ABSTRACT
The capsular polysaccharide (CP) produced by Staphylococcus aureus is a virulence factor that allows the organism to evade uptake and killing by host neutrophils. Polyclonal antibodies to the serotype 5 (CP5) and type 8 (CP8) capsular polysaccharides are opsonic and protect mice against experimental bacteremia provoked by encapsulated staphylococci. Thus, passive immunotherapy using CP antibodies has been considered for the prevention or treatment of invasive antibiotic-resistant S. aureus infections. In this report, we generated monoclonal antibodies (mAbs) against S. aureus CP5 or CP8. Backbone specific mAbs reacted with native and O-deacetylated CPs, whereas O-acetyl specific mAbs reacted only with native CPs. Reference strains of S. aureus and a selection of clinical isolates reacted by colony immunoblot with the CP5 and CP8 mAbs in a serotype-specific manner. The mAbs mediated in vitro CP type-specific opsonophagocytic killing of S. aureus strains, and mice passively immunized with CP5 mAbs were protected against S. aureus bacteremia. Neither CP8-specific mAbs or polyclonal antibodies protected mice against bacteremia provoked by serotype 8 S. aureus clinical isolates, although these same antibodies did protect against a serotype 5 S. aureus strain genetically engineered to produce CP8. We detected soluble CP8 in culture supernatants of serotype 8 clinical isolates and in the plasma of infected animals. Serotype 5 S. aureus released significantly less soluble CP5 in vitro and in vivo. The release of soluble CP8 by S. aureus may contribute to the inability of CP8 vaccines or antibodies to protect against serotype 8 staphylococcal infections.
Introduction
Staphylococcus aureus is a Gram-positive bacterial species that causes multiple infections in humans, ranging from relatively mild infections, such as skin and soft tissue infections, to severe life threatening invasive diseases, such as bacteremia, pneumonia, and endocarditis. Antibiotic therapy to control these infections is currently limited by the widespread emergence of antibiotic resistant S. aureus strains. Whereas immunization to prevent staphylococcal infection would be ideal, multiple efforts to produce an effective vaccine have failed to achieve successful endpoints in clinical trials.Citation1 Current efforts in the vaccine field are focused on multicomponent vaccines that include antigens that provoke opsonic antibodies, neutralize staphylococcal toxins, block bacterial adherence, and elicit an IL-17 response in appropriate T cell populations.Citation2-4 Immunotherapy represents another means of addressing the diminishing antibiotic pipeline, and it has the advantage of potential effectiveness in target populations that are incapable of generating a protective immune response due to chronic conditions or various degrees of immune compromise. Monoclonal antibody (mAb) based products have shown efficacy for therapies against cancer, autoimmune and inflammatory disorders, and more recently, viral diseases and bacterial toxins have been successfully targeted.Citation5-8,9
There is a clear need for improved immunotherapies against staphylococcal infections, especially those caused by methicillin-resistant S. aureus (MRSA) strains. The results of early studies have revealed that targeting a single antigen is not likely to be effective.Citation10-12 Two separate phase 3 clinical trials attempted to prevent sepsis in low-birth-weight premature neonates by passive immunotherapy targeting surface antigens. INH-A21 is a pooled human immunoglobulin preparation enriched for antibodies to the S. aureus cell wall anchored clumping factor A protein; Pagibaximab is a humanized mAb that targeted lipoteichoic acid common to several Gram-positive pathogens.Citation11 Neither product significantly reduced the incidence of staphylococcal sepsis in neonates.
A phase 2 study of tefibazumab, a humanized mAb that binds to clumping factor A, enrolled hospitalized patients with documented S. aureus bacteremia.Citation13 Subjects were randomized to receive either a single dose of tefibazumab plus standard therapy or standard therapy alone. At the conclusion of the trial, composite clinical endpoints between the patients in the tefibazumab group and the placebo group were not significantly different.
Serotype 5 (CP5) or serotype 8 (CP8) capsular polysaccharides are produced by 75–80% of S. aureus clinical isolates,Citation14,15 and capsules have served as effective vaccine targets against other encapsulated bacterial pathogens.Citation16 Staphylococcal CPs elicit opsonic antibodies,Citation17 and opsonophagocytic uptake and killing by neutrophils is a key component for host clearance of S. aureus. AltaStaph is a hyperimmune polyclonal antibody preparation with high levels of vaccine-induced antibodies to S. aureus CP5 and CP8. In a phase 2 study, low-birth-weight neonates were given two intravenous (IV) doses of AltaStaph or placebo.Citation18 The rates of adverse events between the two arms of the study were similar, and the rates of S. aureus bacteremia were nearly identical (∼3%) in both groups. Another phase 2 trial enrolled patients with documented S. aureus bacteremia who received standard therapy plus Altastaph or placebo,Citation19 but the vaccine-induced CP antibodies were insufficient to significantly reduce S. aureus bacteremia in this at-risk population.
Human mAbs that neutralize the cytotoxic effects S. aureus α hemolysin (Hla) have entered phase 2 clinical trials for the prevention or treatment of staphylococcal pneumonia. Hla mAbs reduced the severity and tissue damage associated with staphylococcal skin infections and necrotizing pneumonia in preclinical studies.Citation20-22 A human mAb that neutralizes both Hla and four staphylococcal leukocidinsCitation23 is beginning a phase 2 clinical trial for the prevention of S. aureus pneumonia in mechanically ventilated subjects. Because of the complexity of S. aureus pathogenesis and the diversity of staphylococcal infections, it is likely that immunoprophylaxis with mAbs will need to target multiple staphylococcal antigens.
S. aureus clearance by the host is dependent upon opsonic antibodies that promote uptake and killing of this microbe by host neutrophils. Antibodies to S. aureus CP5 and CP8 are particularly effective in promoting opsonophagocytic killing of S. aureusCitation24-26 and reducing bacteremia in a murine infection model.,Citation25,26 In this report we evaluate mAbs against S. aureus CP5 and CP8 for their specificity in binding to a wide variety of encapsulated clinical isolates and their ability to mediate in vitro opsonophagocytic bacterial killing. We assessed protection against bacteremia provoked by both serotype 5 and 8 S. aureus, and we discovered that serotype 8 S. aureus clinical isolates release soluble CP8 during culture and infection. Soluble CP8 released by S. aureus in vivo may interfere with protection by CP8 antibodies.
Results
Immunization of Balb/c mice with CP5 or CP8 conjugates
Balb/c mice immunized with CP5 or CP8 conjugate vaccines developed high levels of CP5 or CP8 serum antibodies, respectively (Fig. S1A and S1B). Mice given CP5 conjugated to cross-reactive material 197 (CRM197), a genetically detoxified form of diphtheria toxin, showed a serotype-specific antibody response to CP5. As we reported previously,Citation26 serum antibodies from mice immunized with CP8-CRM197 showed not only good binding to purified CP8, but also showed some cross-reactive binding to microtiter plates coated with CP5 (Fig. S1A). After hybridoma fusions were performed, two positive clones were selected for their reactivity with CP5, and three were selected for reactivity with CP8. Each of the mAbs was isotype IgG1κ ().
Table 1. Monoclonal antibodies isolated from mice immunized with CP5 or CP8 conjugate vaccines.
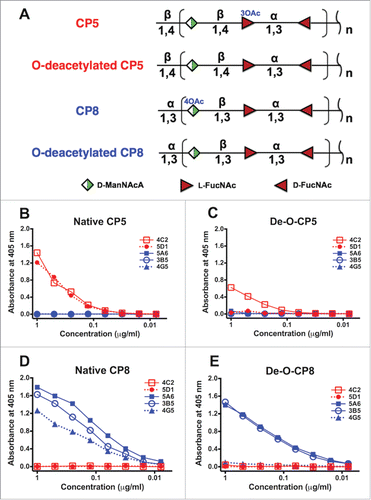
Bacterial polysaccharides are commonly O-acetylated, and the O-acetyl motif is frequently an immunodominant epitope.Citation27-30 To determine the specificity of the mAbs, we tested their binding by ELISA to purified native or chemically O-deacetylated CP5 or CP8. Unlike polyclonal sera,Citation26 none of the mAbs tested showed cross-reactivity between CP5 and CP8 (). mAb CP5–4C2 bound to both native CP5 and O-deacetylated CP5 (). However, the binding to the O-deacetylated CP5 was only about half that of the native antigen, suggesting that the epitope recognized by CP5–4C2 may be influenced by the presence of the O-acetyl group on the N-acetyl L-fucosamine residue. In contrast, mAb CP5–5D1 bound to native CP5 but not O-deacetylated CP5. These results indicate that mAb CP5–4C2 was “backbone specific,” whereas CP5–5D1 was CP5 O-acetyl specific. Likewise, CP8 mAbs 5A6 and 3B5 reacted with both native and O-deacetylated CP8, and as such were backbone specific (). mAb CP8–4G5 showed binding specificity for native CP8, and it did not react with O-deacetylated CP8. Characteristics of the five mAbs are summarized in .
Figure 1. Reactivity of five mAbs with native and O-deacetylated CP5 and CP8. (A) Structural similarities between CP5 and CP8 and their sites of O-acetylation (O-Ac). Both CP5-specific mAbs (4C2 and 5D1) reacted with (B) native CP5, whereas only mAb 4C2 reacted with (C) O-deacetylated CP5 (De-O-CP5). Three CP8-specific mAbs (5A6, 3B5, and 4G5) reacted with (D) native CP8, whereas only 5A6 and 3B5 reacted with (E) O-deacetylated (De-O-CP8) CP8. D-ManNAcA, N-acetyl-D-mannosaminuronic acid; L-FucNAc, N-acetyl-L-fucosamine; D-FucNAc, N-acetyl-D-fucosamine. Each sample was tested in duplicate, and the ELISAs were performed at least twice. The mean values of a representative experiment are shown.
Reactivity of mAbs with serotype 5 and 8 S. aureus isolates
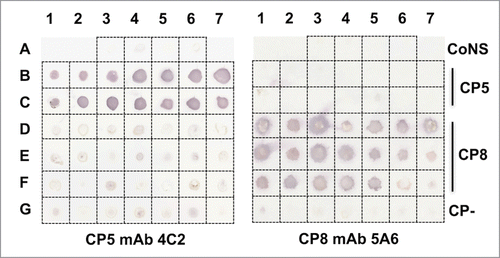
Because S. aureus isolates have been reported to vary in their degree of CP O-acetylation,Citation30 we tested the reactivity of the backbone specific mAbs CP5–4C2 and CP8–5A6 against a range of S. aureus isolates () by a colony immunoblot method. As shown in , neither mAb reacted with representative strains of coagulase-negative staphylococci, including Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus saprophyticus, or Staphylococcus lugdunensis. mAb CP5–4C2 reacted with all 14 serotype 5 S. aureus strains tested, including reference strains (such as Newman and Lowenstein), clinical isolates, and MRSA. Likewise, mAb CP8–5A6 reacted with 19 of 21 serotype 8 strains, including MRSA strains. Serotype 8 Sanger 252 (position F6 on the immunoblot), among the most genetically diverse sequenced S. aureus strains,Citation31 reacted weakly with mAb CP8–5A6. Sanger 252 produces scant CP8, as discussed below and depicted in . S. aureus strain MW2 (position F7 on the immunoblot) also reacted poorly with mAb CP8–5A6, consistent with its minimal reactivity with CP8 polyclonal antibodies.Citation32 MW2 carries a frame-shift mutation in cap8A, resulting in a truncated version of the cap8A gene product. Strain MW2 produces scant CP8 that is reduced in molecular size compared with that of other serotype 8 isolates (J. Lee, unpublished observations).
Table 2. Strains used in this study or tested in the colony immunoblot.
Figure 2. mAbs CP5–4C2 and CP8–5A6 were tested by colony immunoblot for reactivity with S. aureus type 5 and 8 reference strains or clinical isolates. The staphylococcal isolates are listed in . mAb 4C2 reacted with all 14 serotype 5 isolates, whereas mAb 5A6 reacted with 19 of 21 serotype 8 isolates. CoNS, coagulase-negative staphylococci; CP-, capsule negative. The immunoblot was performed three times, and a representative blot is shown.
Opsonophagocytic killing (OPK) of S. aureus mediated by mAbs
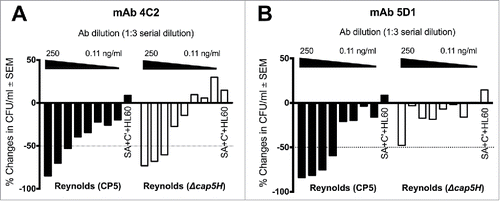
Previous studies have demonstrated that polyclonal antibodies to staphylococcal CPs are opsonic for encapsulated S. aureus.Citation24-26 To determine whether the CP5- and CP8-specific mAbs were functionally opsonic, we performed in vitro OPK assays with agar-grown S. aureus and serial dilutions of either a purified backbone-specific or O-acetyl-specific mAb. Opsonic activity was compared by determining the mAb concentration that yielded 50% killing of the bacterial inoculum. In the presence of HL60 cells and a complement source (guinea pig serum), backbone-specific mAb CP5–4C2 showed equivalent opsonic killing activity against strains S. aureus Reynolds (CP5) and the O-deacetylated Δcap5H mutant (). O-acetyl-specific mAb CP5–5D1 showed good opsonic activity against Reynolds (CP5), but it mediated little opsonic killing against the Δcap5H mutant, with loss of activity at concentrations <250 ng/ml (). These data are consistent with our ELISA results that mAb CP5–5D1 bound only to native O-acetylated CP5.
Figure 3. Opsonic activity of CP5-specific mAbs (A) 4C2 and (B) 5D1 against wild type strain Reynolds (CP5) and its isogenic cap5H deletion mutant that produces wild-type levels of O-deacetylated CP5. The mAbs were serially diluted 3-fold and tested in the OPK assay. The results are expressed as percent changes in the number of CFU/ml after a 2-h incubation with mAb, HL60 cells, and a complement (C’) source. The samples labeled SA+ C’+HL60 contain no mAb. The titer is expressed as the lowest mAb dilution that killed 50% of the inoculum (dotted line). The experiment was performed twice, and a representative experiment is shown.
Because the backbone-specific mAb CP5–4C2 has the advantage of opsonic activity against serotype 5 S. aureus strains independent of CP5 O-acetylation, mAb 4C2 was tested further in OPK assays comparing the heavily encapsulated strain Reynolds (CP5) with strain Newman, which produces little CP5 (see ). Whereas strain Reynolds (CP5) was opsonized for OPK by CP5–4C2 concentrations as low as 40 ng/ml, strain Newman was killed in the assay by mAb CP5–4C2 at concentrations ≥ 333 ng/ml (). Because the target antigen is not expressed abundantly in strain Newman, this may explain its requirement for a higher CP5 mAb concentration to achieve OPK activity. mAb CP5–4C2 (1 µg/ml) was not opsonic for Reynolds (CP-) or USA300 strain LAC (data not shown), since both these strains lack a capsule. There was little to no bacterial killing of the encapsulated S. aureus strains in the presence of complement (C’), bacteria, and phagocytes, but no antibody () or in the presence of antibody alone. Thus the bacterial killing measured in the OPK was not the result of antibody-mediated bacterial agglutination, complement mediated opsonic activity, or mAb bactericidal activity.
Figure 4. Comparative opsonic activity of mAbs against serotype 5 and 8 S. aureus strains. (A) Backbone-specific CP5 mAb 4C2 showed somewhat better opsonic activity against Reynolds (CP5) than strain Newman. (B) Backbone-specific CP8 mAbs 5A6 showed comparable opsonic activity against Reynolds (CP8) and MRSA strain ST80–16. (C) O-acetyl-specific CP8 mAb 4G5 showed greater OPK activity against Reynolds (CP8) than strain ST80–16. The sample labeled 0 antibody contained HL60s, S. aureus, and the complement source. The data shown are means ( ± standard errors) of three to four experiments.
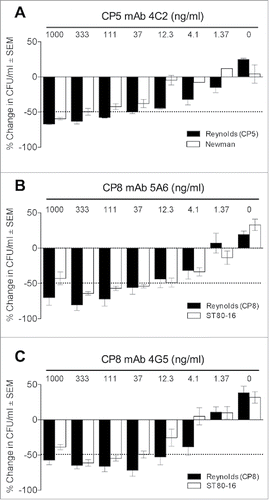
CP8-specific mAb CP8–5A6 is backbone specific ( and ); in OPK assays it was opsonic for both Reynolds (CP8) and MRSA strain ST80–16 at mAb concentrations as low as 12 ng/ml (). Similarly, the O-acetyl specific mAb CP8–4G5 was also opsonic for Reynolds (CP8) at concentrations as low as 12 ng/ml (). However, the opsonic activity of mAb CP8–4G5 against S. aureus ST80–16 was somewhat less, showing 50% killing at a concentration of 37 ng/ml, possibly reflecting a lower level of CP8 O-acetylation than Reynolds (CP8). Moreover, both CP8 mAbs 5A6 and 4G5 showed diminished opsonic activity at the highest concentration tested (1 µg/ml), although the reduction was only significant with strain ST80–16 and CP8 mAb 4G5 (P < 0.05 by one-way ANOVA). The CP- strain LAC was not opsonized for killing by CP8-specific mAbs 5A6 or 4G5 (data not shown).
To determine whether the mAbs would opsonize S. aureus that were not cultivated under conditions that maximize CP production, we performed additional OPK assays with staphylococcal strains cultivated overnight in tryptic soy broth (TSB). As shown in Fig. S2A, broth-grown S. aureus Reynolds (CP5) and Newman were still opsonized for phagocytic killing by mAbs CP5–4C2, although bacterial killing was reduced when compared with bacteria grown on CSA plates (), a growth condition optimal for CP production. Similarly, CP8 mAb 5A6 (Fig. S2B) showed somewhat reduced opsonic activity against broth-grown S. aureus Reynolds (CP8) compared with agar-grown bacteria. Of note, when MRSA strain ST80–16 was grown in TSB for the OPK, the addition of CP8-specific mAb 5A6 to the sample did not enhance the killing obtained in the absence of antibody (0 mAb), suggesting that little or no surface associated CP8 is present on the bacterium under these culture conditions. S. aureus ST80–16 and other isolates were further characterized for CP production in broth culture in experiments detailed below.
The effect of CP-specific antibodies in a mouse model of S. aureus bacteremia
We reported previously that polyclonal antibodies to CP5 significantly reduced bacteremia in mice challenged with Reynolds (CP5), Newman, USA100, or USA200.Citation25,26 Therefore, the CP5 backbone specific mAb 4C2 was evaluated for its ability to protect mice against bacteremia in passive immunization experiments. As shown in , mice injected IV with 100 µg of irrelevant mAb 6C5 developed bacteremia that was measured 2 h after intraperitoneal challenge with Reynolds (CP5). In contrast, mice that received 100 µg CP5 mAb 4C2 or 300 µg of rabbit IgG to CP5 conjugated to Pseudomonas aeruginosa exoprotein A (CP5-Epa) showed significant protection against bacteremia. Likewise, mice given 100 µg of the CP8 backbone-specific mAb 5A6 showed protected against Reynolds (CP8) bacteremia compared with mice given irrelevant mAb 6C5 ().
Figure 5. Passive immunization with CP5- or CP8-specific mAbs in the mouse bacteremia model. Mice were immunized IV with 100 µg of (A) CP5 mAb 4C2 or (B) CP8 mAb 5A6 24 h before challenge by the IP route with 107 CFU (A) S. aureus Reynolds (CP5) or (B) Reynolds (CP8). Control mice were immunized with irrelevant mAb 6C5 or polyclonal CP5 specific rabbit IgG (300 µg), and all mice were bled 2 h after bacterial challenge. The horizontal lines represent median CFU/ml blood for each group of mice. Bacteremia levels in mice administered mAbs 4C2 or 5A6 were compared by the Mann-Whitney U test to levels in control mice given mAb 6C5. ****, P < 0.0001; ***, P < 0.001; **, P < 0.01.
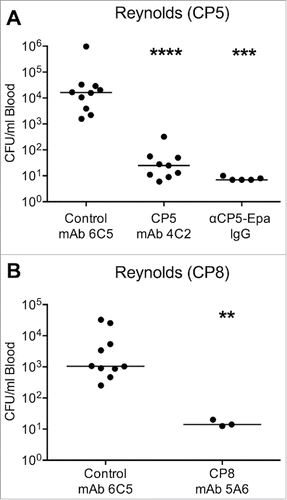
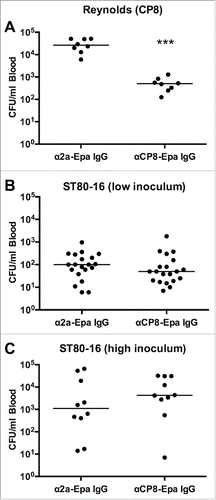
Because there is a paucity of evidence supporting the protective efficacy of CP8 antibodies,Citation33 we examined whether CP8 mAb 5A6 would reduce bacteremia provoked by additional serotype 8 isolates. Compared to mice given isotype control mAbs, mice passively immunized with 100 µg CP8 mAb 5A6 and challenged 24 h later with serotype 8 S. aureus strains ST80–16, MN8, or Wright showed no significant differences in bacteremia levels (Fig. S3). To address whether the CP8 mAbs might be less effective in vivo than CP8-specific polyclonal antibodies, additional mice were passively immunized IV with 1 mg of either polyclonal rabbit IgG to CP8-Epa or polyclonal IgG raised to an irrelevant antigen (Shigella 2a-Epa). Mice given CP8-Epa antibodies and challenged with Reynolds (CP8) showed a median reduction of 98% (1.72 log10) in CFU/ml blood after 2 h compared with mice given the irrelevant IgG (). Consistent with our mAb 5A6 results, however, none of the mice challenged with ST80–16 (low inoculum of 7 × 107 CFU/mouse or high inoculum of 1 × 108 CFU/mouse) were protected against bacteremia by 1 mg polyclonal CP8 antibodies (). These results indicate that the serotype 8 clinical S. aureus isolates are distinct from the genetically engineered Reynolds (CP8) strain as a target for CP8 antibodies.
Figure 6. Passive immunization with polyclonal anti-CP8 antibodies in the mouse bacteremia model. Mice were immunized with 1 mg anti-CP8-Epa or anti-Shigella 2a-Epa IgG 24 h before challenge with 107 CFU (A) S. aureus Reynolds (CP8) or (B, C) two different inocula (low inoculum 7 × 107 CFU/mouse or high inoculum 1 × 108 CFU/mouse) of strain ST80–16. Mice challenged with Reynolds (CP8) were bled 2 h after bacterial challenge, and mice challenged with ST80–16 were bled 1.5 h after bacterial challenge. The horizontal lines represent median CFU/ml blood for each group of mice. Bacteremia levels in mice given CP8-Epa IgG were compared by the Mann-Whitney U test to levels in control mice given 2a-Epa IgG. ***, P < 0.001.
Reynolds (CP8) is not typical of clinical CP8-producing S. aureus isolates
We constructed Reynolds (CP8) from the parental serotype 5 strain Reynolds by genetically swapping the serotype 5-specific cap5HIJK genes from Reynolds with the serotype 8-specific cap8HIJK genes from strain Becker.Citation17 We measured cell-associated CP and soluble CP released into supernatants of overnight cultures of Reynolds (CP5), Reynolds (CP8), as well as 14 additional S. aureus strains. Whereas our ability to reliably measure soluble CP in complex media like TSB was poor, we could readily measure CP5 or CP8 in the supernatants of S. aureus cultivated overnight in RPMI + 1% casamino acids. We report CP levels relative to the isogenic Reynolds (CP5) and Reynolds (CP8) strains (included in every assay) to normalize the results from multiple experiments performed on each strain. As shown in , clinical S. aureus isolates vary markedly in bacterial cell-associated CP levels, and all of the strains produced less CP than Reynolds (CP5) or Reynolds (CP8). Cell-associated CP5 was ∼5-fold higher for the hospital-associated MRSA strains USA100 (NRS 382) and USA200 (NRS 383) strains compared with strains Newman or the MRSA strain ST22. No detectable CP5 was produced by USA300 strain LAC, consistent with previous reports.Citation32,34 Similar variability was seen among the nine serotype 8 strains tested. All produced less cell-associated CP8 than did Reynolds (CP8); Sanger 252 and two of the three ST80 clinical isolates tested produced only trace amounts of cell-associated CP8.
Figure 7. Quantitation of cell-associated and soluble CP5 or CP8 produced by clinical isolates of S. aureus cultivated in RMPI + 1% casamino acids. CP levels were measured by an ELISA inhibition method, and CP5 and CP8 concentrations are expressed relative to those of Reynolds (CP5) and Reynolds (CP8), respectively, which were included as reference strains on every assay. CP levels were compared by one-way ANOVA, and multiple comparisons were made to the reference strains Reynolds (CP5) and Reynolds (CP8). ***, P < 0.001; **, P < 0.01; *, P < 0.05.
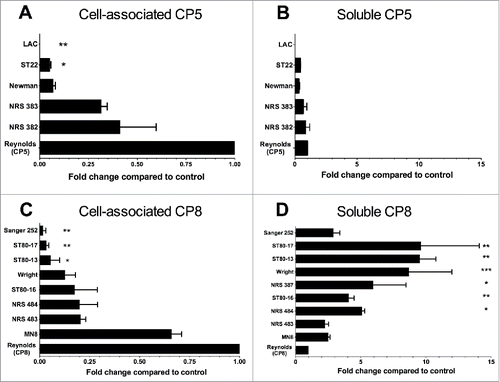
When we measured soluble CP5 in overnight culture supernatants of serotype 5 S. aureus strains (), CP5 levels in the supernatant of most of the isolates mirrored levels produced by Reynolds (CP5). Strain Newman released only 31% of the CP5 levels released by Reynolds (CP5), but Newman also made little cell-associated CP5 ().
Reynolds (CP8) released soluble CP in quantities similar to those produced by the parental strain Reynolds (CP5). In contrast, there was a broad range of soluble CP8 levels detected in the culture supernatants of the serotype 8 clinical isolates (), and all of the isolates released more CP8 than strain Reynolds (CP8). Three of the strains (Sanger 252, NRS 483, and MN8) released >2-fold more CP8 than Reynolds (CP8), but these differences did not reach statistical significance. The remaining isolates released 4- to 10-fold more CP8 than Reynolds (CP8) under these in vitro conditions. We observed no consistent correlations between cell associated and soluble CP produced by the S. aureus isolates examined. Nonetheless, for the CP8+ strains (with the exception of Sanger 252), strains with little cell-associated CP8 (Wright, ST80–13, and ST8–17) showed the highest levels of soluble CP8.
CP antigenemia
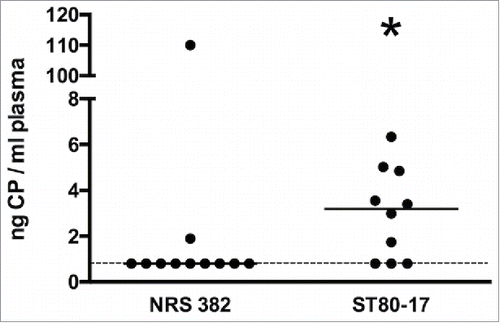
Arbeit and coworkers reported that CP8 was present in the serum of experimental animals infected with serotype 8 S. aureus strains.Citation35,36 To determine whether CP8 was present in higher concentrations than CP5 in the blood of bacteremic animals, we infected groups of 10–11 mice with NRS 382 (CP5+) or ST80–17 (CP8+). When the mice became moribund (1 to 5 d post-challenge), the animals were killed. Quantitative cultures of the infected mouse blood showed that mice infected with the NRS 382 strain had a mean log CFU/ml ( ± SEM) of 5.20 ± 0.44 in the blood, whereas mice infected with ST80–17 had a mean log CFU/ml of 5.21 ± 0.32 S. aureus. Plasma CP levels in both groups were quantified by ELISA. As shown in , CP8 levels in the plasma of ST80–17 infected animals were significantly (P = 0.0309) higher than CP5 levels in the blood of mice infected with NRS 382. Two of 11 animals had detectable CP5 in the mouse plasma (one mouse had 110 ng CP5 per ml blood), whereas 7 of 10 mice infected with ST80–17 had detectable CP8 levels (P = 0.03; Fisher exact test).
Figure 8. Plasma levels of CP5 or CP8 in mice infected IV with ∼2 × 108 CFU of S. aureus NRS382 (CP5+) or ST80–17 (CP8+), respectively. After 1 to 5 days, the animals were bled by cardiac stick, and the plasma extracts were prepared. CP levels were determined by an ELISA inhibition assay, and CP concentrations were compared by the Mann-Whitney U test. *, P < 0.05. The lower limit of CP detection is indicated by a dashed line.
Discussion
CP5 and CP8 are expressed by many S. aureus clinical isolates,Citation14,15,37 and CPs promote bacterial survival in the blood.Citation17,24 CPs are important in immune evasion, allowing the bacterium to evade uptake by professional phagocytes.Citation17,24,25,38 Antibodies to CPs neutralize the antiphagocytic nature of the capsule and effectively mediate uptake and killing by professional phagocytes. Passive immunization with CP5 or CP8 antibodies has shown protection in rodent models of mastitis, bacteremia, endocarditis, and skin abscesses.Citation25,39-41
Despite their failure in clinical trials when used alone in hemodialysis patients,Citation42,43 CP5 and CP8 conjugate vaccines are thought to be important components in a multivalent staphylococcal vaccine.Citation1,25,38,44 Because diverse S. aureus clinical isolates (both methicillin-sensitive and –resistant) produce surface-associated CP5 or CP8,Citation14,15,37 we considered that mAbs to CP5 or CP8 with opsonic activity might be included in a mAb cocktail to prevent or reduce staphylococcal bacteremia. S. aureus USA300, which is highly prevalent in the United States, does not produce a capsule and thus would not be a target for mAbs against CP5 or CP8.Citation34 However, the majority of USA300-associated infections involve superficial wounds or abscesses,Citation45 and USA300 isolates are not common outside of North America.Citation46-48 Among predominant MRSA clones worldwide are the CP8+ lineages ST1, ST30, ST59, ST80, and ST239 and the CP5+ lineages ST5 and ST22.
mAbs against other staphylococcal virulence factors, such as protein A, clumping factor A, and leukocidins have been evaluated in preclinical studies for passive immunotherapeutic purposes.Citation23,49,50 Considering the complexity of the S. aureus disease spectrum, it is likely that a cocktail of mAbs targeting distinct staphylococcal virulence factors may serve to protect against diverse infections, and this cocktail might be delivered therapeutically along with conventional antimicrobial agents.
In the generation of murine mAbs specific for S. aureus CP5 or CP8, we noted that some of the mAbs recognized the immunodominant O-acetyl epitope that decorates the backbone structure of CP5 and CP8. O-acetylation of bacterial polysaccharides is common, and the O-acetyl motif is known to be highly immunogenic in encapsulated bacteria.Citation27-30 Bhasin et al. reported that CP5 O-acetylation rendered S. aureus more resistant to opsonophagocytic killing (OPK) in vitro and enhanced bacterial virulence in a murine infection model.Citation51 S. aureus CP5 and CP8 have similar trisaccharide repeating units.Citation52 However, CP5 is O-acetylated on the N-acetyl fucosamine residue, whereas CP8 is O-acetylated on the N-acetyl mannosaminuronic acid (). Consequently, the O-acetyl-specific CP5 mAb 5D1 and CP8 mAb 4G5 did not cross react between CP5 and CP8. Both O-acetyl and backbone-specific mAbs demonstrated binding and opsonic activity as measured by in vitro assays. However, both CP8 mAbs 5A6 and 4G5 showed diminished in vitro opsonic activity at the highest concentration tested (1 µg/ml), although the reduction was only significant with strain ST80–16 and O-acetyl specific CP8 mAb 4G5. Although this prozone phenomenon has been reported previously,Citation53,54 its mechanism is poorly understood. Some reports have indicated that excess antibody interferes with C3b deposition.Citation55,56 We chose to focus on the backbone-specific CP5 and CP8 mAbs for in vivo protection assays, since S. aureus isolates have been reported to vary in their degree of CP O-acetylation.Citation30
We chose the bacteremia model for our in vivo studies because S. aureus CPs have been shown to markedly enhance bacterial virulence in this model.Citation17,24 Polyclonal antibodies to the staphylococcal CPs protect mice against bacteremia but not lethal pneumonia,Citation25 whereas antibodies to S. aureus Hla protect mice against lethal pneumonia but not bacteremia.Citation21,22,25 We showed previously that polyclonal CP5-specific antibodies protected against experimental bacteremia induced by four different serotype 5 isolates.Citation25 In this study, the CP5 backbone-specific 4C2 mAb protected mice against experimental Reynolds (CP5) bacteremia. Although CP5 mAb 4C2 reacted consistently with 14 serotype 5 S. aureus isolates by colony immunoblot, the protective efficacy of the CP5 mAb 4C2 against bacteremia may be impacted by the fact that its binding to the O-deacetylated CP5 was only ∼50% of its binding to native CP5 by ELISA.
The CP8-specific mAb 5A6 protected against bacteremia when mice were challenged with Reynolds (CP8), a strain constructed from the parental Reynolds (CP5) strain by genetically swapping the serotype 5-specific cap5HIJK genes from Reynolds with the serotype 8-specific cap8HIJK genes from strain Becker.Citation17 The genetic backgrounds of these two strains are identical, and so Reynolds (CP5) and Reynolds (CP8) produce similar levels of cell-associated CP,Citation17 allowing us to evaluate the virulence and biologic activities associated with capsule production in these isogenic strains.Citation17 However, when mice passively immunized with CP8-specific mAb 5A6 were challenged with three other S. aureus isolates (MN8, ST80–16, or Wright), no protection against bacteremia was observed. This finding is consistent with a report by Cook et al.Citation33 in which 800 µg of a CP8 mAb or goat anti-CP8 IgG delivered by the intraperitoneal (IP) route did not protect mice against a lethal intravenous dose of S. aureus MCL8538 (a CP8+ MLST15 skin isolate). Similarly, our data showing that polyclonal CP8 antiserum significantly reduced Reynolds (CP8) bacteremia but not bacteremia provoked by strain ST80–16 suggest that the lack of protective efficacy against clinical S. aureus isolates is not limited to CP8 mAbs.
To address the inconsistencies in the protective efficacy of antibodies to CP5 and CP8, we measured both cell-associated and cell-free (soluble) CP produced by a subset of serotype 5 and 8 S. aureus strains cultivated in broth culture. Predictably, cell-associated CP levels varied markedly among the different staphylococcal strains. Little soluble CP5 was released by the serotype 5 isolates, and there was little variation among the different strains. In contrast, the serotype 8 strains were characterized by the release of 2- to 10-fold more soluble CP than the control strain Reynolds (CP8). To determine whether this finding was relevant to in vivo conditions, we measured CP levels in the plasma of infected mice. Consistent with our in vitro findings, CP8 was detected in the plasma of bacteremic mice at higher levels and more consistently than CP5.
We hypothesize that soluble CP8 could bind opsonic CP8-specific antibodies in vivo and thus compete and interfere with antibody binding to the encapsulated bacterial surface. Only antibodies bound to the bacterial surface are opsonic, mediating uptake and killing by professional phagocytes. As such, released CP8 would compete and interfere with the opsonic function of capsular antibodies. This scenario would reduce the in vivo efficacy of passively administered capsular antibodies, and this premise is consistent with our data demonstrating the inability of CP8 antibodies (polyclonal or mAbs) to protect against experimental bacteremia provoked by clinical CP8+ S. aureus strains. The release of CP by a serotype (ST) 3 strain of Streptococcus pneumoniae, but not by ST1, ST4, ST6B, or ST14 strains, was recently reported by Choi et al.Citation57 Clearly, further work is needed to attain a better understanding of the protective efficacy of CP-specific antibodies, as well as the biosynthetic steps by which CP polymers are anchored (or not) to the cell wall.
Materials and methods
Bacterial strains and growth conditions
The staphylococcal strains used in this study are listed in . The isolates were grown at 37°C on tryptic soy agar for colony immunoblot testing or on Columbia agar + 2% NaCl plates for OPK assays and for challenge in the mouse bacteremia model. To evaluate the influence of culture conditions on OPK results, some of the assays were repeated with S. aureus cultivated overnight with aeration in TSB. For the measurement of S. aureus cell-associated vs. soluble CP, the strains were cultivated for 24 h with aeration in RPMI1640 medium (Mediatech) supplemented with 1% casamino acids (Becton, Dickinson and Co.).
mAbs
Female Balb/c mice (6 to 8 weeks old; Taconic Farms) were immunized every two weeks by the IP route with 2 µg CP5 conjugated to cross-reactive material 197 (CRM197) or CP8-CRM197 (Wyeth-Pfizer; Collegeville, PA) adsorbed to Alhydrogel, as described.Citation26 After the fourth immunization, mouse sera were collected, serially diluted, and tested for binding to CP5 or CP8 by ELISA. Three weeks after the fourth immunization, the mice were administered a final booster dose without adjuvant.
Four days after the final immunization, the splenocytes were fused with a myeloma cell line and then subcloned by standard methods. Hybridoma cells were cultured at 37°C in 5% CO2 in RPMI 1640 and supplemented with 10% heat inactivated fetal bovine serum (Hyclone), L-glutamine, 100 units/ml penicillin, and 100 µg/ml streptomycin (Mediatech). Hybridoma culture supernatants were screened by ELISA for reactivity with CP5 or CP8 that were purified and chemically O-deacetylated as described previously.Citation58 ELISAs were performed on 96-well plates coated with purified CPs (4 µg/ml) coupled to poly-L-lysine as described.Citation59 Antigen-specific clones were subcloned by limiting dilution to yield mAb-secreting hybridomas from single cells. Antibodies from the culture supernatants were purified over protein G columns (GE Healthcare). Protein concentrations were assessed by nanodrop (Thermo Scientific), and mAb purity was confirmed by SDS-PAGE (Fig. S4). CP5 and CP8 ELISAs were used to determine mAb concentrations based on an IgG1 standard curve.Citation60 The colony immunoblot assay Citation61 and the HL60-based OPK assay Citation25 were performed as we described previously.
Quantification of S. aureus CP5 or CP8
To quantify cell-associated CP5 or CP8, ELISA inhibition assays were performed on bacterial cells harvested from 24-h cultures, as described.Citation62,63 To quantify soluble CP, S. aureus culture supernatants were filter-sterilized and then boiled for 10 min to denature proteins and inactivate proteases. Sterile culture medium was used as a negative control. Three-fold serial dilutions of the supernatants or purified CP were then incubated with polyclonal rabbit antibodies specific for CP5 or CP8. ELISA inhibition assays that included a purified CP standard curve were performed as described.Citation63 CP concentrations were calculated from the linear range of the CP standard curve, and cell-associated and soluble CP concentrations were expressed relative to the control strains Reynolds (CP5) and Reynolds (CP8), included in each assay.
Murine bacteremia model
Animal experiments were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the protocols were approved by the Harvard Medical School Standing Committee on Animals. Female Swiss Webster mice (7 to 8 week old; Charles River Laboratories) were injected intravenously with 100 µg mAbs 4C2 (CP5-specific) or 5A6 (CP8-specific) or an irrelevant IgG1 mAb control.Citation64 For certain experiments, mice were injected similarly with 0.3 to 1 mg polyclonal CP5-Epa, CP8-Epa, or Shigella 2a-Epa rabbit IgG, purified by a Protein A affinity chromatography, as described.Citation25 After 24 h the mice were challenged IP with a 500 µl S. aureus inoculum. Heparinized mouse blood was obtained by tail vein puncture at either 1, 1.5. or 2 h (depending on the S. aureus challenge strain) after bacterial inoculation and cultured quantitatively.
For the detection of CP antigenemia, mice were challenged intravenously (IV) with ∼1.6 × 108 CFU of S. aureus NRS 382 (CP5+) or ST80–17 (CP8+). Between 1 and 5 d post-inoculation, the mice were killed by CO2 asphyxiation, and heparinized blood was collected from individual mice by cardiac puncture. After quantitative cultures of the blood were performed, the plasma was separated by centrifugation and stored at −20°C. CP antigenemia was detected by the method described by Arbeit and Nelles.Citation36 Briefly, the plasma was diluted in buffer and then treated with Pronase (Sigma) before autoclaving for 3 min. The samples were clarified by centrifugation and assayed by a capture ELISA. The CP concentrations were determined by comparison with a standard curve of purified CP5 or CP8 added to normal mouse plasma and extracted in a similar fashion.
Statistical analyses
ELISA and OPK data were analyzed using the unpaired two-tailed Student t-test. Quantitative cultures of mouse blood and concentrations of CP in plasma were compared by the Mann-Whitney U test; CP antigenemia was compared by the Fisher's exact test. Cell-associated and supernatant levels of CPs were analyzed by one-way ANOVA. Multiple comparisons were made to CP levels produced by the reference strains Reynolds (CP5) and Reynolds (CP8). The data were analyzed with Prism 6 (GraphPad Software, Inc.), and P values < 0.05 were considered significant.
Abbreviations
| CP | = | capsular polysaccharide |
| CRM197 | = | cross reacting material 197 |
| ELISA | = | enzyme-linked immunosorbent assay |
| Epa | = | Pseudomonas aeruginosa exoprotein A |
| IP | = | intraperitoneal |
| IV | = | intravenous |
| mAb | = | monoclonal antibody |
| MRSA | = | methicillin-resistant S. aureus |
| NARSA | = | Network on Antimicrobial Resistance in S. aureus. |
| OPK | = | opsonophagocytic killing |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
KVIR_S_1270494.zip
Download Zip (1 MB)Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01 AI088754 to JCL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Kelly Shields-Lapierre for technical assistance and Frank Hanses for providing the ST22 and ST80 isolates. We acknowledge the receipt of numerous staphylococcal strains from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) program supported under the National Institute of Allergy and Infectious Diseases/National Institutes of Health Contract No. HHSN272200700055C. J. C. L. received consulting fees in the past from Pfizer, Crucell, and Sanofi Pasteur.
References
- Scully IL, Liberator PA, Jansen KU, Anderson AS. Covering all the bases: Preclinical development of an effective Staphylococcus aureus vaccine. Front Immunol 2014; 5:109; PMID:24715889; http://dx.doi.org/10.3389/fimmu.2014.00109
- Joshi A, Pancari G, Cope L, Bowman EP, Cua D, Proctor RA, McNeely T. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum Vaccin Immunother 2012; 8:336-46; PMID:22327491; http://dx.doi.org/10.4161/hv.18946
- Montgomery CP, Daniels M, Zhao F, Alegre ML, Chong AS, Daum RS. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect Immun 2014; 82:2125-34; PMID:24614654; http://dx.doi.org/10.1128/IAI.01491-14
- Yeaman MR, Filler SG, Chaili S, Barr K, Wang H, Kupferwasser D, Hennessey JP, Fu Y, Schmidt CS, Edwards JE, et al. Mechanisms of NDV-3 vaccine efficacy in MRSA skin versus invasive infection. Proc Natl Acad Sci U S A 2014; 111:E5555-63; PMID:25489065; http://dx.doi.org/10.1073/pnas.1415610111
- Homaira N, Rawlinson W, Snelling TL, Jaffe A. Effectiveness of palivizumab in preventing RSV hospitalization in high risk children: a real-world perspective. Int J Pediatr 2014; 2014:571609; PMID:25548575; http://dx.doi.org/10.1155/2014/571609
- Gentile G, Foà R. Viral infections associated with the clinical use of monoclonal antibodies. Clin Microbiol Infect 2011; 17:1769-75; PMID:22023708; http://dx.doi.org/10.1111/j.1469-0691.2011.03680.x
- Focosi D, Maggi F, Pistello M, Boggi U, Scatena F. Immunosuppressive monoclonal antibodies: current and next generation. Clin Microbiol Infect 2011; 17:1759-68; PMID:21995285; http://dx.doi.org/10.1111/j.1469-0691.2011.03677.x
- Murad JP, Lin OA, Espinosa EV, Khasawneh FT. Current and experimental antibody-based therapeutics: insights, breakthroughs, setbacks and future directions. Curr Mol Med 2013; 13:165-78; PMID:22834842; http://dx.doi.org/10.2174/156652413804486322
- Yang Z, Ramsey J, Hamza T, Zhang Y, Li S, Yfantis HG, Lee D, Hernandez LD, Seghezzi W, Furneisen JM, et al. Mechanisms of protection against Clostridium difficile infection by the monoclonal antitoxin antibodies actoxumab and bezlotoxumab. Infect Immun 2015; 83:822-31; PMID:25486992; http://dx.doi.org/10.1128/IAI.02897-14
- Otto M. Novel targeted immunotherapy approaches for staphylococcal infection. Expert Opin Biol Ther 2010; 10:1049-59; PMID:20528609; http://dx.doi.org/10.1517/14712598.2010.495115
- Schaffer AC, Lee JC. Staphylococcal vaccines and immunotherapies. Infect Dis Clin North Am 2009; 23:153-71; PMID:19135920; http://dx.doi.org/10.1016/j.idc.2008.10.005
- Fowler VG, Jr., Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect 2014; 20(Suppl 5):66-75; PMID:24476315
- Weems JJ, Steinberg JP, Filler S, Baddley JW, Corey GR, Sampathkumar P, Winston L, John JF, Kubin CJ, Talwani R. Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2006; 50:2751-5; PMID:16870768; http://dx.doi.org/10.1128/AAC.00096-06
- Verdier I, Durand G, Bes M, Taylor KL, Lina G, Vandenesch F, Fattom AI, Etienne J, Verdier I, Durand G, et al. Identification of the capsular polysaccharides in Staphylococcus aureus clinical isolates by PCR and agglutination tests. J Clin Microbiol 2007; 45:725-9; PMID:17202275; http://dx.doi.org/10.1128/JCM.01572-06
- Roghmann M, Taylor KL, Gupte A, Zhan M, Johnson JA, Cross A, Edelman R, Fattom AI. Epidemiology of capsular and surface polysaccharide in Staphylococcus aureus infections complicated by bacteraemia. J Hosp Infect 2005; 59:27-32; PMID:15571850; http://dx.doi.org/10.1016/j.jhin.2004.07.014
- Trotter CL, McVernon J, Ramsay ME, Whitney CG, Mulholland EK, Goldblatt D, Hombach J, Kieny MP, subgroup S. Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine 2008; 26:4434-45; PMID:18617296; http://dx.doi.org/10.1016/j.vaccine.2008.05.073
- Watts A, Ke D, Wang Q, Pillay A, Nicholson-Weller A, Lee JC. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect Immun 2005; 73:3502-11; PMID:15908379; http://dx.doi.org/10.1128/IAI.73.6.3502-3511.2005
- Benjamin DK, Schelonka R, White R, Holley HP, Bifano E, Cummings J, Adcock K, Kaufman D, Puppala B, Riedel P, et al. A blinded, randomized, multicenter study of an intravenous Staphylococcus aureus immune globulin. J Perinatol 2006; 26:290-5; PMID:16598296; http://dx.doi.org/10.1038/sj.jp.7211496
- Rupp ME, Holley HP, Jr, Lutz J, Dicpinigaitis PV, Woods CW, Levine DP, Veney N, Fowler VG, Jr, Rupp ME, Holley HP, Jr, et al. Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2007; 51:4249-54; PMID:17893153; http://dx.doi.org/10.1128/AAC.00570-07
- Hilliard JJ, Datta V, Tkaczyk C, Hamilton M, Sadowska A, Jones-Nelson O, O'Day T, Weiss WJ, Szarka S, Nguyen V, et al. Anti-alpha-toxin monoclonal antibody and antibiotic combination therapy improves disease outcome and accelerates healing in a Staphylococcus aureus dermonecrosis model. Antimicrob Agents Chemother 2015; 59:299-309; PMID:25348518; http://dx.doi.org/10.1128/AAC.03918-14
- Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, et al. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 2014; 58:1108-17; PMID:24295977; http://dx.doi.org/10.1128/AAC.02190-13
- Tkaczyk C, Hua L, Varkey R, Shi Y, Dettinger L, Woods R, Barnes A, MacGill RS, Wilson S, Chowdhury P, et al. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin Vaccine Immunol 2012; 19:377-85; PMID:22237895; http://dx.doi.org/10.1128/CVI.05589-11
- Rouha H, Badarau A, Visram ZC, Battles MB, Prinz B, Magyarics Z, Nagy G, Mirkina I, Stulik L, Zerbs M, et al. Five birds, one stone: neutralization of alpha-hemolysin and 4 bi-component leukocidins of Staphylococcus aureus with a single human monoclonal antibody. MAbs 2015; 7:243-54; PMID:25523282; http://dx.doi.org/10.4161/19420862.2014.985132
- Thakker M, Park J-S, Carey V, Lee JC. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun 1998; 66:5183-9; PMID:9784520
- Wacker M, Wang L, Kowarik M, Dowd M, Lipowsky G, Faridmoayer A, Shields K, Park S, Alaimo C, Kelley KA, et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J Infect Dis 2014; 209:1551-61; PMID:24308931; http://dx.doi.org/10.1093/infdis/jit800
- Park S, Gerber S, Lee JC. Antibodies to Staphylococcus aureus serotype 8 capsular polysaccharide react with and protect against serotype 5 and 8 isolates. Infect Immun 2014; 82:5049-55; PMID:25245803; http://dx.doi.org/10.1128/IAI.02373-14
- Szu SC, Li XR, Stone AL, Robbins JB. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect Immun 1991; 59:4555-61; PMID:1937814
- Fusco PC, Farley EK, Huang CH, Moore S, Michon F. Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clin Vaccine Immunol 2007; 14:577-84; PMID:17376859; http://dx.doi.org/10.1128/CVI.00009-07
- Berry DS, Lynn F, Lee CH, Frasch CE, Bash MC. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect Immun 2002; 70:3707-13; PMID:12065513; http://dx.doi.org/10.1128/IAI.70.7.3707-3713.2002
- Fattom AI, Sarwar J, Basham L, Ennifar S, Naso R. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect Immun 1998; 66:4588-92; PMID:9746554
- Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, Enright MC, Foster TJ, Moore CE, Hurst L, Atkin R, et al. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A 2004; 101:9786-91; PMID:15213324; http://dx.doi.org/10.1073/pnas.0402521101
- Montgomery CP, Boyle-Vavra S, Adem PV, Lee JC, Husain AN, Clasen J, Daum RS. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis 2008; 198:561-70; PMID:18598194; http://dx.doi.org/10.1086/590157
- Cook J, Hepler R, Pancari G, Kuklin N, Fan H, Wang XM, Cope L, Tan C, Joyce J, Onishi J, et al. Staphylococcus aureus capsule type 8 antibodies provide inconsistent efficacy in murine models of staphylococcal infection. Hum Vaccin 2009; 5:254-63; PMID:18787395; http://dx.doi.org/10.4161/hv.5.4.6765
- Boyle-Vavra S, Li X, Alam MT, Read TD, Sieth J, Cywes-Bentley C, Dobbins G, David MZ, Kumar N, Eells SJ, et al. USA300 and USA500 clonal lineages of Staphylococcus aureus do not produce a capsular polysaccharide due to conserved mutations in the cap5 locus. MBio 2015; 6:e02585-14; PMID:25852165; http://dx.doi.org/10.1128/mBio.02585-14
- Arbeit RD, Dunn RM. Expression of capsular polysaccharide during experimental focal infection with Staphylococcus aureus. J Infect Dis 1987; 156:947-52; PMID:3680994; http://dx.doi.org/10.1093/infdis/156.6.947
- Arbeit RD, Nelles MJ. Capsular polysaccharide antigenemia in rats with experimental endocarditis due to Staphylococcus aureus. J Infect Dis 1987; 155:242-6; PMID:3805763; http://dx.doi.org/10.1093/infdis/155.2.242
- Hochkeppel HK, Braun DG, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan EL, Boutonnier A, Fournier JM. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol 1987; 25:526-30; PMID:2437148
- Nanra JS, Buitrago SM, Crawford S, Ng J, Fink PS, Hawkins J, Scully IL, McNeil LK, Aste-Amezaga JM, Cooper D, et al. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum Vaccin Immunother 2013; 9:480-7; PMID:23249887; http://dx.doi.org/10.4161/hv.23223
- Skurnik D, Merighi M, Grout M, Gadjeva M, Maira-Litran T, Ericsson M, Goldmann DA, Huang SS, Datta R, Lee JC, et al. Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. J Clin Invest 2010; 120:3220-33; PMID:20739753; http://dx.doi.org/10.1172/JCI42748
- Tuchscherr LP, Buzzola FR, Alvarez LP, Lee JC, Sordelli DO. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infect Immun 2008; 76:5738-44; PMID:18809660; http://dx.doi.org/10.1128/IAI.00874-08
- Lee JC, Park JS, Shepherd SE, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun 1997; 65:4146-51; PMID:9317020
- Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 2002; 346:491-6; PMID:11844850; http://dx.doi.org/10.1056/NEJMoa011297
- Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum Vaccin Immunother 2015; 11:632-41; PMID:25483694; http://dx.doi.org/10.4161/hv.34414
- Levy J, Licini L, Haelterman E, Moris P, Lestrate P, Damaso S, Van Belle P, Boutriau D. Safety and immunogenicity of an investigational 4-component Staphylococcus aureus vaccine with or without AS03B adjuvant: Results of a randomized phase I trial. Hum Vaccin Immunother 2015; 11:620-31; PMID:25715157; http://dx.doi.org/10.1080/21645515.2015.1011021
- Diekema DJ, Richter SS, Heilmann KP, Dohrn CL, Riahi F, Tendolkar S, McDanel JS, Doern GV. Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: results from a nationwide surveillance study. Infect Control Hosp Epidemiol 2014; 35:285-92; PMID:24521595; http://dx.doi.org/10.1086/675283
- van der Mee-Marquet N, Poisson DM, Lavigne JP, Francia T, Tristan A, Vandenesch F, Quentin R, Bertrand X. The incidence of Staphylococcus aureus ST8-USA300 among French pediatric inpatients is rising. Eur J Clin Microbiol Infect Dis 2015; 34:935-42; PMID:25575950; http://dx.doi.org/10.1007/s10096-014-2308-3
- Uhlemann AC, Otto M, Lowy FD, DeLeo FR. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus. Infect Genet Evol 2014; 21:563-74; PMID:23648426; http://dx.doi.org/10.1016/j.meegid.2013.04.030
- Nimmo GR. USA300 abroad: global spread of a virulent strain of community-associated methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect 2012; 18:725-34; PMID:22448902; http://dx.doi.org/10.1111/j.1469-0691.2012.03822.x
- Kim HK, Emolo C, DeDent AC, Falugi F, Missiakas DM, Schneewind O. Protein A-specific monoclonal antibodies and prevention of Staphylococcus aureus disease in mice. Infect Immun 2012; 80:3460-70; PMID:22825452; http://dx.doi.org/10.1128/IAI.00230-12
- Hall AE, Domanski PJ, Patel PR, Vernachio JH, Syribeys PJ, Gorovits EL, Johnson MA, Ross JM, Hutchins JT, Patti JM, et al. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect Immun 2003; 71:6864-70; PMID:14638774; http://dx.doi.org/10.1128/IAI.71.12.6864-6870.2003
- Bhasin N, Albus A, Michon F, Livolsi PJ, Park J-S, Lee JC. Identification of a gene essential for O-acetylation of the Staphylococcus aureus type 5 capsular polysaccharide. Mol Microbiol 1998; 27:9-21; PMID:9466251; http://dx.doi.org/10.1046/j.1365-2958.1998.00646.x
- Jones C. Revised structures for the capsular polysaccharides from Staphylococcus aureus types 5 and 8, components of novel glycoconjugate vaccines. Carbohydr Res 2005; 340:1097-106; PMID:15797125; http://dx.doi.org/10.1016/j.carres.2005.02.001
- Kozel TR, MacGill RS, Percival A, Zhou Q. Biological activities of naturally occurring antibodies reactive with Candida albicans mannan. Infect Immun 2004; 72:209-18; PMID:14688098; http://dx.doi.org/10.1128/IAI.72.1.209-218.2004
- Moss RB. The role of IgG subclass antibodies in chronic infection: the case of cystic fibrosis. N Engl Reg Allergy Proc 1988; 9:57-61; PMID:3362109; http://dx.doi.org/10.2500/108854188778984491
- Han Y, Kozel TR, Zhang MX, MacGill RS, Carroll MC, Cutler JE. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J Immunol 2001; 167:1550-7; PMID:11466376; http://dx.doi.org/10.4049/jimmunol.167.3.1550
- Zhang MX, Kozel TR. Mannan-specific immunoglobulin G antibodies in normal human serum accelerate binding of C3 to Candida albicans via the alternative complement pathway. Infect Immun 1998; 66:4845-50; PMID:9746588
- Choi EH, Zhang F, Lu YJ, Malley R. Capsular polysacharide (CPS) release by serotype 3 pneumococcal strains reduces the protective effect of anti-type 3 CPS antibodies. Clin Vaccine Immunol 2016; 23:162-7; http://dx.doi.org/10.1128/CVI.00591-15
- Tzianabos AO, Wang JY, Lee JC. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc Natl Acad Sci U S A 2001; 98:9365-70; PMID:11470905; http://dx.doi.org/10.1073/pnas.161175598
- Gray BM. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol 1979; 28:187-92.
- Guttormsen HK, Wetzler LM, Finberg RW, Kasper DL. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect Immun 1998; 66:2026-32; PMID:9573085
- Lee JC, Liu MJ, Parsonnet J, Arbeit RD. Expression of type-8 capsular polysaccharide and production of toxic shock syndrome toxin-1 are associated among vaginal isolates of Staphylococcus aureus. J Clin Microbiol 1990; 28:2612-5; PMID:2279990
- Li W, Ulm H, Rausch M, Li X, O'Riordan K, Lee JC, Schneider T, Muller CE. Analysis of the Staphylococcus aureus capsule biosynthesis pathway in vitro: characterization of the UDP-GlcNAc C6 dehydratases CapD and CapE and identification of enzyme inhibitors. Int J Med Microbiol 2014; 304:958-69; PMID:25023075; http://dx.doi.org/10.1016/j.ijmm.2014.06.002
- Lee JC, Takeda S, Livolsi PJ, Paoletti LC. Effects of in vitro and in vivo growth conditions on expression of type-8 capsular polysaccharide by Staphylococcus aureus. Infect Immun 1993; 61:1853-8; PMID:8478074
- Schaffer AC, Solinga RM, Cocchiaro J, Portoles M, Kiser KB, Risley A, Randall SM, Valtulina V, Speziale P, Walsh E, et al. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect Immun 2006; 74:2145-53; PMID:16552044; http://dx.doi.org/10.1128/IAI.74.4.2145-2153.2006
- Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol 2005; 187:2426-38; PMID:15774886; http://dx.doi.org/10.1128/JB.187.7.2426-2438.2005
- Takeuchi F, Watanabe S, Baba T, Yuzawa H, Ito T, Morimoto Y, Kuroda M, Cui L, Takahashi M, Ankai A, et al. Whole-genome sequencing of Staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J Bacteriol 2005; 187:7292-308; PMID:16237012; http://dx.doi.org/10.1128/JB.187.21.7292-7308.2005
- Kuroda M, Yamashita A, Hirakawa H, Kumano M, Morikawa K, Higashide M, Maruyama A, Inose Y, Matoba K, Toh H, et al. Whole genome sequence of Staphylococcus saprophyticus reveals the pathogenesis of uncomplicated urinary tract infection. Proc Natl Acad Sci U S A 2005; 102:13272-7; PMID:16135568; http://dx.doi.org/10.1073/pnas.0502950102
- Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol 1952; 6:95-107; PMID:14927856
- Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 2001; 357:1225-40; PMID:11418146; http://dx.doi.org/10.1016/S0140-6736(00)04403-2
- Baker CJ. Fatal septicemia due to Staphylococcus aureus 502A. Report of a case and review of the infectious complications of bacterial interference programs. Am J Dis Child 1972; 123:45-8; PMID:5010551; http://dx.doi.org/10.1001/archpedi.1972.02110070095012
- Fattom A, Schneerson R, Szu SC, Vann WF, Shiloach J, Karakawa WW, Robbins JB. Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infect Immun 1990; 58:2367-74; PMID:2114365
- Schlievert PM, Kelly JA. Staphylococcal pyrogenic exotoxin type C: further characterization. Ann Intern Med 1982; 96:982-6; PMID:7091979; http://dx.doi.org/10.7326/0003-4819-96-6-982
- Stegger M, Wirth T, Andersen PS, Skov RL, De Grassi A, Simoes PM, Tristan A, Petersen A, Aziz M, Kiil K, et al. Origin and evolution of European community-acquired methicillin-resistant Staphylococcus aureus. MBio 2014; 5:e01044-14; PMID:25161186; http://dx.doi.org/10.1128/mBio.01044-14
- Karakawa WW, Vann WF. Capsular polysaccharides of Staphylococcus aureus. Semin Infect Dis 1982; 4:285-93.
- Drapeau GR, Boily Y, Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem 1972; 247:6720-6; PMID:4627743
- Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A 2009; 106:5883-8; PMID:19293374; http://dx.doi.org/10.1073/pnas.0900743106
- Cassat JE, Dunman PM, McAleese F, Murphy E, Projan SJ, Smeltzer MS. Comparative genomics of Staphylococcus aureus musculoskeletal isolates. J Bacteriol 2005; 187:576-92; PMID:15629929; http://dx.doi.org/10.1128/JB.187.2.576-592.2005
- Herron LL, Chakravarty R, Dwan C, Fitzgerald JR, Musser JM, Retzel E, Kapur V. Genome sequence survey identifies unique sequences and key virulence genes with unusual rates of amino acid substitution in bovine Staphylococcus aureus. Infect Immun 2002; 70:3978-81; PMID:12065548; http://dx.doi.org/10.1128/IAI.70.7.3978-3981.2002
- Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 2002; 359:1819-27; PMID:12044378; http://dx.doi.org/10.1016/S0140-6736(02)08713-5
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 1967; 33:155-66; PMID:4227577; http://dx.doi.org/10.1016/0042-6822(67)90105-5
- Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis 2006; 194:1761-70; PMID:17109350; http://dx.doi.org/10.1086/509506
- Levinson AI, Tar L, Carafa C, Haidar M. Staphylococcus aureus Cowan I. Potent stimulus of immunoglobulin M rheumatoid factor production. J Clin Invest 1986; 78:612-7; PMID:3489006; http://dx.doi.org/10.1172/JCI112617
