Abstract
Candida albicans, one of the pathogenic Candida species, causes high mortality rate in immunocompromised and high-risk surgical patients. In the last decade, only one new class of antifungal drug echinocandin was applied. The increased therapy failures, such as the one caused by multi-drug resistance, demand innovative strategies for new effective antifungal drugs. Synergistic combinations of antifungals and anti-virulence agents highlight the pragmatic strategy to reduce the development of drug resistant and potentially repurpose known antifungals, which bypass the costly and time-consuming pipeline of new drug development. Anti-virulence and synergistic combination provide new options for antifungal drug discovery by counteracting the difficulty or failure of traditional therapy for fungal infections.
Introduction
Candida albicans, one of the leading opportunistic fungal pathogens, caused high mortality rate especially in immunocompromised and high-risk surgical patients.Citation1,2 C. albicans locate in the oral cavity, digestive tract and genital region as the commensal flora in more than half of the healthy population. When given pathogenic opportunity, C. albicans is responsible for more than 50% of human candidiasis, including 2 major types of infections, superficial infections (nonlethal), such as oral or vaginal candidiasis; and systemic infections (∼40% mortality).Citation3,4 Systemic infections caused by C. albicans have become a serious public health threaten in immunocompromised patients, organ transplantations, non-trauma emergency surgery, massive chemotherapy and implantable medical devices during the past several decades.Citation3-6 The development of antifungal drug discovery is relative slower than antibacterial antibiotics, and antifungal drug resistance reduce the efficacy of known antifungals.Citation7 The lack of new antifungal drugs and the limited therapeutic options call for new strategies to find novel antifungal candidates.
Synergistic drug combination has been proved to be a valid and pragmatic strategy to seek drugs with novel mode of actions. It can potentially reduce the dose of single drug usage with increased drug-efficacy, and subsequently lower the drug toxicity. The practice of targeting 2 or more drug targets simultaneously is consistent with the philosophy that a disease is a systematic and complicated outcome caused by multi-effects. Furthermore, the development of drug resistance can be slowed down by the multi-target strategy. There are 3 different phases for synergistic antifungal drug combinations, in vitro testing, in vivo animal model validations, and the clinical trials. Using two or more antifungal drugs to control severe invasive fungal infections has been adopted in clinic for a long time. The first application of synergistic therapy forinvasive candidiasis is flucytosine and amphotericin B. The flucytosine monotherapy usually caused drug resistance and unexpected side effects, while amphotericin B compromised these problems.Citation8,9 This combination was recommended by Infectious Diseases Society of America (IDSA) guidelines for the treatment of candidiasis among patients in selected situations, including those with serious and deep-seated candidal infections involving the central neuron system (CNS) infections, endovascular infections and serious intra-abdominal candidiasis.Citation10 There are also some cases which are well and widely used in clinic ().
Table 1. Footnote*Selected synergistic combinations against C. albicans in vitro and in vivo.
An alternative approach of antifungals is to target virulence factors which opens a new pipeline for antifungal drug discovery.Citation11 It extends the range of potential drug targets from “essential processes (growth)” to “virulence processes (pathogenesis)”. The strategy of targeting pathogen specific virulence can also help to preserve the host normal commensal microbiome.Citation12 In addition, the effective synergistic combinations between anti-virulence agents and low dose of toxic antifungal drugs provide more potent controls against fungal infections, which are more efficacious but less toxic than the use of single drugs. In this review, we summarized the mode of actions of antifungals and fungal drug resistance, highlighted the synergistic screening for new antifungal agents, we also introduced the recent progress on anti-virulence factor research in antifungal drug discovery and prospected the strategy to fight against C. albicans with synergistic combinations of antifungals and anti-virulence agents.
Mode of Actions of Antifungals and Drug Resistance
Currently, the clinical anti-candidiasis therapeutic drugs are limited to few classes including polyenes, azoles, allylamines and echinocandins. These antifungal drugs usually target essential processes of C. albicans which cause the evolution of drug resistance rapidly such as azoles and echinicandins, while the resistance to amphotericin B (a polyene antifungal) is rare. The antifungal drug targets can be confined to the following distinct pathways (): a) ergosterol and ergosterol biosynthesis. Ergosterol is a key component in fungal cell membrane and similar to human cholesterol. It plays an important role in fungal cell growth. Polyene drugs, such as amphotericin B, can bind to ergosterol and lethally cause leak of cell components by forming channels on the fungal cell membranes.Citation13,14 Azoles are another class of antifungals targeting ergosterol biosynthesis. Fluconazole, for instance, functions through targeting lanosterol 14α-demethylase, which is a core enzyme encoded by ERG11 in ergosterol biosynthesis.Citation15,16 b) β(1 3)-D-glucan synthesis. Fungal cell wall containing mannan, chitin, and α- and β-glucansis another attractive drug target because there is no counterpart in mammalian cells. Echinocandins can lead to cell death by inhibiting β(1 3)-D-glucan synthesis and consequently disrupting the fungal cell wall integrity.Citation17 c) Nucleic acids synthesis. Biosynthesis of macromolecules, such as DNA and RNA, are also adopted as antifungal targets. The clinical used antifungal drug, 5-fluorocytosin (5-FC), a fluorinated pyrimidine analog, can be transported into cells and finally converted into 5-fluorodeoxyuridine monophosphate (5-FdUMP) or 5-fluorouracil triphosphate (5-FUTP) to inhibit RNA or DNA synthesis.Citation18 Besides the drug targets list above commonly used in clinic, there are also some other targets identified for antifungal drug discovery. d) Protein synthesis. A potential candidate for new fungicidal development named sordarin is proved that it can inhibit the elongation process of protein synthesis in yeasts by stabilizing the ribosome/EF2 complex but do not affect the protein synthesis machinery in mammalian cells.Citation19 e) Mitosis. The antifungal drug, griseofulvin, used both in animals and humans to treat fungal infections of the skin (commonly known as ringworm) and nails, was reported it can bind to tubulin, interfering with microtubule function, thus inhibiting the fungal cell mitosis.Citation20 f) Mitochondria. The antifungal candidate arylamidine was demonstrated that it can selectively accumulated in C. albicans via transporter-mediated systemsand disrupted yeast mitochondrial function.Citation21,22 Although current antifungals functioning depend on above discussed pathways, more are imperative to be discovered in the future as the pace of antifungal drug resistance continues to increase.
Figure 1. Antifungal drug targets and related drug resistant mechanisms of C. albicans Antifungal drugs and their targets: (A) polyenes, target is ergosterol; (B) azoles, target is ergosterol biosynthesis; (C) echinocandins, target is β(1 3)-D-glucan synthesis; (D) fluoropyrimidine, target is nucleic acids synthesis; (E) sordarins, target is protein synthesis; (F) griseofulvin, target is mitosis; (G) and arylamidines, target is mitochondria. The general drug resistant mechanisms in C. albicans include: 1. target overexpression; 2. targets alteration; 3. drug sequestration; 4. enhanced drug efflux; and 5. blocking of antifungal drug entry.
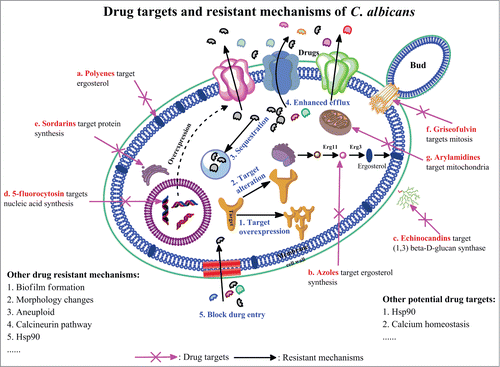
Along with the antibiotics development, drug resistance evolved successively. Several drug resistant mechanisms were found in C. albicans (). a) Target overexpression. Antifungal drug resistant strains can over produce drug targets to blunt the efficacy of antifungals. b) Targets alteration. Under the selective pressure of antifungals, target mutated cells with decreased the binding affinity of antifungals survive and develop as resistant strains. c) Drug sequestration. Fungal pathogens are capable of separating antifungal drugs from their targets, either by keeping drugs out of cells, such as forming biofilms, or by hijacking the invaded drugs into sub-cell structures.Citation23-25 d) Enhanced drug efflux. There are 2 types of drug efflux pumps identified in C. albicans, Candida Drug-Resistance (CDR) pump and Major Facilitator Superfamily (MFS) efflux pump. Both have been implicated in antifungal drug resistance among the putative transporter genes identified in the C. albicans genome.Citation26,27 e) Blocking of antifungal drug entry. Pathogens can set up barriers to reduce or stop the antifungal drug entry to decrease the intracellular drug concentrations. Apart from the mechanisms discussed above, clinical isolates also developed other mechanisms to overcome or bypass the action of antifungals such as chromosome aneuploidy (e.g. increase the copy of target genes on some chromosome) and phenotype transition ().Citation28,29 Moreover, clinical isolates usually occupy more than one drug resistant mechanisms resulting in multi-drug resistance, which cause therapeutic failure of current antifungal drugs.Citation30
Seeking Synergistic Combinations to Fight Against C. albicans
The challenge of decreased public health and lack of new drugs urgently call for new pharmaceutical strategies, one of which is drug combination research. The generally accepted criterion to determine whether a combination is synergistic or not is to calculate the FICI (Fractional Inhibitory Concentration Index) value by the formula: FICI = (MICdrug A in combination/MICdrug A alone) + (MICdrug B in combination/MICdrug B alone) in which A and B mean 2 drugs used in the combination. When FICI > 4, the combination is antagonism, while FICI < 0.5 means synergy, and the combination effect is additive when FICI between 0.5 and 4.Citation31
In order to increase the hit rates of synergistic combinations, sample the unexploited expanse of bioactive chemical space, repurpose the old drugs which are even out of market, and accelerate the antifungal drug development pipeline, we establish a high throughput synergistic screen (HTSS) platform to discovery new antifungal drugs with novel mode of actions.Citation32-34 For the first time, we construct a database named Antifungal Synergistic Drug Combination Database (ASDCD) to assemble published synergistic antifungal combinations.Citation35 Here we highlight some synergistic combinations against C. albicans in in vitro studies, in vivo animal models, and clinical trials (). In accordance with their mode of actions, these synergistic combinations can be categorized into several groups, including 1) antifungal drugs and drug efflux pump inhibitors, 2) antifungal drugs and drug resistant efflux pump reversers which can increase the entrance and accumulation of antifungal drugs, and 3) antifungal drugs and cell wall or cell membrane disrupting agents which enhance antifungal drugs penetrating the cell barriers.
For example, beauvericin, identified from our biodiversity and taxonomy guided marine microbial natural product library, synergized with several azole drugs such as ketoconazole, miconazole against C. albicans including drug resistant isolates by inhibiting the ABC transporters (Tong et al., unpublished).Citation33 Beauvericin was also demonstrated for taking part in the inhibition of FK506 and cyclosporin in calcineurin pathway which is essential for the virulence of C. albicans (Tong et al., unpublished). Berberine, another natural product from our library, was confirmed that it can reverse the efflux function of MDR1, a major facilitator of C. albicans, in fluconazole resistant isolates and further sensitize C. albicans to azole drugs (Sun et al., unpublished). The combinations of azoles with beauvericin or berberine, were confirmed for their effect of killing of C. albicans in both in vitro and systematic infectious mouse model in vivo studies (Tong and Sun et al., unpublished).Citation33
Most of these studies were carried out in vitro, without considering the physicochemical and biopharmaceutical properties of the drug combinations as well as the physiological environment in vivo. Thus, more pre-clinical performances need to be evaluated for clinical usage. However, these synergistic combinations set up the example for synergistic combinations to fight against C. albicans.
Anti-Virulence Factors to Discover New Antifungal Drugs
Virulence factors are attributes of pathogens and generally considered not essential for pathogen survival in vitro but functioning and causing damage to host during infections.Citation36,37 C. albicans express several virulence factors that contribute to its pathogenicity. These factors include environmental adaptation factors, adhesins, morphogenesis, secreted enzymes, phenotype switching, and biofilms.Citation38-40 Virulence factors has also been considered as potent antifungal targets.Citation41-43 The fundamental behind targeting virulence is instead of killing, hindering pathogens to cause any harm to the host. Several advantages can be expected from the drug discovery strategy by targeting virulence factors: a) it extends the range of potential drug targets from ‘essential processes’ to ‘virulence processes’ and enlarges the number of potential drug targets; b) it reduces direct selection on fungal cells which ultimately fosters resistance; c) the strategy of targeting pathogen-specific virulence preserves the host microbiome which is important for normal commensals, whereas broad-spectrum antifungals can cause host microbiota unbalance, such as in gut and mouth.Citation12
Among the virulence factors identified, secreted hydrolytic enzymes, filamentation and the ability to form biofilms are recognized as the main virulence factors contributing to the pathogenesis of candidiasis. Many studies have demonstrated the potency of anti-virulence agents in the antifungal trials with these virulence factors.
Anti-Secreted Hydrolytic Enzymes
One class of the candidates for anti-virulence drugs is protease inhibitors. In the treatment of HIV infections, Hoegl et al. found that the potent HIV protease inhibitors showed a favorable influence on the frequency of mucosal candidiasis in HIV infected patients.Citation44 Further study revealed that this phenomenon were not completely due to partial or total reconstitution of the immune status as originally presumed, but rather due to a direct inhibitory activity of these compounds against secretary aspartic proteases, Saps, a major virulence factor contributed to invasiveness from C. albicans.Citation45 The HIV proteinase inhibitors, such as saquinavir and indinavir, which already showed potency in the cure of candidiasis, can be carefully selected and considered as potential candidates for anticandidal virulence agents.Citation46,47 Phospholipases are another major C. albicans secreted enzymes contributed to invasiveness during infections. Ganendren et al. reported that phospholipases substrates analogs such as alexidine dihydrochloride and 1,12 bis-(tributylphosphonium)-dodecane dibromide had a relatively broad antifungal activity against C. albicans, Cryptococcus neoformans, Aspergillus flavus in vitro.Citation48 These phospholipid inhibitors could be attractive molecules for further development of anticandidal agents.
Anti-Morphogenesis
C. albicans is a polymorphic fungus and is able to transform its morphologies between yeast and filamentous forms. Filamentation not only represents a virulence trait itself, but it is also coordinately regulated with other virulence factors associated with cellular morphology.Citation36,49 The evidence of filamentation in C. albicans virulence was derived from the gene disrupted strains locked in yeast morphology.Citation50 By using tet-NRG1 strain with a tetracycline-regulatable promoter system (morphology is controlled by the presence or absence of doxycycline), Saville et al. demonstrated that the filamentation of C. albicans was associated with virulence and mortality.Citation51 Moreover, the authors provided a proof of concept that inhibition of filamentation represents an attractive target for the development of new antifungal drugs. An increasing number of small molecules has been reported that are able to modulate morphogenetic conversions and inhibit filamentation. These are mainly regulators of the yeast-to-hyphae transition for C. albicans such as phenazines and homoserine lactones from Pseudomonas aeruginosa, mutanobactins from Streptococcus mutans and capric acid secreted by Saccharomyces boulardii, farnesol and other autoregulatory alcohols that act as quorum sensing molecules produced by C. albicans itself, retigeric acid, and bisbibenzyls.Citation52-58
Anti-Adhesion
Biofilms are structural microbial communities attached to a surface or encased in a matrix of material. They provide the potential to initiate or prolong infections by providing a safe environment for cells for local tissue invading, new infection sites seeding and drug resisting.Citation7 C. albicans is one of the biofilm forming species and most clinical manifestations of candidiasis are linked to biofilm formation.Citation59,60 The exopolymeric anti-adhesion strategy can be either targeting biofilm matrix or cell dispersion. Martins et al. demonstrated that addition of DNase improves the anti-biofilm activity of some antifungal drugs as extracellular DNA is a component of the C. albicans biofilm matrix.Citation61 Another possible strategy is to target dispersion, as cells dispersed from the biofilms are responsible for dissemination, extravasation and establishment of deep-seated candidiasis.Citation62 Molecules targeting the specific genes or proteins in the regulatory of yeast cell dispersions could be consider as a candidate for anti-biofilm agent. It is notable that, because of the intimate link between filamentation and biofilms, drugs that modulate C. albicans morphogenesis also could potentially inhibit the development biofilms. This assumption could be also applicable to other virulence factors as the pathogenicity of C. albicans is multifactorial and delicate to the host and environment conditions.
Combinations of Anti-Virulence Agents With Antifungals
Though promising, the approach of targeting virulence is still at the start of the antifungal drug development pipeline. One of the drawbacks of the anti-virulence agent application is that most of the genes encoding the major virulence factors are non-essential and C. albicans express different virulence factors during the pathogenic process. A pragmatic strategy is to identify anti-virulence agents in conjunction with antifungal therapy, which not only increases the clearance of fungal pathogens, but also reduces the pathogenicity and decreases the toxicity of antifungal drugs by lowering their dosages. Here we highlight some important applications of anti-virulence agents combined with antifungals in in vitro studies.
Synergistic Activities of Inhibitors from Calcineurin Pathway
Calcineurin is proved essential for virulence of C. albicans.Citation63 Mutations from catalytic subunit Cmp1 and regulatory subunit Cnb1 cause the hypersensitivity to environmental stresses and are avirulent in mouse model of disseminated candidiasis.Citation64,65 The deletion of genes from the calcineurin pathway resulted in loss of tolerance to several antifungal agents.Citation64,66 CyclosporineA (CsA) and FK506, 2 immunosuppressive drugs which can inhibit calcineurin signaling by binding to the cyclophilin and FKBP12 respectively, have been proved for their repurposing usage in antifungal treatment by synergizing with various antifungals which mainly are azoles.Citation67 These synergistic effects possibly result from the cell membrane damage and accumulation of toxic sterols when C. albicans is treated by azoles, while calcineurin pathway is essential for the response to these stresses.Citation68 This proved concept opens the chapter to combine inhibitors from these 2 pathways: calcineurin signaling pathway and egosterol biosynthesis pathway. For example, radicicol and geldenamycin show potent synergistic antifungal activities with azoles by inhibiting the functions of Hsp90, a molecular chaperone to calcineurin.Citation69 The inhibitors of PKC1 (regulates cell wall integrity) render C. albicans hypersensitive to azoles and echinocandins by regulating the PKV signaling cascade involved with calcineurin and Hsp90.Citation70 Another small heat shock protein Hsp21, similar to Hsp90, which plays important roles in environmental adaption and virulence potentiates antifungal drug tolerance in C. albicans, while null mutants become sensitive to antifungal drugs including terbinafine, clotrimazole, bifonazole, nocadazole and caspofungin.Citation71,72 These results indicated the synergistic potential between antifungal drugs and Hsp21 inhibitors.
Anti-Biofilm Agents Sensitize C. albicans to Antifungals
Biofilms formed by C. albicans are resistant to most of the commonly used antifungal drugs.Citation73 Susceptibility studies have revealed that biofilms formed by C. albicans can be resistant to various antifungal drugs even thousand times than the planktonic cells.Citation74,75 Currently, there is not a signal antifungal antibiotic was found effective against biofilm related C. albicans infections at low concentrations. Meanwhile, higher concentrations of the antifungal drugs are not advisable because of side effects due to toxicity. Thus, combination of drugs with different mode of actions inhibiting multiple cellular targets would be a wise strategy against biofilms. In 2002, for the first time, Khun et al. demonstrated the unique combination activity of lipid formulations of amphotericin B and echinocandins against Candida biofilms.Citation76 More efforts have been taken in this prospective area since then and a summary of these works can be found in the recent review article by Bink et al.Citation77
In antifungal combination studies, it is interesting that the results of combination against planktonic cells are not always match with that of the biofilms but the utilization of anti-biofilm agents usually sensitize C. albicans to antifungals. For example, combination of fluconazole and amphotericin B has synergistic effects on planktonic growth of C. albicans but does not alter the activity of amphotericin B against biofilms.Citation78 While in another research, Mohd et al. found that the phytocompound eugenol, a potential anti-biofilm agent against pre-formed biofilms and the formation of biofilms alone, exhibited a synergistic interaction with fluconazole against biofilms.Citation79 The SMIC (sessile MICs) of fluconazole could be reduced down to 32-fold. The tested compounds were added before the biofilm formed and inhibit its development which would be of interest for combating recalcitrant infections involving Candida biofilms. The results showed a varying level of attenuation of biofilm formation by planktonic Candida cells in the presence of anti-biofilm compounds and drugs in a dose-dependent manner. The authors suggested that the anti-biofilm agent, eugenol, target cell membranes in both planktonic (higher sterol content) and sessile (lower sterol content) cells of C. albicans, and that their mode of action remains unaffected by the phenotypic variation in the ergosterol content exhibited by planktonic and sessile cells.Citation79 Other candidates for combination therapies such as chloroquine and cyclosporine also showed potency in the sensitization of C. albicans to conventional antifungal drugs.Citation80,81 It is notable that most of the reports dealing with studies of C. albicans biofilm susceptibility for antifungal combination treatment were in vitro. Studies in vivo and clinical are need to perform imperatively for real applicable combination treatments of candidiasis.
Host Model in Synergistic Antifungal Drug Discovery
Despite the increasing knowledge of C. albicans virulence and the discovering of synergistic combinations, it has been not possible to harness all the information for the development of new drugs and therapies for candidiasis. Moreover, the great majority of these combination studies were done in vitro, lacking of evidence for the effects in vivo and clinical, which limited the eventual development of anti-candidiasis drugs with some general concerns about potency and potential toxicity. Thus, host models are necessary to be introduced for comprehensively studying and understanding virulence factors and their interactions with anti-virulence agents. In antifungal drug discovery, besides screening antifungal compounds in vitro, an alternative approach is to screen molecules based on host-pathogen interactions in vivo. Breger et al. developed a high-throughput in vivo assay with Caenorhabditis elegans for antifungal screening.Citation82 In a screen of 1266 compounds with known pharmaceutical activities, 15 were identified that prolonged survival of nematodes infected with C. albicans and inhibited filamentation and biofilm formation of the fungus. Considering C.elegans is invertebrate organism with only innate immunity, other vertebrate animals should be employed as host model in synergistic antifungal study in the future. The utilization of such host model in anti-infection drug discovery can help to identify not only antifungal or anti-virulence agents but also host immunomodulatory active compounds which could also expand the library of synergistic combinations.
Conclusion
The antifungal drug discovery pipeline has declined substantially over the past decades. The concept of synergistic therapy breaks the historical paradigm, “one-drug-one-target dogma”, by targeting different targets and pathways in the disease network. And the synergistic combination of anti-virulence agents and antifungal drugs was proved to be a promising way to combat C. albicans, especially the drug-resistant strains, by targeting both pathogenic process and the cell growth. However, the discovery of such synergistic combinations based on experimental methods by testing a large number of combinations, which is a formidable challenge in terms of costs and time-consuming. Therefore, discovery of synergistic drug combinations based upon known combinations and advancements of fungal pathogen genomics with computational prediction science prospects a new direction in antifungal drug discovery and therapy.
To date, rare synergistic combinations have been proved for the efficacy and safety based on animal models and clinical trials. More pre-clinical evaluation and investigations need to be carried out in the future and the mode of actions of these synergistic combinations should be deciphered. Though there is a long road from in vitro assay to clinical usage, we believe that an approach abiding by the integral concept of incorporating disease process and synergistic bioprospecting strategy gives a promising prospect for the fight against fungal pathogens, even the multi-drug resistant ones.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by China Ocean Mineral Resources R & D Association (Grant No. DY125-15-T-07) and the National Program on Key Basic Research Project (973 program, 2013CB734000), in part by grants from the National Natural Science Foundation of China [31430002, 31400090, 31320103911, 81302678 and 31125002], and the Ministry of Science and Technology of the People's Republic of China [2011ZX09102-011-11, 2013ZX10005004-005], LZ is an awardee for the National Distinguished Young Scholar Program in China.
References
- Dixon DM, McNeil MM, Cohen ML, Gellin BG, La Montagne JR. Fungal infections: a growing threat. Public Health Rep 1996; 111:226; PMID:8643813
- Saral R. Candida and Aspergillus infections in immunocompromised patients: an overview. Rev Infect Dis 1991; 13:487-92; PMID:1866554; http://dx.doi.org/10.1093/clinids/13.3.487
- Pfaller M, Diekema D. Epidemiology of invasive candidiasis: a persistent public health problem. ClinMicrobiol Rev 2007; 20:133-63; PMID:17223626
- Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. CritRev Microbiol 2010; 36:1-53; PMID:20088682
- León C, Ostrosky-Zeichner L, Schuster M. What's new in the clinical and diagnostic management of invasive candidiasis in critically ill patients. Intensive Care Med 2014; 40(6):808-19; http://dx.doi.org/10.1007/s00134-014-3281-0
- Kourkoumpetis T, Manolakaki D, Velmahos G, Chang Y, Alam HB, De Moya MM, Sailhamer EA, Mylonakis E. Candida infection and colonization among non-trauma emergency surgery patients. Virulence 2010; 1(5): 359-66; PMID:21178471; http://dx.doi.org/10.4161/viru.1.5.12795
- Pierce CG, Lopez-Ribot JL. Candidiasis drug discovery and development: new approaches targeting virulence for discovering and identifying new drugs. Exp Opin Drug Dis 2013; 8:1117-26; PMID:23738751; http://dx.doi.org/10.1517/17460441.2013.807245
- Hatipoglu N, Hatipoglu H. Combination antifungal therapy for invasive fungal infections in children and adults. Expert Rev Anti Infect Ther 2013; 11:523-35; PMID:23627858; http://dx.doi.org/10.1586/eri.13.29
- Baddley JW, Poppas PG. Antifungal combination therapy. Drugs 2005; 65:1461-80; PMID:16033288; http://dx.doi.org/10.2165/00003495-200565110-00002
- Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, Edwards JE. Guidelines for treatment of candidiasis. Clin Infect Dis 2004; 38:161-89; PMID:14699449; http://dx.doi.org/10.1086/380796
- Gauwerky K, Borelli C, Korting HC. Targeting virulence: a new paradigm for antifungals. Drug Discov Today 2009; 14:214-22; PMID:19152839; http://dx.doi.org/10.1016/j.drudis.2008.11.013
- Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol 2007; 3:541-8; PMID:17710100; http://dx.doi.org/10.1038/nchembio.2007.24
- Hirano M, Takeuchi Y, Matsumori N, Murata M, Ide T. Channels formed by amphotericin B covalent dimers exhibit rectification. J Membrane Biol 2011; 240:159-64; PMID:21424544; http://dx.doi.org/10.1007/s00232-011-9354-x
- Matsumori N, Masuda R, Murata M. Amphotericin B covalent dimers bearing a tartarate linkage. Chem Biodivers 2004; 1:346-52; PMID:17191852; http://dx.doi.org/10.1002/cbdv.200490030
- Lupetti A, Danesi R, Campa M, Del Tacca M, Kelly S. Molecular basis of resistance to azole antifungals. Trends Mol Med 2002; 8:76-81; PMID:11815273; http://dx.doi.org/10.1016/S1471-4914(02)02280-3
- White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 1998; 11:382-402; PMID:9564569
- Holt SL, Drew RH. Echinocandins: Addressing outstanding questions surrounding treatment of invasive fungal infections. Am J Health-Syst Ph 2011; 68:1207-20; PMID:21690427; http://dx.doi.org/10.2146/ajhp100456
- Morschhäuser J. Regulation of multidrug resistance in pathogenic fungi. Fungal Genet Biol 2010; 47:94-106; http://dx.doi.org/10.1016/j.fgb.2009.08.002
- Domínguez JM, Kelly VA, Kinsman OS, Marriott MS, de las Heras FG, Martín JJ. Sordarins: a new class of antifungals with selective inhibition of the protein synthesis elongation cycle in yeasts. Antimicrob Agents Chemother 1998; 42:2274-8
- Borgers M. Mechanism of action of antifungal drugs, with special reference to the imidazole derivatives. Rev Infect Dis 1980; 2:520-34; PMID:7003674; http://dx.doi.org/10.1093/clinids/2.4.520
- Nishikawa H, Yamada E, Shibata T, Uchihashi S, Fan H, Hayakawa H, Nomura N, Mitsuyama J. Uptake of T-2307, a novel arylamidine, in Candida albicans. J Antimicrob Chemoth 2010; 65(8):1681-87; PMID:20513704; http://dx.doi.org/10.1093/jac/dkq177
- Shibata T, Takahashi T, Yamada E, Kimura A, Nishikawa H, Hayakawa H, Nomura N, Mitsuyama J. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother 2012; 56:5892-7; PMID:22948882; http://dx.doi.org/10.1128/AAC.05954-11
- Mitchell K, Taff H, Cuevas M, Reinicke E, Sanchez H, Andes D. Role of matrix β-1, 3 glucan in antifungal resistance of non-albicans Candida biofilms. Antimicrob Agents Chemother 2013; 57:1918-20; PMID:23318790; http://dx.doi.org/10.1128/AAC.02378-12
- Duvvuri M, Krise JP. Intracellular drug sequestration events associated with the emergence of multidrug resistance: a mechanistic review. Front Biosci 2005; 10:1499-509; PMID:15769640; http://dx.doi.org/10.2741/1634
- Maebashi K, Kudoh M, Nishiyama Y, Makimura K, Uchida K, Mori T, Yamaguchi H. A novel mechanism of fluconazole resistance associated with fluconazole sequestration in Candida albicans isolates from a myelofibrosis patient. Microbiol Immunol 2002; 46:317-26; PMID:12139391; http://dx.doi.org/10.1111/j.1348-0421.2002.tb02702.x
- Cowen LE. The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat Rev Microbiol 2008; 6:187-98; PMID:18246082; http://dx.doi.org/10.1038/nrmicro1835
- Paulsen IT. Multidrug efflux pumps and resistance: regulation and evolution. Curr Opin Microbiol 2003; 6:446-51; PMID:14572535; http://dx.doi.org/10.1016/j.mib.2003.08.005
- Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 2006, 313(5785): 367-370; PMID:16857942
- Selmecki, A., Gerami-Nejad M., Paulson C., Forche A., Berman J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol 2008; 68:624-641; PMID:18363649; http://dx.doi.org/10.1111/j.1365-2958.2008.06176.x
- Perea S, López-Ribot JL, Kirkpatrick WR, McAtee RK, Santillán RA, Martínez M, Calabrese D, Sanglard D, Patterson TF. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 2001; 45:2676-84; PMID:11557454; http://dx.doi.org/10.1128/AAC.45.10.2676-2684.2001
- Odds F. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Ch 2003; 52:1-1; PMID:12805255; http://dx.doi.org/10.1093/jac/dkg301
- Liu X, Ashforth E, Ren B, Song F, Dai H, Liu M, Wang J, Xie Q, Zhang L. Bioprospecting microbial natural product libraries from the marine environment for drug discovery. J Antibiot 2010; 63:415-22; PMID:20606699; http://dx.doi.org/10.1038/ja.2010.56
- Zhang L, Yan K, Zhang Y, Huang R, Bian J, Zheng C, Sun H, Chen Z, Sun N, An R. High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. P Natl Acad Sci USA 2007; 104:4606-11.
- Ashforth EJ, Fu C, Liu X, Dai H, Song F, Guo H, Zhang L. Bioprospecting for antituberculosis leads from microbial metabolites. Nat Prod Rep 2010; 27:1709-19; PMID:20922218; http://dx.doi.org/10.1039/c0np00008f
- Chen X, Ren B, Chen M, Liu M-X, Ren W, Wang Q-X, Zhang L-X, Yan G-Y. ASDCD: antifungal synergistic drug combination database. PloS One 2014; 9:e86499; PMID:24475134; http://dx.doi.org/10.1371/journal.pone.0086499
- Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol 2001; 9:327-35; PMID:11435107; http://dx.doi.org/10.1016/S0966-842X(01)02094-7
- Jones BD, Falkow S. Salmonellosis: Host immune responses and bacterial virulence determinants1. Annu RevImmunol 1996; 14:533-61; PMID:8717524; http://dx.doi.org/10.1146/annurev.immunol.14.1.533
- Garcia-Vidal C, Viasus D, Carratala J. Pathogenesis of invasive fungal infections. Curr Opin Infect Dis 2013; 26:270-6; PMID:23449139; http://dx.doi.org/10.1097/QCO.0b013e32835fb920
- Gozalbo D, Roig P, Villamon E, Gil ML. Candida and candidiasis: the cell wall as a potential molecular target for antifungal therapy. Curr Drug Targets Infect Disord 2004; 4:117-35; PMID:15180460; http://dx.doi.org/10.2174/1568005043341046
- Cassone A, Bernardis F, Torososantucci A. An outline of the role of anti-candida antibodies within the context of passive immunization and protection from candidiasis. Curr Mol Med 2005; 5:377-82; PMID:15977993; http://dx.doi.org/10.2174/1566524054022549
- De Backer MD, Magee PT, Pla J. Recent developments in molecular genetics of Candida albicans. Annu Re Microbiol 2000; 54:463-98; PMID:11018135; http://dx.doi.org/10.1146/annurev.micro.54.1.463
- Odds FC, Gow NA, Brown AJ. Fungal virulence studies come of age. Genome Biol 2001; 2:1009.1-.4.
- Casadevall A, Pirofski L. Host-pathogen interactions: the attributes of virulence. J Infect Dis 2001; 184:337-44; PMID:11443560; http://dx.doi.org/10.1086/322044
- Hoeg L, Thoma-Greber E, Röcken M, Korting H. HIV protease inhibitors influence the prevalence of oral candidosis in HIVinfected patients: a 2-year study. Mycoses 1998; 41:321-5; PMID:9861838; http://dx.doi.org/10.1111/j.1439-0507.1998.tb00345.x
- Korting HC, Schaller M, Eder G, Hamm G, Böhmer U, Hube B. Effects of the human immunodeficiency virus (HIV) proteinase inhibitors saquinavir and indinavir on in vitro activities of secreted aspartyl proteinases of Candida albicans isolates from HIV-infected patients. Antimicrob Agents Chemother 1999; 43:2038-42; PMID:10428932
- Bein M, Schaller M, Korting HC. The secreted aspartic proteinases as a new target in the therapy of candidiasis. Curr Drug Targets 2002; 3(5): 351-357; PMID:12182226; http://dx.doi.org/10.2174/1389450023347542
- Casolari C, Rossi T, Baggio G, Coppi A, Zandomeneghi G, Ruberto AI, Farina C, Fabio G, Zanca A, Castelli M. Interaction between saquinavir and antimycotic drugs on C. albicans and C. neoformans strains. Pharmacol Res 2004; 50(6): 605-610; PMID:15501699; http://dx.doi.org/10.1016/j.phrs.2004.06.008
- Ganendren R, Widmer F, Singhal V, Wilson C, Sorrell T, Wright L. In vitro antifungal activities of inhibitors of phospholipases from the fungal pathogen Cryptococcus neoformans. Antimicrob Agents Chemother 2004; 48:1561-9; PMID:15105106; http://dx.doi.org/10.1128/AAC.48.5.1561-1569.2004
- Soll DR. Candida commensalism and virulence: the evolution of phenotypic plasticity. Acta Trop 2002; 81:101-10; PMID:11801217; http://dx.doi.org/10.1016/S0001-706X(01)00200-5
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell 1997; 90:939-49; PMID:9298905; http://dx.doi.org/10.1016/S0092-8674(00)80358-X
- Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2003; 2:1053-60; PMID:14555488; http://dx.doi.org/10.1128/EC.2.5.1053-1060.2003
- Martins M, Henriques M, Azeredo J, Rocha SM, Coimbra MA, Oliveira R. Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells. Eukaryot Cell 2007; 6:2429-36; PMID:17981993; http://dx.doi.org/10.1128/EC.00252-07
- Joyner PM, Liu J, Zhang Z, Merritt J, Qi F, Cichewicz RH. Mutanobactin A from the human oral pathogen Streptococcus mutans is a cross-kingdom regulator of the yeast-mycelium transition. Org Biomol Chem 2010; 8:5486-9; PMID:20852771; http://dx.doi.org/10.1039/c0ob00579g
- Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. App Environ Microb 2001; 67:2982-92; PMID:11425711; http://dx.doi.org/10.1128/AEM.67.7.2982-2992.2001
- Chang W, Li Y, Zhang L, Cheng A, Lou H. Retigeric acid B attenuates the virulence of Candida albicans via inhibiting adenylyl cyclase activity targeted by enhanced farnesol production. PloS One 2012; 7:e41624; PMID:22848547; http://dx.doi.org/10.1371/journal.pone.0041624
- Gibson J, Sood A, Hogan DA. Pseudomonas aeruginosa-Candida albicans interactions: localization and fungal toxicity of a phenazine derivative. Appl Environ Microbiol 2009; 75:504-13; PMID:19011064; http://dx.doi.org/10.1128/AEM.01037-08
- Hogan DA, Vik Å, Kolter R. A Pseudomonas aeruginosa quorumsensing molecule influences Candida albicans morphology. Mol Microbiol 2004; 54:1212-23; PMID:15554963; http://dx.doi.org/10.1111/j.1365-2958.2004.04349.x
- Zhang L, Chang W, Sun B, Groh M, Speicher A, Lou H. Bisbibenzyls, a new type of antifungal agent, inhibit morphogenesis switch and biofilm formation through upregulation of DPP3 in Candida albicans. PloS One 2011; 6:e28953; PMID:22174935; http://dx.doi.org/10.1371/journal.pone.0028953
- Nett J, Andes D. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr Opin Microbiol 2006; 9:340-5; PMID:16815078; http://dx.doi.org/10.1016/j.mib.2006.06.007
- Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Our current understanding of fungal biofilms. Crit Rev Microbiol 2009; 35:340-55; PMID:19863383; http://dx.doi.org/10.3109/10408410903241436
- Martins M, Henriques M, Lopez-Ribot JL, Oliveira R. Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms. Mycoses 2012; 55:80-5; PMID:21668524; http://dx.doi.org/10.1111/j.1439-0507.2011.02047.x
- Uppuluri P, Chaturvedi AK, Srinivasan A, Banerjee M, Ramasubramaniam AK, Köhler JR, Kadosh D, Lopez-Ribot JL. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog 2010; 6:e1000828; PMID:20360962; http://dx.doi.org/10.1371/journal.ppat.1000828
- Bader T, Bodendorfer B, Schröppel K, Morschhäuser J. Calcineurin is essential for virulence in Candida albicans. Infect Immun 2003; 71:5344-54; PMID:12933882; http://dx.doi.org/10.1128/IAI.71.9.5344-5354.2003
- Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol 2003; 48:959-76; PMID:12753189; http://dx.doi.org/10.1046/j.1365-2958.2003.03495.x
- Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, Cardenas ME, Perfect JR, McCusker JH, Heitman J. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J 2002; 21:546-59; PMID:11847103; http://dx.doi.org/10.1093/emboj/21.4.546
- Blankenship JR, Heitman J. Calcineurin is required for Candida albicans to survive calcium stress in serum. Infect Immun 2005; 73:5767-74; PMID:16113294; http://dx.doi.org/10.1128/IAI.73.9.5767-5774.2005
- Steinbach WJ, Reedy JL, Cramer RA, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol 2007; 5:418-30; PMID:17505522; http://dx.doi.org/10.1038/nrmicro1680
- Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 1999; 12:501-17; PMID:10515900
- Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 2005; 309:2185-9; PMID:16195452; http://dx.doi.org/10.1126/science.1118370
- LaFayette SL, Collins C, Zaas AK, Schell WA, Betancourt-Quiroz M, Gunatilaka AL, Perfect JR, Cowen LE. PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog 2010; 6:e1001069; PMID:20865172; http://dx.doi.org/10.1371/journal.ppat.1001069
- Mayer FL, Wilson D, Jacobsen ID, Miramón P, Slesiona S, Bohovych IM, Brown AJ, Hube B. Small but crucial: the novel small heat shock protein Hsp21 mediates stress adaptation and virulence in Candida albicans. PloS One 2012; 7:e38584; PMID:22685587; http://dx.doi.org/10.1371/journal.pone.0038584
- Mayer FL, Wilson D, Hube B. Hsp21 potentiates antifungal drug tolerance in Candida albicans. PloS One 2013; 8:e60417; PMID:23533680; http://dx.doi.org/10.1371/journal.pone.0060417
- Tobudic S, Kratzer C, Lassnigg A, Presterl E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses 2012; 55:199-204; PMID:21793943; http://dx.doi.org/10.1111/j.1439-0507.2011.02076.x
- Baillie GS, Douglas LJ. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Ch 2000; 46:397-403; PMID:10980166; http://dx.doi.org/10.1093/jac/46.3.397
- Ramage G, Saville SP, Thomas DP, López-Ribot JL. Candida biofilms: an update. Eukaryot Cell 2005; 4:633-8; PMID:15821123; http://dx.doi.org/10.1128/EC.4.4.633-638.2005
- Kuhn D, George T, Chandra J, Mukherjee P, Ghannoum M. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 2002; 46:1773-80; PMID:12019089; http://dx.doi.org/10.1128/AAC.46.6.1773-1780.2002
- Bink A, Pellens K, Cammue B, Thevissen K. Anti-biofilm strategies: how to eradicate Candida biofilms. Open Mycol J 2011; 5:29-38; http://dx.doi.org/10.2174/1874437001105010029
- Bachmann SP, Ramage G, VandeWalle K, Patterson TF, Wickes BL, López-Ribot JL. Antifungal Combinations against Candida albicans Biofilms In vitro. Antimicrob Agents Chemother 2003; 47:3657-9; PMID:14576141; http://dx.doi.org/10.1128/AAC.47.11.3657-3659.2003
- Khan MSA, Ahmad I. Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. J Antimicrob Chemoth 2012; 67:618-21; PMID:22167241; http://dx.doi.org/10.1093/jac/dkr512
- Shinde RB, Chauhan NM, Raut JS, Karuppayil SM. Sensitization of Candida albicans biofilms to various antifungal drugs by cyclosporine A. Ann Clin Microbiol Antimicrob 2012; 11:1-6; PMID:22236533; http://dx.doi.org/10.1186/1476-0711-11-27
- Shinde RB, Raut JS, Chauhan NM, Karuppayil SM. Chloroquine sensitizes biofilms of Candida albicans to antifungal azoles. Braz J Infect Dis. 2013; 17:395-400; PMID:23602464; http://dx.doi.org/10.1016/j.bjid.2012.11.002
- Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, Mylonakis E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog 2007; 3:e18; PMID:17274686; http://dx.doi.org/10.1371/journal.ppat.0030018
- Cury AE, Hirschfeld MPM. Interactions between amphotericin B and nitroimidazoles against Candida albicans. Mycoses 1997; 40:187-92; PMID:9476486; http://dx.doi.org/10.1111/j.1439-0507.1997.tb00212.x
- Van't Hof W, Reijnders IM, Helmerhorst EJ, Walgreen-Weterings E, Simoons-Smit IM, Veerman EC, Amerongen AVN. Synergistic effects of low doses of histatin 5 and its analogues on amphotericin B anti-mycotic activity. AntonVan Leeuwenhoek 2000; 78:163-9; PMID:11204768; http://dx.doi.org/10.1023/A:1026572128004
- Zhang H, Wang K, Zhang G, Ho HI, Gao A. Synergistic anti-candidal activity of tetrandrine on ketoconazole: an experimental study. Planta Med 2010; 76:53-61; PMID:19644794; http://dx.doi.org/10.1055/s-0029-1185973
- Ahmad A, Khan A, Manzoor N. Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur J Pharm Sci 2013; 48:80-6; PMID:23111348; http://dx.doi.org/10.1016/j.ejps.2012.09.016
- Zhang J, Liu W, Tan J, Sun Y, Wan Z, Li R. Antifungal activity of geldanamycin alone or in combination with fluconazole against Candida species. Mycopathologia 2013; 175:273-9; PMID:23341047; http://dx.doi.org/10.1007/s11046-012-9612-1
- Sharma M, Manoharlal R, Negi AS, Prasad R. Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res 2010; 10:570-8; PMID:20528949
- Quan H, Cao Y-Y, Xu Z, Zhao J-X, Gao P-H, Qin X-F, Jiang Y-Y. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob Agents Chemother 2006; 50:1096-9; PMID:16495278; http://dx.doi.org/10.1128/AAC.50.3.1096-1099.2006
- Iwazaki RS, Endo EH, Ueda-Nakamura T, Nakamura CV, Garcia LB, Dias Filho BP. In vitro antifungal activity of the berberine and its synergism with fluconazole. Antonie Van Leeuwenhoek 2010; 97:201-5; PMID:19882381; http://dx.doi.org/10.1007/s10482-009-9394-8
- Li D-D, Xu Y, Zhang D-Z, Quan H, Mylonakis E, Hu D-D, Li M-B, Zhao L-X, Zhu L-H, Wang Y. Fluconazole assists berberine to kill fluconazole-resistant Candida albicans. Antimicrob Agents Chemother 2013:AAC. 00499-13
- Jansen G, Lee AY, Epp E, Fredette A, Surprenant J, Harcus D, Scott M, Tan E, Nishimura T, Whiteway M. Chemogenomic profiling predicts antifungal synergies. Mol Syst Biol 2009; 5:338; PMID:20029371; http://dx.doi.org/10.1038/msb.2009.95
- Chen Y-L, Lehman VN, Averette AF, Perfect JR, Heitman J. Posaconazole exhibits in vitro and in vivo synergistic antifungal activity with caspofungin or FK506 against Candida albicans. PloS One 2013; 8:e57672; PMID:23472097; http://dx.doi.org/10.1371/journal.pone.0057672
- Barchiesi F, Di Francesco LF, Compagnucci P, Arzeni D, Giacometti A, Scalise G. Invitro interaction of terbinafine with amphotericin B, fluconazole and itraconazole against clinical isolates of Candida albicans. J Antimicrob Chemoth 1998; 41:59-65; PMID:9511038; http://dx.doi.org/10.1093/jac/41.1.59
- Dupont B, Drouhet E. In vitro synergy and antagonism of antifungal agents against yeast-like fungi. Postgrad Med J 1979; 55:683-6; PMID:523360; http://dx.doi.org/10.1136/pgmj.55.647.683
- Chin N, Weitzman I, Della-Latta P. In vitro activity of fluvastatin, a cholesterol-lowering agent, and synergy with flucanazole and itraconazole against Candida species and Cryptococcus neoformans. Antimicrob Agents Chemother 1997; 41:850-2; PMID:9087504
- Sun S, Li Y, Guo Q, Shi C, Yu J, Ma L. In vitro interactions between tacrolimus and azoles against Candida albicans determined by different methods. Antimicrob Agents Chemother 2008; 52:409-17; PMID:18056277; http://dx.doi.org/10.1128/AAC.01070-07
- Sun L, Sun S, Cheng A, Wu X, Zhang Y, Lou H. In vitro activities of retigeric acid B alone and in combination with azole antifungal agents against Candida albicans. Antimicrob Agents Chemother 2009; 53:1586-91; PMID:19171796; http://dx.doi.org/10.1128/AAC.00940-08
- Kobayashi T, Kakeya H, Miyazaki T, Izumikawa K, Yanagihara K, Ohno H, Yamamoto Y, Tashiro T, Kohno S. Synergistic antifungal effect of lactoferrin with azole antifungals against Candida albicans and a proposal for a new treatment method for invasive candidiasis. JPN J Infect Dis 2011; 64:292-6; PMID:21788703
- Sharma M, Prasad R. The quorum-sensing molecule farnesol is a modulator of drug efflux mediated by ABC multidrug transporters and synergizes with drugs in Candida albicans. Antimicrob Agents Chemother 2011; 55:4834-43; PMID:21768514; http://dx.doi.org/10.1128/AAC.00344-11
- Guo Q, Sun S, Yu J, Li Y, Cao L. Synergistic activity of azoles with amiodarone against clinically resistant Candida albicans tested by chequerboard and time-kill methods. J Med Microbiol 2008; 57:457-62; PMID:18349365; http://dx.doi.org/10.1099/jmm.0.47651-0
- Khan MSA, Malik A, Ahmad I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med Mycol 2012; 50:33-42; PMID:21756200; http://dx.doi.org/10.3109/13693786.2011.582890
- Jin J, Guo N, Zhang J, Ding Y, Tang X, Liang J, Li L, Deng X, Yu L. The synergy of honokiol and fluconazole against clinical isolates of azole‐resistant Candida albicans. Lett App Microbiol 2010; 51:351-7; PMID:20681969; http://dx.doi.org/10.1111/j.1472-765X.2010.02900.x
- Liu W, Li LP, Zhang JD, Li Q, Shen H, Chen SM, He LJ, Yan L, Xu GT, An MM. Synergistic antifungal effect of glabridin and fluconazole. PloS One 2014; 9:e103442; PMID:25058485; http://dx.doi.org/10.1371/journal.pone.0103442
- Simonetti G, Simonetti N, Villa A. Increase of activity of tioconazole against resistant microorganisms by the addition of butylated hydroxyanisole. Int J Antimicrob Ag 2003; 22:439-43; PMID:14522107; http://dx.doi.org/10.1016/S0924-8579(03)00120-1
- Fu Z, Lu H, Zhu Z, Yan L, Jiang Y, Cao Y. Combination of baicalein and amphotericin B accelerates Candida albicans apoptosis. Biol Pharm Bull 2010; 34:214-8; PMID:21415530; http://dx.doi.org/10.1248/bpb.34.214
- Sharma M, Manoharlal R, Shukla S, Puri N, Prasad T, Ambudkar SV, Prasad R. Curcumin modulates efflux mediated by yeast ABC multidrug transporters and is synergistic with Antifungals. Antimicrob Agents Chemother 2009; 53:3256-65; PMID:19470507; http://dx.doi.org/10.1128/AAC.01497-08
- Chaturvedi V, Ramani R, Andes D, Diekema DJ, Pfaller MA, Ghannoum MA, Knapp C, Lockhart SR, Ostrosky-Zeichner L, Walsh TJ, et al. Multilaboratory testing of two-drug combinations of antifungals against Candida albicans, Candidaglabrata, and Candidaparapsilosis. Antimicrob Agents Chemother 2011; 55:1543-8; PMID:21282457; http://dx.doi.org/10.1128/AAC.01510-09
- Shi WN, Chen ZZ, Chen X, Cao LL, Liu P, Sun SJ. The combination of minocycline and fluconazole causes synergistic growth inhibition against Candida albicans: an in vitro interaction of antifungal and antibacterial agents. FEMS Yeast Res 2010; 10:885-93; PMID:20707818; http://dx.doi.org/10.1111/j.1567-1364.2010.00664.x
- Huang S, Cao YY, Dai BD, Sun XR, Zhu ZY, Cao YB, Wang Y, Gao PH, Jiang YY. In vitro synergism of fluconazole and baicalein against clinical isolates of Candida albicans resistant to fluconazole. Biol Pharm Bull 2008; 31:2234-6; PMID:19043205; http://dx.doi.org/10.1248/bpb.31.2234
- Sasaki E, Maesaki S, Miyazaki Y, Yanagihara K, Tomono K, Tashiro T, Kohno S. Synergistic effect of ofloxacin and fluconazole against azole-resistant Candida albicans. J Infect Chemother 2000; 6:151-4; PMID:11810556; http://dx.doi.org/10.1007/s101560070014
- Uppuluri P, Nett J, Heitman J, Andes D. Synergistic effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob Agents Chemother 2008; 52:1127-32; PMID:18180354; http://dx.doi.org/10.1128/AAC.01397-07
- Wei GX, Xu X, Wu CD. In vitro synergism between berberine and miconazole against planktonic and biofilm Candida cultures. Arch Oral Biol 2011; 56:565-72; PMID:21272859; http://dx.doi.org/10.1016/j.archoralbio.2010.11.021
- Zhou YB, Wang GG, Li YT, Liu Y, Song Y, Zheng WS, Zhang N, Hu XY, Yan SK, Jia JH. In vitro interactions between aspirin and amphotericin B against planktonic cells and biofilm cells of Candida albicans and C. parapsilosis. Antimicrob Agents Chemother 2012; 56:3250-60; PMID:22391539; http://dx.doi.org/10.1128/AAC.06082-11
- Troskie AM, Rautenbach M, Delattin N, Vosloo JA, Dathe M, Cammue BP, Thevissen K. Synergistic activity of the tyrocidines, antimicrobial cyclodecapeptides from Bacillus aneurinolyticus, with amphotericin B and caspofungin against Candida albicans biofilms. Antimicrob Agents Chemother 2014; 58:3697-707; PMID:24752256; http://dx.doi.org/10.1128/AAC.02381-14
- Delattin N, De Brucker K, Vandamme K, Meert E, Marchand A, Chaltin P, Cammue BP, Thevissen K. Repurposing as a means to increase the activity of amphotericin B and caspofungin against Candida albicans biofilms. J Antimicrob Chemother 2014; 69:1035-44; PMID:24284780; http://dx.doi.org/10.1093/jac/dkt449
- Venkatesh M, Rong L, Raad I, Versalovic J. Novel synergistic antibiofilm combinations for salvage of infected catheters. J Med Microbiol 2009; 58:936-44; PMID:19502361; http://dx.doi.org/10.1099/jmm.0.009761-0
- Pemmaraju SC, Pruthi PA, Prasad R, Pruthi V. Candida albicans biofilm inhibition by synergistic action of terpenes and fluconazole. Indian J Exp Biol 2013; 51:1032-7; PMID:24416942
- Gao Y, Li H, Liu S, Zhang X, Sun S. Synergistic effect of fluconazole and doxycycline against Candida albicans biofilms resulting from calcium fluctuation and downregulation of fluconazole-inducible efflux pump gene overexpression. J Med Microbiol 2014; 63:956-61; PMID:24809386; http://dx.doi.org/10.1099/jmm.0.072421-0
- Monteiro DR, Silva S, Negri M, Gorup LF, de Camargo ER, Oliveira R, Barbosa DB, Henriques M. Antifungal activity of silver nanoparticles in combination with nystatin and chlorhexidine digluconate against Candida albicans and Candidaglabrata biofilms. Mycoses 2013; 56:672-80; PMID:23773119; http://dx.doi.org/10.1111/myc.12093
- Gao Y, Zhang C, Lu C, Liu P, Li Y, Li H, Sun S. Synergistic effect of doxycycline and fluconazole against Candida albicans biofilms and the impact of calcium channel blockers. FEMS Yeast Res 2013; 13:453-62; PMID:23577622; http://dx.doi.org/10.1111/1567-1364.12048
- You JL, Du L, King JB, Hall BE, Cichewicz RH. Small molecule suppressors of Candida albicans biofilm formation synergistically enhance the antifungal activity of amphotericin B against clinical Candida Isolates. ACS Chem Biol 2013; 8:840-8; PMID:23387427; http://dx.doi.org/10.1021/cb400009f
- Yu Q, Ding X, Xu N, Cheng X, Qian K, Zhang B, Xing L, Li M. In vitro activity of verapamil alone and in combination with fluconazole or tunicamycin against Candida albicans biofilms. Int J Antimicrob Agents 2013; 41:179-82; PMID:23265915; http://dx.doi.org/10.1016/j.ijantimicag.2012.10.009
- Shinde RB, Chauhan NM, Raut JS, Karuppayil SM. Sensitization of Candida albicans biofilms to various antifungal drugs by cyclosporine A. Ann Clin Microb Anti 2012; 11:27; PMID:23035934
- Del Pozo JL, Frances ML, Hernaez S, Serrera A, Alonso M, Rubio MF. Effect of amphotericin B alone or in combination with rifampicin or clarithromycin against Candida species biofilms. Int J Artif Organs 2011; 34:766-70; PMID:22094555; http://dx.doi.org/10.5301/ijao.5000023
- Chen YL, Lehman VN, Averette AF, Perfect JR, Heitman J. Posaconazole exhibits in vitro and in vivo synergistic antifungal activity with caspofungin or FK506 against Candida albicans. PloS One 2013; 8:e57672; PMID:23472097; http://dx.doi.org/10.1371/journal.pone.0057672
- Hossain MA, Reyes GH, Long LA, Mukherjee PK, Ghannoum MA. Efficacy of caspofungin combined with amphotericin B against azole-resistant Candida albicans. J Antimicrob Chemother 2003; 51:1427-9; PMID:12716772; http://dx.doi.org/10.1093/jac/dkg230
- Zhang H, Wang K, Zhang G, Ho HI, Gao A. Synergistic anti-candidal activity of tetrandrine on ketoconazole: an experimental study. Planta Med 2010; 76:53-61; PMID:19644794; http://dx.doi.org/10.1055/s-0029-1185973
- Han Y, Lee JH. Berberine synergy with amphotericin B against disseminated candidiasis in mice. Biol Pharm Bull 2005; 28:541-4; PMID:15744087; http://dx.doi.org/10.1248/bpb.28.541
- Hanson LH, Perlman AM, Clemons KV, Stevens DA. Synergy between cilofungin and amphotericin B in a murine model of candidiasis. Antimicrob Agents Chemother 1991; 35:1334-7; PMID:1929290; http://dx.doi.org/10.1128/AAC.35.7.1334
- MacCallum DM, Desbois AP, Coote PJ. Enhanced efficacy of synergistic combinations of antimicrobial peptides with caspofungin versus Candida albicans in insect and murine models of systemic infection. Eur J Clin Microbiol 2013; 32:1055-62; PMID:23572153; http://dx.doi.org/10.1007/s10096-013-1850-8
- Hector RF, Schaller K. Positive interaction of nikkomycins and azoles against Candida albicansin vitro and in vivo. Antimicrob Agents Chemother 1992; 36:1284-9; PMID:1416829; http://dx.doi.org/10.1128/AAC.36.6.1284
- Polak A. Combination therapy of experimental candidiasis, cryptococcosis, aspergillosis and wangiellosis in mice. Chemotherapy 1987; 33:381-95; PMID:2822362; http://dx.doi.org/10.1159/000238524
- Kujath P, Lerch K, Kochendorfer P, Boos C. Comparative study of the efficacy of fluconazole versus amphotericin B/flucytosine in surgical patients with systemic mycoses. Infection 1993; 21:376-82; PMID:8132367; http://dx.doi.org/10.1007/BF01728917
- AbeleHorn M, Kopp A, Sternberg U, Ohly A, Dauber A, Russwurm W, Buchinger W, Nagengast O, Emmerling P. A randomized study comparing fluconazole with amphotericin B/5-flucytosine for the treatment of systemic Candida infections in intensive care patients. Infection 1996; 24:426-32; PMID:9007589; http://dx.doi.org/10.1007/BF01713042
- Casado JL, Quereda C, Oliva J, Navas E, Moreno A, Pintado V, Cobo J, Corral I. Candidal meningitis in HIV-infected patients: Analysis of 14 cases. Clin Infec Dis 1997; 25:673-6; PMID:9314460; http://dx.doi.org/10.1086/513746
- Pina-Vaz C, Sansonetty F, Rodrigues AG, Martinez-De-Oliveira J, Fonseca AF, Mardh PA. Antifungal activity of ibuprofen alone and in combination with fluconazole against Candida species. J Med Microbiol 2000; 49:831-40; PMID:10966233
- Scheven M, Junemann K, Schramm H, Huhn W. Successful treatment of a Candidaalbicans sepsis with a combination of flucytosine and fluconazole. Mycoses 1992; 35:315-6; PMID:1302806; http://dx.doi.org/10.1111/j.1439-0507.1992.tb00886.x
- Rex JH, Pappas PG, Karchmer AW, Sobel J, Edwards JE, Hadley S, Brass C, Vazquez JA, Chapman SW, Horowitz HW, et al. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis 2003; 36:1221-8; PMID:12746765; http://dx.doi.org/10.1086/374850
- Ghannoum MA, Elewski B. Successful treatment of fluconazole-resistant oropharyngeal candidiasis by a combination of fluconazole and terbinafine. Clin Diagn Lab Immun 1999; 6:921-3; PMID:10548586
- Pachl J, Svoboda P, Jacobs F, Vandewoude K, Van der Hoven B, Spronk P, Masterson G, Malbrain M, Aoun M, Garbino J, et al. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis 2006; 42:1404-13; PMID:16619152; http://dx.doi.org/10.1086/503428
