ABSTRACT
Work has shown that increased exposure to air pollutants independently contributes to obesity and type 2 diabetes risk, yet the exact mechanisms underlying these associations have not been fully characterized. The current review summarizes recent findings regarding the impact of inhaled and ingested air pollutants on the gut microbiota. Animal and human studies provide evidence that air pollutants, such as particulate matter, nitrogen oxides, and ozone, have the potential to alter the gut microbiota. Further, studies suggest that such exposure-induced alterations to the gut microbiota may contribute to increased risk for obesity and type 2 diabetes through inflammatory pathways. Future work is needed to fully understand the complex interactions between air pollution, the gut microbiome, and human health. Additionally, advanced sequencing methods for gut microbiome research present unique opportunities to study the underlying pathways that link increased air pollution exposure with obesity and type 2 diabetes risk.
Introduction
Globally, approximately 39% of adults are overweight and 13% are obese.Citation1 These high rates of overweight and obesity have profound implications for global health as excess adiposity increases the risk of numerous diseases, including cardiovascular disease and type 2 diabetes.Citation2 By 2030 an estimated 552 million people worldwide will have diabetes,Citation3 and an estimated 90-95% of these cases will be type 2 diabetes.Citation4 Although low levels of physical activity and a Western diet are the primary risk factors for obesity and type 2 diabetes, genetics,Citation5 low socioeconomic status, and geographic locationCitation6 also contribute to the etiology of these diseases. Furthermore, exposure to ambient and traffic-related air pollution has been shown to influence the development of obesityCitation7,Citation8 and type 2 diabetes.Citation9–Citation12 For example, a recent study reported that substantial geographical variation in the global burden of diabetes was attributable to air pollution, with elevated concentrations of particulate matter <2.5 µm in diameter (PM2.5) contributing to approximatly 3.2 million incident cases of diabetes.Citation13
Based on current evidence, exposure to air pollutants may contribute to obesity and type 2 diabetes through inflammatory pathways and alterations to metabolism. For example, animal models indicate that exposure to traffic-related air pollution and ambient particulate matter (PM) result in inflammation in the lungs and adipose tissue.Citation14–Citation16 Animal models also illustrate an increase in white adipose tissue and inhibition of lipolysis due to increased exposure to polycyclic aromatic hydrocarbons (PAH),Citation17 which are byproducts of traffic-related fuel combustion. Furthermore, certain components of PM such as transition metals and lipopolysaccharides may penetrate the systemic vasculature and activate inflammatory pathways via toll-like receptors.Citation18 In addition to the above mentioned hypothesized mechanisms, a growing body of literature suggests that air pollutants may alter the composition and function of gut bacteria.Citation19-Citation25
The gut microbiome plays a vital role in human health by facilitating an array of metabolic and immune processes through microbial-host exchanges of metabolites,Citation26,Citation27 breakdown of complex dietary carbohydrates,Citation28 and synthesis of essential vitamins.Citation29 Within a healthy gut, the intestinal mucosal barrier limits the translocation of microbes and microbial products from the lumen into systemic circulation.Citation30 Notably, obesity and type 2 diabetes are characterized by distinct gut microbial profiles, including decreased microbial diversity and alterations in the relative abundance of Firmicutes and Bacteroidetes,Citation31–Citation37 which may alter bacterial fermentation pathways that decrease the production of short-chain fatty acids (SCFAs) and increase inflammation.Citation38–Citation40 These alterations can also induce bacterial overgrowth, breakdown of the intestinal barrier, bacterial and metabolite translocation, and/or chronic endotoxemia.Citation41–Citation43 Therefore, exposure to air pollutants may contribute to increased risk for obesity and type 2 diabetes through alterations to gut microbial composition and function.
Although the relationships between air pollutants and the gut microbiota are a relatively new field of study, a growing body of work suggests that increased exposure to air pollutants may impact obesity and type 2 diabetes via the gut microbiota.Citation24,Citation25 In 2014, a literature review summarized the evidence linking exposure to air pollutants with the gut microbiota and inflammatory diseases.Citation44 The current review provides an updated summary of studies published between February of 2000 and June of 2019 in order to synthesize recent evidence linking increased exposure to air pollutants with alterations to the gut microbiota in both human studies and animal models. The review further discusses the potential health implications of such exposure-induced alterations to the gut microbiota in regard to obesity and type 2 diabetes.
Methods
A comprehensive examination of the literature was conducted to summarize findings related to exposure to air pollutants and the gut microbiota that were published between February of 2000 and June of 2019. Searches were performed between October 2018 and October 2019. PubMed and ProQuest were searched for articles that contained terms in the title and/or abstract that were relevant to the current review. The terms included: “gut microbiome” or “gut microbiota” AND (“ambient air pollution,” or “particulate matter,” or “traffic-related air pollution,” or “nanoparticles”). Bibliographies of relevant articles were also examined as well as papers previously known to the authors.
As shown in Supplemental Figure 1, PubMed and ProQuest yielded 40 references that were read and evaluated for their relevance to the current review. Of these 40 references, 18 were excluded since they did not examine air pollution and 6 were excluded as they did not include assessment of the gut microbiota. Ten references were excluded for being literature reviews; however, these reviews were used to identify two additional references that were relevant to the current review. This process resulted in eight references that we included in the current review (five animal studies and three human studies). Lastly, if a reference included both animal and human studies, each was reported separately in their respective sections.
Figure 1. Hypothesized mechanisms linking exposure to air pollutants with the gut microbiota and increased risk for obesity and type 2 diabetes.
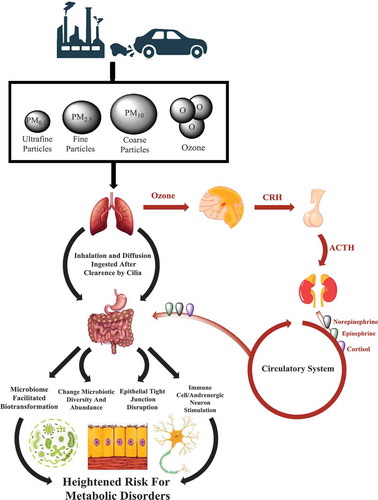
Studies included in this review examined the gut microbiota using 16S rRNA amplicon sequencing or phenotypic indicators of bacterial function such as SCFA production. Studies also examined a wide range of air pollutants, including ambient nitrogen dioxide (NO2), ozone (O3) and PM of various size fractions, including PM <10 µm (PM10), <5 µm (PM5), <2.5 µm (PM2.5), and <0.1 µm in aerodynamic diameter (ultrafine/nanoparticles). Nitrogen oxides (NOx), sulfur dioxide (SO2) and PAHs were also included. Many of these particles are released by vehicles via the burning of fossil fuels, as well as from other sources, like the degradation of tires and brakes.
Potential mechanisms by which air pollutants impact the gut microbiota
Epidemiological studies have provided preliminary evidence that exposure to airborne particles may alter the gut microbiota and gut health. For example, several studies have found associations between elevated levels of air pollution exposure and gastrointestinal diseases, including inflammatory bowel disease (IBD),Citation55,Citation56 irritable bowel syndrome,Citation57 appendicitis,Citation58,Citation59 and gastroenteric disorders in infants.Citation60 Cigarette smoke, which contains particulates that are present in ambient and traffic-related air pollution,Citation61 has also been shown to alter the composition of the gut microbiota in humanCitation62-Citation64 and animal studies.Citation65,Citation66
Upon human exposure to air pollutants, there are several potential routes of interactions between air pollutants and the gut microbiome, which may alter gut bacterial composition as well as the luminal environment. For example, the gastrointestinal (GI) tract is exposed to air pollutants through inhalation and ingestion. Following inhalation, large particles are deposited in the upper airway, trachea, or larger bronchi while smaller particles, such as PM2.5, can reach the bronchioles and alveolar spaces where they are sequestered by alveolar macrophages.Citation45–Citation48 Particles in the mucus layer, as well as those isolated by macrophages, are transported to the oropharynx via mucociliary clearance and are subsequently ingested, providing a pathway for airborne particles to reach the GI tract.Citation45–Citation48 In addition to inhalation, PM can be directly ingested through contaminated food and water.Citation67-Citation70 It has been estimated that humans ingest an average of 1012–1014 particles a day on a Western diet.Citation71,Citation72 Thus, the GI tract is exposed to large amounts of PM, which has the potential to exert pro- and anti-bacterial effects on gut bacteria that may alter gut physiology, including immune responses, metabolism, and intestinal permeabilityCitation19,Citation27,Citation73 (). For example, studies suggest that components of air pollution, such as elemental carbon, black carbon, nitrates, sulfates, and toxic metals like lead and arsenicCitation74 may alter host bacterial communities through antimicrobial properties and the formation of biofilms.Citation75–Citation79
Ozone is another central component of ambient air pollution that is produced by the reaction of NOx, volatile organic compounds, and sunlight.Citation80 Although numerous studies have demonstrated the deleterious effects of increased O3 exposure on respiratory health, few have examined the impact of O3 on the central nervous system and gut health. Prior work suggests that increased O3 exposure may impact the hypothalamic-pituitary-adrenal (HPA) axis, resulting in increased corticosteroid levelsCitation49 and alterations to the gut microbiota.Citation81 Further, O3 exposure has been shown to increase plasma adrenocorticotropic hormone and corticosterone concentrations.Citation50 PM and SO2 exposure have also been shown to stimulate the autonomic nervous system,Citation53,Citation54 which may impact the gut microbiota through changes to GI tract motility and secretion. Lastly, reciprocal communication through the gut-brain axisCitation82 may increase the production of norepinephrine at the gut lamina propria, thereby contributing to increased proliferation of gram-negative bacteria in the lumen.Citation83
Exposure to air pollutants alters the composition of the gut microbiota in animal models
A number of rodent studies have investigated the effects of inhaled and ingested air pollutants on measures of gut microbial diversity, and a summary of these five studies can be found in .Citation19-Citation23 A higher diversity may be indictive of a healthier gut microbiota and these measures include alpha and beta diversity, which are measures of species richness and evenness within a sample and differences in microbial abundances between multiple samples, respectively.Citation84 In one study, 12 low-density lipoprotein receptor knock out mice were fed a high-fat diet and exposed to ambient ultrafine particles for 10 weeks (3 d/week). As a result of this exposure, gut microbial alpha diversity decreased and beta diversity increased.Citation20 Another study exposed 10 mice to PM2.5 for 3 weeks using concentrated air from Chicago, and found increased alpha diversity in the small intestine, colon, and feces, as well as increased beta diversity throughout the GI tract.Citation22 In another study, 10 mice were exposed to PM2.5 concentrated air from Shanghai for 1 y. While PM2.5 exposure did not alter gut microbial alpha diversity, there were decreases in measures of gut bacterial community richness (i.e., ACE and Chao-1) when compared to mice exposed to filtered air.Citation23 These mixed findings may be due to regional differences in the chemical composition of PM2.5 as well as differences in the duration of exposure.
Table 1. Summary of studies that examined air pollutants and the gut microbiota in murine models.
In addition to measures of gut microbial diversity, PM exposure has also been shown to alter the relative abundance of gut bacterial taxa in murine modelsCitation19,Citation22,Citation23 (). In one study, the relative abundance of bacterial taxa was measured in mice exposed to PM2.5 concentrated air from Shanghai for 1 y. Increased exposure resulted in an increase of nine bacterial taxa, a decrease of 15 bacterial taxa, as well as impaired insulin and glucose tolerance.Citation23 In another study, mice exposed to inhaled PM2.5 for 3 weeks displayed a decrease in the relative abundance of Firmicutes and several bacterial families within the Firmicutes phyla, such as Staphylococcaceae. At the same time, exposed mice exhibited an increase in the relative abundance of bacteria belonging to the Bacteroidetes phyla, including bacteria in the Rikenellaceae family.Citation22 In another study, seven interleukin-10 knock out mice (IL-10−/−) and eight wild type (WT) mice were gavaged with ambient PM10 collected from Ontario, Canada for 35 d. In WT and IL-10−/− mice, ingestion of PM10 increased in the relative abundance of the Verrucomicrobia phyla relative to the control mice. Both groups of mice exposed to PM10 also had alterations in cecal concentrations of SCFAs (e.g., increased isovalerate and isobutyrate, and decreased butyrate and valerate), which are important mediators of gut barrier integrity and predominantly produced by bacteria in the gut.Citation19
Figure 2. Gut bacterial phyla associated with exposure to air pollutants in animal and human studies.
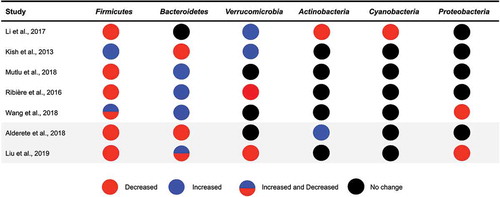
Another study examined PM smaller than 0.1 µm in diameter,Citation20 which is the smallest fraction of PM and may have differential effects on physiology when compared to larger particles. This study orally exposed 12 mice to ultrafine particles 3 d a week for 10 weeks and found that the relative abundance of Verrucomicrobia was significantly higher in mice exposed to ultrafine particles than the control group. Additionally, mice exposed to ultrafine particles had a lower relative abundance of Actinobacteria, Cyanobacteria, and Firmicutes when compared to the control group.Citation20 While numerous studies have examined the associations between exposure to PMCitation19,Citation22,Citation23 or ultrafine PMCitation20 and the gut microbiota in rodents, only one study has specifically focused on exposures from traffic sources.Citation21 This study exposed 11 mice to benzo[a]pyrene (BaP) via oral gavage for 28 d. While BaP exposure did not impact alpha diversity, it did alter the relative abundance of 15 bacterial families, including an increase in the relative abundance of Paraprevotella, Alcaligenaceae, and Bacteroides as well as a decrease in the relative abundance of Lactobacillus and Oscillospira compared to baseline.Citation21 BaP is a PAH that is commonly produced in automobile exhaust fumes and the burning of biomasses and is representative of an exposure that is common in traffic-related air pollution.Citation17 Thus, results from this study suggest that exposure to components of traffic-related air pollution, in addition to PM, may alter the gut microbiota in mice.
Alterations to the gut microbiota may mediate the ssociations between air pollution, Obesity, and type 2 diabetes in animal models
In addition to gut microbial profiles, exposure to air pollutants was associated with several markers of inflammation as well as glucose intolerance in animal models. Several studies found that PM exposure was associated with increased pro-inflammatory cytokine expression in the murine intestine.Citation19,Citation20,Citation22 All of these studies observed increased expression of tumor necrosis factor-alpha (TNF-α) in mice exposed to PM. The gut microbiota may also contribute to the regulation of circulating levels of TNF-α,Citation85 and importantly TNF-α has been found to be associated with obesity and markers of insulin resistance and type 2 diabetes.Citation85–Citation88 These findings suggest a potentially novel gut microbial mediated link between air pollution and obesity and type 2 diabetes. Additionally, histological evidence of inflammation of the intestine was found in mice exposed to ultrafine particles and BaP.Citation20,Citation21 One study found that alterations to bacterial richness (Chao-1 estimator) mediated the observed associations between PM exposure and glucose intolerance.Citation23 Results from these studies suggest that exposure to air pollutants may induce changes in the gut microbiota and increase systemic markers of inflammation that may contribute to the risk for type 2 diabetes.
Summary of animal studies
Studies in rodents demonstrate that increased exposure to air pollutants, through inhalation or ingestion, have the potential to alter the gut microbiota, intestinal inflammation, and risk for type 2 diabetes and obesity.Citation19-Citation23 These animal studies demonstrate important exposure-induced alterations to the diversity and relative abundance of gut bacterial taxa. Despite the strengths of these experimental studies, they are limited by the use of 16S rRNA sequencing, which prevents examination of exposure-induced effects on gut bacterial species or bacterial function. Moving forward, the use of stool shotgun sequencing and fecal metabolomic profiling would allow for detailed characterization of the impact of air pollutants on gut bacterial composition (down to the species level) as well as elucidate the impact of air pollution exposure on gut microbial function. To date, only one study has included a mediation analysis and found that the gut microbiota mediated the observed associations between exposure to PM2.5 and glucose intolerance.Citation23 Including these analyses in future studies will help to determine if the gut microbiota mediates the associations between air pollution exposure and obesity or type 2 diabetes risk.
The use of murine models has allowed for experimental studies that are not feasible in humans. While these investigations are a useful tool for predicting the possible effects of environmental exposures on the human gut microbiota, there are limitations to using animal studies to infer the impact of air pollutants on the human gut. For example, only 15% of the genera of microbes in the distal gut of mice have been identified in the human gut.Citation89 Moreover, there are differences in the anatomy of the human and murine GI tract as humans lack a forestomach and mice lack an appendix and have different distributions of goblet and Paneth cells.Citation90 These differences in the composition of the gut microbiota and anatomy of the GI tract have the potential to impact microbe, host, and environment interactions. Given this, it is important to examine the associations between increased exposure to air pollutants and the gut microbiome in humans.
Exposure to air pollutants is associated with the composition of the gut microbiota in humans
Three human studies have examined the associations between exposure to air pollutants and the gut microbiota, which are summarized in . Two of these were epidemiological studies that examined the associations between exposure to traffic-related air pollutantsCitation24 and PMCitation25 with the gut microbiota, respectively. The other was an experimental study that examined the effects of nanoparticles on the gut microbiota in a human model colon.Citation92 As described below, these studies suggest that exposure to PM and nanoparticles may impact the composition of the gut microbiota as well as the intestinal immune response.
Table 2. Summary of studies that examined air pollutants and the gut microbiota in humans.
To our knowledge, associations between traffic-related air pollution exposure and the gut microbiota have only been examined in one study that was conducted in 43 overweight and obese adolescents (17–19 y of age) from Southern California.Citation24 This study estimated individual exposure to traffic-related air pollution using residential addresses. Results from this study found that traffic-related air pollution exposure was positively correlated with fasting glucose levels and gut microbial taxa that have been linked with obesity and insulin resistance, including Bacteroidaceae and Coriobacteriaceae.Citation93,Citation94 Additionally, the gut microbial taxa that were correlated with traffic-related air pollution exposure accounted for 24% and 29% of the correlation between exposure to air pollutants and fasting glucose levels.Citation24 Similar results were found in a recent study that examined 5,828 adults from south China.Citation25 This study used a spatiotemporal land-use regression model to estimate exposure to ambient air pollutants and found that exposure to PM2.5 and PM1 was associated with a decreased gut microbial alpha diversity. PM exposure was also negatively associated with the gut microbial taxa Firmicutes, Proteobacteria, and Verrucomicrobia and was associated positively and negatively with several taxa within the Bacteroidetes phyla. Additionally, impaired fasting glucose was found to be positively associated with PM2.5 and PM1 exposure and further analyses revealed that this association was partially mediated by gut microbial diversity.Citation25 These findings suggest that air pollution exposure may increase susceptibility to obesity and type 2 diabetes risk through alterations in the composition of the gut microbiota.
Similar to larger PM, nanoparticles can be inhaled, ingested, and impact gut health and the gut microbiota. Nanoparticles are smaller than 0.1 µm in size, allowing for a greater surface area to volume ratio of the particle that contributes to increased reactivity.Citation92 Only one study analyzed the impact of nanoparticles on the gut microbiota in humans.Citation91 This study examined zinc oxide (ZnO), cerium dioxide (CeO2), and titanium dioxide (TiO2) nanoparticles, which are manmade and are present in air pollution.Citation95–Citation97 Additionally, TiO2 has been used as a photocatalyst to clean pollutants from the air via oxidation of organic compounds.Citation98 In this study, each nanoparticle was introduced separately to a model colon from human donors at levels that are similar to human exposures. The model colon was comprised of a reactor, wherein a dialysis tube was encapsulated by a glass tube, which was maintained at conditions similar to the proximal colon (e.g., pH, temp). This reactor was inoculated with a microbial community obtained from human feces. These exposures altered SCFA production and resulted in a microbial community that was different from the non-exposed group.Citation91 These results offer preliminary evidence that ingested nanoparticles that are found in air pollutants have the potential to modulate the human gut microbiota.
Summary of human studies
To date, only two human studies have examined the associations between exposure to air pollutants and the gut microbiota.Citation24,Citation25 Each of these studies suggest that air pollutants may increase the risk for obesity and type 2 diabetes via alterations to the gut microbiota. These studies considered potentially important confounders by adjusting for factors known to be associated with exposure to air pollutants, the gut microbiota, and risk for obesity and type 2 diabetes (e.g., diet, body fat percent). However, it is important to note that residual confounding may have still been present and could partially account for some of the observed associations in these studies. These investigations estimated human exposure to air pollutants based on residential addresses and may have also been limited by exposure misclassification, yet exposure misclassification should be random across participants (non-differential) and likely would bias their reported estimates toward the null.Citation99 Lastly, one study found that the ingestion of nanoparticles alters gut bacterial composition and SCFA production in a human model colon.Citation91 While these studies support epidemiological findings, further research is needed to determine the specific impact of air pollutants on the human gut microbiome, including the composition and functional potential of gut bacteria. For this reason, human studies should include personal exposure monitoring, detailed measures of possible confounders (e.g., diet, socioeconomic status), repeated fecal sampling, metabolomic profiling, and detailed shotgun sequencing samples to fully characterize the gut microbiome.
Air pollutants and the gut microbiota: implications for obesity and type 2 diabetes
As summarized in this review, early evidence from animalCitation19-Citation23,Citation73 and human studiesCitation24,Citation25,Citation91 suggest that exposure to air pollutants may decrease gut bacterial diversity and alter the relative abundance of gut bacterial taxa (e.g., Firmicutes), both of which have been linked with obesity and type 2 diabetes. While not true in all cohorts,Citation32 most studies have shown that the composition of the gut microbiota is altered during obesity and type 2 diabetes where there is decreased microbial diversity, increased Firmicutes, and decreased Bacteroidetes.Citation31,Citation33-Citation36 Further, mouse studies have shown that obesity and metabolic dysfunction are independently transmissible through fecal transplants.Citation37,Citation100,Citation101
In addition to altering the composition of the gut microbiota, increased exposure to air pollutants may also modify gut bacterial function, including the production of gut bacterial-derived metabolites involved in biological processes related to obesity and type 2 diabetes.Citation20,Citation102 Indeed, studies have shown that specific gut bacteria are involved in SCFA,Citation103 lipid,Citation104 amino acid,Citation102,Citation108 bile acid,Citation109,Citation110 and tryptophan metabolism,Citation43,Citation111 which have been linked with gut barrier integrity, satiety, body weight, adipose tissue inflammation, and type 2 diabetes. Bacteria in the gut also play an important role in xenobiotic metabolism of environmental toxicants.Citation51,Citation52 For example, the human colon microbiota has been shown to biotransform PAH to estrogenic metabolites,Citation51 which has the potential to modulate pathways related to insulin resistance and obesity.Citation112,Citation113
Conclusions
Animal and human studies provide evidence that exposure to air pollutants, either through inhalation or ingestion, contributes to alterations to the gut microbiota. This is supported by numerous experimental studies that have observed important shifts in the composition of the gut microbiota (i.e., diversity and relative abundance), decreased gut barrier integrity, and increased inflammation in the murine GI tract. Epidemiological studies have also found that exposure to air pollutants is associated with gut microbial diversity and the relative abundance of bacterial taxa. Additionally, the gut microbiome is known to influence numerous physiological processes related to the development of disease and specific gut microbial profiles have been observed in patients with obesity and type 2 diabetes. Together, results from these studies suggest that the associations between exposure to air pollutants and obesity and type 2 diabetes may be partially mediated by exposure-induced changes to the gut microbiota. A better understanding of these effects has the potential to reduce the global burden of disease through public health policies aimed at improving air quality. Additionally, interventions targeting the human gut microbiota (i.e., pre- and probiotic supplementation) have been shown to alter gut bacterial phylaCitation114,Citation115 that have also been linked with exposure to air pollutants (e.g., Firmicutes, Proteobacteria, Actinobacteria).Citation24,Citation25
Despite recent advances in our understanding of the effects of air pollution exposure on the gut microbiota using animal models, few studies have examined the impact of inhaled pollutants on the gut microbiota in humans. Additionally, there are currently no longitudinal studies that have examined the chronic and dynamic impacts of air pollution on the gut microbiome, and their subsequent implications for obesity and type 2 diabetes risk over the life course. In order to understand the mechanisms linking air pollution exposure with alterations in gut bacteria, future studies should perform a detailed characterization of the gut microbiome using whole genome sequencing. Additionally, pairing genomic sequencing with fecal metabolomic profiling may allow for a more in-depth understanding of the impact of air pollutants on the composition and function of the gut microbiome. In summary, there is emerging evidence that links air pollution exposure with alterations in the gut microbiota that may have broad implications for human health. However, future studies are needed to fully characterize the effects of air pollution on the composition and function of gut bacteria.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (276.3 KB)Acknowledgments
This work was supported by NIH NIEHS R00ES027853.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- World Health Organization. Obesity and overweight. 2019. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight..
- Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015 Jul;33(7):673–689. doi:https://doi.org/10.1007/s40273-014-0243-x.
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011 Dec;94(3):311–321. doi:https://doi.org/10.1016/j.diabres.2011.10.029.
- Centers for Disease Control and Prevention (CDC). National Diabetes Statistics Report, 2017. 2017 Jul 19:1–20.
- Ali O. Genetics of type 2 diabetes. World J Diabetes. 2013 Aug;4(4):114–123. doi:https://doi.org/10.4239/wjd.v4.i4.114.
- Sung B, Etemadifar A. Multilevel analysis of socio-demographic disparities in adulthood obesity across the United States geographic regions. Osong Public Health Res Perspect. 2019 Jun;10(3):137–144. doi:https://doi.org/10.24171/j.phrp.2019.10.3.04.
- Jerrett M, McConnell R, Chang CCR, Wolch J, Reynolds K, Lurmann F, Gilliland F, Berhane K. Automobile traffic around the home and attained body mass index: A longitudinal cohort study of children aged 10–18 years. Prev Med. 2010 Jan;50(Suppl 1):S50–8. doi:https://doi.org/10.1016/j.ypmed.2009.09.026.
- McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang -C-C, Lurmann F, Berhane K. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California children’s health study. Environmental Health Perspectives. 2015 Apr;123(4):360–366. doi:https://doi.org/10.1289/ehp.1307031.
- Yang B-Y, Qian Z, Li S, Chen G, Bloom MS, Elliott M, Syberg KW, Heinrich J, Markevych I, Wang S-Q, et al. Ambient air pollution in relation to diabetes and glucose-homoeostasis markers in China: a cross-sectional study with findings from the 33 communities chinese health study. Lancet Planet Health. 2018 Feb;2(2):e64–e73. doi:https://doi.org/10.1016/S2542-5196(18)30001-9.
- Lucht SA, Hennig F, Matthiessen C, Ohlwein S, Icks A, Moebus S, Jöckel KH, Jakobs H, Hoffmann B. Air pollution and glucose metabolism: an analysis in non-diabetic participants of the heinz nixdorf recall study. Environ Health Perspect. 2018 Apr;126(4):047001. doi:https://doi.org/10.1289/EHP2561.
- Alderete TL, Habre R, Toledo-Corral CM, Berhane K, Chen Z, Lurmann FW, Weigensberg MJ, Goran MI, Gilliland FD. Longitudinal associations between ambient air pollution with insulin sensitivity, β-cell function, and adiposity in Los Angeles Latino children. Diabetes. 2017Jul;66(7):1789–1796. doi:https://doi.org/10.2337/db16-1416.
- Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, Habre R, Bastain TM, Lurmann F, Wilson JP, et al. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care. 2016 Apr;39(4):547–554. doi:https://doi.org/10.2337/dc15-1795.
- Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. The 2016 global and national burden of diabetes mellitus attributable to PM2.5 air pollution. Lancet Planet Health. 2018 Jun;2(7):e301–12. doi:https://doi.org/10.1016/S2542-5196(18)30140-2.
- Wei Y, Zhang JJ, Li Z, Gow A, Chung KF, Hu M, Sun Z, Zeng L, Zhu T, Jia G, et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing. Faseb J. 2016 Jun;30(6):2115–2122. doi:https://doi.org/10.1096/fj.201500142.
- Liu C, Xu X, Bai Y, Wang TY, Rao X, Wang A, Sun L, Ying Z, Gushchina L, Maiseyeu A, Morishita M, Sun Q, Harkema JR, Rajagopalan S. Air pollution–mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environ Health Perspect. 2014 Jan;122(1):17–26.
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009. Feb;119(4):538–546.
- Irigaray P, Ogier V, Jacquenet S, Notet V, Sibille P, Mejean L, Bihain BE, Yen FT. Benzo[a]pyrene impairs beta-adrenergic stimulation of adipose tissue lipolysis and causes weight gain in mice. A novel molecular mechanism of toxicity for a common food pollutant. Febs J. 2006 Apr;273(7):1362–1372. doi:https://doi.org/10.1111/j.1742-4658.2006.05159.x.
- Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012 Dec;61(12):3037–3045. doi:https://doi.org/10.2337/db12-0190.
- Kish L, Hotte N, Kaplan GG, Vincent R, Tso R, Gänzle M, Rioux KP, Thiesen A, Barkema HW, Wine E, et al. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS One. 2013;8(4):e62220. doi:https://doi.org/10.1371/journal.pone.0062220.
- Li R, Yang J, Saffari A, Jacobs J, Baek KI, Hough G, Larauche MH, Ma J, Jen N, Moussaoui N et al. Ambient ultrafine particle ingestion alters gut microbiota in association with increased atherogenic lipid metabolites. Sci Rep. 2017 Feb;7(1):42906. doi:https://doi.org/10.1038/srep42906.
- Ribière C, Peyret P, Parisot N, Darcha C, Déchelotte PJ, Barnich N, Peyretaillade E, Boucher D. Oral exposure to environmental pollutant benzo[a]pyrene impacts the intestinal epithelium and induces gut microbial shifts in murine model. Sci Rep. 2016 Aug;6(1):31027. doi:https://doi.org/10.1038/srep31027.
- Mutlu EA, Comba IY, Cho T, Engen PA, Yazıcı C, Soberanes S, Hamanaka RB, Niğdelioğlu R, Meliton AY, Ghio AJ, et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ Pollut. 2018 Sep;240:817–830. doi:https://doi.org/10.1016/j.envpol.2018.04.130.
- Wang W, Zhou J, Chen M, Huang X, Xie X, Li W, Cao Q, Kan H, Xu Y, Ying Z. Exposure to concentrated ambient PM2.5 alters the composition of gut microbiota in a murine model. Part Fibre Toxicol, 2018 Apr;15(1):17. doi:https://doi.org/10.1186/s12989-018-0252-6.
- Alderete TL, Jones RB, Chen Z, Kim JS, Habre R, Lurmann F, Gilliland FD, Goran MI. Exposure to traffic-related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environ Res. 2018 Feb;161:472–478. doi:https://doi.org/10.1016/j.envres.2017.11.046.
- Liu T, Chen X, Xu Y, Wu W, Tang W, Chen Z, Ji G, Peng J, Jiang Q, Xiao J, et al. Gut microbiota partially mediates the effects of fine particulate matter on type 2 diabetes: evidence from a population-based epidemiological study. Environ Int. 2019 Jun;130:104882. doi:https://doi.org/10.1016/j.envint.2019.05.076.
- Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, Mujagic Z, Masclee AAM, Jonkers DMAE, Oosting M, et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet. 2019 Apr;51(4):600–605. doi:https://doi.org/10.1038/s41588-019-0350-x.
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012 Jun;336(6086):1262–1267. doi:https://doi.org/10.1126/science.1223813.
- Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012 Jul;3(4):289–306. doi:https://doi.org/10.4161/gmic.19897.
- Hill MJ. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. 1997 Mar;6(Suppl 1):S43–5. doi:https://doi.org/10.1097/00008469-199703001-00009.
- Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014 Mar;5(1):3–17. doi:https://doi.org/10.3920/BM2012.0065.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006 Dec;444(7122):1027–1031. doi:https://doi.org/10.1038/nature05414.
- Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014 Nov;588(22):4223–4233. doi:https://doi.org/10.1016/j.febslet.2014.09.039.
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006 Dec;444(7122):1022–1023. doi:https://doi.org/10.1038/4441022a.
- Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga–Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012 Oct;143(4):913–917. doi:https://doi.org/10.1053/j.gastro.2012.06.031.
- Ross MC, Muzny DM, McCormick JB, Gibbs RA, Fisher-Hoch SP, Petrosino JF. 16S gut community of the cameron county hispanic cohort. Microbiome. 2015 Mar;3:7. doi:https://doi.org/10.1186/s40168-015-0072-y.
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012 Oct;490(7418):55–60. doi:https://doi.org/10.1038/nature11450.
- Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y, Harmelin A, Kolodkin-Gal I, Shapiro H, Halpern Z, Segal E, Elinav E. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014 Oct;514(7521):181–6. doi:https://doi.org/10.1038/nature13793.
- Plöger S, Stumpff F, Penner GB, Schulzke J-D, Gäbel G, Martens H, Shen Z, Günzel D, Aschenbach JR. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012 Jul;1258(1):52–59. doi:https://doi.org/10.1111/j.1749-6632.2012.06553.x.
- Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke J-D, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014 Nov;14(1):189. doi:https://doi.org/10.1186/s12876-014-0189-7.
- Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011 May;141(5):769–776. doi:https://doi.org/10.3945/jn.110.135657.
- Amar J, Lange C, Payros G, Garret C, Chabo C, Lantieri O, Courtney M, Marre M, Charles MA, Balkau B, et al. Blood microbiota dysbiosis is associated with the onset of cardiovascular events in a large general population: the D.E.S.I.R. study. PLoS ONE. 2013;8(1):e54461. doi:https://doi.org/10.1371/journal.pone.0054461.
- Païssé S, Valle C, Servant F, Courtney M, Burcelin R, Amar J, Lelouvier B. Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion. 2016 May;56(5):1138–1147. doi:https://doi.org/10.1111/trf.13477.
- Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, Joannas L, Basavappa MG, Spencer SP, Clark ML et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci Transl Med. 2019 Jun;11(496):eaav1892. doi:https://doi.org/10.1126/scitranslmed.aav1892.
- Salim SY, Kaplan GG, Madsen KL. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes. 2014 Mar;5(2):215–219. doi:https://doi.org/10.4161/gmic.27251.
- Möller W, Häussinger K, Winkler-Heil R, Stahlhofen W, Meyer T, Hofmann W, Heyder J. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J Appl Physiol. 2004 Dec;97(6):2200–2206. doi:https://doi.org/10.1152/japplphysiol.00970.2003.
- Oberdörster G. Lung dosimetry: pulmonary clearance of inhaled particles. Aerosol Sci Technol. 1993;18(3):279–289. doi:https://doi.org/10.1080/02786829308959605.
- Stuart BO. Deposition and clearance of inhaled particles.. Environ Health Perspect. 1984 Apr;55:369–390. doi:https://doi.org/10.1289/ehp.8455369.
- Kreyling WG, Blanchard JD, Godleski JJ, Haeussermann S, Heyder J, Hutzler P, Schulz H, Sweeney TD, Takenaka S, Ziesenis A, et al. Anatomic localization of 24- and 96-h particle retention in canine airways. J Appl Physiol. 1999 Jul;87(1):269–284. doi:https://doi.org/10.1152/jappl.1999.87.1.269.
- Thomas J, Guénette J, Thomson EM. Stress axis variability is associated with differential ozone-induced lung inflammatory signaling and injury biomarker response. Environ Res. 2018 Nov;167:751–758. doi:https://doi.org/10.1016/j.envres.2018.09.007.
- Thomson EM, Vladisavljevic D, Mohottalage S, Kumarathasan P, Vincent R. Mapping acute systemic effects of inhaled particulate matter and ozone: multiorgan gene expression and glucocorticoid activity. Toxicol Sci. 2013 Jun;135(1):169–181. doi:https://doi.org/10.1093/toxsci/kft137.
- Van de Wiele T, Vanhaecke L, Boeckaert C, Peru K, Headley J, Verstraete W, Siciliano S. Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites. Environ Health Perspect. 2005 Jan;113(1):6–10. doi:https://doi.org/10.1289/ehp.7259.
- Pinyayev TS, Kohan MJ, Herbin-Davis K, Creed JT, Thomas DJ. Preabsorptive metabolism of sodium arsenate by anaerobic microbiota of mouse cecum forms a variety of methylated and thiolated arsenicals. Chem Res Toxicol. 2011 Apr;24(4):475–477. doi:https://doi.org/10.1021/tx200040w.
- Peters A, Perz S, Döring A, Stieber J, Koenig W, Wichmann HE. Increases in heart rate during an air pollution episode. Am J Epidemiol. 1999 Nov;150(10):1094–1098. doi:https://doi.org/10.1093/oxfordjournals.aje.a009934.
- Ibald-Mulli A, Stieber J, Wichmann HE, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health. 2001 Apr;91(4):571–577. doi:https://doi.org/10.2105/ajph.91.4.571.
- Kaplan GG, Hubbard J, Korzenik J, Sands BE, Panaccione R, Ghosh S, Wheeler AJ, Villeneuve PJ. The inflammatory bowel diseases and ambient air pollution: a novel association. Am J Gastroenterol. 2010 Nov;105(11):2412–2419. doi:https://doi.org/10.1038/ajg.2010.252.
- Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. Ambient air pollution correlates with hospitalizations for inflammatory bowel disease: an ecologic analysis. Inflamm Bowel Dis. 2011 May;17(5):1138–1145. doi:https://doi.org/10.1002/ibd.21455.
- Kaplan GG, Szyszkowicz M, Fichna J, Rowe BH, Porada E, Vincent R, Madsen K, Ghosh S, Storr M. Non-specific abdominal pain and air pollution: a novel association. PLoS ONE. 2012 Oct;7(10):e47669. doi:https://doi.org/10.1371/journal.pone.0047669.
- Kaplan GG, Dixon E, Panaccione R, Fong A, Chen L, Szyszkowicz M, Wheeler A, MacLean A, Buie WD, Leung T et al. Effect of ambient air pollution on the incidence of appendicitis. Can Med Assoc J. 2009 Oct;181(9):591–597. doi:https://doi.org/10.1503/cmaj.082068.
- Kaplan GG, Tanyingoh D, Dixon E, Johnson M, Wheeler AJ, Myers RP, Bertazzon S, Saini V, Madsen K, Ghosh S, et al. Ambient ozone concentrations and the risk of perforated and nonperforated appendicitis: a multicity case-crossover study. Environ Health Perspect. 2013 Aug;121(8):939–943. doi:https://doi.org/10.1289/ehp.1206085.
- Orazzo F, Nespoli L, Ito K, Tassinari D, Giardina D, Funis M, Cecchi A, Trapani C, Forgeschi G, Vignini M, et al. Air pollution, aeroallergens, and emergency room visits for acute respiratory diseases and gastroenteric disorders among young children in six Italian cities. Environ Health Perspectives. 2009 Nov;117(11):1780–1785. doi:https://doi.org/10.1289/ehp.0900599.
- Harris JE Cigarette smoke components and disease: cigarette smoke is more than a triad of tar, nicotine, and carbon monoxide. In: National Cancer Institute. The FTC cigarette test method for determining tar, nicotine, and carbon monoxide yields of US cigarettes: report of the NCI Expert Committee. Bethesda, Maryland: National Institutes of Health, 1996. (NIH Publication No 96-4028.)
- Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ, Steurer-Stey C, Frei A, Frei P, Scharl M, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8(3):e59260. doi:https://doi.org/10.1371/journal.pone.0059260.
- Benjamin JL, Hedin CRH, Koutsoumpas A, Ng SC, McCarthy NE, Prescott NJ, Pessoa-Lopes P, Mathew CG, Sanderson J, Hart AL, et al. Smokers with active Crohnʼs disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflammatory Bowel Dis. 2012 Jun;18(6):1092–1100. doi:https://doi.org/10.1002/ibd.21864.
- Stewart CJ, Auchtung TA, Ajami NJ, Velasquez K, Smith DP, De La Garza R, Salas R, Petrosino JF. Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: a pilot study. PeerJ. 2018 Apr;6:e4693. doi:https://doi.org/10.7717/peerj.4693.
- Allais L, Kerckhof F-M, Verschuere S, Bracke KR, De Smet R, Laukens D, Van den Abbeele P, De Vos M, Boon N, Brusselle GG, et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ Microbiol. 2016 May;18(5):1352–1363. doi:https://doi.org/10.1111/1462-2920.12934.
- Kim M, Gu B, Madison MC, Song HW, Norwood K, Hill AA, Wu W-J, Corry D, Kheradmand F, Diehl GE, et al. Cigarette smoke induces intestinal inflammation via a Th17 cell-neutrophil axis. Front Immunol. 2019 Jan;10:75. doi:https://doi.org/10.3389/fimmu.2019.00075.
- Beamish LA, Osornio-Vargas AR, Wine E. Air pollution: an environmental factor contributing to intestinal disease. J Crohns Colitis. 2011 Aug;5(4):279–286. doi:https://doi.org/10.1016/j.crohns.2011.02.017.
- Scientific Committee on Food (SCF). Opinion of the scientific committee on food on the risks to human health of polycyclic aromatic hydrocarbons in food. Brussels: Scientific Committee on Food; 2002.
- De Brouwere K, Buekers J, Cornelis C, Schlekat CE, Oller AR. Assessment of indirect human exposure to environmental sources of nickel: oral exposure and risk characterization for systemic effects. Sci Total Environ. 2012 Mar;419(419):25–36. doi:https://doi.org/10.1016/j.scitotenv.2011.12.049.
- Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008 Jan;151(2):362–367. doi:https://doi.org/10.1016/j.envpol.2007.06.012.
- Lomer MCE, Hutchinson C, Volkert S, Greenfield SM, Catterall A, Thompson RPH, Powell JJ. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn’s disease. Br J Nutr. 2004 Dec;92(6):947–955. doi:https://doi.org/10.1079/BJN20041276.
- Lomer MCE, Thompson RPH, Powell JJ. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn’s disease. Proc Nutr Soc. 2002 Feb;61(1):123–130. doi:https://doi.org/10.1079/PNS2001134.
- Mutlu EA, Engen PA, Soberanes S, Urich D, Forsyth CB, Nigdelioglu R, Chiarella SE, Radigan KA, Gonzalez A, Jakate S, et al. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxicol. 2011 Jun;8:19. doi:https://doi.org/10.1186/1743-8977-8-19.
- Adams K, Greenbaum DS, Shaikh R, van Erp AM, Russell AG. Particulate matter components, sources, and health: systematic approaches to testing effects. Journal of the Air. 2015 May;65(5):544–558. doi:https://doi.org/10.1080/10962247.2014.1001884.
- Li X, Brejnrod AD, Ernst M, Rykær M, Herschend J, Olsen NMC, Dorrestein PC, Rensing C, Sørensen SJ. Heavy metal exposure causes changes in the metabolic health-associated gut microbiome and metabolites. Environ Int. 2019 May;126:454–467. doi:https://doi.org/10.1016/j.envint.2019.02.048.
- Gao B, Chi L, Mahbub R, Bian X, Tu P, Ru H, Lu K. Multi-omics reveals that lead exposure disturbs gut microbiome development, key metabolites, and metabolic pathways. Chem Res Toxicol. 2017 Apr;30(4):996–1005. doi:https://doi.org/10.1021/acs.chemrestox.6b00401.
- Hussey SJK, Purves J, Allcock N, Fernandes VE, Monks PS, Ketley JM, Andrew PW, Morrissey JA. Air pollution alters Staphylococcus aureus and Streptococcus pneumoniae biofilms, antibiotic tolerance and colonisation. Environ Microbiol. 2017 May;19(5):1868–1880. doi:https://doi.org/10.1111/1462-2920.13686.
- Yasuyuki M, Kunihiro K, Kurissery S, Kanavillil N, Sato Y, Kikuchi Y. Antibacterial properties of nine pure metals: a laboratory study using Staphylococcus aureus and Escherichia coli. Biofouling. 2010 Oct;26(7):851–858. doi:https://doi.org/10.1080/08927014.2010.527000.
- Roslund MI, Rantala S, Oikarinen S, Puhakka R, Hui N, Parajuli A, Laitinen OH, Hyöty H, Rantalainen A-L, Sinkkonen A, et al. Endocrine disruption and commensal bacteria alteration associated with gaseous and soil PAH contamination among daycare children. Environ Int. 2019 Jun;130(130):104894. doi:https://doi.org/10.1016/j.envint.2019.06.004.
- U.S. EPA. Integrated science assessment (ISA) of ozone and related photochemical oxidants. Washington (DC): U.S. Environmental Protection Agency; 2013. Final Report, Feb 2013. EPA/600/R-10/076F.
- Petrosus E, Silva EB, Lay D, Eicher SD. Effects of orally administered cortisol and norepinephrine on weanling piglet gut microbial populations and Salmonella passage. J Anim Sci. 2018 Nov;96(11):4543–4551. doi:https://doi.org/10.1093/jas/sky312.
- Lyte M. Microbial endocrinology and the microbiota-gut-brain axis. Adv Exp Med Biol. 2014;817:3–24. doi:https://doi.org/10.1007/978-1-4939-0897-4_1.
- Lyte M, Ernst S. Catecholamine induced growth of gram negative bacteria. Life Sci. 1992;50(3):203–212. doi:https://doi.org/10.1016/0024-3205(92)90273-r.
- Morgan XC, Huttenhower C, Lewitter F, Kann M. Chapter 12: human microbiome analysis. Lewitter F, Kann M, editors. PLoS Comp Biol. 2012;8(12):e1002808. doi:https://doi.org/10.1371/journal.pcbi.1002808.
- Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Ter Horst R, Jansen T, Jacobs L, Bonder MJ et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016 Nov;167(4):1125–1128. doi:https://doi.org/10.1016/j.cell.2016.10.020.
- Swaroop JJ, Naidu JN, Rajarajeswari D. Association of TNF-α with insulin resistance in type 2 diabetes mellitus. Indian J Med Res. 2012;135(1):127–130. doi:https://doi.org/10.4103/0971-5916.93435.
- Aguirre M, Venema K. Does the gut microbiota contribute to obesity? going beyond the gut feeling. Microorganisms. 2015 Apr;3(2):213–235. doi:https://doi.org/10.3390/microorganisms3020213.
- Moon Y-S, Kim D-H, Song D-K. Serum tumor necrosis factor-α levels and components of the metabolic syndrome in obese adolescents. Metabolism. 2004 Jul;53(7):863–867. doi:https://doi.org/10.1016/j.metabol.2004.02.007.
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Nat Acad Sci. 2005 Aug;102(31):11070–11075. doi:https://doi.org/10.1073/pnas.0504978102.
- Nguyen TLA, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015 Jan;8(1):1–16. doi:https://doi.org/10.1242/dmm.017400.
- Taylor AA, Marcus IM, Guysi RL, Walker SL. Metal oxide nanoparticles induce minimal phenotypic changes in a model colon gut microbiota. Environ Eng Sci. 2015 Jul;32(7):602–612. doi:https://doi.org/10.1089/ees.2014.0518.
- Kohane DS. Microparticles and nanoparticles for drug delivery. Biotechnol Bioeng. 2007 Feb;96(2):203–209. doi:https://doi.org/10.1002/bit.21301.
- Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, Berry D. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in F irmicutes populations. Environ Microbiol. 2017 Jan;19(1):95–105. doi:https://doi.org/10.1111/1462-2920.13463.
- Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, SPRING Trial Group. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes. 2016 Aug;65(8):2214–2223. doi:https://doi.org/10.2337/db16-0278.
- Shah SNA, Shah Z, Hussain M, Khan M. Hazardous effects of titanium dioxide nanoparticles in ecosystem. Bioinorg Chem Appl. 2017;2017:4101735. doi:https://doi.org/10.1155/2017/4101735.
- Dahle J, Arai Y. Environmental geochemistry of cerium: applications and toxicology of cerium oxide nanoparticles. Int J Environ Res Public Health. 2015 Feb;12(2):1253–1278. doi:https://doi.org/10.3390/ijerph120201253.
- Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015 Apr;7(3):219–242. doi: https://doi.org/10.1007/s40820-015-0040-x.
- Truffier-Boutry D, Fiorentino B, Bartolomei V, Soulas R, Sicardy O, Benayad A, Damlencourt J-F, Pépin-Donat B, Lombard C, Gandolfo A, Wortham H, Brochard G, Audemard A, Porcar L, Gebela G, Gligorovski S. Characterization of photocatalytic paints: a relationship between the photocatalytic properties – release of nanoparticles and volatile organic compounds. Environ Sci Nano. 2017;4(10):1998–2009. https://doi.org/10.1039/C7EN00467B
- É Nerriere, Zmirou-Navier D, Blanchard O, Momas I, Ladner J, Le Moullec Y, Personnaz M-B, Lameloise P, Delmas V, Target A, Desqueyrouxj H. Can we use fixed ambient air monitors to estimate population long-term exposure to air pollutants? The case of spatial variability in the Genotox ER study. Environ Res. 2005 Jan;97(1):32–42. doi:https://doi.org/10.1016/j.envres.2004.07.009.
- Duca FA, Sakar Y, Lepage P, Devime F, Langelier B, Doré J, Covasa M. Replication of obesity and associated signaling pathways through transfer of microbiota from obese-prone rats. Diabetes. 2014 May;63(5):1624–1636. doi:https://doi.org/10.2337/db13-1526.
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013 Sep;341(6150):1241214. doi:https://doi.org/10.1126/science.1241214.
- Zierer J, Jackson MA, Kastenmüller G, Mangino M, Long T, Telenti A, Mohney RP, Small KS, Bell JT, Steves CJ, et al. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet. 2018 Jun;50(6):790–795. doi:https://doi.org/10.1038/s41588-018-0135-7.
- Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017 Jun 1;474(11):1823–1836. doi:https://doi.org/10.1042/BCJ20160510.
- Fu J, Bonder MJ, Cenit MC, Tigchelaar EF, Maatman A, Dekens JAM, Brandsma E, Marczynska J, Imhann F, Weersma RK et al. The Gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circulation Research. 2015 Oct;117(9):817–824. doi:https://doi.org/10.1161/CIRCRESAHA.115.306807.
- Ma N, Dietary Amino MX. Acids and the gut‐microbiome‐immune axis: physiological metabolism and therapeutic prospects. Compr Rev Food Sci Food Saf. 2018 Dec;18(1):221–242. doi:https://doi.org/10.1111/1541-4337.12401.
- She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007 Dec;293(6):E1552–63. doi:https://doi.org/10.1152/ajpendo.00134.2007.
- Butte NF, Liu Y, Zakeri IF, Mohney RP, Mehta N, Voruganti VS, Göring H, Cole SA, Comuzzie AG. Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am J Clin Nutr. 2015 Aug;102(2):256–267. doi:https://doi.org/10.3945/ajcn.115.111872.
- McCormack SE, Shaham O, McCarthy MA, Deik AA, Wang TJ, Gerszten RE, Clish CB, Mootha VK, Grinspoon SK, Fleischman A, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013 Feb;8(1):52–61. doi:https://doi.org/10.1111/j.2047-6310.2012.00087.x.
- Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009 Sep;10(3):167–177. doi:https://doi.org/10.1016/j.cmet.2009.08.001.
- Kriaa A, Bourgin M, Potiron A, Mkaouar H, Jablaoui A, Gérard P, Maguin E, Rhimi M. Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. J Lipid Res. 2019 Feb, 60(2), 323–332. DOI: https://doi.org/10.1194/jlr.R088989
- Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013 Aug;39(2):372–385. doi:https://doi.org/10.1016/j.immuni.2013.08.003.
- Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006 Mar;116(3):561–570. doi:https://doi.org/10.1172/JCI27987.
- Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids. 2014 Nov;90:13–29. doi:https://doi.org/10.1016/j.steroids.2014.06.012.
- Nicolucci AC, Hume MP, Martínez I, Mayengbam S, Walter J, Reimer RA. Prebiotics reduce body fat and alter intestinal microbiota in children who are overweight or with obesity. Gastroenterology. 2017 Sep;153(3):711–722. doi:https://doi.org/10.1053/j.gastro.2017.05.055.
- Grazul H, Kanda LL, Gondek D. Impact of probiotic supplements on microbiome diversity following antibiotic treatment of mice. Gut Microbes. 2016;7(2):101–114. doi:https://doi.org/10.1080/19490976.2016.1138197.
