ABSTRACT
This study focuses on the analysis of a post-medieval assemblage of glass vessels and tableware, recovered from an Ottoman bathhouse in Kyparissia, SW Peloponnese, Greece. The chemical composition of the samples was estimated using SEM/EDS, whereas minor and trace elements were identified by qualitative XRF analysis. Raman spectroscopy was applied in order to examine the connectivity of the silicate glass matrix in more detail. The acquired data indicate significant chemical variations among the samples, especially in regards to the alkali source and the decolourant used. Moreover, one high-lead glass has also been identified. The provenance analysis, which was based on the statistical treatment of the resulted and published analytical data, indicated that the different glass types originated both from the eastern regions of the Ottoman Empire and from glassmaking centres in central and northern Europe. The determination of technology and provenance of the assemblage provided with valuable new information regarding the production and trading network of glass in mainland Greece during the Ottoman rule.
Introduction
Since the invention of glass at the beginning of the 2nd millennium BC, the model of glass production remained similar for almost three millennia: glassmaking and glassworking were two distinct processes which took place in different workshops. Glassmaking initially occurred in few and specialized workshops, namely the Syropalestine region, Mesopotamia and Egypt. In later times and especially since the Classical period, glassmaking is considered to have expanded in other Mediterranean regions, like Greece and Italy, as well as Britain (Henderson, Citation2013). The produced glass was then transferred in the form of ingots or cullets for long distances throughout the Mediterranean Sea and Europe to the glassworking workshops, where the final objects were formed. The number and the geographic distribution of glassworking workshops varied significantly according to the time period.
However, at some point during the Middle Ages (Veritá Citation2013) the production model changed, with the introduction of local workshops which carried out both the activities of glassmaking and glassworking. Along with a major shift in the 9th c. towards the use of plant ash instead of mineral natron as the major alkali source, a period of continuous experimentation with the use of local easily accessible raw materials started. At the same time, the production of specialised glasses was still based on the use of traditional raw materials of high quality, which were often transferred to large distances. The new raw materials used, along with the aesthetic need for the production of very clear colourless glass, led to the invention of new preparation methods for the raw materials, the use of significantly different recipes and the introduction of new manufacturing processes. As a result, the glass produced in Europe and Middle East between the 15th and 19th c. shows a remarkable variation in regards to its visual and chemical characteristics.
Although glassmaking and glassworking in the European centres has attracted extensive attention from scholars, production and use of glass of the same period from the Ottoman Empire remains largely unknown. The present study is a first analytically based attempt to examine an assemblage of Ottoman glass from Kyparissia, southern Greece, focusing specifically in determining the chemical composition and the likely provenance of the studied glasses.
Glass in the 15th-19th centuries
Between the 15th and the 17th c. Venice was one of the larger and more famous glassworking centres in Europe, primarily due to the high quality of the produced glass, the aesthetic superiority of the arts and the large variance of objects produced (Verità, Citation2014). Venetian glassworkers followed the Islamic tradition and produced glass of the soda-lime-silica type, with plant-ash rich in soda serving as the alkali source. The plant-ash was shipped from specific regions in Egypt, Syria and (after the 16th c.) Spain; siliceous pebbles were collected from the river beds of Ticino and Adige (Cagno, Citation2008). The main decolourant used was manganese and, from the 17th c. onwards, arsenic. Venetians followed very strict recipes and produced three different types of colourless glass of varying quality: ‘vetro comune’, ‘vitrum blanchum’ and ‘cristallo’, which was the most superior of them (Verità, Citation2013).
During the 16th c. a number of Venetian glassmakers disregarded the movement prohibition, which was imposed in order to protect the secrets of the Venetian glass industry, and fled to cities of central and north Europe. There, they established glassmaking workshops following the Venetian recipes and using similar raw materials; the produced glass is generally known as ‘façon de Venise’. Significant workshops of the kind were located in Antwerp, Amsterdam and London and continued producing glass until the 18th c. Although it is generally impossible to distinguish the original Venetian glass based on its macroscopic traits or the chemical composition of the major elements (Janssens, Citation2013), analyses of both major and trace elements have proven successful (e.g. De Raedt, et. al., Citation2001; Henderson, et. al., Citation2005).
The above mentioned glass types prevailed for several centuries for the production of luxurious objects. However, during the medieval (since the 8th-9th c.) and post-medieval period the prevailing glass type for the production of everyday objects and glass panes was ‘forest glass’. Forest glass is a naturally coloured type of K-rich glass, produced with the addition of forest plant-ash rich in potassium as the alkali source (Dungworth, et al., Citation2006). The large variation in the composition of the ashes depending on the specific species used, or even on the part of the same plant, led to a varied composition in the glass produced.
A special sub-category of forest glass is the High Alumina Low Alkali (HLLA) glass. HLLA is naturally coloured and its alkali source probably was plant-ash rich in potassium. It is easily distinguished chemically by the relatively low concentration of alkalis (Na2O + K2O < 10 wt%) and the very high concentration in calcium (CaO > 20 wt%) (Dungworth, et al., Citation2006). HLLA glass was produced in Germany since the 14th c. and France and Britain since the 16th c., in some cases completely replacing the production of forest glass (Barrera and Velde, Citation1989; Dungworth and Clark, Citation2004). Its production continued until the end of the 19th c.; it was initially used for both bottles and glass panes, and later on exclusively for bottles.
Another type of common glass is mixed alkali glass, which is characterized by the simultaneous presence of relatively high concentrations of sodium and potassium. According to the categorization presented by Dungworth et al. (Citation2006), Na-rich glass contains Na2O > 12 wt% and K2O < 5 wt%, whereas K-rich glass contains K2O between 10-15 wt% and Na2O < 5 wt%. All the intermediate compositions are characterized as mixed alkali. This type of glass, produced in various centres in central and northern Europe, presents a high degree of chemical variability.
During the 17th c. two new types of clear colourless glass were invented in central Europe, the so-called crystal and chalk glass, threatening the Venetian monopoly of clear glass. According to Kunicki-Goldfinger (Citation2005) the technological roots of these glass types can be located in France or Netherlands and later spread to many regions in central Europe. Crystal glass, which should not be mistaken for English lead crystal or Venetian cristallo, is a K-rich glass. Its main raw materials were siliceous pebbles, saltpetre (potassium nitrate) as the alkali source, chalk and arsenic as the decolourant (Kunicki-Goldfinger, Citation2005). Chalk glass, often called ‘Bohemian glass’ is a similar type of glass, invented in Bohemia. It is of slightly lower quality than crystal glass, with similar raw materials with the exception of the alkali source. In this case the flux added was potash (potassium carbonate), sometimes combined with saltpetre (Kunicki-Goldfinger, Citation2003).
The last significant type of European glass in this period is the English colourless lead glass, invented by George Ravenscroft in 1673/1674. Until this time, lead had only been used in strongly coloured glasses and various types of glassy materials (Charleston, Citation1960). The colourless lead glass, elsewhere referred to as ‘flint glass’ or ‘lead crystal’, contained between 15 and 40 wt% lead and was made from fine white sand and saltpetre (Dungworth, et al., Citation2006). Its use spread very quickly in Britain and the rest of Europe, due to its high quality and low production cost. By the beginning of the 18th c. imitations of English lead glasses were produced in Norway, Normandy and elsewhere.
Finally, the Islamic glass, which prevailed both in the Middle East and in Europe between the 7th and 14th c., was now going through a period of decline. Glass in the Islamic world was primarily imported from Europe and especially Venice and Bohemia. In the Ottoman Empire, what little glass was locally produced was primarily following the European forms and patterns (Soudavar Diba, Citation1983). In the 19th c. the area of Beykoz in Istanbul enlarged its glass production; the local glass was strongly influenced by Venetian and Bohemian glasses.
The archaeological site of Kyparissia
The assemblage studied here was recovered in a small Ottoman public bathhouse (hamam) in Kyparissia, southern Greece. Kyparissia is located in SW Peloponnese, at the foothills of mount Aigaleo, allowing the strategic control of inland pathways. At the same time, its natural port facilitated the maritime trade with the west. Kyparissia was conquered by the Franks after the 4th Crusade (1201-1204). After a period of successive conquerors, Kyparissia was conquered by the Ottomans in 1459. The Ottoman rule continued until 1821, with a 30-year interval of Venetian occupation.
The bathhouse was built to the west of the central cobblestone which led to the castle. The structure was revealed when a house, built on top of the remaining parts of the bathhouse, was demolished. The restoration work was limited to the debris removal and to the excavation of the main parts of the bathhouse without expanding to the rest of the building. The exact time of construction is unknown. However, a bathhouse was mentioned by the Ottoman traveler Evliyâ Çelebi at 1668; assuming that his testimony is a reference to this bathhouse, we have a date that serves as a terminus ante quem. Generally speaking, the Ottoman public buildings would likely be built shortly after the regime change, at the beginning of the Ottoman occupation at the 2nd half of the 15th c. However, certain aspects of the building (e.g. its simplified construction, the lack of decorated internal surfaces and the extended use of bricks), in conjunction with the recovered pottery, suggest a later date for its construction, likely around the first half of the 17th c. (Germanidou and Gerolymou, Citation2015).
During the excavation a wide variety of findings was recovered, most of which was not directly related to the usage period of the bathhouse. Among the findings, it is interesting to note the presence of glazed pottery dated to the end of the 16th or beginning of 17th c., two Venetian copper coins dated to the period 1684-1710 and one Ottoman silver coin from the 18th c. (Germanidou and Gerolymou, Citation2015).
Materials and methods
A number of glass sherds were additionally recovered from the bathhouse. The assemblage presented in this paper consists of 42 glass sherds from small vessels or tableware (). The majority of the samples are colourless, or colourless with a slight yellow, pink or grey tinge. The coloured samples have a green (1), light blue (3) and blue hue (2). All the items have thick walls and demonstrate almost no decorative patterns.
Table 1. Basic characteristics of the assemblage of Ottoman glass from Kyparissia.
Optical microscopy using a fibre optics system (FOM/i-scope, Moritex) was performed on all samples aiming at an initial documentation of their preservation state. Overall, the microscopic examination showed limited visual alterations; iridescence was the prevailing corrosion pattern visible in all samples. Non-destructive X-Ray Fluorescence (p-XRF) analysis was applied on all samples using a portable Bruker Tracer III SD set up, with a beam diameter of 3 mm; data quantification was made using S1PXRF software and a custom-built calibration curve, created in collaboration with the scientific personnel of Bruker. A detailed description of the calibration curve and the accuracy and precision achieved for the analysis of glass is provided elsewhere (Palamara, et al., Citation2016). In order to optimise the analytical range, two settings were used: (1) an unfiltered low-energy excitation mode (high voltage set at 15 kV and current of 24 μΑ, analyses carried out under vacuum) was used for the analysis of major and minor elements with an atomic number, Z, between 11 and 29; and (2) an Al/Ti filtered (0.012 inches Al plus 0.001 inches Ti) high-energy excitation mode (high voltage set at 40 kV and current of 12 μA) was used for the analysis of minor and trace elements with an atomic number Z > 29. The collection time of each measurement was 300 sec. The samples were not cleaned prior to the analysis, however care was taken to select areas with clear glass, flat surface and thick walls, based on the results of the FOM examination.
The initial quantification of the XRF data revealed alkali values significantly lower than those expected, coupled with very high silica values (exceeding 90 wt% for the majority of the samples) thus pointing to an overall significant alteration of the chemical composition of the external surfaces due to corrosion (Zacharias and Palamara, Citation2016). Therefore, the XRF analyses were only used qualitatively for the identification of minor and trace quantities of colourants and decolourants, which are not expected to be affected by the corrosion processes.
For the better evaluation of the pristine glass of the samples, fresh cuts were measured by Scanning Electron Microscopy coupled with an Energy Dispersive Spectrometer (SEM/EDS). Microsamples were cut off, embedded in resin and polished. The polished samples were analyzed under a SEM type JEOL JSM–6510LV coupled with an Oxford Instruments EDS. The analytical data were obtained by INKA software. The bulk analyses were conducted at high vacuum, 20 kV accelerating voltage and with a count time of 300 sec. Each sample was measured at least three times. The accuracy and precision of the applied SEM/EDS setting has been described in detail elsewhere (Moropoulou, et al., Citation2016; Palamara, et al., Citation2016). Briefly, all major oxides in glass present high precision (error < 5%), with the exception of CaO which presents a deviation from the true value of approximately 10-15%. Naturally, for alkali concentration lower than 5 wt%, the precision of the analysis lowers significantly. The achieved accuracy of the analysis usually varies, with deviations from the mean value between 5 and 10% for all major oxides.
Raman was additionally employed in order to examine the connectivity of the silicate glass matrix in detail, and therefore the production technology. Attempts to measure Raman spectra with laser excitation in the visible led to strong fluorescence backgrounds. This problem was largely resolved with excitation in the near-infrared at 1064 nm, using an FT-Raman spectrometer (Bruker RFS100) acquired with a Nd:YAG laser. All Raman spectra were measured at room temperature with 4 cm−1 resolution. Each spectrum represents the average of 400 scans with an effective frequency range from ca. 80 to 3,500 cm−1. The analysed surfaces were cleaned but not polished prior to the analysis. Compositional profiles of the fresh cuts conducted by SEM/EDS, suggested that the external corroded layers had a width of 2-4 μm. Therefore, taking into consideration the relatively large penetration depth of the Raman beam (Möncke, et al., Citation2013), the acquired Raman spectra are considered to probe the pristine glass matrix.
Chemical characterization
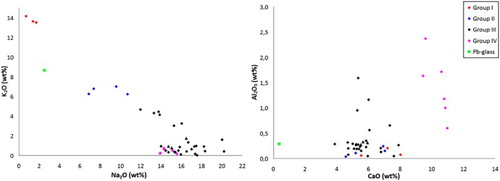
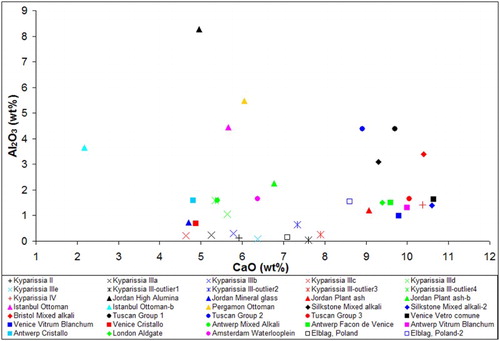
The mean value and standard deviation of the major elements of the glass fragments were determined by SEM/EDS (Supplemental Material 1). The chemical composition of the samples demonstrates significant variability for all major elements, suggesting the simultaneous presence of many different glass types. The concentrations of sodium versus potassium and calcium versus alumina are presented in the two biplots of . It should be highlighted that four samples present significant quantities of lead; these samples will be discussed in detail below in a separate section.
According to Dungworth et al. (Citation2006) medieval and post-medieval glass can be categorised in four types based on its alkali concentration: (a) Na-rich glass, with Na2O > 12 wt% and K2O < 5 wt%; (b) K-rich glass, with Κ2Ο between 10 and 15 wt% and Na2O < 5 wt%; (c) mixed alkali glass, with intermediate relatively high values for both sodium and potassium; and (d) HLLA glass, which is easily differentiated by the overall concentration in alkalis (Na2O + K2O < 10 wt%). Therefore, the majority of the samples of the present assemblage are Na-rich, three samples are K-rich and 4 belong to the mixed alkali group (). No HLLA glass is found among the analysed samples.
Taking also into consideration the composition of alumina and calcium (), we can divide the samples of the assemblage into 4 distinct groups of glass and one outlier. One of the groups can be further divided into several sub-groups. The mean value of the concentration of the major elements for each of these groups is given in .
Group I: Comprises of 3 K-rich glasses. All samples are clear and colourless. They are characterized by very low Al2O3 levels (<0.25 wt%), but their CaO concentration varies significantly (between 5.5 and 8 wt%). The very low magnesium and phosphorus concentration of the samples suggests that the potassium source is saltpetre or potash, instead of K-rich plant-ash. The lack of a green tinge also suggests that this group does not belong to the forest glass type, but is either crystal or chalk glass. This categorization has implications on both the dating of the samples and the likely provenance, since both these glass types were in use mostly in central Europe and only after the 2nd half of the 17th c. (Mádl and Kunicki-Goldfinger, Citation2006).
Group II: Comprises of 4 mixed alkali glasses. All the samples of this group are colourless or naturally coloured with a yellow tinge. The variability of all major oxides is high in this group. The samples have characteristically high silica concentration (>77 wt%), calcium values vary between 4.6 and 7 wt% and the Na2O/K2O ratio varies between 1.1 and 1.7.
Group III: Comprises of 28 Na-rich glasses. The majority of the samples are colourless or naturally coloured with a yellow, pink or grey tinge. The group also includes three light blue and one dark blue sample. Three samples with low-lead concentration also belong to this group.
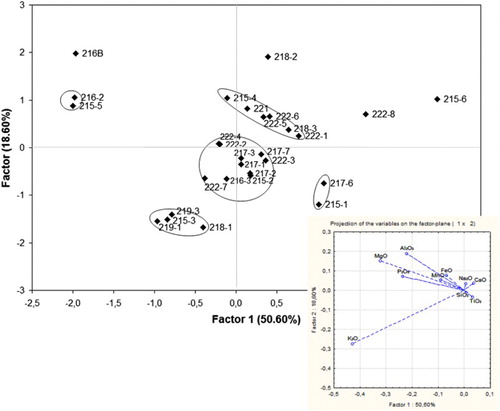
The alkali source for this group is plant-ash rich in sodium. The concentration of the major elements presents high variability within this group. Apart from the difference in raw materials and recipes used, the observed compositional heterogeneity could also be the result of recycling. PCA analysis was carried out for Group III samples based on all major elements (). The PCA plot indicates the presence of at least three sub-groups, with 4, 6 and 7 samples respectively. Two more couples of samples may form distinct sub-groups, while four samples are outliers. The small number of samples and the absence of data for the trace elements make it impossible to clearly distinguish between sub-groups. However, taking into consideration the large number of workshops operating during this period, often using the same recipe and local raw materials, the presence of different Na-rich sub-groups is worth further examination.
Group IV: Comprises of 6 Na-rich glasses. The silica source in this groups is again plant-ash rich in sodium. Four samples are colourless or naturally coloured with a green tinge. The other samples show green and dark blue colours. The composition of Na2O, K2O and MgO is similar to that of Group III samples. However, the samples of this group demonstrate a characteristically high and homogeneous concentration of calcium (approximately 11 wt%). The concentration of alumina is elevated compared to the other samples of the assemblage, but varies greatly from 0.5 to 2.5 wt%.
High-Pb glass: Sample 219-4 is distinguished by its high concentration in lead and will be further discussed below. The sample is colourless and clear, and does not present any decorative patterns or otherwise unique morphological characteristics.
Table 2. Mean value (μ) and standard deviation (s) for the concentration (in wt%) of the major oxides for each group and sub-group of the assemblage.
Low- and high-lead groups
As has already been mentioned, in four of the samples of this assemblage lead was identified in varying concentrations. The three low-lead glasses belong to Group III, are colourless and have very similar chemical composition, with a lead concentration between 2.1 and 2.6 wt%. When lead is present in the glass in such small quantities it does not serve as the main network modifier and it seems generally improbable that it could significantly affect physical properties of glass, such as melting temperature, viscosity etc. (Dungworth, et al., Citation2006). Small quantities of lead (<5 wt%) have been identified in various colourless glasses of the period under study, which do not present otherwise significant differences in their chemical composition. However, it is not certain whether the introduction of lead in these cases was done on purpose or was accidental.
Sample 219-4, also colourless, presents a high concentration in lead (23 wt%) and can therefore be described as lead glass. The primary alkali is potassium, although sodium is also present in relatively high concentration. As expected for lead glasses, the concentration of all other oxides is very low (<0.4 wt%).
Colourants and decolourants
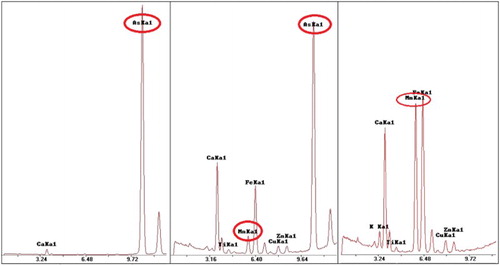
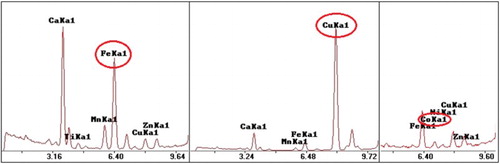
Based on the qualitative XRF analysis of the coloured samples, the colourant used in each case was identified. More specifically, cobalt oxide was added for the production of blue glass, copper oxide for the light blue glass and iron oxide for the green glass (). The use of these colourants is typical and well documented in literature.
Figure 3. Representative XRF spectra for samples of green (left), light blue (centre) and blue colour (right) (samples 218-4, 217-1 and 216-1, respectively).
Glass workers of the period under study could achieve a very good control over the raw materials and the manufacturing process. Furnace control was achieved by the careful consideration of a number of parameters during firing, such as fuel selection, air supply, crucible shape, maximum temperature and the time that the melt was held at the maximum temperature (Henderson, et. al. Citation2005). Additionally, the use of new and more efficient furnaces, as well as the replacement of wood with mineral fuels in the early 17th c., allowed the consistent production of better quality glass (Charleston, Citation1978; Crossley, Citation1991).
As a result, medieval and post-medieval colourless glass was produced with the addition of very limited quantities of decolourants. The most common decolourant was manganese, added in the form of pyrolusite (MnO2). From the 17th c. onwards, arsenic trioxide was also used (Verità, Citation2013). In Venice, As2O3 was used either on its own or along with small quantities of manganese, whereas in central Europe it was widely used for the production of clear colourless glass (crystal glass, chalk glass) (Kunicki-Goldfinger, et al., Citation2003).
Among the samples of the present assemblage 26 are colourless and 9 are naturally coloured with a yellow, green, pink or grey tinge. Despite the lack of quantitative data for the minor and trace elements, a number of interesting observations can be made from the qualitative XRF analysis, as described below. It should be highlighted that no strong correlation was found between the visual characteristics of the colourless and naturally coloured samples and the identification of each decolourant.
The spectra of 8 samples present strong arsenic peaks, whereas manganese is absent (). All these samples are colourless and very clear. It should be noted that all Group I samples belong to this category.
For the majority of the samples, both colourless and naturally coloured, the XRF spectra show the simultaneous presence of arsenic and manganese (). It should be highlighted that the use of both decolourants has been reported primarily for 18th c. glasses (Verità, Citation2013).
The spectra of 5 samples present strong manganese peaks, whereas arsenic is absent (). These samples belong to Groups II, III and IV; therefore no conclusions can be drawn regarding the exclusive use of manganese for a specific glass type. Only one of the samples (sample UN) is fully colourless and clear, whereas the other 4 samples demonstrate a strong grey tinge. Manganese in high quantities is known to act as a colourant, producing grey or pink colour.
No other decolourant, such as antimony, was identified in any of the samples of the assemblage.
Provenance
The difficulties in identifying the likely provenance of archaeological and historical glass have been thoroughly discussed in literature (Pollard and Heron, Citation2008; Gratuze, Citation2013). In this particular case, the study of provenance is even more difficult due to a number of additional factors, the most important of which are the following: (a) there are no previously published data for Ottoman glasses in the Greek region; (b) a large number of different glass types were produced during this period in Europe and Middle East; (c) the published work on post-medieval glass may come from the analysis of luxurious vessels, tableware, glass panes or glassworking waste; (d) the production of glass was mainly taking place in smaller local workshops, which in many cases used the same recipe but local raw materials; and (e) glass recycling was very common during the period under study.
Given the above mentioned issues, the provenance study of the present assemblage focused solely on the comparison with well defined assemblages, which represent significant workshops or technologically characteristic glass types. Further and more detailed analysis using a larger dataset of published data from smaller workshops was not possible within the limitations of the present study. The assemblages used for the comparative analysis belong to the K-rich, Na-rich, mixed alkali and lead glass types. No HLLA glasses were used, since the alkali content clearly discards this group. The examination for each glass type was carried our separately in order to facilitate the comparison. More specifically, the following reference data was used:
Na-rich glass: 16th-19th c. glass from Pergamon, Turkey (Rehren, et al., Citation2015); 12th-19th c. glass from Jordan (Boulogne, et al., Citation2009); 14th-16th c. glass from Tuscany, Italy (Cagno, et al., Citation2010); 15th-19th c. glass from Istanbul, Turkey (Canav-Özgümüş, Citation2012); 16th-17th c. glass from Antwerp, Belgium (Janssens, et al., Citation2013); 16th-18th c. glass from Venice, Italy (Verità, Citation2013); 14th-19th c. glass from Elblag, Poland (Kunicki-Goldfinger, et al., Citation2008).
Mixed alkali glass: 14th-16th c. glass from Tuscany, Italy (Cagno, et al., Citation2010); 17th-18th c. glass from Silkstone and Bristol, Britain (Dungworth, Citation2013); 16th-17th c. glass from Antwerp, Belgium, Amsterdam, Netherlands and Aldgate, Britain (Janssens, et al., Citation2013).
K-rich glass: 16th c. glass from Idenhurst, Britain (Dungworth, Citation2013); 13th-18th c. glass from Spessart and Lubeck, Germany (Kuisma-Kursula, et al., Citation1997); 11th-15th c. glass from Hoexter, Germany (Wedepohl and Simon, Citation2010); 15th-16th c. glass from Belgium (Schalm, et al., Citation2004); 18th c. glass from Lauenstein, Germany, Naliboki, Belarus and Bohemia, Czech Republic (Kunicki-Goldfinger, et al., Citation2003); 17th c. glass from Prague, Czech Republic (Mádl and Kunicki-Goldfinger, Citation2006); 15th-19th c. glass from Istanbul, Turkey (Canav-Özgümüş, Citation2012).
Lead glass: 17th c. glass from Britain (Dungworth and Brain, Citation2013).
Glass with low lead content: 18th c. glass from Naliboki, Belarus and Altmunde, Dresden and Zachlin, Germany (Kunicki-Goldfinger, et al., Citation2003); 14th-18th c. glass from Poznan, Poland (Kunicki-Goldfinger, et al., Citation2008).
Na-rich and mixed alkali glass
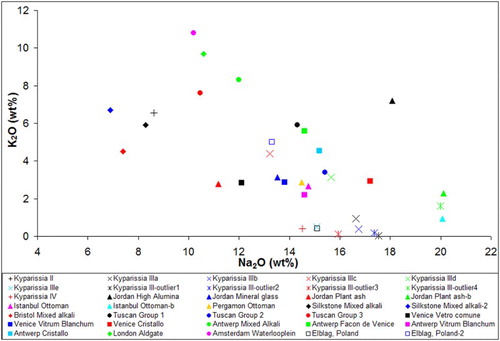
The comparison of the Na-rich and mixed alkali glasses of the present assemblage with the published data clearly shows that the majority of the Kyparissia samples present very high concentrations of silica. The SEM/EDS analyses were carried out on pristine glass, therefore the elevated levels of silica cannot be attributed to corrosion effects. Based on and the following hypotheses can be made regarding the likely provenance of the samples:
Group II (mixed alkali glass) does not fit well with the mixed alkali glasses from Britain and central Europe. Contrary to other published data, Group II samples demonstrate particularly low concentration of magnesium and alumina.
Sub-group ΙΙΙe and outlier 222-8 seem to coincide chemically with 18th-19th c. glasses from Poland.
The composition of Sub-group IΙΙd shows similarities with Venetian cristallo; however, it is impossible to differentiate between original cristallo and façon de Venise based on the major oxides. Despite the fact that Venetian cristallo is primarily related to high quality and valuable artefacts, there are known examples of its use for everyday objects. Such a characteristic example is a large number of wine glasses made of Venetian cristallo, which were found in a 16th c. tavern in Salzburg, Austria (Whitehouse Citation2012).
Sub-group ΙΙΙc strongly correlates to an assemblage of Islamic glasses found in Jordan. The likely location of production for these glasses is not provided by the authors. Based on their typological traits, the glasses were dated between the 13th and the 19th c.; however, Boulogne and Henderson (Citation2009) consider a later date more likely.
Outlier 216Β presents characteristically high sodium and magnesium values (20% and 4.7 wt%, respectively), which fit well with two Islamic glasses recovered in Jordan. The high concentration of alumina was explained by Boulogne and Henderson (Citation2009) by the use of beach sand with alkali feldspar impurities. According to the authors, these samples belong to the Islamic glass group Raqqa 4, which was produced in Syria. However, according to Henderson (Citation2003), this glass type was produced for a period of approximately 3 centuries, between 800 and 1100 AD. The observed compositional similarities between sample 216B and these two samples could however be indicative of a common technology and therefore an eastern provenance can be suggested for this sample.
The likely provenance of Sub-groups IIIa and IIIb was not identified. The samples of these groups present very low levels of alumina and magnesium, differentiating them from published data.
Group IV presents calcium, sodium and alumina values similar to vitrum blanchum groups, produced both in Venice and in Antwerp. They are however very low in potassium and magnesium, therefore the attribution to any of these groups is not certain. The very low concentration in both magnesium and phosphorus oxide could be the result of purification of the plant-ashes used, a pretreatment process which involved solution, filtration and evaporation (Henderson, et. al., Citation2005).
K-rich glass
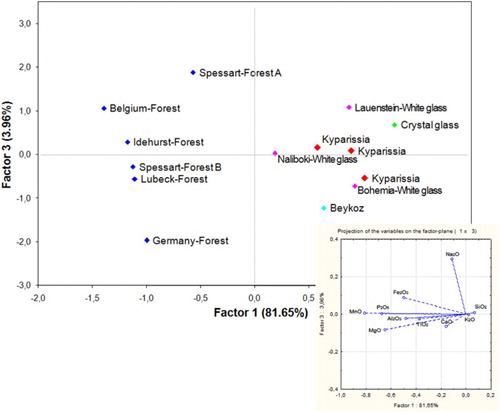
The K-rich glasses were compared using PCA statistical analysis based on all major oxides. The analysis clearly shows that Group I samples have many chemical similarities with 18th c. chalk glass from central Europe and 19th c. glass from Beykoz (in Istanbul, Turkey), whereas they are distinctly different from forest glasses from Britain and central Europe (). It is therefore confirmed that the alkali source of Group I samples is not plant ash, but potash possibly intermixed with saltpetre. Given the great similarities between Beykoz glass and chalk glass it is difficult to determine the exact provenance, though a provenance from Istanbul seems more likely. In any case, Group I samples can be dated after the end of the 17th c., when this type of glass was invented.
Lead glass
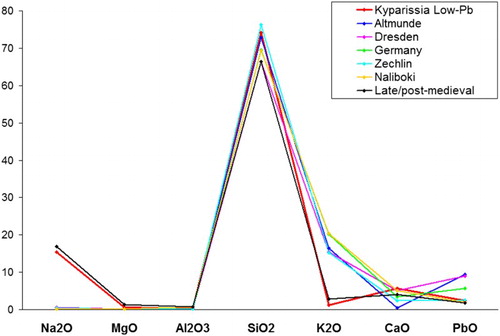
shows the comparison of the concentration of major oxides between the lead glass of the present assemblage (sample 219-4) and the three main English lead glass types of the period. These three types were successively produced in Britain for well determined periods; over the course of time the potassium content decreased and the lead content increased (Dungworth and Brain, Citation2013). Sample 219-4 shows an intermediate composition between Groups 1 and 2; however, the potassium concentration is closer to Group 3. Therefore, either the composition of this sample belongs to an intermediate production recipe from an English workshop, or it was produced elsewhere, in a workshop roughly following the English recipes. In any case, this glass can be securely dated after the second half of the 17th c., when lead glass was invented.
Figure 8. Comparison of the concentration (in wt%) of major oxides for lead glasses from Kyparissia (sample 219-4) and Britain.
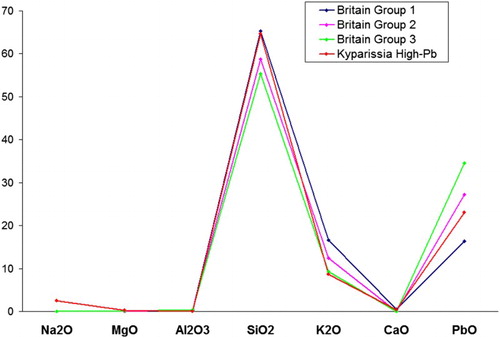
As was mentioned before, the low lead content that was identified in three samples was not necessarily added in purpose. In order to check the hypothesis that there was a distinct chemical group with low amounts of added lead, the provenance of the low-Pb samples was examined separately in comparison with other samples of the same period which also present PbO < 10 wt% (). The majority of the low-lead glasses mentioned in literature belongs to the K-rich type and has very low calcium concentrations. However, a single glass from Poland, dated to a vast period between the 14th and 18th c., shows remarkably similar composition to the Kyparissia samples.
Glass matrix
A number of recent studies have focused on exploring the potential of using Raman spectroscopy for the identification of different glass types (Colomban, Citation2003; Baert, et al., Citation2011; Ricciardi, et al., Citation2009; Tournie, et al., Citation2012). The Raman spectra of silica-based glasses show main bands due to the stretching of Si-O-Si bridging bonds, ν(Si-O-Si), and the Si-O stretching in Qn silicate units, ν(Qn), where Qn indicates a silicate tetrahedron with n bridging and 4-n non-oxygen atoms (Kamitsos and Risen, Citation1984). The energy and intensity of the ν(Si-O-Si) and ν(Qn) Raman bands change with chemical composition and certain parameters of glass production technology like firing and/or glass quenching conditions (Möncke, Citation2006), thus allowing the discrimination between different glass types. This approach has many significant advantages: the analysis can be conducted in situ with portable Raman spectrometers and the analytical process is quick and non-destructive. It can therefore be very helpful in the analysis of glass artefacts that cannot be sampled, such as stained glass, or for the preliminary categorization of large assemblages.
Raman spectroscopy was applied on representative samples of each chemical group and colour category in order to examine the glass matrix and identify the likely differences within and between groups. The comparison of the acquired spectra for colourless glasses of the same type showed that there is very strong homogeneity within each group. However, between different types of colourless glass, certain small differences were noted (). The Q3 band appears at slightly higher energies for K-rich glasses (Group I) compared to Na-rich (III, IV) glasses (1107 cm-1 and ∼1090 cm-1, respectively). This difference has also been noted by Baert et al. (Citation2011). Mixed alkali glasses present an intermediate behaviour between the two types (Q3 at 1100 cm–1). Moreover, it is interesting to note that in Group IV spectra bands Q2 and Q1 are also present with varying intensities, at ca. 994 and 947 cm-1, respectively. The increased breakage of the silicate network is manifested by the upshift of ν(Si-O-Si) to 558 cm-1, and is most probably related to the increased concentration of alkaline earths on this glass type.
Figure 10. Representative Raman spectra from colourless glass of each major group (I: sample 217-4; II: sample 217-5; III: sample 222-6; III + low Pb: sample222-7; IV: sample 219-5; High-Pb: sample 219-4).
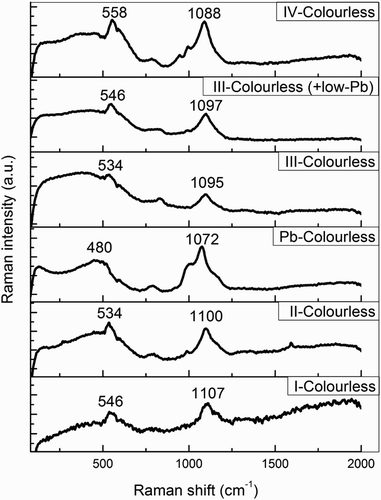
The high-lead glass (219-4) has an overall different Raman spectrum, since in this case lead may also act as a network former. Both the ν(Q3) and the ν(Si-O-Si) bands are broader, as in binary lead-silicate glasses (Feller, et al., Citation2010) and appear in significantly lower energies than in a typical silica spectrum (at 1072 and 480 cm-1, respectively). The spectra of low-lead glasses show no significant differences compared to the other Group III glasses. This is expected, since such a low amount of lead should not cause any significant changes in the glass matrix.
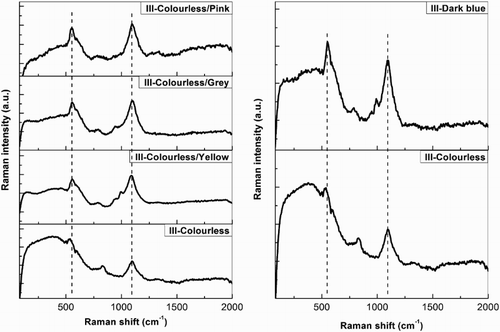
shows a comparison between Group III glasses with different colours (colourless, naturally coloured with yellow, grey and pink tinge and strongly coloured-blue). It is interesting to note that both the blue and the naturally coloured glasses show a greater degree of depolymerization than colourless glasses, since bands Q1 and/or Q2 appear with varying intensity. This observation can be explained by the presence of increased amounts of oxides of metallic ions, which can break up the Si-O-Si bridges in the silicate network, in the naturally coloured and strongly coloured (blue) glasses.
Figure 11. Comparison of representative Raman spectra from colourless versus naturally coloured Group III glasses (left) and colourless versus coloured Group III glasses (right) (III-colourless: sample 222-6; III-colourless/yellow: sample 215-4; III-colourless/grey: sample 215-5; III–colourless/pink: sample 215-6; III-dark blue: sample 221).
Concluding remarks
The present paper focuses on the chemical and spectroscopic analysis of an assemblage of everyday glass objects, recovered in an Ottoman bathhouse in Kyparissia, Greece. The chemical analysis demonstrated an unexpected number of different glass types, including Na-rich, K-rich, mixed alkali and lead glasses. Based on the chemical composition of these groups, an effort was made to identify the likely provenance of the glasses. Moreover, in some cases the technological analysis also allowed the indirect dating of the samples, thus providing with useful information on the likely construction date of the bathhouse, which has not yet been determined.
Application of Raman spectroscopy supported the grouping which resulted by the EDS analysis and enabled a more detailed examination of the glass matrix, focusing especially on the observed differences within and between glasses of different chemical groups. Further processing of the acquired spectra, such as the measurement of the polymerization index, is under way in order to assist in the identification not only of the composition but also of technological parameters of the glass production.
The main aspects of the different glass types of the assemblage are summarized below:
Chalk glass: Three K-rich glasses are chemically similar with the chalk glass type. The alkali source is potash and/or saltpetre and the decolourant used is arsenic. Glass of this type was produced in central Europe (especially in Poland and the Czech Republic) after the end of the 17th c. and in Istanbul during the 19th c. Though it is impossible to identify the provenance with certainty, based on the available data Istanbul seems a more likely origin.
Mixed alkali glass: A small number of samples belong to the mixed alkali type, though their composition differs from published assemblages of mixed alkali glasses from England, Germany and France.
Na-rich glass: Glasses of this type demonstrate high chemical variability forming a number of different sub-groups, although in every case the alkali source used is plant-ash rich in sodium. The identification of a likely provenance was not possible for all sub-groups. One sub-group is chemically similar to glasses produced in Poland in the 18th and 19th c. A second sub-group can be considered Islamic glass and shows significant similarities with glass recovered in Jordan, with a likely Syrian provenance. Finally, one sub-group has chemical similarities with cristallo, though it cannot be determined whether it was authentic Venetian cristallo or façon de Venise.
Na-rich glass with high calcium and alumina content: This group presents significant chemical similarities with the vitrum blanchum type, produced both in Venice and in other workshops. Identifying the exact provenance was not possible based on the available data.
Na-rich glass with low-lead quantities (∼2 wt%.): The addition of such low quantities of lead in the glass may not have been deliberate, although all three samples of this type present strikingly similar compositions. Moreover, their composition resembles that of a glass from Poland, dated between the 14th and 18th c.
Lead glass: One lead glass shows high quantities of lead (23 wt%), and an overall chemical composition that generally resembles the English lead glasses, without however falling clearly within one of the well-defined chemical groups which prevailed between the 17th and 19th c. Perhaps this glass was produced in northern or central Europe, from a workshop producing lead glass roughly following the English recipe.
The construction of the bathhouse is roughly estimated between the mid. 15th c. and the late 17th c. A number of technological aspects suggest that the majority of the glasses were produced during the 18th c.: (a) the K-rich and lead glasses can definitively be dated after the end of the 17th c., when these types of glass were invented; (b) a number of Na-rich samples fit well with glasses dated to the 18th or 19th c.; and (c) the simultaneous use of both arsenic and manganese as a decolourant, which occurs in the majority of the samples, was common primarily during the 18th c. Though it is not possible to exclude that some of the samples have an earlier date, the so far available data corroborates the hypothesis of a late construction date for the bathhouse, as suggested by Germanidou and Gerolymou (Citation2015).
It is not possible to determine whether the glasses were traded as empty vessels, for their content, or even moved as personal objects of travelers. In any case, the determination of the likely provenance of the glass objects from Kyparissia reveals the existence of a remarkable glass trading network, not only within the Ottoman Empire, but also with many of the large European centres of the period. This proves the significant role of Kyparissia as a port with direct and strong contact with the western European centres. Given the complete lack of available information regarding glassmaking in the Greek region during the Ottoman occupation, the local production of glass somewhere in Greece cannot be excluded and should be further examined in the future. Overall, the present work highlights the need of further analyses of Ottoman glasses, which have so far received limited attention by researchers of related fields.
Supplementary Table
Download MS Word (163.5 KB)Acknowledgements
The authors acknowledge permits granted by the Greek Ministry of Culture and Sports to enable the study of the assemblage.
Notes on Contributors
Eleni Palamara (EP) is a Postdoctoral Researcher in the Department of History, Archaeology and Cultural Resources Management of the University of the Peloponnese, Greece.
Nikolaos Zacharias (NZ) is a Professor and the Director of the Laboratory of Archaeometry at the University of the Peloponnese, Greece.
Sophia Germanidou (SG) works as an archaeologist in the Ephorate of Antiquities of Messinia (Hellenic Ministry of Culture) in Kalamata, Greece.
Konstantina Gerolymou (KG) works as an archaeologist in the Ephorate of Antiquities of Messinia (Hellenic Ministry of Culture) in Kalamata, Greece.
Dimitrios Palles (DP) is an Application Scientist of the Theoretical and Physical Chemistry Institute of the National Hellenic Research Foundation in Athens, Greece.
Efstratios I. Kamitsos (EIK) is a Research Director of the Theoretical and Physical Chemistry Institute of the National Hellenic Research Foundation in Athens, Greece.
References
- Baert, K., W. Meulebroeck, H. Wouters, A. Ceglia, K. Nys, H. Thienpont and H. Terryn. 2011. “Raman spectroscopy as a rapid screening method for ancient plain window glass.” Journal of Raman Spectroscopy, 42: 1055–1061. doi: 10.1002/jrs.2799
- Barrera, J. and B. Velde. 1989. “A study of French medieval glass composition.” Journal of Glass Studies, 31: 48–54.
- Boulogne, S. and J. Henderson. 2009. “Indian glass in the Middle East? Medieval and Ottoman glass from central Jordan.” Journal of Glass Studies, 59: 53–75.
- Cagno, S., K. Janssens and M. Mendera. 2008. “Compositional analysis of Tuscan glass samples: In search of raw materials fingerprints.” Analytical and Bioanalytical Chemistry, 391(4): 1389–1395. doi: 10.1007/s00216-008-1945-8
- Cagno, S., M. Mendera, T. Jeffries and K. Janssens. 2010. “Raw materials for medieval to post-medieval Tuscan glassmaking: New insight from LA-ICP-MS analyses.” Journal of Archaeological Science, 37: 3030–3036. doi: 10.1016/j.jas.2010.06.030
- Canav-Özgümüş, U. 2012. “Recent glass finds in Istanbul.” In Annales du 18e Congrès de l’Association Internationale pour l’Histoire du Verre, edited by D. Ignatiadou and A. Antonaras, 326–332. Thessaloniki: Ziti Publications.
- Charleston, R.J. 1960. “Lead in glass.” Archaeometry, 3(1): 1–4. doi: 10.1111/j.1475-4754.1960.tb00508.x
- Charleston, R.J. 1978. “Glass furnaces through the ages.” Journal of Glass Studies, 20: 9–33.
- Colomban, Ph. 2003. “Polymerization degree and Raman identification of ancient glasses used for jewelry, ceramic enamels and mosaics.” Journal of Non-Crystalline Solids, 323: 180–187. doi: 10.1016/S0022-3093(03)00303-X
- Crossley, D. 1991. “Current Research on English Glass Furnaces.” In Archeologia e storia della produzione del vetro preindustriale, edited by M. Mendera, 411–422. Firenze: University of Siena.
- De Raedt, I., K. Janssens, J. Veeckman, L. Vincze, B. Vekermans and Jeffries, T.E. 2001. “Trace analysis for distinguishing between Venetian and Façon-de-Venise glass vessels of the 16th and 17th century.” Journal of Analytical Atomic Spectrometry, 4: 1012–1017. doi: 10.1039/B102597J
- Dungworth, D. 2006. “Composition of early eighteenth century window glass from Silkstone, Yorkshire.” English Heritage: Research Department Report 18/2006.
- Dungworth, D. 2013. “Innovations in the 17th-century glass industry: the introduction of kelp (seaweed) ash in Britain.” In Les Innovations Verrières et Leur Devenir, edited by S. Lagabrielle and C. Maitte, 119–123. Verre et Histoire.
- Dungworth, D. and C. Brain. 2013. “Seventeenth- and eighteenth- century English lead glass.” In Modern methods for analysing archaeological and historical glass, edited by K. Janssens, 573–589. Chichester: John Wiley and Sons Ltd.
- Dungworth, D. and C. Clark. 2004. “SEM-EDS analysis of Wealden glass.” English Heritage: Centre for Archaeology Report 54/2004.
- Dungworth, D., T. Cromwell, D. Ashurst, C. Cumberpatch, D. Higgins and H. Willmott. 2006. “Glass and pottery manufacture at Silkstone, Yorkshire.” Post-Medieval Archaeology, 40(1): 160–190. doi: 10.1179/174581306X143089
- Feller, S., G. Lodden, A. Riley, T. Edwards, J. Croskrey, A. Schue, D. Liss, D. Stentz, S. Blair, M. Kelley, G. Smith, S. Singleton, M. Affatigato, D. Holland, M.E. Smith, E.I. Kamitsos, C.P.E. Varsamis and E. Ioannou. 2010. “A multispectroscopic structural study of lead silicate glasses over an extended range of compositions.” Journal of Non-Crystalline Solids, 356: 304–313. doi: 10.1016/j.jnoncrysol.2009.12.003
- Germanidou, S. and K. Gerolimou. 2015. “The hamam of Kyparissia, western Messinia: an unknown Ottoman bath and its structure within the frame of local Ottoman architecture and topography.” In “Against Gravity - Bulding Practices in the Pre-Industrial World”, Philadelphia, 20-22 March 2015, edited by R. Ousterhout, D. Borbonus and E. Dumser.
- Gratuze, B. 2013. “Provenance analysis of glass artefacts. Venetian soda glass.” In Modern methods for analysing archaeological and historical glass, edited by K. Janssens, 311–343. Chichester: John Wiley and Sons Ltd.
- Henderson, J. 2003. “Glass trade and chemical analysis: a possible model for Islamic glass production.” In Échanges et commerce du verre dans le monde antique, edited by D. Foy and M.-D. Nenna, 109–123. Montagnae: Éditions Monique Mergoil.
- Henderson, J. 2013. Ancient Glass: An Interdisciplinary Exploration. NY: Cambridge University Press.
- Henderson, J., P. Adams and J. Mann. 2005. “Medieval and Post-Medieval Glass Finewares from Lincoln: an Investigation of the Relationships between Technology, Chemical Compositions, Typology and Value.” Archaeological Journal, 162 (1): 256–322. doi: 10.1080/00665983.2005.11020626
- Janssens, K., S. Cagno, I. De Raedt and P. Degryse. 2013. “Transfer of glass manufacturing technology in the sixteenth and seventeenth centuries from Southern to Northern Europe.” In Modern methods for analysing archaeological and historical glass, edited by K. Janssens, 537–562. Chichester: John Wiley and Sons Ltd,.
- Kamitsos, E.I. and W.M. Risen. 1984. “Vibrational spectra of single and mixed alkali pentasilicate glasses.” Journal of Non Crystalline Solids, 65: 333–354. doi: 10.1016/0022-3093(84)90057-7
- Kuisma-Kursula, P., J. Räisänen and H. Matiskainen. 1997. “Chemical analyses of European forest glass.” Journal of Glass Studies, 39: 57–68.
- Kunicki-Goldfinger, J., L. Kierzek, P. Dzierżanowski and A.J. Kasrzak. 2005. “Central European crystal glass of the first half of the eighteenth century.” In: Annales du 16e Congrès de l'Association Internationale pour l'Histoire du Verre. Nottingham: AIHV, pp. 258–262.
- Kunicki-Goldfinger, J., J. Kierzek, A.J. Kasprzak and B. Malozewska-Bucko. 2003. “Analyses of 18th century central European colourless glass vessels.” In: Annales du 15e Congrès de l'Association Internationale pour l'Histoire du Verre. Nottingham: AIHV, pp. 224–229.
- Kunicki-Goldfinger, J., E. Pańczyk, P. Dierżanowski and L. Walliś. 2008. “Trace element characterization of medieval and post-medieval glass objects by means of INAA and EPMA.” Journal of Radioanalytical and Nuclear Chemistry, 278(2): 307–311. doi: 10.1007/s10967-008-9507-2
- Mádl, M. and J. Kunicki-Goldfinger. 2006. “Eiland: Georg Gundelach and the glassworks on the Dĕčín Estate of Count Maximilian Thun-Hohenstein.” Journal of glass studies, 48: 225–247.
- Möncke, D., D. Ehrt, C.P.E. Varsamis, E.I. Kamitsos and A.G. Kalampounias. 2006. “Thermal history of a low alkali borosilicate glass probed by infrared and Raman spectroscopy.” Glass Technology: European Journal of Glass Science and Technology, Part A, 47(5): 133–137.
- Möncke, D., D. Palles, N. Zacharias, M. Kaparou, L. Kamitsos E. I. and Wondraczek. 2013. “Formation of an outer borosilicate glass layer on late Bronze Age Mycenaean blue vitreous relief fragments.” Physics and Chemistry of Glasses: European Journal of Glass Science and Technology, 54 (1): 52–59.
- Moropoulou, A., N. Zacharias, E.T. Delegou, B. Maróti and Zs. Kasztovszky. 2016. “Analytical and technological examination of glass tesserae from Hagia Sophia.” Microchemical Journal, 126: 170–184. doi: 10.1016/j.microc.2015.11.020
- Palamara, E., N. Zacharias, M. Xanthopoulou, Zs Kasztovszky., I. Kovács, D. Palles and E.I. Kamitsos. 2016. “Technology issues of Byzantine glazed pottery from Corinth, Greece.” Microchemical Journal, 129: 137–150. doi: 10.1016/j.microc.2016.06.008
- Pollard, M. and Heron C. 2008. Archaeological Chemistry (2nd Edition). Cambridge: Royal Society of Chemistry.
- Rehren, Th., P. Connolly, N. Schibille and H. Schwarzer. 2015. “Changes in glass consumption in Pergamon (Turkey) from Hellenistic to late Byzantine and Islamic times.” Journal of Archaeological Science, 55: 266–279. doi: 10.1016/j.jas.2014.12.025
- Ricciardi, P., Ph Colomban., A. Tournié and V. Milande. 2009. “Nondestructive on-site identification of ancient glasses: Genuine artifacts, embellished pieces or forgeries?” Journal of Raman Spectroscopy, 40: 604–617. doi: 10.1002/jrs.2165
- Schalm, O., D. Caluwé, H. Wouters, K. Janssens, F. Verhaeghe and M. Pieters. 2004. “Chemical composition and deteriaration of glass exavated in the 15th-16th century fishermen town of Raversijde (Belgium).” Spectrochimica Acta, 59 (B): 1647–1656. doi: 10.1016/j.sab.2004.07.012
- Soudavar Diba, L. 1983. “Glass and glassmaking in the Eastern Islamic lands: Seventeenth to ninenteenth century.” Journal of Glass Studies, 25: 187–193.
- Tournié, A., L.C. Prinsloo and Ph. Colomban. 2012. “Raman classification of glass beads excavated on Mapungubwe hill and K2, two archaeological sites in South Africa.” Journal of Raman Spectroscopy, 43: 532–542. doi: 10.1002/jrs.3069
- Verità, M. 2013. “Venetian soda glass.” In: Modern methods for analysing archaeological and historical glass, edited by K. Janssens, 515–536. Chichester: John Wiley and Sons Ltd.
- Verità, M. 2014. “Secrets and innovations of Venetian glass between the 15th and the 17th centuries: Raw materials, glass melting and artifacts.” In: Study Days on Venetian Glass, approximately 1600’s, edited by R. Barovier and C. Tonini, Volume 172, 53–68. Venice: Atti dell’Istituto Veneto di Scienze, Lettere ed Arti.
- Wedepohl, K.H. and K. Simon. 2010. “The chemical composition of medieval wood ash glass from Central Europe.” Chemie der Erde, 70: 89–97. doi: 10.1016/j.chemer.2009.12.006
- Whitehouse, D. 2012. Glass. A short history. London: The British Museum Press.
- Zacharias, N. and E. Palamara. 2016. “Chapter 12: Glass Corrosion: Issues and Approaches for Archaeological Science.” In Recent Advances in the Scientific Research on Ancient Glass and Glaze, edited by F. Gan, Q. Li and J. Henderson, 233–248. World Scientific.