ABSTRACT
Most fungi are capable of disseminating into the central nervous system (CNS) commonly being observed in immunocompromised hosts. Microglia play a critical role in responding to these infections regulating inflammatory processes proficient at controlling CNS colonization by these eukaryotic microorganisms. Nonetheless, it is this inflammatory state that paradoxically yields cerebral mycotic meningoencephalitis and abscess formation. As peripheral macrophages and fungi have been investigated aiding our understanding of peripheral disease, ascertaining the key interactions between fungi and microglia may uncover greater abilities to treat invasive fungal infections of the brain. Here, we present the current knowledge of microglial physiology. Due to the existing literature, we have described to greater extent the opportunistic mycotic interactions with these surveillance cells of the CNS, highlighting the need for greater efforts to study other cerebral fungal infections such as those caused by geographically restricted dimorphic and rare fungi.
Introduction
The Central Nervous System (CNS) is protected from toxins, drugs, and pathogens by an anatomical blood brain barrier (BBB) and intrinsic immune system. Microglia is the major surveillance cell of the CNS with phagocytic abilities, similar to myeloid-derived peripheral macrophages, which respond to local injury and infectious agents. Most fungi are capable of disseminating from the lungs to the CNS, particularly in immunocompromised individuals including AIDS and neutropenic patients.Citation1,2 Although there are shared characteristics between various fungi and their interaction with host's immunity, each fungus has varying structural and virulence factors which result in differing immune responses.Citation3
After inoculation of a healthy subject, most fungal infections are self-limited due to the individual's immunity. Local immune cells, such as macrophages, dendritic cells, and neutrophils first initiate the fungal defense through phagocytosis, release of cytokines, and initiation of the complement, humoral and cell mediated defenses, keeping the fungi localized and further eliminating it. In fact, the patient's immunosuppression and fungal immunity evasion mechanisms that have likely been acquired from interacting with other organisms in the environment,Citation4 enable fungal opportunism and potential dissemination to distal sites, such as the invasion of the CNS, contributing to the conditions associated to this process including: meningitis, encephalitis, abscess formation, and death.
Fungi are ubiquitous eukaryotic organisms found in the environment in association with soil, animal feces, plant debris, or water, only requiring moisture and organic matter for survival. The main route of transmission is through inhalation as many fungi in the form of spores enter into the respiratory system. Some fungi are found commensally on skin and mucosal flora only becoming a threat to human health during immunosuppression. Moreover, most fungal infections are confined to the skin, respiratory and intestinal tracts, and vaginal mucosa. However, in severe cases, systemic mycosis can arise and disseminate hematogenously into the CNS.Citation5,6 Direct contiguous spread from the orbits, petromastoid region, and the paranasal sinuses can also occur in some patients.Citation3 Additionally, fungal infection with or without dissemination may occur during trauma, prosthetic valve placement, and intensive care or intracranial procedures.Citation3 For example, outbreaks of fungal meningitis caused by the extremely rare human pathogens, Exserohilum rostratumCitation7 and Exophiala (Wangiella) dermatitidisCitation8 in patients receiving contaminated injectable steroids highlight the opportunistic nature of fungi. These eukaryotic microbes can be divided into 2 categories as they relate to their usual infectivity in relation to the human host immune system. The opportunistic mycoses such as candidiasis, aspergillosis, cryptococcosis, and mucormycosis, most exclusively are diseases afflicting immunosuppressed hosts. Also, thermally dimorphic fungi that live as filamentous mold in the environment and morphologically switch to yeasts or spherules at mammalian body temperature causing deep organ systemic infections. Fungi belonging to this group include Histoplasma capsulatum, Blastomyces dermatitidis, Coccidiodes immitis, and Paracoccidioides brasiliensis.
In this review, we will discuss the existing knowledge of microglial physiology, including their origin, morphological characteristics, and their ability to recognize and respond to the CNS infections. We are especially interested in describing the current knowledge about the evolving interactions between fungi and resident microglia of the CNS as this is crucial to understand the brain's capabilities of defending itself against infectious fungi. Putting in perspective the information available about how microglial cells recognize and combat fungal infections is important to influence and encourage further investigation to mitigate the number of clinical cases involving individuals with impaired immunity and afflicted with CNS mycoses.
Microglia, guardians of the CNS against fungi
Origin
Microglial cells are known to comprise approximately 10–20% of the glial population in the CNS.Citation9 They can be found throughout the brain parenchyma, most commonly in the hippocampus and the retina.Citation10 The comparison between microglia and macrophage has facilitated our understanding of microglial physiology. However, distinctions must be made between them, since microglial cells are entirely maintained by self-replication and derive from a different ontogeny.Citation11 Microglia precursors have been identified as early as embryonic day 8.5-9.5 in the fetal yolk sac.Citation12,13-15 These CD45− and CKIT cells in the yolk sac give rise to CX3CR1, CD45+ microglia within the CNS.Citation16,17 CX3CR1 is a key regulatory receptor for maintenance of this population and has been characterized to have exclusivity for microglia in the CNS, even though is also expressed by peripheral cells including monocytes, dendritic cells, and natural killer (NK) cells.Citation18 In contrast to bone marrow-derived cells, microglia develops prior to the development of the fetal liver which becomes responsible for the first circulating myeloid cells.Citation19 Recent RNA sequencing studies identified 29 specific genes that differentiate microglia from other cells in the CNS and peripheral monocyte-derived macrophages.Citation18 In addition, microglial cells have a sensome or transcriptomic signature, which regulate a unique cluster of transcripts encoding proteins that are important for detecting endogenous recognition molecules and microbial antigens. Aging plays an important role in the regulation of the sensome by increasing the expression of microglial specific antimicrobial genes necessary for neuroprotection.Citation20
Murine microglial maintenance and proliferation is dependent on the IRF8 transcription factor PU-1 and colony stimulating factor 1 receptor (CSF1R).Citation21 Particularly, IL-34 produced by neurons acts on the CSF1R,Citation17,22 thus not requiring ligands such as c-Myb and CSF-1, which are essential for bone marrow-derived macrophage preservation.Citation11 Perivascular microglial cells, also known as perivascular macrophages, are bone marrow-derived cells produced as early as embryonic day 13.5 and remain adjacent to the basement membrane of small CNS parenchymal vessels. Similarly, macrophages aside the choroid plexus or meninges are also derivatives of this myeloid population.Citation21,23 This specific population of cells is continuously being maintained from the periphery, while self-replication characteristics of parenchymal microglia safeguard their maintenance.Citation11,12,24 In pathological conditions, microglia and perivascular macrophages release chemokines that regulate the neuroinflammatory response by increased recruiting of dendritic cells, neutrophils, and lymphocytes from peripheral tissue.Citation10,21,25 It is conceivable to think that the interaction of microglia and perivascular macrophages may be necessary to bridge the immunological communication between cells in the CNS and cells in the peripheral tissues. However, future and rigorous investigations are needed to unequivocally establish this connection.
States of existence
In the CNS of healthy individuals, microglial cells morphologically show refined branched processes oriented radially to a small elliptical soma ().Citation25,26 These cells share similar characteristics to peripheral macrophages consistently monitoring their microenvironment, awaiting foreign pathogens and neurological insults, maintaining healthy synaptic junctions, and responding to apoptotic neuronal death.Citation27 Under homeostatic conditions, neurons and astrocytes communicate with microglia via paracrine and autocrine pathways by expressing receptors (e.g. CX3CR1, CD200R, CD45, etc.) that recognize ligands (e.g., fractalkine (CX3CL1), CD200, CD22, etc.) that keep microglia in a ramified state.Citation28 When neuronal death occurs, the release of adenosine triphosphate and calcium activates microglia to morphologically change into their active form. Many activation signals trigger innate immune responses. Pattern recognition receptors (PRRs) are maintained on their surface or intracellularly and allow for recognition of foreign antigens known as pathogen-associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs). PRRs that are responsive to fungal antigens among peripheral macrophages and naive microglia include major histocompatibility complex II (MHC II), CD45, Toll-like receptors (TLRs), complement receptors (CR-3; CD11b/CD18), CD14, and CSF1R.Citation29
Figure 1. States of existence of microglia. (A) Naive microglia in the neocortical region of CX3CR1-GFP mice exhibits delicate, branched processes oriented radially to a small elliptical soma. (B) Interactions of microglia with Cryptococcus neoformans strain H99 in a brain lesion of a CX3CR1-GFP mouse intratracheally infected for 14 days. Microglia became reactive or amoeboid or phagocytic-like in shape upon interaction with yeast cells (white arrows). During this phase, there is hypertrophy of the soma including shortened and fewer processes. Scale bar: 50 µm.
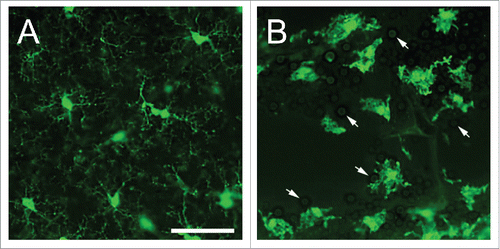
Upon antigenic interaction, naïve microglia switch reactivity into an activated state taking on an amoeboid and phagocytic-like shape, with hypertrophy of the cell soma, retraction of ramifications, upregulation or de novo synthesis of cell surface or intracellular molecules ().Citation13,21,24 This cellular transformation occurs in steps which may include hyper-ramification in order to improve motility and locomotion.Citation25 Additionally, fungal recognition by TLRs, Dectin-1, mannoproteins, and scavenger receptors on these cells lead to release of distinctive cytokines, such as IFN-γ, TNF-α, IL-1β, IL-6, and IL-12, which enhance phagocytosis and production of free radicals, in the form of nitric oxide (NO) and superoxide anion.Citation30,31 Intracellular and extracellular defense against fungi by microglia depend on cytokine release, such as IFN-γ, complement activation,Citation32 and opsonization of antigens.Citation33 For example, microglia expresses the S100B protein which surrounds the phagosome of opsonized Cryptococcus neoformans, which in the presence of IFN-γ stimulates transcription of the inducible NO synthase gene resulting in increased secretion of NO.Citation34 These cells express a greater number of PRRs, including TLRs and MHC II, allowing for increased communication with other immune cells through the secretion of cytokines and chemokines.Citation35 Nucleotide binding oligomerization domains-like receptors on microglia stimulate the production of IL-1β and IL-18 which assist in the recruitment of neutrophils into the CNS infection site.Citation36
Although the phenotypic variations of microglial cells are complex, they are mainly involved in anti-inflammatory and pro-inflammatory processes. Depending on the stimuli, microglia can polarize into multiple phenotypes and switch activity states.Citation37 Similarly to the dual role of macrophages in the periphery after Th1 or Th2 lymphocyte stimulation, microglia have also being implicated in the M1 and M2 classification to describe and simplify their activation states.Citation35,38 The M1 cell, or “classical activated” microglia, acts as antigen presenting cell. In contrast, M2 microglial cells downregulate inflammation aiming to minimize the potentially neurotoxic effects of the immune response.Citation39 However, recent evidence suggests that the typical and perpetuated polarization comparison of microglia to macrophages and its terminology is insufficient and controversial, only reflecting individual bias and reductionism with limited in vivo applications.Citation24 For instance, elegant studies demonstrated that microglial and monocyte-derived macrophage gene expression profiles, functions, tissue life-span, and tridimensional morphology are considerably different despite of exhibiting similar number of cells during tissue inflammation.Citation40,41 These findings were later validated by showing that microglia's transcriptomic activity is unambiguously different to those of peripheral monocyte-derived macrophages, regardless of their anatomical origin.Citation18 Likewise, numerous studies have demonstrated microglia's own biological identity including the regulation of synaptic pruning and plasticity,Citation42-44 the spatial distribution of axonal projections,Citation45,46 and neuronal homeostasis and survival.Citation47,48 Alternatively, a current hypothesis proposes that microglial reactivity may be stimulated by damaged neurons with deficient signaling, the presence of circulatory plasma molecules in the CNS due to the BBB disruption, and peripheral leukocyte signaling mediated by cytokines after interactions with microbes or their antigens.Citation24 Major efforts in specifically dissecting the biology of microglia should focused on using epigenomics, comparative transcriptomics, proteomics, and other multidimensional technologies such as computational biology and 2-photon imaging.Citation24
Following activation of PRR due to fungal PAMPs recognition, adaptor molecules are important to proper functioning of signal transduction pathways that lead to inflammatory responses (). Myd88 is the key adaptor molecule in the TLR activation pathway against fungi and associates with the cytoplasmic part of TLR,Citation12 and subsequently recruits members of the IL-1β receptor associated kinase (IRAK) family, most importantly IRAK2 and IRAK4, which through TRAF6 downstream signaling leads to the translocation of NF-κB, and ultimately the release of inflammatory cytokines and interferon inducible genes.Citation9,12,36,49 Following Dectin-1 receptor activation by fungal cell wall antigens, Syk-CARD9 is the key adaptor molecule,Citation50 independent of Myd88 pathways, leading to NF-κB expression and Th17 responses.Citation51
Figure 2. Microglial activation after interaction with opsonized or non-opsonized fungal antigens (e.g., glucorunoxylomannan (GXM) and β-glucans). Pattern recognition receptors (TLR-2/4, Dectin-1 and 2, CR-3, CD45, CD86, MHC II, FcγR1, CX3CR1 and CCR2) on the surface of microglial cells or intracellularly (TLR-9) recognize fungal antigens triggering effector signal transduction pathways eventually leading to NF-κB activation and the production of cytokine and chemokine production.
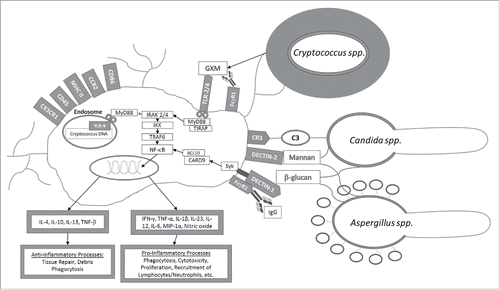
TLR-2, 4, and 9, are associated with recognition of most fungal antigens, including the C. neoformans polysaccharide capsule, Candida albicans pseudohyphae, and Aspergillus spp. conidia.Citation52 On the surface of microglia cells, the TLR-4 predominates, which is known to induce pro-inflammatory processes favoring the development of a Th1 response that is critical in protection against fungi.Citation53,54 For example, knockout mice for the TLR-4 receptor are susceptible to disseminated candidiasis and reduced clearance of conidias produced by Aspergillus. TLR-2, Dectin-1, and CR-3 on the surface of microglia and macrophages recognize carbohydrates such as mannose and β-glucans on the surface of A. fumigatus and C. albicans.Citation49 In this regard, fungal PAMPs are restricted to complex carbohydrates in the cell wall, including chitin, mannoproteins, phospholipomannan, and β-glucans, such as zymosan, which may subsequently activate microglia yielding pro-lymphocytic and humoral responses to control fungal infections.Citation52
Currently, there is limited knowledge on the modulation of anti-inflammatory processes by microglia in the setting of fungal infection. In this regard, stressed mice infected with C. neoformans, stimulate production of anti-inflammatory chemokines such as CCL-2 by microglia, increasing animal susceptibility to disease.Citation55 In contrast, certain fungi are also capable of using their interaction with TLR and anti-inflammatory response to evade host defenses. For example, C. albicans induces immunosuppression by activating TLR-2, leading to the release of IL-10, an anti-inflammatory cytokine that activates CD4+CD25+ T regulatory cells.Citation51
Fungal interaction with the BBB and CNS invasion
Fungi, particularly yeast cells, can spread hematogenously and penetrate into the brain parenchyma transcellularly, paracellularly, or inside of circulating macrophages using the Trojan horse mechanism. Upon exposure to the cerebral microcirculation, fungal cells interact with microglia, as well as astrocytes and endothelial cells, causing leptomeningitis, encephalitis, and granulomas.Citation56 The BBB aims to protect the CNS from pathogen invasion, particularly those being transported by the bloodstream. Activated PRRs on the surface of luminal endothelial cells of the BBB release pro-inflammatory cytokines leading to the activation of microglia and subsequent defense mechanisms.Citation57 However, disruption of the BBB via trauma, surgery or in AIDS patients increases the risk of CNS invasion. Microglial activation and released cytokines such as TNF-α have also been shown to disrupt the tight junctions of the BBB.Citation58 Yeast cells may also infect the mucosa and paranasal sinuses, but they do not usually spread intracranially. In contrast, hyphae are capable of contiguous spread through nearby sources such as the cribriform plate or periorbital and paranasal sinuses. Similarly, Aspergillus and the zygomycetes can form abscesses in nearby lobar locations, encephalitis, and vasculitis. Cerebral abscesses are typically localized, enhancing masses with degrees of surrounding cerebral edema. In this regard, focal neurologic deficits, seizures, and dementia are common manifestations of fungal CNS diseases.
Opportunistic fungal infections
Cryptococcosis. The most common cerebral manifestation of cryptococcosis, which is caused by C. neoformans and C. gattii, after inhalation of their infectious particles, is meningoencephalitis, which is characterized by granuloma or cryptococcoma formation. Cerebral cryptococcosis caused by C. neoformans occurs in 90% of infected immunocompromised hosts,Citation59 whereas C. gatti preferentially infects apparently healthy hosts.Citation60 The mortality rate of C. neoformans cerebral infection has been reported to be 30%.Citation61 CNS penetration by Cryptococcus spp occurs via a transcytosis, paracellular, and the Trojan horse pathways.Citation6,62
Post-mortem neuropathological examinations of patients' brains have demonstrated that C. neoformans' main virulence factor, its polysaccharide capsule which is extensively released during infection, is ingested and localizes inside of microglia.Citation63,64 In the periphery, the capsular polysaccharide can interfere with phagocytosis, antigen presentation, leukocyte migration and proliferation, and specific antibody responses, and can enhance HIV replication.Citation65,66 Alternatively, the black pigment melanin may play a role in anti-phagocytic activity of C. neoformans.Citation67 In avirulent nonmelanogenic C. neoformans infected mice produced numerous key cytokines as described above without fatality, the virulent melanogenic fungi produces little or no cytokine secretion in mice with massive tissue damage and a number of fatalities.Citation68
The microglial response is critical against C. neoformans after CNS invasion. Fungal PAMPs are recognized by microglial cells and astrocytes. Cytokine and antimicrobial molecules, that include IFN-γ, TNF-α, IL-1β, IL-4, IL-6, IL-10, IL-12, IL-23, NO, and macrophage inflammatory protein-1α (MIP-1α) upon exposure to fungal antigens, recruit peripheral CD4+ and CD8+ T cells, peripheral macrophages, and neutrophils that are able to seed into the CNS.Citation69,70 CD40 and IL-2 enhanced the host cell response to C. neoformans in intracerebrally injected mice by up-regulating CD45, CD11 and MHC II on the surface of microglia as well as infiltration of these cells to the site of injection.Citation71 Experiments performed with IFN-γ knockout mice revealed that this cytokine is essential for both microglial cell activation and the anti-cryptococcal efficacy especially after anti-CD40/IL-2 administration. In spite of the observed increased in the levels of circulating IFN-γ and microglial reactivity early after treatment, minimal levels of IFN-γ were detected in brain homogenates. Additionally, the phagocytic function of microglia reduces C. neoformans growth and increases expression of MHC II in the presence of CD4+ cells using murine cell lines.Citation72 In another study, alongside IL-12, the addition of IL-23p19 enhances the microglial response to C. neoformans.Citation73 Differences in response to NO by animal models have been observed. In contrast to murine microglia, human microglia may not be capable of killing C. neoformans probably due to insufficient production of NO as compared to the high levels of NO production by murine microglia.Citation74
Since macrophage physiology and iron metabolism are intertwined, the influence of increased iron levels on the effector functions of untreated and IFN-γ plus lipopolysaccharide (LPS) microglial cells infected with C. neoformans was investigated in vitro using the murine cell line BV-2.Citation75 A high iron milieu augmented and decreased the anti-cryptococcal activity of basal and IFN-γ plus LPS-treated BV-2 cells, respectively but had no effect on their phagocytic activity. Likewise, mice supplemented with iron and intracerebrally infected with C. neoformans showed increased fungal burden and reduced IL-12 production throughout the infection and low levels of IFN-γ especially in the late stages of the infection relative to untreated control animals.Citation76
In healthy individuals, the brain is devoid of antibodies given that the BBB is intact and prevents these relatively large molecules from entering the CNS.Citation77,78 Yet, in cerebral inflammation, the BBB is disrupted and becomes increasingly permeable to these opsonins which pass through to help fight infections, augmenting the microglial anti-cryptococcal activity. Peripheral macrophages have shown that opsonization by IgM and complement activation is important for efficient phagocytosis of cryptococci.Citation79 For example, increased survival of healthy mice and immunosuppressed mice infected with opsonized cryptococcal cells was observed compared to unopsonized yeast cells.Citation33,80,81 Within the brain, the capabilities of a monoclonal anti-capsular IgG enhance microglial cell phagocytosis of cryptococci.Citation82 C. neoformans monoclonal antibody immune complex promotes chemokine production and phagocytosis in primary human microglia via activation of Fcγ receptor for IgG. Additionally, disruption of phosphatidylinositol-3 kinase pathway inhibits phagocytosis independent of an effect on the MIP-1α secretion.Citation83 Interestingly, opsonization is required for human microglia to ingest cryptococci whereas murine and porcine microglia can phagocytize the yeast cells independently of opsonins participation.Citation84 Furthermore, G-protein coupled receptors (GPCR), particularly GPR34 which is highly expressed in murine retinal and cortical microglia, play a crucial role in cryptococcal phagocytosis. GPR34 knockout microglia showed impaired phagocytosis of cryptococcal cells.Citation85
Studies with complement deficient animals indicate that the complement system plays a critical role in resistance to cryptococcosis.Citation86,87 The complement system is an important regulator of the inflammatory response against C. neoformans and contributes to host resistance by opsonization of the yeast to facilitate adhesion and phagocytosis by peripheral phagocytic cells.Citation32,88 Although all complement components can be locally produced by resident brain cells, including astrocytes, neurons, oligodendrocytes, and microglia, often in response to injury, developmental signals, or infection,Citation77 there is no data available on the role of complement in controlling cerebral cryptococcosis by either microglia or peripheral macrophages. For instance, C1q is expressed in microglia and abundantly found in brain tissue, playing a major role in neurodegenerative diseases such as Alzheimer's disease.Citation89 This is an exciting and promising area of investigation considering the predilection of C. neoformans for the CNS and the importance microglia and complement may play in preventing fungal brain colonization.Citation90
Aspergillosis
A. fumigatus is recognized as the most frequent species to cause aspergillosis and the most common cause of fungal brain abscesses, followed by A. flavus, A. niger, and A. oxyzaei. Cerebral aspergillosis is associated with 90–100% mortality, but only 10–20% of cases of invasive aspergillosis compromise the brain.Citation91 Numerous factors such as the site of infection, virulence of the strain, associated immunodeficiency state, as well as limited treatment options relate to these poor outcomes.Citation92 Patients with tuberculosis, neutropenia, asthma or chronic obstructive pulmonary disease, chronic granulomatous disease, cancer, and those taking prolonged immunosuppressant medications are most susceptible.Citation91,93 Nosocomial outbreaks have occurred especially during building renovations or constructions when air conditioning ducts become heavily contaminated and an ideal environment for hyphal growth and dispersion of the infectious conidia.Citation94 After inhalation, dissemination to the CNS occurs through the bloodstream and contiguous spread from the orbits, periorbital regions, middle ear, or paranasal sinuses.Citation95
Similar to CNS granuloma formation in murine models of cryptococcosis, cerebral aspergillosis granuloma is surrounded by microglia, leukocytes, and necrotic neurons.Citation96 When stimulated by Aspergillus β-glucans, Dectin-1 leads to the release of TNF-α, IL-1β, IL-6, IL-8, IL-12, and CXCL-1.Citation97 In Dectin-1 knockout mice, an increased mortality rate and impairment of cytokine production in the presence of the mold is observed. TLR-2 can recognize both, the conidial and hyphal forms of Aspergillus spp, but TLR-4 only recognizes the hyphal form.Citation98 IL-6 knockout mice are more susceptible to invasive aspergillosis than wild-type animals.Citation99 This is important because IL-6 is a neuroinflammatory cytokine released by cells of the brain, including microglia, astrocytes, and neurons. IL-6 plays a role in sequestering intracellular free iron, an important nutritional source for microbial growth within phagocytes.Citation100
The virulence factors of Aspergillus spp may enhance the evasion of immunity and penetration of the CNS.Citation101 For example, mycotoxins, such as gliotoxin, can damage and kill microglial cells, astrocytes, neurons via apoptosis. Mycotoxins also inhibit phagocytosis, reduce Reactive Oxygen Species (ROS) production by neutrophils and inhibit T cell responses.Citation95,102 Secretion of gliotoxin makes the fungal conidia less susceptible to opsonization increasing the fungus propensity to invade the CNS through endothelial cell endocytosis.Citation90,102 When this mold is cultured in human cerebrospinal fluid (CSF) secretes Alp-1, an alkaline protease that can cleave and inactivate complement proteins.Citation103 For example, A. fumigatus, the most frequent cause of cerebral aspergillosis, destroyed complement activity more efficiently than other Aspergillus sppCitation104 The degradation of complement in CSF results in a drastic reduction of the capacity to opsonize fungal hyphae. The Aspergillus-derived protease diminishes the amount of CR-3, a surface molecule to mediate eradication of opsonized pathogens, on granulocytes and microglia. Furthermore, a reduction in CR-3 expression of microglia cell surface causes a significant reduction in phagocytosis of fungal cells. Moreover, supplementation of CSF with nitrogen sources rescues the complement proteins and abolishes any Alp-1 induced cleavage, representing a potential therapy for aspergillosis treatment.
Candidiasis
C. albicans is a commensal most commonly known to cause oral and vaginal candidiasis in patients with immunodeficiency. C. albicans can disseminate to all the organs including the brain leading to meningitis, even though brain abscesses can occur in 50% of patients. Mortality due to invasive candidiasis ranges from 20 to 50%.Citation105 Microglial cells are the principal effector cells in invasive cerebral candidiasis, and when administered intracerebrally can limit infection and tissue damage.Citation106,107 Notably, during systemic candidiasis, accumulation of neutrophils predominate in all organs except the brain, where microglia is the major cell type detected interacting with the fungus.Citation106 In the retina of C. albicans infected mice, microglia become reactive 3 days post-infection, undergoing significant morphological transformations, increased expression of MHC II, CD11b, and CD45, and close association with blood vessels.Citation108
Biofilm formation seems to protect C. albicans from microglial damage impairing fungal cell phagocytosis, cytokine release, and NO production.Citation109 The β-glucans on the surface of C. albicans are detected by TLR-2 and 4, as well as Dectin-1 expressed on the surface of retinal and intraparenchymal microglia.Citation110-112 However, these carbohydrates may attenuate TLR-mediated NF-κB activation, decreasing the capacity of microglia to release inflammatory cytokines in response to the pathogen.Citation113 CARD9, an adaptor molecule linked to Dectin-1 receptors, responds to the cell wall structures of the yeast, especially carbohydrates, and has been associated with cytokines production by microglia leading to the recruitment of neutrophils. This observation was confirmed in a CARD9 knockout mouse that shows reduced neutrophil recruitment and increased CNS fungal burden.Citation31
Mucormycosis
Rhizopus and Mucor are zygomycetes known to cause CNS infection. Rhinocerebral infection is a complication of mucormycosis which is suspected in patients with a triad of nasoorbital infection, diabetic ketoacidosis, and meningoencephalitis.Citation114 Cerebral infection occurs in 33–50% of all cases, and 70% of these cases are associated to patients with diabetic ketoacidosis which display cellular defective phagocytosis function.Citation114 In the diabetic murine model, the monocytes are dysfunctional, incapable of suppressing spore germination in serum.Citation114 Other populations at risk are patients with compromised immunity due to chemotherapy and a known history of haematopoietic stem cell transplantation (HSCT).Citation115,116 These patients have suppressed expression of Dectin-1 and TLR-2, due to possible polymorphisms, which may facilitate their susceptibility to acquire this fungal infection. In fact, patients with mucormycosis have shown a deficient population of CD4+ T cells that is responsive to IL-6 stimulation.Citation116 Additionally, the adoptive transfer of NK cells is promising alternative therapy that may restore host immunity after HSCT, decreasing the patients' susceptibility to mucormycosis.Citation115 IL-2 pre-stimulation of NK cells effectively killed a broad range of mucormycetes. Surprisingly, NK cells displayed a reduced production of IFN-γ, which is important augmenting the fungicidal activity of macrophages.Citation117 Furthermore, neutrophils in healthy individuals can be chemotactically recruited by monocytes and induce damage to the pathogen by initiating ROS production. In the ketoacidotic state, this process is impaired, enabling contiguous spread from the sinuses, through the cribriform plate and into the brain. These fungi also cause frontal lobe abscess and cavernous sinus thrombosis.Citation118 Although there is dearth of information in the setting of mucormycosis, the brain parenchyma of a locked in syndrome patient infected by Mucor spp showed early ischemic damage in the presence of complete neuronal loss, microglial activation, edema, and vascular engulfment.Citation119
Dimorphic and rare fungi
Dimorphic fungi cause geographically restricted mycoses, corresponding to the areas in the world where warm temperature and dry conditions exist. These fungal pathogens found in the environment in association with soil and bird or pigeon excreta, primarily infect the skin, bone, or lung parenchyma in immunocompromised hosts or individuals that practice outdoor activities (e.g., farming, hiking, spelunking, etc.) in endemic geographical regions that can progressively disseminate to the CNS. The prevalence of CNS invasion by the majority of these fungi is 5–25% with clinical manifestations that may include subacute and chronic meningitis, focal brain or spinal cord lesions, or encephalitis. Despite the importance in understanding the direct interaction between dimorphic fungi and microglia, there is limited information available in this area. Only data on the H. capsulatum cell wall protein Yps3p has been described to interact with TLR-2 receptors on microglia promoting the release of CCL-2 after activation of the NF-κB pathway.Citation120 Likewise, the β-glucan zymosan appears to be engulfed by both CR-3 and Dectin-1 PRRs on microglia regardless of complement opsonization. This slightly differs from macrophages as Dectin-1 predominately mediates phagocytosis of opsonized zymosan, while CR-3 predominates in non-opsonized carbohydrate.Citation52 Similarly, rodents intracerebrally infected with P. brasiliensis demonstrated progressive neuroinflammation characterized by substantial infiltration of peripheral phagocytic cells and increased chemokine expression that resulted in granulomatous meningoencephalitis.Citation121
Following the inhalation of conidia, the rare, but invasive pigmented or black fungi, including Cladophialophora bantiana, Exophiala dermatitidis, and Ramichloridium mackenziei, have been isolated as the causative agent of primary cerebral phaeohyphomycosis in individuals with both, competent and compromised immunity lacking medical intervention.Citation122 Melanin production may interfere with microglial recognition and eradication of fungi in the brain parenchyma resulting in high mortality due to meningoencephalitis and granulomatous disease, in which the fungus located within the giant cells walled in by fibrosis and reactive gliosis.Citation123 Therapy against black fungi in the setting of CNS involvement using combinations of amphotericin B, 5-flucytosine, and itraconazole has demonstrated improved survival.Citation124
Fusarium verticilliodes is a fungus that produces mycotoxins, commonly contaminating corn and other grains, leading to diseases of both animals and humans.Citation125 Fumonisin B1, the fungus most common mycotoxin, has been associated with cases of cerebral fungal invasion leading to neuronal axon demyelination. Fumonisin B1 is cytotoxic to microglia causing the accumulation of phospholipids in the cell membrane and altering cellular respiration by impairing mitochondrial function.Citation126,127
Cases of CNS infection due to Penicillium marneffei are becoming increasingly a common opportunistic infection in patients with AIDS in Southeast Asia.Citation128 In contrast, recent studies suggest the neuroprotective role of Penicillium spp related components in fermented dairy products. Particularly, dehydroergosterol and oleamide show reduced microglial-induced inflammation, an important manifestation in the pathogenesis of dementia and Alzheimer's disease.Citation129 Furthermore, oleamide reduce Aβ accumulation via enhanced microglial phagocytosis, and suppresses microglial inflammation after Aβ deposition in the hippocampus.Citation130 Although these findings are provocative, further validation is necessary.
Conclusion
We have highlighted the role microglia play in fungal brain infection. Uncovering the innate abilities of microglial mediated phagocytosis, the factors that impair and enhance this process, and the transduction once fungal antigenic detection occurs will provide a greater path toward decreasing the burden of pathologies caused by fungi. It is apparent that to optimize microglial function against fungi, the presence of opsonins and T cells is crucial, yet as we have described fungal virulence and immune deficiencies may hamper the host's ability to properly attenuate these pathogens. Still in the face of fungi, many areas of research must be investigated, such as astrocyte and microglia interactions, improved models of research that duplicate actual disease processes, such as anti-fungal processes in the setting of T cell deficiency as is seen in HIV/AIDS, and solid organ transplant recipients on long-lasting corticosteroid therapy, that does not include systemic fungi that also lead to brain disease. Due to the worldwide prevalence and the challenges encountered once these vulnerable populations acquire invasive fungal diseases, the urge to study the opportunistic neurotropism of fungi is imperative for prophylaxis and patient care, including the development of efficacious anti-fungal therapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
G.W.K. and L.R.M. wrote the manuscript and prepared the diagram presented in . R.L.R. photographed and prepared the images presented in .
Funding
L.R.M. was supported by the NYIT College of Osteopathic Medicine intramural funds and the National Institute of General Medical Sciences of the US NIH under award number R15GM117501.
References
- Rohatgi S, Pirofski LA. Host immunity to Cryptococcus neoformans. Future Microbiol 2015; 10:565-81; PMID:25865194; http://dx.doi.org/10.2217/fmb.14.132
- Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 2010; 36:1-53; PMID:20088682; http://dx.doi.org/10.3109/10408410903241444
- Jacobs CS, Etherton MR, Lyons JL. Fungal infections of the central nervous system. Curr Infect Dis Rep 2014; 16:449; PMID:25348744; http://dx.doi.org/10.1007/s11908-014-0449-2
- Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A 2001; 98:15245-50; PMID:11742090; http://dx.doi.org/10.1073/pnas.261418798
- Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997-2009. PLoS One 2013; 8:e56269; PMID:23457543; http://dx.doi.org/10.1371/journal.pone.0056269
- Shi M, Mody CH. Fungal Infection in the Brain: What We Learned from Intravital Imaging. Front Immunol 2016; 7:292; PMID:27532000
- Casadevall A, Pirofski LA. Exserohilum rostratum fungal meningitis associated with methylprednisolone injections. Future Microbiol 2013; 8:135-7; PMID:23374119; http://dx.doi.org/10.2217/fmb.12.138
- Centers for Disease C, Prevention. Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy–United States, July–November 2002. MMWR Morb Mortal Wkly Rep 2002; 51:1109-12; PMID:12530707
- Fu R, Shen Q, Xu P, Luo JJ, Tang Y. Phagocytosis of microglia in the central nervous system diseases. Mol Neurobiol 2014; 49:1422-34; PMID:24395130; http://dx.doi.org/10.1007/s12035-013-8620-6
- Li L, Eter N, Heiduschka P. The microglia in healthy and diseased retina. Exp Eye Res 2015; 136:116-30; PMID:25952657; http://dx.doi.org/10.1016/j.exer.2015.04.020
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012; 336:86-90; PMID:22442384; http://dx.doi.org/10.1126/science.1219179
- Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci 2010; 17:6-10; PMID:19926287; http://dx.doi.org/10.1016/j.jocn.2009.05.006
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 2009; 27:119-45; PMID:19302036; http://dx.doi.org/10.1146/annurev.immunol.021908.132528
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res 1999; 117:145-52; PMID:10567732; http://dx.doi.org/10.1016/S0165-3806(99)00113-3
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010; 330:841-5; PMID:20966214; http://dx.doi.org/10.1126/science.1194637
- Casano AM, Peri F. Microglia: multitasking specialists of the brain. Dev Cell 2015; 32:469-77; PMID:25710533; http://dx.doi.org/10.1016/j.devcel.2015.01.018
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 2011; 11:775-87; PMID:22025055; http://dx.doi.org/10.1038/nri3086
- Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O'Keeffe S, Phatnani HP, Muratet M, Carroll MC, Levy S, Tavazoie S, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 2013; 4:385-401; PMID:23850290; http://dx.doi.org/10.1016/j.celrep.2013.06.018
- Waisman A, Ginhoux F, Greter M, Bruttger J. Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol 2015; 36:625-36; PMID:26431940; http://dx.doi.org/10.1016/j.it.2015.08.005
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci 2013; 16:1896-905; PMID:24162652; http://dx.doi.org/10.1038/nn.3554
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 2014; 15:300-12; PMID:24713688; http://dx.doi.org/10.1038/nrn3722
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 2012; 37:1050-60; PMID:23177320; http://dx.doi.org/10.1016/j.immuni.2012.11.001
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013; 38:792-804; PMID:23601688; http://dx.doi.org/10.1016/j.immuni.2013.04.004
- Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 2016; 19:987-91; PMID:27459405; http://dx.doi.org/10.1038/nn.4338
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev 2011; 91:461-553; PMID:21527731; http://dx.doi.org/10.1152/physrev.00011.2010
- Verkhratsky A, Butt A. Glial Neurobiology: A Textbook. John Wiley & Sons, 2007.
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005; 308:1314-8; PMID:15831717; http://dx.doi.org/10.1126/science.1110647
- Benarroch EE. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology 2013; 81:1079-88; PMID:23946308; http://dx.doi.org/10.1212/WNL.0b013e3182a4a577
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 2002; 40:133-9; PMID:12379901; http://dx.doi.org/10.1002/glia.10154
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 2004; 173:3916-24; PMID:15356140; http://dx.doi.org/10.4049/jimmunol.173.6.3916
- Drummond RA, Collar AL, Swamydas M, Rodriguez CA, Lim JK, Mendez LM, Fink DL, Hsu AP, Zhai B, Karauzum H, et al. CARD9-dependent neutrophil recruitment protects against fungal invasion of the central nervous system. PLoS Pathog 2015; 11:e1005293; PMID:26679537; http://dx.doi.org/10.1371/journal.ppat.1005293
- Kozel TR, Highison B, Stratton CJ. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun 1984; 43:574-9; PMID:6363293
- Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun 1995; 63:4211-8; PMID:7591049
- Adami C, Sorci G, Blasi E, Agneletti AL, Bistoni F, Donato R. S100B expression in and effects on microglia. Glia 2001; 33:131-42; PMID:11180510; http://dx.doi.org/10.1002/1098-1136(200102)33:2%3c131::AID-GLIA1012%3e3.0.CO;2-D
- Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol 2015; 131:65-86; PMID:26067058; http://dx.doi.org/10.1016/j.pneurobio.2015.05.003
- Barichello T, Generoso JS, Simoes LR, Goularte JA, Petronilho F, Saigal P, Badawy M, Quevedo J. Role of microglial activation in the pathophysiology of bacterial meningitis. Mol Neurobiol 2016; 53:1770-81; PMID:25744564; http://dx.doi.org/10.1007/s12035-015-9107-4
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 2007; 10:1387-94; PMID:17965659; http://dx.doi.org/10.1038/nn1997
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41:14-20; PMID:25035950; http://dx.doi.org/10.1016/j.immuni.2014.06.008
- Cherry JD, Olschowka JA, O'Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 2014; 11:98; PMID:24889886; http://dx.doi.org/10.1186/1742-2094-11-98
- Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 2014; 211:1533-49; PMID:25002752; http://dx.doi.org/10.1084/jem.20132477
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 2011; 14:1142-9; PMID:21804537; http://dx.doi.org/10.1038/nn.2887
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013; 155:1596-609; PMID:24360280; http://dx.doi.org/10.1016/j.cell.2013.11.030
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012; 74:691-705; PMID:22632727; http://dx.doi.org/10.1016/j.neuron.2012.03.026
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007; 131:1164-78; PMID:18083105; http://dx.doi.org/10.1016/j.cell.2007.10.036
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, Garel S. Microglia modulate wiring of the embryonic forebrain. Cell Rep 2014; 8:1271-9; PMID:25159150; http://dx.doi.org/10.1016/j.celrep.2014.07.042
- Pont-Lezica L, Beumer W, Colasse S, Drexhage H, Versnel M, Bessis A. Microglia shape corpus callosum axon tract fasciculation: functional impact of prenatal inflammation. Eur J Neurosci 2014; 39:1551-7; PMID:24593277; http://dx.doi.org/10.1111/ejn.12508
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 2013; 16:543-51; PMID:23525041; http://dx.doi.org/10.1038/nn.3358
- Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell 2012; 23:1189-202; PMID:23201120; http://dx.doi.org/10.1016/j.devcel.2012.10.027
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 2009; 22:240-73, Table of Contents; PMID:19366914; http://dx.doi.org/10.1128/CMR.00046-08
- Gross O, Gewies A, Finger K, Schafer M, Sparwasser T, Peschel C, Forster I, Ruland J. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 2006; 442:651-6; PMID:16862125; http://dx.doi.org/10.1038/nature04926
- van de Veerdonk FL, Kullberg BJ, van der Meer JW, Gow NA, Netea MG. Host-microbe interactions: innate pattern recognition of fungal pathogens. Curr Opin Microbiol 2008; 11:305-12; PMID:18602019; http://dx.doi.org/10.1016/j.mib.2008.06.002
- Hadas S, Reichert F, Rotshenker S. Dissimilar and similar functional properties of complement receptor-3 in microglia and macrophages in combating yeast pathogens by phagocytosis. Glia 2010; 58:823-30; PMID:20091776
- Hill JO, Aguirre KM. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J Immunol 1994; 152:2344-50; PMID:7907637
- Huang SH, Jong AY. Cellular mechanisms of microbial proteins contributing to invasion of the blood-brain barrier. Cell Microbiol 2001; 3:277-87; PMID:11298651; http://dx.doi.org/10.1046/j.1462-5822.2001.00116.x
- Shimoda M, Jones VC, Kobayashi M, Suzuki F. Microglial cells from psychologically stressed mice as an accelerator of cerebral cryptococcosis. Immunol Cell Biol 2006; 84:551-6; PMID:16956390; http://dx.doi.org/10.1111/j.1440-1711.2006.01466.x
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol 2009; 9:429-39; PMID:19461673; http://dx.doi.org/10.1038/nri2565
- Chen Z, Trapp BD. Microglia and neuroprotection. J Neurochem 2016; 136 Suppl 1:10-7; PMID:25693054; http://dx.doi.org/10.1111/jnc.13062
- da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FR. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci 2014; 8:362; PMID:25404894; http://dx.doi.org/10.3389/fncel.2014.00362
- Speed B, Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis 1995; 21:28-34; discussion 5-6; PMID:7578756; http://dx.doi.org/10.1093/clinids/21.1.28
- Correa Mdo P, Severo LC, Oliveira Fde M, Irion K, Londero AT. The spectrum of computerized tomography (CT) findings in central nervous system (CNS) infection due to Cryptococcus neoformans var. gattii in immunocompetent children. Rev Inst Med Trop Sao Paulo 2002; 44:283-7.
- Mitchell TG, Perfect JR. Cryptococcosis in the era of AIDS–100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev 1995; 8:515-48; PMID:8665468
- May RC, Stone NR, Wiesner DL, Bicanic T, Nielsen K. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol 2016; 14:106-17; PMID:26685750; http://dx.doi.org/10.1038/nrmicro.2015.6
- Lee SC, Dickson DW, Casadevall A. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenetic implications. Hum Pathol 1996; 27:839-47; PMID:8760020; http://dx.doi.org/10.1016/S0046-8177(96)90459-1
- Lee SC, Casadevall A, Dickson DW. Immunohistochemical localization of capsular polysaccharide antigen in the central nervous system cells in cryptococcal meningoencephalitis. Am J Pathol 1996; 148:1267-74; PMID:8644867
- Hole C, Wormley FL, Jr. Innate host defenses against Cryptococcus neoformans. J Microbiol 2016; 54:202-11; PMID:26920880; http://dx.doi.org/10.1007/s12275-016-5625-7
- Urai M, Kaneko Y, Ueno K, Okubo Y, Aizawa T, Fukazawa H, Sugita T, Ohno H, Shibuya K, Kinjo Y, et al. Evasion of innate immune responses by the highly virulent cryptococcus gattii by altering capsule glucuronoxylomannan structure. Front Cell Infect Microbiol 2015; 5:101; PMID:26779451
- Almeida F, Wolf JM, Casadevall A. Virulence-associated enzymes of cryptococcus neoformans. Eukaryot Cell 2015; 14:1173-85; PMID:26453651; http://dx.doi.org/10.1128/EC.00103-15
- Barluzzi R, Brozzetti A, Delfino D, Bistoni F, Blasi E. Role of the capsule in microglial cell-Cryptococcus neoformans interaction: impairment of antifungal activity but not of secretory functions. Med Mycol 1998; 36:189-97; PMID:9776834
- Buchanan KL, Doyle HA. Requirement for CD4(+) T lymphocytes in host resistance against Cryptococcus neoformans in the central nervous system of immunized mice. Infect Immun 2000; 68:456-62; PMID:10639404; http://dx.doi.org/10.1128/IAI.68.2.456-462.2000
- Goldman D, Song X, Kitai R, Casadevall A, Zhao ML, Lee SC. Cryptococcus neoformans induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta in human microglia: role of specific antibody and soluble capsular polysaccharide. Infect Immun 2001; 69:1808-15; PMID:11179358; http://dx.doi.org/10.1128/IAI.69.3.1808-1815.2001
- Zhou Q, Gault RA, Kozel TR, Murphy WJ. Protection from direct cerebral cryptococcus infection by interferon-gamma-dependent activation of microglial cells. J Immunol 2007; 178:5753-61; PMID:17442959; http://dx.doi.org/10.4049/jimmunol.178.9.5753
- Aguirre K, Crowe J, Haas A, Smith J. Resistance to Cryptococcus neoformans infection in the absence of CD4+ T cells. Med Mycol 2004; 42:15-25; PMID:14982110
- Kleinschek MA, Muller U, Brodie SJ, Stenzel W, Kohler G, Blumenschein WM, Straubinger RK, McClanahan T, Kastelein RA, Alber G. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. J Immunol 2006; 176:1098-106; PMID:16393998; http://dx.doi.org/10.4049/jimmunol.176.2.1098
- Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol 1992; 149:2736-41; PMID:1383325
- Saleppico S, Boelaert JR, Omodeo Sale F, Mazzolla R, Morucci P, Bistoni F, Blasi E. Differential effects of iron load on basal and interferon-gamma plus lipopolysaccharide enhance anticryptococcal activity by the murine microglial cell line BV-2. J Neuroimmunol 1999; 93:102-7; PMID:10378873; http://dx.doi.org/10.1016/S0165-5728(98)00206-9
- Barluzzi R, Saleppico S, Nocentini A, Boelaert JR, Neglia R, Bistoni F, Blasi E. Iron overload exacerbates experimental meningoencephalitis by Cryptococcus neoformans. J Neuroimmunol 2002; 132:140-6; PMID:12417444; http://dx.doi.org/10.1016/S0165-5728(02)00324-7
- Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol 2011; 48:1592-603; PMID:21546088; http://dx.doi.org/10.1016/j.molimm.2011.04.003
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004; 16:1-13; PMID:15207256; http://dx.doi.org/10.1016/j.nbd.2003.12.016
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol 2006; 16:2161-5; PMID:17084702; http://dx.doi.org/10.1016/j.cub.2006.09.061
- Lipovsky MM, Juliana AE, Gekker G, Hu S, Hoepelman AI, Peterson PK. Effect of cytokines on anticryptococcal activity of human microglial cells. Clin Diagn Lab Immunol 1998; 5:410-1; PMID:9606001
- Blasi E, Barluzzi R, Mazzolla R, Mosci P, Bistoni F. Experimental model of intracerebral infection with Cryptococcus neoformans: roles of phagocytes and opsonization. Infect Immun 1992; 60:3682-8; PMID:1500177
- Lee SC, Kress Y, Dickson DW, Casadevall A. Human microglia mediate anti-Cryptococcus neoformans activity in the presence of specific antibody. J Neuroimmunol 1995; 62:43-52; PMID:7499491; http://dx.doi.org/10.1016/0165-5728(95)00097-L
- Song X, Tanaka S, Cox D, Lee SC. Fcgamma receptor signaling in primary human microglia: differential roles of PI-3K and Ras/ERK MAPK pathways in phagocytosis and chemokine induction. J Leukoc Biol 2004; 75:1147-55; PMID:14982949; http://dx.doi.org/10.1189/jlb.0403128
- Lipovsky MM, Gekker G, Anderson WR, Molitor TW, Peterson PK, Hoepelman AI. Phagocytosis of nonopsonized Cryptococcus neoformans by swine microglia involves CD14 receptors. Clin Immunol Immunopathol 1997; 84:208-11; PMID:9245554; http://dx.doi.org/10.1006/clin.1997.4381
- Preissler J, Grosche A, Lede V, Le Duc D, Krugel K, Matyash V, Szulzewsky F, Kallendrusch S, Immig K, Kettenmann H, et al. Altered microglial phagocytosis in GPR34-deficient mice. Glia 2015; 63:206-15; PMID:25142016; http://dx.doi.org/10.1002/glia.22744
- Rhodes JC. Contribution of complement component C5 to the pathogenesis of experimental murine cryptococcosis. Sabouraudia 1985; 23:225-34; PMID:4023888; http://dx.doi.org/10.1080/00362178585380331
- Macher AM, Bennett JE, Gadek JE, Frank MM. Complement depletion in cryptococcal sepsis. J Immunol 1978; 120:1686-90; PMID:351055
- Kozel TR. Activation of the complement system by the capsule of Cryptococcus neoformans. Curr Top Med Mycol 1993; 5:1-26; PMID:8242797
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016; 352:712-6; PMID:27033548; http://dx.doi.org/10.1126/science.aad8373
- Rambach G, Maier H, Vago G, Mohsenipour I, Lass-Florl C, Defant A, Wurzner R, Dierich MP, Speth C. Complement induction and complement evasion in patients with cerebral aspergillosis. Microbes Infect 2008; 10:1567-76; PMID:18977454; http://dx.doi.org/10.1016/j.micinf.2008.09.011
- Ruhnke M, Kofla G, Otto K, Schwartz S. CNS aspergillosis: recognition, diagnosis and management. CNS Drugs 2007; 21:659-76; PMID:17630818; http://dx.doi.org/10.2165/00023210-200721080-00004
- Saghrouni F, Ben Youssef Y, Gheith S, Bouabid Z, Ben Abdeljelil J, Khammari I, Fathallah A, Khlif A, Ben Said M. Twenty-nine cases of invasive aspergillosis in neutropenic patients. Med Mal Infect 2011; 41:657-62; PMID:22036518; http://dx.doi.org/10.1016/j.medmal.2011.09.011
- Jantunen E, Salonen J, Juvonen E, Koivunen E, Siitonen T, Lehtinen T, Kuittinen O, Leppa S, Anttila VJ, Itala M, et al. Invasive fungal infections in autologous stem cell transplant recipients: a nation-wide study of 1188 transplanted patients. Eur J Haematol 2004; 73:174-8; PMID:15287914; http://dx.doi.org/10.1111/j.1600-0609.2004.00273.x
- Musial CE, Cockerill FR, 3rd, Roberts GD. Fungal infections of the immunocompromised host: clinical and laboratory aspects. Clin Microbiol Rev 1988; 1:349-64; PMID:3069198; http://dx.doi.org/10.1128/CMR.1.4.349
- Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin Microbiol Rev 2009; 22:447-65; PMID:19597008; http://dx.doi.org/10.1128/CMR.00055-08
- Anand R, Shankar J, Tiwary BN, Singh AP. Aspergillus flavus induces granulomatous cerebral aspergillosis in mice with display of distinct cytokine profile. Cytokine 2015; 72:166-72; PMID:25647272; http://dx.doi.org/10.1016/j.cyto.2015.01.006
- Mezger M, Kneitz S, Wozniok I, Kurzai O, Einsele H, Loeffler J. Proinflammatory response of immature human dendritic cells is mediated by dectin-1 after exposure to Aspergillus fumigatus germ tubes. J Infect Dis 2008; 197:924-31; PMID:18279049; http://dx.doi.org/10.1086/528694
- Chai LY, Vonk AG, Kullberg BJ, Verweij PE, Verschueren I, van der Meer JW, Joosten LA, Latge JP, Netea MG. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect 2011; 13:151-9; PMID:20971208; http://dx.doi.org/10.1016/j.micinf.2010.10.005
- Cenci E, Mencacci A, Casagrande A, Mosci P, Bistoni F, Romani L. Impaired antifungal effector activity but not inflammatory cell recruitment in interleukin-6-deficient mice with invasive pulmonary aspergillosis. J Infect Dis 2001; 184:610-7; PMID:11494166; http://dx.doi.org/10.1086/322793
- Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 2012; 8:1254-66; PMID:23136554; http://dx.doi.org/10.7150/ijbs.4679
- Rambach G, Hagleitner M, Mohsenipour I, Lass-Florl C, Maier H, Wurzner R, Dierich MP, Speth C. Antifungal activity of the local complement system in cerebral aspergillosis. Microbes Infect 2005; 7:1285-95; PMID:16027023; http://dx.doi.org/10.1016/j.micinf.2005.04.014
- Tomee JF, Kauffman HF. Putative virulence factors of Aspergillus fumigatus. Clin Exp Allergy 2000; 30:476-84; PMID:10718844; http://dx.doi.org/10.1046/j.1365-2222.2000.00796.x
- Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, Laviolette M, Panettieri RA, Jr., Druey KM. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun 2015; 6:6763; PMID:25865874; http://dx.doi.org/10.1038/ncomms7763
- Rambach G, Dum D, Mohsenipour I, Hagleitner M, Wurzner R, Lass-Florl C, Speth C. Secretion of a fungal protease represents a complement evasion mechanism in cerebral aspergillosis. Mol Immunol 2010; 47:1438-49; PMID:20303595; http://dx.doi.org/10.1016/j.molimm.2010.02.010
- Long B, Koyfman A. Mucormycosis: what emergency physicians need to know? Am J Emerg Med 2015; 33:1823-5; PMID:26452511; http://dx.doi.org/10.1016/j.ajem.2015.08.037
- Lionakis MS, Lim JK, Lee CC, Murphy PM. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun 2011; 3:180-99; PMID:21063074; http://dx.doi.org/10.1159/000321157
- Blasi E, Mazzolla R, Barluzzi R, Mosci P, Bartoli A, Bistoni F. Intracerebral transfer of an in vitro established microglial cell line: local induction of a protective state against lethal challenge with Candida albicans. J Neuroimmunol 1991; 32:249-57; PMID:2033118; http://dx.doi.org/10.1016/0165-5728(91)90195-D
- Maneu V, Noailles A, Megias J, Gomez-Vicente V, Carpena N, Gil ML, Gozalbo D, Cuenca N. Retinal microglia are activated by systemic fungal infection. Invest Ophthalmol Vis Sci 2014; 55:3578-85; PMID:24833742; http://dx.doi.org/10.1167/iovs.14-14051
- Orsi CF, Borghi E, Colombari B, Neglia RG, Quaglino D, Ardizzoni A, Morace G, Blasi E. Impact of Candida albicans hyphal wall protein 1 (HWP1) genotype on biofilm production and fungal susceptibility to microglial cells. Microb Pathog 2014; 69-70:20-7; PMID:24685698; http://dx.doi.org/10.1016/j.micpath.2014.03.003
- Shah VB, Huang Y, Keshwara R, Ozment-Skelton T, Williams DL, Keshvara L. Beta-glucan activates microglia without inducing cytokine production in Dectin-1-dependent manner. J Immunol 2008; 180:2777-85; PMID:18292498; http://dx.doi.org/10.4049/jimmunol.180.5.2777
- Gil ML, Gozalbo D. Role of Toll-like receptors in systemic Candida albicans infections. Front Biosci (Landmark Ed) 2009; 14:570-82; PMID:19273086; http://dx.doi.org/10.2741/3263
- Maneu V, Yanez A, Murciano C, Molina A, Gil ML, Gozalbo D. Dectin-1 mediates in vitro phagocytosis of Candida albicans yeast cells by retinal microglia. FEMS Immunol Med Microbiol 2011; 63:148-50; PMID:21668824; http://dx.doi.org/10.1111/j.1574-695X.2011.00829.x
- Shah VB, Williams DL, Keshvara L. beta-Glucan attenuates TLR2- and TLR4-mediated cytokine production by microglia. Neurosci Lett 2009; 458:111-5; PMID:19393720; http://dx.doi.org/10.1016/j.neulet.2009.04.039
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev 2000; 13:236-301; PMID:10756000; http://dx.doi.org/10.1128/CMR.13.2.236-301.2000
- Schmidt S, Schneider A, Demir A, Lass-Florl C, Lehrnbecher T. Natural killer cell-mediated damage of clinical isolates of mucormycetes. Mycoses 2016; 59:34-8; PMID:26578394; http://dx.doi.org/10.1111/myc.12431
- Camargo JF, Bhimji A, Kumar D, Kaul R, Pavan R, Schuh A, Seftel M, Lipton JH, Gupta V, Humar A, et al. Impaired T cell responsiveness to interleukin-6 in hematological patients with invasive aspergillosis. PLoS One 2015; 10:e0123171; PMID:25835547; http://dx.doi.org/10.1371/journal.pone.0123171
- Kawakami K, Koguchi Y, Qureshi MH, Yara S, Kinjo Y, Uezu K, Saito A. NK cells eliminate Cryptococcus neoformans by potentiating the fungicidal activity of macrophages rather than by directly killing them upon stimulation with IL-12 and IL-18. Microbiol Immunol 2000; 44:1043-50; PMID:11220678; http://dx.doi.org/10.1111/j.1348-0421.2000.tb02601.x
- Roilides E, Kontoyiannis DP, Walsh TJ. Host defenses against zygomycetes. Clin Infect Dis 2012; 54 Suppl 1:S61-6; PMID:22247447; http://dx.doi.org/10.1093/cid/cir869
- Maffini F, Cocorocchio E, Pruneri G, Bonomo G, Peccatori F, Chiapparini L, Vincenzo SD, Martinelli G, Viale G. Locked-in syndrome after basilary artery thrombosis by mucormycosis masquerading as meningoencephalitis in a lymphoma patient. Ecancermedicalscience 2013; 7:382; PMID:24386011
- Aravalli RN, Hu S, Woods JP, Lokensgard JR. Histoplasma capsulatum yeast phase-specific protein Yps3p induces Toll-like receptor 2 signaling. J Neuroinflammation 2008; 5:30; PMID:18606009; http://dx.doi.org/10.1186/1742-2094-5-30
- Pedroso VS, Vilela MC, Santos PC, Cisalpino PS, Rachid MA, Teixeira AL. Traffic of leukocytes and cytokine up-regulation in the central nervous system in a murine model of neuroparacoccidioidomycosis. Mycopathologia 2013; 176:191-9; PMID:23877333; http://dx.doi.org/10.1007/s11046-013-9679-3
- Revankar SG, Sutton DA, Rinaldi MG. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin Infect Dis 2004; 38:206-16; PMID:14699452; http://dx.doi.org/10.1086/380635
- Kantarcioglu AS, de Hoog GS. Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses 2004; 47:4-13; PMID:14998393; http://dx.doi.org/10.1046/j.1439-0507.2003.00956.x
- Deng S, Pan W, Liao W, de Hoog GS, Gerrits van den Ende AH, Vitale RG, Rafati H, Ilkit M, Van der Lee AH, Rijs AJ, et al. Combination of amphotericin B and flucytosine against neurotropic species of melanized fungi causing primary cerebral phaeohyphomycosis. Antimicrob Agents Chemother 2016; 60:2346-51; PMID:26833164; http://dx.doi.org/10.1128/AAC.02526-15
- Osuchowski MF, Sharma RP. Fumonisin B1 induces necrotic cell death in BV-2 cells and murine cultured astrocytes and is antiproliferative in BV-2 cells while N2A cells and primary cortical neurons are resistant. Neurotoxicology 2005; 26:981-92; PMID:16005069; http://dx.doi.org/10.1016/j.neuro.2005.05.001
- Osuchowski MF, Edwards GL, Sharma RP. Fumonisin B1-induced neurodegeneration in mice after intracerebroventricular infusion is concurrent with disruption of sphingolipid metabolism and activation of proinflammatory signaling. Neurotoxicology 2005; 26:211-21; PMID:15713342; http://dx.doi.org/10.1016/j.neuro.2004.10.001
- Osuchowski MF, He Q, Sharma RP. Endotoxin exposure alters brain and liver effects of fumonisin B1 in BALB/c mice: implication of blood brain barrier. Food Chem Toxicol 2005; 43:1389-97; PMID:15913876; http://dx.doi.org/10.1016/j.fct.2005.03.014
- Le T, Huu Chi N, Kim Cuc NT, Manh Sieu TP, Shikuma CM, Farrar J, Day JN. AIDS-associated Penicillium marneffei infection of the central nervous system. Clin Infect Dis 2010; 51:1458-62; PMID:21054180; http://dx.doi.org/10.1086/657400
- Ano Y, Kutsukake T, Hoshi A, Yoshida A, Nakayama H. Identification of a novel dehydroergosterol enhancing microglial anti-inflammatory activity in a dairy product fermented with Penicillium candidum. PLoS One 2015; 10:e0116598; PMID:25760331; http://dx.doi.org/10.1371/journal.pone.0116598
- Ano Y, Ozawa M, Kutsukake T, Sugiyama S, Uchida K, Yoshida A, Nakayama H. Preventive effects of a fermented dairy product against Alzheimer's disease and identification of a novel oleamide with enhanced microglial phagocytosis and anti-inflammatory activity. PLoS One 2015; 10:e0118512; PMID:25760987; http://dx.doi.org/10.1371/journal.pone.0118512
