ABSTRACT
Honey adulteration has been on the rise with the increasing demand for high-quality honey. The ladders-shape melting temperature isothermal amplification (LMTIA) was a newly developed nucleic acid amplification technique, and the aim of this study was to establish a method combining the LMTIA with the proofreading enzyme-mediated probe cleavage (Proof-man probe) for detecting the thaumatin-like protein (TLP) genes of Brassica rapa var. oleifera in honey. The LMTIA primers was selected as a target to be designed, the temperature of the Proof-man LMTIA reaction was optimized, and the specificity, sensitivity, and applicability to the honey samples were determined. The results showed that the Proof-man LMTIA method was highly specific with a detection sensitivity of 30-40 copies genomic DNA/reaction at the optimal temperature of 60°C, and the amplification could be completed within 20 min. This method can detect the adulteration of low-price B. rapa honey into high-price honey.
1. Introduction
Honey is a naturally sweet substance that is collected by bees from nectar, honeydew, or manna secreted by the nectar glands of plants, which is mixed with the secretions of the salivary glands of the bees and then matured in the hive through a variety of transformations (Pita-Calvo & Vázquez, Citation2017). As a natural sweetener, it contains sugars, polyphenolic compounds, vitamins, minerals, and other nutrients (Fratianni et al., Citation2023; Mežnarić et al., Citation2022; Palma-Morales et al., Citation2023). These nutrients gave honey a variety of bioactive effects, such as anti-inflammatory, antibacterial, antioxidant (Palma-Morales et al., Citation2023; Poulsen-Silva et al., Citation2023; Ranneh et al., Citation2021). Depending on the botanical origin, honey can be classified as monofloral or multifloral, if arising predominantly from a single or from several plant species, respectively (Soares et al., Citation2017). Honey can result from a broad variety of plant species. The plant sources of monofloral honey include B. rapa, acacia, sunflower, linden acacia and so on (Machado et al., Citation2020; Pauliuc et al., Citation2022). Monofloral honeys are more preferred for their flavor and aroma and also have special pharmacological properties.
Consumers have increased the demand for honey and their quality, which has increased the commercial value of honey. The adulteration of honey occurs frequently (Brar et al., Citation2023; de Souza et al., Citation2021). Common forms of honey adulteration include the mixing of syrup into honey. Honey adulteration mixed with syrup is similar to natural honey in composition, but the nutritional and medicinal values will be compromised (Ali et al., Citation2022; Tosun & Keles, Citation2021). Due to the high yield and low-price of B. rapa, the B. rapa honey is often used as a low-quality honey adulterated with high-quality honey. The type of adulteration is selling high-price honey mixed with low-price honey for a higher profit (Calle et al., Citation2023; Ropciuc et al., Citation2023; Wu et al., Citation2023; You et al., Citation2021) Honey adulteration caused a lower quality of honey, disrupted the market order and affected the health benefits of consumers.
In order to protect consumers and promote fair competition among producers, it is increasingly necessary to assess the authenticity of honey, especially with regard to botanical origin. Currently, the main methods for detecting whether honey is adulterated include physical and chemical index analysis techniques (Ciursa & Oroian, Citation2021; Gonçalves et al., Citation2023), chromatographic techniques (Tosun & Keles, Citation2021), isotope mass spectrometry (El Hawari et al., Citation2021), DNA-based methods (Soares et al., Citation2023; Sobrino-Gregorio et al., Citation2019; Wang et al., Citation2020). Physical and chemical index analysis is simple to operate and the results are easy to analyze, while the technique is not accurate and cannot be accurately analyzed for the adulteration of complex constituents. Chromatographic and spectroscopic techniques are highly accurate (Oroian et al., Citation2017), but most of these techniques are time-consuming, expensive instrumentation and require highly skilled operators. PCR-based methods are highly specific and sensitive to achieve quantitative analysis of adulterated syrups. The PCR amplification process was completed in 1 hour, while the Proof-man LMTIA amplification process was completed in 20 minutes, it is a time-consuming method compared with the Proofman-LMTIA (Song et al., Citation2023; Xiao et al., Citation2023). So it is urgent to establish a rapid, effective and specific technique for detection of the honey adulterated with different origins.
Nucleic acid detection techniques have been widely used for species adulteration and species identification. Ladder-shape melting temperature isothermal amplification (LMTIA) is a newly developed nucleic acid amplification technique (Wang et al., Citation2021). The single-strand DNA templates are supplied by the difference in melting temperatures (Tm) between the primer and its target sequence rather than thermal temperature or enzyme catalysis. A pair of primers reacts with the Bst DNA polymerase and the primers recognize a ladder-type region of the target sequence, enabling stable amplification of nucleic acids at a constant temperature. The LMTIA technique has the advantages of short reaction time, high sensitivity, and low requirements for target sequence length (Wang et al., Citation2021). Currently, it has been successfully applied in the fields of food adulteration (Wang et al., Citation2022a, Citation2022b; Xiao et al., Citation2023), virus (Wang et al., Citation2022c) and pathogenic bacteria detection. Proofman probe is a new type of probe (Ding et al., Citation2021), which is designed according to the loop primer of LMTIA, marked with fluorescent and quenching groups at both ends of the sequence. The real-time target substance monitoring through collection of fluorescent signals by cutting out mismatched bases and releasing fluorescent groups by the ultra-fidelity DNA polymerase. Botanical source of honey is greatly related to its price, the suppliers probably adulterate honey from different nectar sources to increase their profits (Jaafar et al., Citation2020). Thus, the detection of plant sources in different honeys is important for the detection the adulteration of honey. Nuclear genes are expressed at stable and constant copy numbers in different species and tissues, avoiding interference with species diversity. Single-copy thaumatin-like protein (TLP) gene and multi-copy internal transcribed spacer (ITS) gene were used as targets for designing selection primers. Both systems have good specificity, stable reactions, and no false-positive results were observed (Xiao et al., Citation2024). In this study, the TLP gene have high sensitivity to be used as target sequences. The primers and probes were designed with the specific TLP gene as target. A rapid method for the detection of the thaumatin-like protein (TLP) gene from Brassica rapa var. oleifera in honey by combining the Proofman probe with LMTIA technology. In order to provide a new method for detection of the adulteration of mixed honey on the market.
2. Materials and methods
2.1. Materials, reagents, and instruments
Materials: Fresh plant of Brassica rapa var. oleifera is picked on the Xuchang University, the rice, linden, acacia, wattle, cassava, corn, and dates are purchased from the Xuchang Supermarket. Apple honey, lychee honey, jujube honey, honeysuckle honey, paulownia honey, and multifloral honey were provided by manufacturers in Yan’an, Shenzhen, Aksu, Lankao, Nanping, and Yichun, respectively.
Reagents: 2×Mix Premix: Shandong Merit Biotechnology Co., LTD, Heze, Shandong Province, China; GPV8 Ultra-fidelity DNA polymerase: General Biological Systems Co., LTD, Chuzhou, Anhui Province, China; Plant and animal genome DNA extraction kit: Beijing Tiangen Biochemical Technology Co., LTD, Beijing, China; Food Genome Extraction Kit: MACHEREY-NAGEL GmbH & Co. KG, Duren, Germany; Primers and Probes: General Biological Systems Co., LTD, Chuzhou, Anhui Province, China.
Main instruments: Gentier 96E Automatic Medical PCR Analysis System: Xian Tianlong Technology Co., LTD, Xi’an, Shanxi Province; Nano Drop One Ultra Micro Nucleic Acid/Protein Analyzer: Thermo Fisher Scientific Inc., Waltham, Ma, U.S.A.; SCI-VS Adjustable Mixer: Thermo Fisher Scientific Inc., Waltham, Ma, U.S.A.; TGL-16C Table Low speed Centrifuge: Shanghai Anting Scientific Instrument Factory, Shanghai, China; Dry Thermostat (Metal Bath): Hangzhou Ruicheng Instrument Co., LTD, Hangzhou, China.
2.2. Target sequence selection and LMTIA primer design
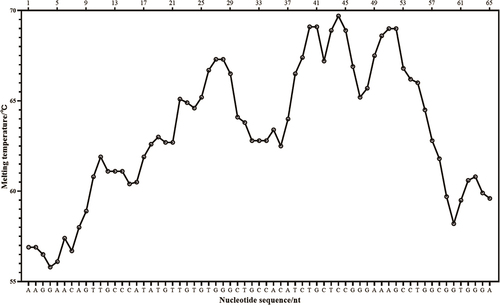
The TLP gene of B. rapa was selected as the target sequence. The selected sequences were analyzed using the software Oligo7 (Molecular Biology Insights, Inc. Colorado Springs, CO, U.S.A.). The Fragments with the ladder-shaped melting temperature curve and the GC base content of 40% to 60% in the DNA are selected as the target sequences. The target sequence was aligned in the GenBank in order to select the highly specific fragment. The sequence with the melting temperature curve is selected as shown in . The LMTIA primers and Proofman probes were designed with the online software Primer3Plus (http://www.primer3plus.com/). The target sequence was selected , and the primer and Proofman probes sequences were listed as in .
Table1. The thaumatin-like protein gene of Brassica rapa var. oleifera target sequences.
Table 2. Sequences of LMTIA primers and Proofman probe.
2.3. DNA preparation
The genomic DNAs (gDNAs) were extracted using the Plant Genome Extraction Kit (Tiangen Biotechnology (Beijing) Co., Ltd.) by following the manufacturer’s protocol. These gDNAs will be used for temperature optimization, specificity determination, and sensitivity determination of the Proofman-LMTIA assay, as well as the DNAs from the honey samples. Using the Plant Genomic DNA Extraction Kit (Tengen), the amount of DNA extracted is 3–30 micrograms per 100 mg of plant tissue. The amount of DNA extracted from different sources of plant tissue material may differ. Using the ultra-micro nucleic acid protein analyzer to know the purity and concentration of extracted DNA, with the DNA concentration expressed in nanograms per microliter and the purity based on the A260/A280 ratio. The B. rapa DNA concentration is 30 ng/μL. The extracted B. rapaDNA was diluted in the following gradients: 10 ng/μL, 1 ng/μL, 0.1 ng/μL, 0.01 ng/μL, 0.001 ng/μL, and 0.001 ng/μL. The gDNAs from the artificially prepared samples were used for the detection limit determination of the Proofman-LMTIA assay.
2.4. Temperature optimization of the Proofman-LMTIA reaction
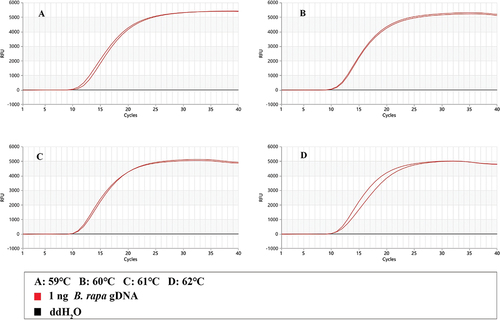
The Proofman-LMTIA reaction temperature was optimized using 10 μL reaction system containing 1.6 μM each of primer P and primer D, 0.4 μM primer LB, 0.1 μM Proofman probe, 2× General LMTIA Reaction Mix (1.0 mM dNTPs, 20 mM Tris-HCl [pH 8.8], 10 mM KCl, 10 mM (NH4)2SO4, 6 mM MgSO4, 0.1% Triton™ X-100), 0.32 U/μL Bst DNA polymerase and 1 ng gDNAs of B. rapa as template. Upon the pre-experiment (Supplementary Materials), the reaction system was overlaid with 20 μL of liquid paraffin to avoid aerosol contamination. The reaction system was heated at 59°C, 60°C, 61°C, and 62°C in the StepOne™ System Real-Time PCR System (Applied Biosystems, CA, U.S.A.). This system was also used to collect fluorescent signals with 30 sec intervals, and 40 signals were collected.
2.5. Specificity determination of the Proofman-LMTIA assay
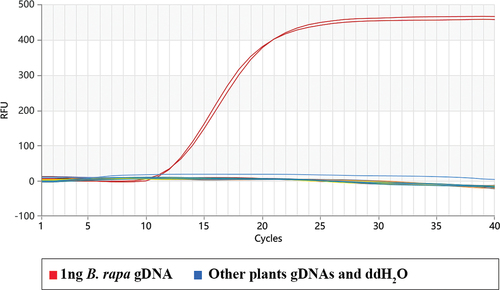
The specificity of the newly developed Proofman-LMTIA assay was determined using 1ng gDNAs of the B. rapa, the rice, linden, acacia, wattle, cassava, corn, and dates, and the reaction systems were heated at the optimized temperature in the StepOne™ System Real-Time PCR System (Applied Biosystems, CA, U.S.A.). This system was also used to collect fluorescent signals with 30 sec intervals, and 40 signals were collected.
2.6. Sensitivity determination of the Proofman-LMTIA assay
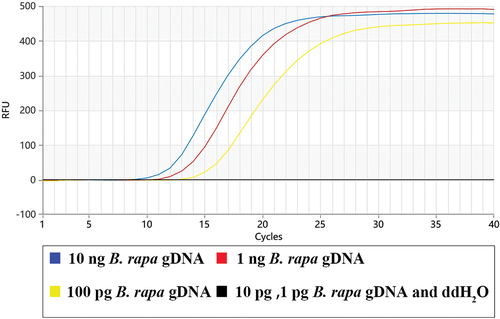
The sensitivity of the newly developed Proofman-LMTIA assay was determined using the gDNAs of the B. rapa ranging from 10 ng to 1 pg, and the reaction mixtures were heated at the optimized temperature in the StepOne™ System Real-Time PCR System (Applied Biosystems, CA, U.S.A.). This system was also used to collect fluorescent signals with 30 sec intervals, and 40 signals were collected.
2.7. Determination of the honey samples with the Proofman-LMTIA assay
The DNAs of apple honey, lychee honey, mountain flower B. rapa honey, jujube honey, paulownia honey, and multifloral honey samples were extracted using the Plant Genome Extraction Kit, and these DNAs were determined with the established Proofman-LMTIA assay, while ddH2O was used as the negative control and the 1 ng B. rapa gDNAs was used as the positive control. The StepOne™ System Real-Time PCR System was also used to collect the fluorescent signals with 30 sec intervals, and 40 signals were collected.
3. Results
3.1. Proofman-LMTIA reaction temperature optimization
As shown in , in the Proofman-LMTIA reaction, the results of all positive controls (with 1ng gDNAs of B. rapa as the template) were positive and the results of all negative controls (DNA template substituted by ddH2O) were negative at 59°C, 60°C, 61°C, and 62°C. In terms of the amplification efficiency, the repeatability as well as the results of temperature optimization in the the pre-experiment (Supplementary Materials), the temperature 60°C was selected as the optimal temperature of the Proofman-LMTIA assay.
3.2. Specificity of the Proofman-LMTIA assay
The reaction system was 10 μL, with 8 μL of the mixed solution (including primer, probe, Mix, and enzyme), and 2 μL of negative and positive controls with the gDNAs concentration was 1 ng. As shown in the specific determination result of the Proofman–LMTIA method was that the amplification of the B. rapa genomic DNA occurred, and no amplification of the other seven plant species gDNAs and the negative control occurred. The experimental results proved the established Proofman-LMTIA assay was of high specificity for the selected genetic fragment of Brassica rapa var. oleifera. This method had not non-specific reaction with the gDNAs of other species and was specific to the B. rapa genes in honey.
3.3. Sensitivity of the Proofman-LMTIA assay
The sensitivity of the Proofman-LMTIA assay was determined using the rape-derived gDNAs in the range of 10 ng ~ 1 pg. As shown in , the systems with the 10 ng, 1 ng, and 0.1 ng gDNAs had amplification, while the systems with 0.01 ng, 0.001 ng and the negative control had no amplification. The sensitivity of the Proofman-LMTIA assay was 0.1 ng/μL B. rapa gDNAs. According to the genome size, 0.1ng/μL was equivalent to 30-40 copies genomic DNA/reaction and the amplification could be finished within 20 min.
3.4. Detection of the honey samples with the Proofman-LMTIA assay
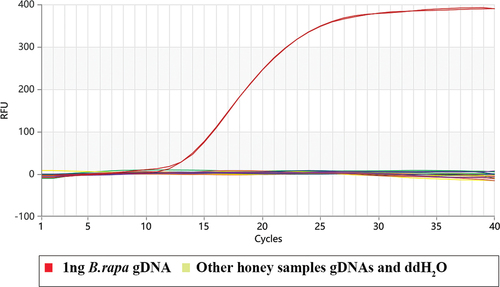
The results of the samples determined with the Proofman-LMTIA assay were shown in , only the positive control had amplification, while the other DNAs from the marketed honey as well as the negative control had no amplification. Among them, the DNAs of Mountain Flower B. rapa honey either had no amplification, indicating that Mountain Flower B. rapa honey does not contain B. rapa genes, which was different from its ingredient list.
4. Discussion
The B. rapa honey is often considered a low-quality honey that would be used to mix with high-quality honey for adulteration. WANG et al. used the real-time LAMP method for authentication of B. rapa honey and the detection limit was 10 pg (Wang et al., Citation2020), while the detection limit of the Proofman–LMTIA method was 100 pg, the difference may be for that the TLP gene in the Proofman–LMTIA method is single-copy gene while the ITS gene in the real-time LAMP method has 200–300 copies in a single cell. Moreover, the nucleic acid amplification time was shortened from 60 min in the LAMP method to 20 min in the Proofman–LMTIA method. You et al. have detected the adulteration of acacia honey with canola honey by the droplet digital polymerase chain reaction (ddPCR) (You et al., Citation2021). Compared with the RT-PCR, the developed duplex ddPCR could simultaneously detect two nectar sources at the same time. The ddPCR can detect 1% adulteration. But the detection of pollen by the ddPCR method to determine honey adulteration has some limitations. These pollen detection methods cannot be applied to detect the naked residual DNA that is dissolved in honey (Truong et al., Citation2021). Sobrino-Gregorio et al. have detected and quantified the presence of rice molasses in honey by the conventional and real-time PCR (Sobrino-Gregorio et al., Citation2019). The technology enables quantitative detection of rice molasses adulteration, but the reaction time consuming. In this experiment, the Proofman probe was combined with the LMTIA technology for detecting B. rapa genes in honey. The reaction time was shortened from more than 1 hour to less than 20 minutes. The technology offers the faster alternative compared to traditional PCR methods. This method was highly specific with 30-40 copies of genomic DNA/reaction at the optimal temperature of 60°C. This Proofman-LMTIA technique has been successfully applied in the rapid detection of pathogenic bacteria Listeria monocytogenes contamination in food (Song et al., Citation2023), rapid discrimination of Panax quinquefolium and Panax ginseng (Zhang et al., Citation2023), and rapid detection of soy-derived components in dairy products (Xiao et al., Citation2023). The limitation of LMTIA technology is that the target sequence should have ladder – shape melting temperature curve. Currently, the detection with the technology is qualitative rather than quantitative.
The results of this experiment showed that the LMTIA technique can detect oleaginous source components in honey. According to its principle, the established Proofman-LMTIA system can also detect other food products containing B. rapa components.
5. Conclusion
In this study, the Proofman-LMTIA assay had been established for rapid detection of rape-derived genes in honey. The LMTIA primers and Proofman probe were designed for the target sequence of thaumatin-like protein gene with high specificity. The sensitivity of the Proofman-LMTIA assay 30–40 copies genomic DNA/reaction of Brassica rapa var. oleifera at the optimal temperature of 60°C, and the whole amplification could be finished within 20 min. The new method can not only be used for the detection of B. rapa genes in honey but also applicable to the detection of B. rapa genes in other foods.
Supplemental Material
Download MS Word (88.5 KB)Acknowledgements
The authors wish to thank all members from the Key Laboratory of Biomarker Based Rapid-detection Technology for Food Safety of Henan Province, China, and Dr. Yanhong Liu from US Department of Agriculture for critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support this study are available from the corresponding author upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19476337.2024.2351912
Additional information
Funding
References
- Ali, H., Rafique, K., Ullah, R., Saleem, M., & Ahmad, I. (2022). Classification of Sidr honey and detection of sugar adulteration using right angle fluorescence spectroscopy and chemometrics. European Food Research and Technology, 248(7), 1823–7. https://doi.org/10.1007/s00217-022-04008-9
- Brar, D. S., Nayik, G. A., Aggarwal, A. K., Kaur, S., Nanda, V., Saxena, S., Gautam, S., Ramniwas, S., & Tolcha, T. D. (2023). Chemical and functional characteristics to detect sugar syrup adulteration in honey from different botanical origins. International Journal of Food Properties, 26(1), 1390–1413. https://doi.org/10.1080/10942912.2023.2218066
- Calle, J. L. P., Punta-Sánchez, I., González de Peredo, A. V., Ruiz-Rodríguez, A., Ferreiro-González, M., & Palma, M. (2023). Rapid and automated method for detecting and quantifying adulterations in high-quality honey using vis-NIRs in combination with machine learning. Foods, 12(13), 2491. https://doi.org/10.3390/foods12132491
- Ciursa, P., & Oroian, M. (2021). Voltammetric e-tongue for honey adulteration detection. Sensors, 21(15), 5059. https://doi.org/10.3390/s21155059
- de Souza, R. R., Fernandes, D. D. D. S., & Diniz, P. H. G. D. (2021). Honey authentication in terms of its adulteration with sugar syrups using UV–vis spectroscopy and one-class classifiers. Food Chemistry, 365, 130467. https://doi.org/10.1016/j.foodchem.2021.130467
- Ding, S., Chen, G., Wei, Y., Dong, J., Du, F., Cui, X., Huang, X., & Tang, Z. (2021). Sequence-specific and multiplex detection of COVID-19 virus (SARS-CoV-2) using proofreading enzyme-mediated probe cleavage coupled with isothermal amplification. Biosensors and Bioelectronics, 178, 113041. https://doi.org/10.1016/j.bios.2021.113041
- El Hawari, K., Al Iskandarani, M., Jaber, F., Ezzeddine, R., Ziller, L., Perini, M., Bontempo, L., Pellegrini, M., & Camin, F. (2021). Evaluation of honey authenticity in Lebanon by analysis of carbon stable isotope ratio using elemental analyzer and liquid chromatography coupled to isotope ratio mass spectrometry. Journal of Mass Spectrometry, 56(6). https://doi.org/10.1002/jms.4730
- Fratianni, F., Amato, G., d’Acierno, A., Ombra, M. N., De Feo, V., Coppola, R., & Nazzaro, F. (2023). In vitro prospective healthy and nutritional benefits of different citrus monofloral honeys. Scientific Reports, 13(1). https://doi.org/10.1038/s41598-023-27802-1
- Gonçalves, W. B., Teixeira, W. S. R., Cervantes, E. P., Mioni, M. D. S. R., Sampaio, A. N. D. C. E., Martins, O. A., Gruber, J., & Pereira, J. G. (2023). Application of an electronic nose as a new technology for rapid detection of adulteration in honey. Applied Sciences, 13(8). https://doi.org/10.3390/app13084881
- Jaafar, M., Othman, M., Yaacob, M., Talip, B., Ilyas, M., Ngajikin, N., & Fauzi, N. (2020). A review on honey adulteration and the available detection approaches. International Journal of Integrated Engineering, 12(2), 125–131. https://doi.org/10.30880/ijie.2020.12.02.015
- Machado, A. M., Miguel, M. G., Vilas-Boas, M., & Figueiredo, A. C. (2020). Honey volatiles as a fingerprint for botanical origin—A review on their occurrence on monofloral honeys. Molecules, 25(2), 374. https://doi.org/10.3390/molecules25020374
- Mežnarić, S., Brčić Karačonji, I., Crnković, G., Lesar, A., Pavlešić, T., Vučković, D., & Gobin, I. (2022). Combined inhibitory effect of fir (Abies alba mill.) honeydew honey and probiotic bacteria Lactiplantibacillus plantarum on the growth of salmonella enterica serotype typhimurium. Antibiotics, 11(2), 145. https://doi.org/10.3390/antibiotics11020145
- Oroian, M., Ropciuc, S., & Paduret, S. (2017). Honey adulteration detection using raman spectroscopy. Food Analytical Methods, 11(4), 959–968. https://doi.org/10.1007/s12161-017-1072-2
- Palma-Morales, M., Huertas, J. R., & Rodríguez-Pérez, C. (2023). A comprehensive review of the effect of honey on human health. Nutrients, 15(13), 3056. https://doi.org/10.3390/nu15133056
- Pauliuc, D., Dranca, F., Ropciuc, S., & Oroian, M. (2022). Advanced characterization of monofloral honeys from Romania. Agriculture, 12(4), 526. https://doi.org/10.3390/agriculture12040526
- Pita-Calvo, C., & Vázquez, M. (2017). Differences between honeydew and blossom honeys: A review. Trends in Food Science & Technology, 59, 79–87. https://doi.org/10.1016/j.tifs.2016.11.015
- Poulsen-Silva, E., Gordillo-Fuenzalida, F., Velásquez, P., Llancalahuen, F. M., Carvajal, R., Cabaña-Brunod, M., & Otero, M. C. (2023). Antimicrobial, antioxidant, and anti-inflammatory properties of monofloral honeys from Chile. Antioxidants, 12(9), 1785. https://doi.org/10.3390/antiox12091785
- Ranneh, Y., Akim, A. M., Hamid, H. A., Khazaai, H., Fadel, A., Zakaria, Z. A., Albujja, M., & Bakar, M. F. A. (2021). Honey and its nutritional and anti-inflammatory value. BMC Complementary Medicine and Therapies, 21(1). https://doi.org/10.1186/s12906-020-03170-5
- Ropciuc, S., Dranca, F., Pauliuc, D., & Oroian, M. (2023). Honey authentication and adulteration detection using emission – excitation spectra combined with chemometrics. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 293, 122459. https://doi.org/10.1016/j.saa.2023.122459
- Soares, S., Amaral, J. S., Oliveira, M. B. P. P., & Mafra, I. (2017). A comprehensive review on the main honey authentication issues: Production and origin. Comprehensive Reviews in Food Science and Food Safety, 16(5), 1072–1100. https://doi.org/10.1111/1541-4337.12278
- Soares, S., Rodrigues, F., & Delerue-Matos, C. (2023). Towards DNA-Based methods analysis for honey: An update. Molecules, 28(5), 2106. https://doi.org/10.3390/molecules28052106
- Sobrino-Gregorio, L., Vilanova, S., Prohens, J., & Escriche, I. (2019). Detection of honey adulteration by conventional and real-time PCR. Food Control, 95, 57–62. https://doi.org/10.1016/j.foodcont.2018.07.037
- Song, C., Wang, B., Wang, Y., Liu, J., & Wang, D. (2023). Detection of listeria monocytogenes in food using the proofman-LMTIA assay. Molecules, 28(14), 5457. https://doi.org/10.3390/molecules28145457
- Tosun, M., & Keles, F. (2021). Investigation methods for detecting honey samples adulterated with sucrose syrup. Journal of Food Composition and Analysis, 101, 101. https://doi.org/10.1016/j.jfca.2021.103941
- Truong, A. T., Kim, S., & Yoon, B. (2021). Determination of honey adulterated with corn syrup by quantitative amplification of maize residual DNA using ultra‐rapid real‐time PCR. Journal of the Science of Food and Agriculture, 102(2), 774–781. https://doi.org/10.1002/jsfa.11411
- Wang, Y., Wang, B., & Wang, D. (2022a). Detection of chicken adulteration in beef via ladder-shape melting temperature isothermal amplification (LMTIA) assay. Biotechnology & Biotechnological Equipment, 36(1), 339–345. https://doi.org/10.1080/13102818.2022.2081514
- Wang, Y., Wang, B., & Wang, D. (2022b). Detection of pork adulteration in beef with ladder-shape melting temperature isothermal amplification (LMTIA) assay. CyTA - Journal of Food, 20(1), 244–250. https://doi.org/10.1080/19476337.2022.2129791
- Wang, Y., Wang, B., Xu, D., Zhang, M., Zhang, X., & Wang, D. (2022c). Development of a ladder-shape melting temperature isothermal amplification (LMTIA) assay for detection of African swine fever virus (ASFV). Journal of Veterinary Science, 23(4), e51. https://doi.org/10.4142/jvs.22001
- Wang, D., Wang, Y., Zhang, M., Zhang, Y., Sun, J., Song, C., Xiao, F., Ping, Y., Pan, C., Hu, Y., Wang, C., & Liu, Y. (2021). Ladder-shape melting temperature isothermal amplification of nucleic acids. BioTechniques, 71(1), 358–369. https://doi.org/10.2144/btn-2020-0173
- Wang, Y., Zhang, M., Wang, D., Zhang, Y., Jiao, X., & Liu, Y. (2020). Development of a real-time LAMP assay for monofloral honey authentication using rape honey. CyTA - Journal of Food, 18(1), 309–314. https://doi.org/10.1080/19476337.2020.1749135
- Wu, X., Xu, B., Luo, H., Ma, R., Du, Z., Zhang, X., Liu, H., & Zhang, Y. (2023). Adulteration quantification of cheap honey in high-quality Manuka honey by two-dimensional correlation spectroscopy combined with deep learning. Food Control, 154, 154. https://doi.org/10.1016/j.foodcont.2023.110010
- Xiao, F., Gu, M., Zhang, Y., Xian, Y., Zheng, Y., Zhang, Y., Sun, J., Ding, C., Zhang, G., & Wang, D. (2023). Detection of soybean-derived components in dairy products using proofreading enzyme-mediated probe cleavage coupled with ladder-shape melting temperature isothermal amplification (proofman–LMTIA). Molecules, 28(4), 1685. https://doi.org/10.3390/molecules28041685
- Xiao, F., Zhang, Y., Gu, M., Xi, X., Zhang, Y., Sun, J., Wei, Q., Hu, B., Zhang, G., & Wang, D. (2024). Comparative study on the target sequences of thaumatin-like protein (TLP) and internal transcribed spacer (ITS) in the detection of corn component in edible oil by ladder-shape melting temperature isothermal amplification (LMTIA). Lwt, 197, 115903. https://doi.org/10.1016/j.lwt.2024.115903
- You, Z., Mei, Y., Wang, X., Chen, X., & Xu, J. (2021). Droplet digital polymerase chain reaction (ddPCR) for rapid screening of adulterants in honey: A case study on acacia honey adulterated with canola honey. Food Control, 130, 130. https://doi.org/10.1016/j.foodcont.2021.108234
- Zhang, X., Li, Z., Zhang, Y., Xu, D., Zhang, L., Xiao, F., & Wang, D. (2023). Rapid Discrimination of Panax quinquefolium and Panax ginseng using the proofman-duplex-LMTIA technique. Molecules, 28(19), 6872. https://doi.org/10.3390/molecules28196872