Diversity of plant sterols metabolism: The impact on human health, sport, and accumulation of contaminating sterols. Nutrients, 13(5), 1623. https://doi.org/10.3390/nu13051623
- Kudumula, N., Divya, N., Sravika, N., Priya, S., Anusha, P., & Jyotsna, M. S. (2021). Molecular properties, bioactivity scores, and toxicity predictions of the phytoconstituents present in Bauhinia Acuminata. International Journal of Scientific Research and Management (IJSRM), 9(7), 408–414. https://doi.org/10.18535/ijsrm/v9i07.mp02
- Lagarda, M. J., García-Llatas, G., & Farré, R. (2006). Analysis of phytosterols in foods. Journal of Pharmaceutical and Biomedical Analysis, 41(5), 1486–1496. https://doi.org/10.1016/j.jpba.2006.02.052
- Liang, Y. T., Chen, J., Jiao, R., Peng, C., Zuo, Y., Lei, L., Chen, J., Liu, Y., Wang, X., Ma, K. Y., Huang, Y., & Chen, Z. Y. (2015). Cholesterol-lowering activity of sesamin is associated with down-regulation on genes of sterol transporters involved in cholesterol absorption. Journal of Agricultural and Food Chemistry, 63(11), 2963–2969. https://doi.org/10.1021/jf5063606
- Liaudanskas, M., Viškelis, P., Raudonis, R., Kviklys, D., Uselis, N., & Janulis, V. (2014). Phenolic composition and antioxidant activity of Malus domestica leaves. Scientific World Journal, 2014, 1–10. https://doi.org/10.1155/2014/306217
- Li, Z., Cheng, B., Yong, B., Liu, T., Peng, Y., Zhang, X., Ma, X., Huang, L., Liu, W., & Nie, G. (2019). Metabolomics and physiological analyses reveal β-sitosterol as an important plant growth regulator inducing tolerance to water stress in white clover. Planta, 250(6), 2033–2046. https://doi.org/10.1007/s00425-019-03277-1
- Lima, R. D. S., & Block, J. M. (2019). Coconut oil: What do we really know about it so far? Food Quality and Safety, 3(2), 61–72. https://doi.org/10.1093/fqsafe/fyz004
- Liu, Y., Li, Z., Li, W., Chen, X., Yang, L., Lu, S., Zhou, S., Li, M., Xiong, W., Zhang, X., Liu, Y., & Zhou, J. (2024). Discovery of β-sitosterol’s effects on molecular changes in rat diabetic wounds and its impact on angiogenesis and macrophages. International Immunopharmacology, 126, 111283. https://doi.org/10.1016/j.intimp.2023.111283
- Li, J., Yu, H., Yang, Y., Drummond, C. J., & Conn, C. E. (2021). Effect of crystallization state on the gel properties of oleogels based on β-sitosterol. Food Biophysics, 16(1), 48–57. https://doi.org/10.1007/s11483-020-09648-6
- Loizou, S., Lekakis, I., Chrousos, G. P., & Moutsatsou, P. (2010). β‐sitosterol exhibits anti‐inflammatory activity in human aortic endothelial cells. Molecular Nutrition & Food Research, 54(4), 551–558. https://doi.org/10.1002/mnfr.200900012
- Marangoni, F., & Poli, A. (2010). Phytosterols and cardiovascular health. Pharmacological Research, 61(3), 193–199. https://doi.org/10.1016/j.phrs.2010.01.001
- Matsuoka, R. (2022). Property of phytosterols and development of its containing mayonnaise-type dressing. Foods, 11(8), 1141. https://doi.org/10.3390/foods11081141
- Mel’nikov, S. M., ten Hoorn, J. W. S., & Eijkelenboom, A. P. (2004). Effect of phytosterols and phytostanols on the solubilization of cholesterol by dietary mixed micelles: An in vitro study. Chemistry and Physics of Lipids, 127(2), 121–141. https://doi.org/10.1016/j.chemphyslip.2003.09.015
- Mishra, A., Das, S., & Kumari, S. (2024). Potential role of herbal plants and beta sitosterol as a bioactive constituent in circumventing Alzheimer’s disease. Plant Science Today, 11(1), 454–465. https://doi.org/10.14719/pst.2420
- Moreau, R. A., Whitaker, B. D., & Hicks, K. B. (2002). Phytosterols, phytostanols, and their conjugates in foods: Structural diversity, quantitative analysis, and health-promoting uses. Progress in Lipid Research, 41(6), 457–500. https://doi.org/10.1016/S0163-7827(02)00006-1
- Nandi, S., Nag, A., Khatua, S., Sen, S., Chakraborty, N., Naskar, A., Sharifi‐Rad, J., Sharifi‐Rad, J., & Acharya, K. (2023). Anticancer activity and other biomedical properties of β‐sitosterol: Bridging phytochemistry and current pharmacological evidence for future translational approaches. Phytotherapy Research, 38(2), 592–619. https://doi.org/10.1002/ptr.8061
- Patel, K., Kumar, V., Verma, A., Rahman, M., & Patel, D. K. (2017). β-sitosterol: Bioactive compounds in foods, their role in health promotion and disease prevention “a concise report of its phytopharmaceutical importance”. Current Traditional Medicine, 3(3), 168–177. https://doi.org/10.2174/2215083803666170615111759
- Perveen, A. N. J. U. M., & Qaiser, M. U. H. A. M. M. A. D. (2010). Pollen flora of Pakistan–LXV. Berberidaceae. Pakistan Journal of Botany, 42(1), 1–6.
- Piironen, V., Toivo, J., & Lampi, A. M. (2002). Plant sterols in cereals and cereal products. Cereal Chemistry, 79(1), 148–154. https://doi.org/10.1094/CCHEM.2002.79.1.148
- Ponnulakshmi, R., Shyamaladevi, B., Vijayalakshmi, P., & Selvaraj, J. (2019). In silico and in vivo analysis to identify the antidiabetic activity of β-sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicology Mechanisms and Methods, 29(4), 276–290. https://doi.org/10.1080/15376516.2018.1545815
- Reddy, C. S. S., Priyadharshini, R., & Selvaraj Jayaraman, D. P. S. (2022). Chemopreventive anticancer activity of beta sitosterol in human prostate cancer cells (Pc-3) and Emt-mediated signaling regulation: An in vitro study. Journal of Pharmaceutical Negative Results, 13(9), 1514–1521.
- Rubis, B., Paszel, A., Kaczmarek, M., Rudzinska, M., Jelen, H., & Rybczynska, M. (2008). Beneficial or harmful influence of phytosterols on human cells? British Journal of Nutrition, 100(6), 1183–1191. https://doi.org/10.1017/S0007114508981423
- Ryan, E., Galvin, K., O’Connor, T. P., Maguire, A. R., & O’Brien, N. M. (2007). Phytosterol, squalene, tocopherol content and fatty acid profile of selected seeds, grains, and legumes. Plant Foods for Human Nutrition, 62(3), 85–91. https://doi.org/10.1007/s11130-007-0046-8
- Sajfrtova, M., Ličková, I., Wimmerová, M., Sovová, H., & Wimmer, Z. (2010). β-sitosterol: Supercritical carbon dioxide extraction from sea buckthorn (Hippophae rhamnoides L.) seeds. International Journal of Molecular Sciences, 11(4), 1842–1850. https://doi.org/10.3390/ijms11041842
- Sakul, A. A., & Okur, M. E. (2021). β-sitosterol and its antinociceptive mechanism action. Journal of Faculty of Pharmacy of Ankara University, 45(2), 238–252.
- Sharma, N., Tan, M. A., & An, S. S. A. (2021). Phytosterols: Potential metabolic modulators in neurodegenerative diseases. International Journal of Molecular Sciences, 22(22), 12255. https://doi.org/10.3390/ijms222212255
- Shi, C., Wu, F., Zhu, X., & Xu, J. (2013). Incorporation of β-sitosterol into the membrane increases resistance to oxidative stress and lipid peroxidation via estrogen receptor-mediated PI3K/GSK3β signaling. Biochimica et Biophysica Acta (BBA)-General Subjects, 1830(3), 2538–2544. https://doi.org/10.1016/j.bbagen.2012.12.012
- Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2021). Cancer statistics, 2021. CA: A Cancer Journal for Clinicians, 71(1), 7–33. https://doi.org/10.3322/caac.21654
- Singh, J., & Kakkar, P. (2009). Antihyperglycemic and antioxidant effect of berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. Journal of Ethnopharmacology, 123(1), 22–26. https://doi.org/10.1016/j.jep.2009.02.038
- Tang, X., Yan, T., Wang, S., Liu, Q., Yang, Q., Zhang, Y., & Yang, L. (2024). Treatment with β-sitosterol ameliorates the effects of cerebral ischemia/reperfusion injury by suppressing cholesterol overload, endoplasmic reticulum stress, and apoptosis. Neural Regeneration Research, 19(3), 642–649. https://doi.org/10.4103/1673-5374.380904
- Tasyriq, M., Najmuldeen, I. A., In, L. L., Mohamad, K., Awang, K., & Hasima, N. (2012). 7α-Hydroxy-β-sitosterol from Chisocheton tomentosus induces apoptosis via dysregulation of cellular Bax/Bcl-2 ratio and cell cycle arrest by downregulating ERK1/2 activation. Evidence-Based Complementary & Alternative Medicine: eCAM, 2012, 1–12. https://doi.org/10.1155/2012/765316
- Vecka, M., Staňková, B., Kutová, S., Tomášová, P., Tvrzická, E., & Žák, A. (2019). Comprehensive sterol and fatty acid analysis in nineteen nuts, seeds, and kernel. SN Applied Sciences, 1(12), 1531. https://doi.org/10.1007/s42452-019-1576-z
- Vlahakis, C., & Hazebroek, J. (2000). Phytosterol accumulation in canola, sunflower, and soybean oils: Effects of genetics, planting location, and temperature. Journal of the American Oil Chemists’ Society, 77, 49–53. https://doi.org/10.1007/s11746-000-0008-6
- Wang, H., Wang, Z., Zhang, Z., Liu, J., & Hong, L. (2023). β-sitosterol as a promising anticancer agent for chemoprevention and chemotherapy: Mechanisms of action and future prospects. Advances in Nutrition, 14(5), 1085–1110. https://doi.org/10.1016/j.advnut.2023.05.013
- Xu, G., Guan, L., Sun, J., & Chen, Z. Y. (2009). Oxidation of cholesterol and β-sitosterol and prevention by natural antioxidants. Journal of Agricultural and Food Chemistry, 57(19), 9284–9292. https://doi.org/10.1021/jf902552s
- Yang, R., Xue, L., Zhang, L., Wang, X., Qi, X., Jiang, J., Yu, P., Wang, X., Zhang, W., Zhang, Q., & Li, P. (2019). Phytosterol contents of edible oils and their contributions to estimated phytosterol intake in the Chinese diet. Foods, 8(8), 334. https://doi.org/10.3390/foods8080334
- Ye, J. C., Chang, W. C., Hsieh, D. J. Y., & Hsiao, M. W. (2010). Extraction and analysis of β-sitosterol in herbal medicines. Journal of Medicinal Plants Research, 4(7), 522–527.
- Ye, J. Y., Li, L., Hao, Q. M., Qin, Y., & Ma, C. S. (2020). β-sitosterol treatment attenuates cognitive deficits and prevents amyloid plaque deposition in amyloid protein precursor/presenilin 1 mice. The Korean Journal of Physiology & Pharmacology, 24(1), 39–46. https://doi.org/10.4196/kjpp.2020.24.1.39
- Yin, Y., Liu, X., Liu, J., Cai, E., Zhao, Y., Li, H., & Gao, Y. (2018). The effect of β-sitosterol and its derivatives on depression by the modification of 5-HT, DA and GABA-ergic systems in mice. RSC advances, 8(2), 671–680. https://doi.org/10.1039/C7RA11364A
- Yuan, C., Zhang, X., Long, X., Jin, J., & Jin, R. (2019). Effect of β-sitosterol self-microemulsion and β-sitosterol ester with linoleic acid on lipid-lowering in hyperlipidemic mice. Lipids in Health and Disease, 18(1), 1–11. https://doi.org/10.1186/s12944-019-1096-2
- Yu, Y., Cao, Y., Huang, W., Liu, Y., Lu, Y., & Zhao, J. (2021). β-sitosterol ameliorates endometrium receptivity in PCOS-like mice: The mediation of gut microbiota. Frontiers in Nutrition, 8, 667130. https://doi.org/10.3389/fnut.2021.667130
- Yuk, J. E., Woo, J. S., Yun, C. Y., Lee, J. S., Kim, J. H., Song, G. Y., Young, E. J., Hur, K. I., & Kim, I. S. (2007). Effects of lactose-β-sitosterol and β-sitosterol on ovalbumin-induced lung inflammation in actively sensitized mice. International Immunopharmacology, 7(12), 1517–1527. https://doi.org/10.1016/j.intimp.2007.07.026
- Zaheed, O., Samson, J., & Dean, K. (2020). A bioinformatics approach to identify novel long, non-coding RNAs in breast cancer cell lines from an existing RNA-sequencing dataset. Non-Coding RNA Research, 5(2), 48–59. https://doi.org/10.1016/j.ncrna.2020.02.004
- Zhang, P., Liu, N., Xue, M., Zhang, M., Liu, W., Xu, C., Fan, Y., Meng, Y., Zhang, Q., & Zhou, Y. (2023). Anti-inflammatory and antioxidant properties of β-sitosterol in copper sulfate-induced inflammation in Zebrafish (Danio rerio). Antioxidants, 12(2), 391. https://doi.org/10.3390/antiox12020391
- Zhang, C. R., Schutzki, R. E., & Nair, M. G. (2013). Antioxidant and anti-inflammatory compounds in the popular landscape plant berberis thunbergii var. atropurpurea. Natural Product Communications, 8(2), 1934578X1300800207. https://doi.org/10.1177/1934578X1300800207