?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A method, improved d-SPE extration couple with ultra performance liquid chromatography tandem-mass spectrometry (UPLC-MS/MS), was developed for the determination of pentachlorophenol and its sodium salt residue in eggs. The sodium pentachlorophenol in the eggs was converted firstly into pentachlorophenol under acidic conditions, extracted by 1.5% (v/v) acetic acid/acetonitril, and the extraction solution was purified finally by disperse solid phase extraction. The composition of dispersive sorbents was optimized based on the recoveries and matrix effects. The recoveries at fortification levels of 0.5, 5 and 50.0 μg/kg in eggs ranged from 72.2% to 89.8% with the relative standard deviations of 0.2%~0.8%. The limits of quantification (LOQ) was 0.5 μg/kg. Market samples were quantified, and PCP residues were found, but all the detection levels were below the method LOQ. We demonstrated that UPLC – MS/MS in combination with modified d-SPE can be used to routinely monitor PCP residues in egg samples.
KEYWORDS:
1. Introduction
Sodium pentachlorophenate (Na-PCP) is a highly toxic organchlorine compound, easy to convert into the form of pentachlorophenol (PCP) under acidic conditions or in aqueous solutions, commonly used as a herbicide, wood preservative, and pesticide. Na-PCP has high water solubility (330,000 mg/L at 25°C) and is easy to diffuse through water carrier, thereby affecting ecological safety and causing bioaccumulation (Mäenpää et al., Citation2004; NIH, Citation2022). In China, Na-PCP was once widely introduced as snacides that have been sprayed around lake or river to kill Oncomelania Hupensis Gredler for stopping the spread of schistosomiasis in 1960s. Meanwhile, as a herbicide in rice fields, Na-PCP has also been widely used. It is well known that some Persistent Organic Pollutants (POPs) were impurities from Na-PCP product, and those POPs are seriously threatening human health (Cheng et al., Citation2015; Weber et al., Citation2008).
From 2019, the Chinese Ministry of Agriculture and Rural Affairs has mandated that Na-PCP is prohibited from all food animals and cannot be detected in any animal source foods(MARA, Citation2019). However, some investigations confirmed that PCP&Na-PCP residues can be found in livestock and poultry products even if not directly used before (SAMR, Citation2022); surveys found that the detection rate of PCP&Na-PCP in eggs and egg products reached 22.58% (Wen-Jing et al., Citation2018), 16.7% (Zhang et al., Citation2023), respectively. These phenomenon maybe due to the residue of PCP&Na-PCP in environment can be transferred from plants to animals via the food chain (Dougherty, Citation1978). For this reason, feeding laying hens with contaminated feeding stuff, such as grain, crop and water, could cause PCP&Na-PCP residue in eggs. In recent years, the implementation of Rules for China National Food Safety Spot-Check Supervision has clearly stipulated that PCP&Na-PCP in livestock and poultry meat and its by-products is a mandatory inspection item (SAMR, Citation2023).
Currently, the detection methods for PCP&Na-PCP in food mainly include gas chromatography (Ge et al., Citation2007; Zhao, Citation2014), gas chromatography-mass spectrometry (GC-MS)(Zhang et al., Citation2023), gas chromatography-tandem mass spectrometry (GC-MS/MS) (Czech et al., Citation2016), high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) (Zeng et al., Citation2021), and ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS)(Yanhong et al., Citation2019). PCP need to be derivated before gas chromatography-based analysis, but it was directly detected by LC-MS/MS which has the characteristics of high sensitivity, strong selectivity, and simple pre-treatment, and is widely used for the determination of Na-PCP&PCP. There are two national standards for the detection of PCP&Na-PCP in animal derived food, namely GC-MS and LC-MS/MS methods (MARA, Citation2013, Citation2016), which are applicable to animal derived meat. The former requires derivatization treatment, while the latter uses solid phase extraction (SPE) method for extraction and purification, both the experimental procedures are complex and costly. The QuEChERS method was first reported in 2003 and has become the preferred method for analyzing pesticide residues in fruits and vegetables(Anastassiades et al., Citation2003; Lehotay, Citation2006; Tripathy et al., Citation2022), as well as the “template” method for dispersed solid phase extraction (d-SPE). Because of using of low milligram range sorbent amount, it (d-SPE) also can be called dispersive micro-solid phase extraction (D-μSPE) (Zarabi et al., Citation2021), which can select different dispersed adsorbents based on the physicochemical properties of the analyte and the type of matrix (Khiltash et al., Citation2023; Mohammadnia et al., Citation2020; Wilkowska & Biziuk, Citation2011). There also have been many reports on the detection method of drug residues in animal derived foods using d-SPE (Abdallah et al., Citation2015; Stubbings & Bigwood, Citation2009), but barely reports on detecting PCP&Na-PCP residue in eggs.
In this article, our purpose is to try using d-SPE purification method combined with UPLC-MS/MS to develop a rapid determination method for trace amounts of Na-PCP&PCP in eggs.
2. Materials and methods
2.1. Chemicals, reagents and equipments
A certified standard solution of sodium pentachlorophenol (Na-PCP) was obtained from Agro-Environment protection Institute (MARA, China), and its concentration was at 100 mg/L. The acetonitrile (HPLC grade) and methanol (HPLC grade), acetic acid (HPLC grade), anhydrous magnesium sulfate (Guaranteed reagent, GR), sodium chloride (Guaranteed reagent, GR), 15 mL screwcap centrifuge tube and 0.22 μm filter membrane were all purchased from ANPEL Laboratory Technologies (Shanghai, China). The extraction purified adsorbent of C18 (Octadecyl bonded silica gel, average particle size 50–70 μm, average pore size 40–50 Å) and PSA (Primary-secondary amine, average particle size 55–75 μm, average pore size 45–55 Å) were bought from Agilent Technologies (Shanghai, China). Ultrapure water was obtained by using a MilliQ UF-Plus system (Millipore, Germany) with a resistivity of at least 18.2 MΩ.cm at 25°C.
Equipments deployed in this study included: a vortex shaker (Vortex 3, IKA-Werke GmbH & CO. KG Janke & Kunkel-Str., Staufen, Germany), a dispersers (T 18 digital, IKA-Werke GmbH & CO. KG Janke & Kunkel-Str., Staufen, Germany), a high-speed refrigerated centrifuge (H2050R, Hunan Xiangyi Laboratory Instrument Development Co., Ltd, Changsha, China), an electronic balance (JA12002, Shanghai Sunny Hengping Scientific Instrument Co., Ltd, China) and pipettes (Research plus, Eppendorf China, Shanghai, China).
2.2. Standard solution
The concentration of stock standard was at 10,000 μg/L level, and the working standard solutions were prepared at two concentration levels (1000 and 100 μg/L) in acetonitrile. Calibration standards were prepared in both acetonitrile solutions and blank extracts of egg at the concentration levels corresponding to 0.5, 1, 2, 5, 10, 20 and 50 μg/L.
2.3. Analytic condition of LC-MS/MS
The liquid-chromatography (LC) coupled with a triple quadrupole mass spectrometer (Model: LCMS-8050, SHIMADZU Corporation, Japan) with electron spray ionization (ESI) source was deployed to determine PCP&Na-PCP in eggs. The ACQUITY UPLC BEH-C8 column (100 mm × 2.1 mm, 1.7 μm particle size, Waters Corporation, U.S.A.) was used for chromatographic separation.
For the LC mobile phase, methanol and 0.1% acetic acid aqueous solution (V/V) were adopted as mobile phases A and B. The gradient elution program and flow rate were listed at , and the column temperature was set at 40°C. The ESI was operated in negative mode, and scanned with multiple reaction monitoring (MRM); other operating conditions were as follows: spray gas flow rate was 3 mL/min; both heating and drying gas flow rate were 10 mL/min; interface temperature was 350°C; DL temperature was 250°C; heating block temperature was 400°C; desolvation temperature was 600°C. Using argon as the collision gas in which working pressure was at 17 kPa. The sample injection volume was 2 μL.
Table 1. Mobile phase conditions of LC for PCP&Na-PCP.
2.4. Sample preparation
2.5 g homogenized sample was transferred to a 15 mL polytetrafluoroethylene (PTFE) screwcap centrifuge tube, 5.0 mL acetonitrile containing 1.5% acetic acid was added in it, and extracted by shaking violently for about 1 min, subsequently adding 1 g sodium chloride and 200.0 mg anhydrous magnesium sulfate, shaking same as previous step, and 9000 rpm/min centrifugation for 5 min. Finally, transferred 2.0 mL supernatant into a 15 mL screwcap centrifuge tube (PTFE), and purified by adding 200 mg of anhydrous magnesium sulfate and 400 mg of C18, vortex agitation for about 1 min, and centrifuged at 9000 rpm/min for 5 min. The acetonitrile phase (about 1.5 mL) after cleanup was passed through the 0.22 μm filter membrane, and waited for detection by LC-MS/MS.
2.5. Method validation
Method validation was conducted by linearity, precision, recovery, and limit of quantification (LOQ), according to the SANTE/11312/2021(V2) (applying from 01/01/2024). Linearity was determined by 5 concentration level matrix-matched standards (n = 3) 0.5, 5, 10, 50, and 100 μg/kg. The recoveries and precisions were carried out in spiked experiment, which spiked PCP&Na-PCP in blank sample at three concentration levels, that was 0.5, 5, and 50 μg/kg, and each level should be repeated six times (n = 6) to calculate the relative standard deviation (RSD) for determining precision.
Matrix effect could be expressed to suppression and/or enhancement, and should be evaluated in this study. Matrix-matched standard curve and solvent standard curve were prepared in identical concentration point, which were 0.25, 0.5, 5, 10, 50 and 100 ug/kg. Matrix-matched standards were fortified with the PCP and blank extracts, and the medium of solvent standard was acetonitrile. We used Equation. 1 to evaluate the matrix effect (%ME). It was considered weak matrix effect if between 20% and −20%, and indicated an enhancing effect when %ME was more than 20% or suppressing effect when %ME was less than −20%.
Equation. 1:
3. Results
3.1. Conditions of LC-MS/MS
In the acidic conditions Na-PCP transforms into PCP, a kind of chlorophenol and an acid compound with stable properties, and it can be obtaining high sensitivity under negative ion mode of mass spectrometry ([M-H]−). The PCP Standard solution was scanned by flow injection under the mode of ESI− to optimize fragmentation voltages, precursor ions, collision energies, and product ions, and the optimum conditions for MS in terms of for PCP was given in .
Table 2. MS conditions for PCP&Na-PCP.
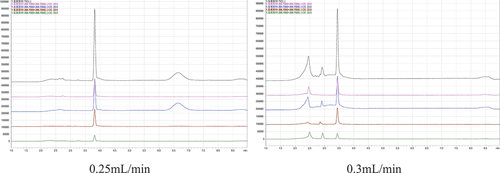
When the pH > 4.93, a part of PCP exists in ionic state. Adding acid to the mobile phase can reduce the pH value of the solution and the activity of residual silica hydroxyl groups in the chromatographic column, thus reducing the secondary interaction between the target compound and the silica hydroxyl groups. Using ammonium acetate formic acid aqueous solution/methanol and C18 column as mobile phase and separated column were reported in some papers (MARA, Citation2016; Peters & Gebbink, Citation2019). In this study, a good chromatographic performance was achieved by using the C8 column and acetic acid aqueous solution/methanol mobile phase, and we found that the flow rate of 0.25 mL/min can obtain a better peak shape than 0.3 mL/min under the same chromatographic conditions, the TIC chromatogram is shown in .
3.2. Optimization of sample preparation
3.2.1. Acidification of extraction solvents
The usual solvent used for determining drug residues in food matrixs was acetonitrile because its extracts contained fewer co-extracts than acetone and ethyl-acetate, therefore it was obvious preference for the extract solvent (Wilkowska & Biziuk, Citation2011). Furthermore, the addition of acetic acid facilitated the conversion of bound pentachlorophenol in eggs to a free state, while assisting acetonitrile to precipitate proteins more efficiently. In our study, we investigated extraction efficiency of three different percentage contents of acetic acid in acetonitrile (v/v), they were 1.0%, 1.5%, 2.0% separately. The PCP&Na-PCP was spiked in egg samples (repeated 6 times, n = 6) at 5.0 μg/kg level, and extracted by three different percentage acetic acid/acetonitrile (v/v), and treated with step of 2.4. The average recovery of PCP&Na-PCP were 68%, 80% and 76% in 1.0%, 1.5%, 2.0% acetic acid/acetonitrile (v/v) individually. The results showed little difference in the recovery rates of PCP&Na-PCP under three different percentage acetic acid/acetonitrile (v/v). From a practical perspective, it was more suitable to choose 1.5% (v/v) which the volume percentage of acetic acid in acetonitrile.
3.2.2. Purification sorbent
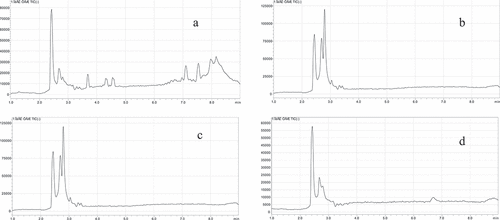
We have tried to treat the egg sample with the method GB 23,200.92–2016 (MARA, Citation2016), but the SPE column was blocked by the extraction solution of the sample during the purification process. Therefore, a new cleanup treatment will be required. The d-SPE, dispersive solid-phase extraction method, was introduced to our experiment. The selection of purification sorbent of d-SPE was a key procedure, and two kinds of sorbent were selected in this paper. PSA sorbent can remove various polar organic acids, polar pigments, some sugars and fatty acids; C18 is effective at removing non-polar interfering substances like lipids; remaining water in the extract was wiped out by MgSO4 to improve the PCP concentration in acetonitrile phase. These two kinds of sorbents of PSA and C18 were investigated by using with single or different combination in spiked experiment. The PCP was spiked at 10 ug/kg in eggs, the extraction procedure followed step of 2.4, and were cleaned by 400 mg C18, 400 mg PSA, C18+ PSA (200 mg +200 mg) respectively, repeated 6 times for each treatment. Without cleanup steps, the base line of egg blank matrix TIC (total ion chromatogram) was highest among these TICs and had some interfering peaks (see ). The combination of C18 and PSA was the best effect of cleanup, but the spiked recovery was below the qualified level (56.0%). When using C18 and PSA separately, the purification effect of these two materials was similar. However, the spiked recovery of PSA was lowest (51.5%), C18 was highest (78.2%). Therefore, C18 was chosen as the sample purification agent in the pretreatment.
3.3. Validation results
The method validation was evaluated by determining the recovery, precision, lineary and LOQ. As shown in , the recovery and precision (RSD) were 72.2% and 0.2% at 0.5 μg/kg concentration level, 83.1% and 0.8% at 5 μg/kg concentration level, 89.8% and 0.5% at 50 μg/kg concentration level. An excellent linearity was observed at matrix-matched concentration point from 0.5 μg/kg to 100 μg/kg. The r2 and LOQ value was 0.9999 and 0.5 μg/kg in as well. The linear equations of matrix-matched standard curve and solvent standard curve were y = 52327×-8540 and y = 53508× + 21048 respectively. According to the Equation.1, the %ME value was −2.2%, and indicated that the matrix effect of PCP was barely any effect in egg matrix.
Table 3. Recovery (%), relative standard deviation (RSD, %), r2 and LOQ of egg spiked with PCP&Na-PCP.
Compared with the method deployed SPE cleanup coupled with LC-MS/MS determination, the recovery (86.4%-102.5%), LOQ (1 μg/kg) and RSD (2.9%-12.1%) in the seafood; the recovery (66.7%-109%) and LOQ (1 μg/kg) in the animal-derived food(MARA, Citation2016; Yan et al., Citation2023), our proposal method has lower LOQ. It demonstrated that the method we developed can be applied successfully to analysis PCP residue in eggs with acceptable LOQ and recoveries.
3.4. Real sample analysis
The sample of 40 eggs were purchased from different markets and analyzed using the method described in this article. A trace amount of PCP was detected in three of these samples, but the content concentration of all positive samples was below the LOQ of this method.
4. Discussion
According to the United Nations Food and Agriculture Organization (FAO), in 2022, China’s egg production accounted for 37.6% of the world’s production, with a per capita egg count of 428 and an average daily consumption of 1 egg per person (FAO, Citation2022). Therefore, egg production and sales in China are both high level. However, some studies have shown that long-term exposure to a dietary environment containing PCP&Na-PCP can lead to a certain positive correlation with cancer (Cheng et al., Citation2015; Cui et al., Citation2017; Yan et al., Citation2023). We also found that the detection rate of PCP&Na-PCP in eggs was 16.7%, relatively high (Zhang et al., Citation2023), resulted in the dietary risk of consuming contaminated eggs.
By the literature reviewed, the majority of studies are focused on the residual levels of PCP in the food (animal-derived meat) and environment, predominantly determined by GC instruments (Cui et al., Citation2017; Czech et al., Citation2016; Ge et al., Citation2007; Xin et al., Citation2022; Zhang et al., Citation2023), which included GC, GC-MS and GC-MS/MS. These methods can acquire a good methodological effects for analyzing PCP&Na-PCP residue in food. However, the disadvantage of these methods often requires complex sample pre-treatment (such as Liquid-liquid extraction (LLE), accelerated solvent extraction (ASE), SPE cleanup), at last, the analyte needs to be derivatived for detection (Ge et al., Citation2007; MARA, Citation2013; Zhang et al., Citation2023; Zhao, Citation2014). The increase in sample pre-treatment steps will, on the one hand, lead to longer analysis time and higher costs for individual samples; On the other hand, it will also affect the accuracy of the method. The method references built on the LC instruments (LC-MSMS) that we reviewed, especially for sample cleanup procedures, which aslo cleaned by LLE and SPE for animal-derived meat. However, egg samples contains very high protein and lecithin, it needs a special cleanup process to determine, and the egg matrix was excluded from the reference methods we mentioned above. Aslo, processing egg samples with two National Standards(MARA, Citation2013, Citation2016), we found that the SPE and derivatization methods are not suitable. Consequently, we optimized the extraction solvent and purification materials based on d-SPE (QuEChERS) method, and developed a operable method for PCP&Na-PCP in eggs. This method is simple, easy to operate, fast, economical, and meets the requirements of residual analysis methods.
5. Conclusions
Optimizing the solid phase extraction methods is a key procedure in analytical chemistry. More and more studies will be conducted on the composition of adsorbents about the d-SPE extraction method due to the introduction of new solid phase adsorbents. In this work, a method for the determination of trace amounts of pentachlorophenol and its sodium salt in eggs by d-SPE-UPLC-MS/MS was established. Sample was extracted and purified by the 1.5% (v/v) acetic acid/acetonitril and the sorbent of C18. The advantages of this method is simple to operate, environment friendly, fast and accurate, and the technical indicators meet the requirements of residue analysis and regulations, and it is suitable for screening PCP&Na-PCP in a large number of egg samples.
Author contributions
Conceptualization, N.C.; methodology, N.C. and X.M.; validation, X.Q. and M.X.; writing—original draft preparation, N.C.; writing—review and editing, N.C. sampling, S.F.; All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We thank YueQiong Kang and JunYing Yang from the Agricultural product quality and safety inspection and testing center of MARA (Chongqing) for providing the experimental guidance in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available on request from the corresponding author.
Additional information
Funding
References
- Abdallah, H., Arnaudguilhem, C., Lobinski, R., & Jaber, F. (2015). A multi-residue analysis of sulphonamides in edible animal tissues using QuEChERS extraction and HPLC-MS/MS. Analytical Methods, 7(4), 1549–6. https://doi.org/10.1039/C4AY01727G
- Anastassiades, M., Lehotay, S. J., Stajnbaher, D., & Schenck, F. J. (2003). Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. Journal of AOAC International, 86(2), 412. https://doi.org/10.1093/jaoac/86.2.412
- Cheng, P., Zhang, Q., Shan, X., Shen, D., Wang, B., Tang, Z., Jin, Y., Zhang, C., & Huang, F. (2015). Cancer risks and long-term community-level exposure to pentachlorophenol in contaminated areas, China. Environmental Science and Pollution Research, 22(2), 1309–1317. https://doi.org/10.1007/s11356-014-3469-4
- Cui, Y., Liang, L., Zhong, Q., He, Q., Shan, X., Chen, K., & Huang, F. (2017). The association of cancer risks with pentachlorophenol exposure: Focusing on community population in the areas along certain section of Yangtze River in China 2017/01/18 ed. Environmental Pollution, 224, 729–738. https://doi.org/10.1016/j.envpol.2016.12.011
- Czech, T., Bonilla, N. B., Gambus, F., González, R. R., Marín-Sáez, J., Vidal, J. M., & Frenich, A. G. (2016). Fast analysis of 4-tertoctylphenol, pentachlorophenol and 4-nonylphenol in river sediments by QuEChERS extraction procedure combined with GC-QqQ-MS/MS. Science of the Total Environment, 557, 681–687. https://doi.org/10.1016/j.scitotenv.2016.03.135
- Dougherty, R. C. (1978). Human exposure to pentachlorophenol. In K. Ranga Rao (Ed.), Pentachlorophenol: Chemistry, pharmacology, and environmental toxicology (pp. 351–361). Springer. https://link.springer.com/chapter/10.1007/978-1-4615-8948-8_30
- FAO. (2022). Crops and Livestock Products. Retrieved January 28, from https://www.fao.org/faostat/en/#data/QCL
- Ge, J., Pan, J., Fei, Z., Wu, G., & Giesy, J. P. (2007). Concentrations of pentachlorophenol (PCP) in fish and shrimp in Jiangsu Province, China. Chemosphere, 69(1), 164–169. https://doi.org/10.1016/j.chemosphere.2007.04.025
- Khiltash, S., Heydari, R., & Ramezani, M. (2023). Graphene oxide/polydopamine-polyacrylamide nanocomposite as a sorbent for dispersive micro-solid phase extraction of diazinon from environmental and food samples and its determination by HPLC-UV detection. International Journal of Environmental Analytical Chemistry, 103(19), 7431–7446. https://doi.org/10.1080/03067319.2021.1971211
- Lehotay, S. J. (2006). Quick, easy, cheap, effective, rugged, and safe approach for determining pesticide residues. In J. L. M. Vidal and A. G. Frenich (Eds.), Pesticide protocols (pp. 239–261). Humana Press. https://doi.org/10.1385/1-59259-929-x:239
- Mäenpää, K. A., Penttinen, O.-P., & Kukkonen, J. V. (2004). Pentachlorophenol (PCP) bioaccumulation and effect on heat production on salmon eggs at different stages of development. Aquatic Toxicology (Amsterdam, Netherlands), 68(1), 75–85. https://doi.org/10.1016/j.aquatox.2004.02.004
- MARA. (2013). Determination of sodium pentachlorophenol residues in animal derived food by gas chromatography–mass spectrometric method (Vol. GB 29708-2013).
- MARA. (2016). Determination of pentachlorophenol residue in animal-derived foods liquid chromatography - mass spectrometry (Vol. GB 23200.92-2016).
- MARA. (2019). No 250 bulletin of the MARA of PRC. Retrieved January 25, from http://www.moa.gov.cn/gk/tzgg_1/gg/202001/t20200106_6334375.htm
- Mohammadnia, M., Heydari, R., & Sohrabi, M. R. (2020). Determination of 2,4-Dichlorophenoxyacetic acid in food and water samples using a modified graphene oxide sorbent and high-performance liquid chromatography. Journal of Environmental Science and Health, Part B, 55(4), 293–300. https://doi.org/10.1080/03601234.2019.1692613
- NIH. (2022). Physical and chemical properties of pentachlorophenol. Retrieved January 25, from https://www.ncbi.nlm.nih.gov/books/NBK590415/table/ch4.tab2/
- Peters, R., & Gebbink, W. (2019). Determination of pentachlorophenol in feed materials and compound feed by LC-MS/MS (No. 2019.009). Wageningen Food Safety Research.
- SAMR. (2022). Food safety sampling inspection announcement results query system. Retrieved January 25, from https://spcjsac.gsxt.gov.cn/
- SAMR. (2023). Enforcement regulation for national food safety spot-check supervision. Retrieved January 25, from https://scjgj.cq.gov.cn/zz/bnq/zwgk/fdzdgknr_146781/jdjc_146793/spyp/jcjh/202311/W020231123556489714936.pdf
- Stubbings, G., & Bigwood, T. (2009). The development and validation of a multiclass liquid chromatography tandem mass spectrometry (LC–MS/MS) procedure for the determination of veterinary drug residues in animal tissue using a QuEChERS (QUick, Easy, CHeap, Effective, Rugged and Safe) approach. Analytica Chimica Acta, 637(1–2), 68–78. https://doi.org/10.1016/j.aca.2009.01.029
- Tripathy, V., Sharma, K. K., Sharma, K., Gupta, R., Yadav, R., Singh, G., Aggarwal, A., & Walia, S. (2022). Monitoring and dietary risk assessment of pesticide residues in brinjal, capsicum, tomato, and cucurbits grown in Northern and Western regions of India. Journal of Food Composition & Analysis, 110, 104543. https://doi.org/10.1016/j.jfca.2022.104543
- Weber, R., Gaus, C., Tysklind, M., Johnston, P., Forter, M., Hollert, H., Heinisch, E., Holoubek, I., Lloyd-Smith, M., Masunaga, S., Moccarelli, P., Santillo, D., Seike, N., Symons, R., Torres, J. P. M., Verta, M., Varbelow, G., Vijgen, J. … Zennegg, M. (2008). Dioxin- and POP-contaminated sites - Contemporary and future relevance and challenges: Overview on background, aims and scope of the series [Review]. Environmental Science and Pollution Research, 15(5), 363–393. https://doi.org/10.1007/s11356-008-0024-1
- Wen-Jing, G., Yu, M., Xiao-Ming, Y., Biao, G., & Ru-Pu, Y. (2018). Analysis of food risk monitoring results in Kaifeng in 2017. Chinese Journal of Health Laboratory Technology. https://CNKI:SUN:ZWJZ.0.2018-22-039
- Wilkowska, A., & Biziuk, M. (2011). Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chemistry, 125(3), 803–812. https://doi.org/10.1016/j.foodchem.2010.09.094
- Xin, L., Jiang, D., Liang, F., Zhang, H., Wei, K., & Wang, J. (2022). A new method for detecting environmental pollutant sodium pentachlorophenate (PCP-Na) based on PAX solid-phase extraction and UPLC-MS/MS. Water, Air, and Soil Pollution, 233(2), 51. https://doi.org/10.1007/s11270-022-05511-1
- Yan, X., Zhao, Q., Yan, Z., Chen, X., He, P., Li, S., & Fang, Y. (2023). Determination of pentachlorophenol in seafood samples from Zhejiang Province using pass-through SPE-UPLC-MS/MS: Occurrence and human dietary exposure risk. Molecules, 28(17), 6394. https://doi.org/10.3390/molecules28176394
- Yanhong, C., Sui, C., Anhua, S., Jingchu, H., Yueming, C., Jia, L., & Yu, W. (2019). Establishment of trace pentachlorophenol and its sodium salt in animal-origin foods by automated solid phase extraction-coupled with ultra performance liquid chromatography-tandem mass spectrometry. Food & Machinery, 35(7), 80–86. https://doi.org/10.13652/j.issn.1003-5788.2019.07.014
- Zarabi, S., Heydari, R., & Mohammadi, S. Z. (2021). Dispersive micro-solid phase extraction in micro-channel. Microchemical Journal, 170, 106676. https://doi.org/10.1016/j.microc.2021.106676
- Zeng, Y.-T., Liao, J.-L., Yang, C.-M., Li, S.-S., Li, X.-Y., & Gao, B.-D. (2021). Determination of pentachlorophenol sodium in Huanghou by high performance liquid chromatography-tandem mass spectrometry. Journal of Food Safety and Quality, 12(9), 3709–3714. https://doi.org/10.19812/j.cnki.jfsq11-5956/ts.2021.09.039
- Zhang, Y., Mhungu, F., Zhang, W., Wang, Y., Li, H., Liu, Y., Li, Y., Gan, P., Pan, X., Huang, J., Zhong, X., Song, S., Liu, Y., & Chen, K. (2023). Probabilistic risk assessment of dietary exposure to pentachlorophenol in Guangzhou, China. Food Additives & Contaminants: Part A, 40(2), 262–270. https://doi.org/10.1080/19440049.2022.2163301
- Zhao, D. (2014). Determination of pentachlorophenol residue in meat and fish by gas chromatography–electron capture detection and gas chromatography–mass spectrometry with accelerated solvent extraction. Journal of Chromatographic Science, 52(5), 429–435. https://doi.org/10.1093/chromsci/bmt054