Introduction
Visceral leishmaniasis caused by the anthroponotic Leishmania donovani (VL) and the zoonotic L.infantum (ZVL) are among the most lethal Neglected Tropical Diseases (NTD). They are transmitted by Phlebotominae sand flies (Phlebotomus sp and Lutzomyia sp) and are widely distributed in tropical and subtropical areas [Citation1]. Human vaccination has been elusive [Citation2] and the marketed vaccines for canine infections caused by L.infantum have several shortcomings [Citation3]. Environmental control of this vector-borne disease is unpractical [Citation4]. Moreover, indiscriminate culling of canine population has not yielded the expected benefits to limit the human infections in areas where ZVL is prevalent [Citation5] and it is not acceptable by ethical reasons. Main control system is chemotherapy in both humans and dogs although available treatments have important drawbacks including toxicity and less toxic presentations are highly priced [Citation6]. Emergence of resistant isolates and treatment failures are frequent [Citation7,Citation8] and the drug discovery pipeline is slim [Citation9]. Thus, it is clear that there are research areas where a substantial effort has to be made, namely immune response and chemotherapy.
While in vitro and ex vivo models (e.g. macrophage lines; bone-marrow-derived or peritoneal macrophages) are useful for some experimental purposes they have important limitations in all aspects related to immunology, pathology or chemotherapy; thus animal models of the disease are needed. BALB/c mice have been extensively used in drug screening and immunological studies. However, despite the obvious advantages of this species (price, size and housing, genetic homogeneity, availability of reagents) and their receptivity to L.donovani and L.infantum infections by different routes, murine infections do not reproduce the human or canine visceral infections and minor or none clinical signs are observed in infected mice [Citation10–Citation12]. Hamster (Mesocricetus auratus) is probably the best rodent model for VL since they develop chronic leishmanial infections with clinical and lesions comparable to those found in humans and dogs [Citation12–Citation14]. Besides using infected sand flies [Citation15] to initiate infections several routes of infection have been used in hamster including intraperitoneal (IP), intracardiac (IC), intradermal (ID) and intravenous (IV). IV inoculations, in absence of developed tail, are quite limited (e.g. lingual vein) [Citation16]. IP injection is a frequently used via because it is easily performed and does not require anesthesia [Citation17,Citation18] although on occasion it can yield suboptimal and non-consistent infections [Citation19]. ID injection, although more closely related to the natural inoculation by sand flies, does not guarantee visceral infection of inoculated hamsters [Citation19]. By its part, IC route allows accurate infective dosage [Citation13,Citation17,Citation20–Citation22] but it demands highly skilled personnel and the risk of early death of animals after inoculation cannot be ruled out. Retro-orbital (RO) inoculation is a well-described technique in mice for drug administration, bone marrow transplantation or gene therapy [Citation23]. Recently this route has been used in mice to inoculate L.infantum [Citation24] but as far as we know this route of infection has not been tested or reported in hamster. This manuscript presents a comparative study of IP, IC, and RO inoculation routes of L.donovani by determining clinical course and lesions, biochemical and immunological profile of infected hamsters, and parasite burdens in target organs.
Material and methods
Animals
Male Syrian hamsters (Mesocricetus auratus) were purchased from Janvier Labs (France) when they were 4 weeks old. Animals were allocated in the animal facilities of the Instituto de Investigación Hospital 12 de octubre (ES280790001164) under controlled temperature and light/darkness cycle, and provided with pelleted food and water ad libitum. Suitable nesting and activity materials were also included in the cages for environmental enrichment.
Leishmania Strain and inoculum preparation
Freshly isolated L.donovani (MHOM/SD/43/124) from hamsters were transformed and maintained in laboratory conditions by subpassage of promastigotes in culture flasks at 27°C in RPMI-1640 medium (Lonza) supplemented with 10% heat-inactivated (56°C, 30 min) fetal bovine serum (Gibco), 1% L-glutamine (Lonza) and 100 U/mL penicillin+100 mg/mL streptomycin (Lonza). Stationary phase promastigotes of subpassage 8th were washed three times with phosphate saline buffer (PBS) and resuspended in saline serum at a final concentration of 5 × 108 promastigotes/mL.
Infection and follow-up
After a quarantine period, animals were divided in a stratified way (live weight, lw) into four matched groups of six. Animals were anesthetized with Isoflurane 2–5% and inoculated with 108 promastigotes/animal in 0.2 mL saline serum by either intraperitoneal (IP) (n = 6), intracardiac (IC) (n = 6) or retro-orbital (RO) (n = 6) injection or kept as uninfected control animals (n = 5). IC and RO injections were performed with 29G needle and IP with 25G needles. IP and IC inoculations were carried following standard procedures. RO injection followed the technique described by Yardeni et al. [Citation23],for mice. Hamsters’ eye was carefully protruded by downward pressure on the peri-orbital area to facilitate access to the ocular venous sinus. An ophthalmic anesthetic (tetracaine chlorhydrate 1mg/mL + oxybuprocaine chlorhydrate 4mg/mL) was applied for pain prevention. Along infection hamsters were weekly weighed and blood samples were taken by cava vein puncture [Citation25] every 2 weeks. Two weeks before ending the experiment one of the hamsters from the IP group died by a process unrelated to the leishmanial infection. Thus, the data on live weight and antibody response of this animal were included up to this time. Twenty-six weeks after inoculation, animals were euthanatized by anesthetic overdose (Isoflurane 20%), blood samples were taken by IC puncture and dissection was performed for organs extraction.
Specific antibody response
Specific peripheral immune response was evaluated by ELISA. Antibodies (IgG, IgG1, and IgG2) were determined following the protocol described by Corral et al. [Citation18],with some modifications. Briefly, after setting optimal assay conditions in a checkerboard manner, 96-well plates (Maxisorp®, Nunc) were coated with 50µL/well of 50µg/mL soluble L. donovani extract (4ºC, overnight). Sera samples obtained at different time points along the experiment were added at 1/50 dilution, 50µL/well. Goat anti-hamster IgG (H+L)-HRP (Southern Biotech), mouse anti-Armenian hamster IgG1 and anti-Armenian hamster IgG2 heavy chain (biotin) (Abcam) at dilutions of 1/4000, 1/1000 and 1/4000, respectively, were employed as secondary antibodies. Plates for IgG1 and IgG2 determination included further addition of HRP-conjugated streptavidin (Southern Biotech), 50µL/well, 1/2000 diluted and incubated for 30 min at RT. O-phenylenediamine (1mg/mL) (Sigma) plus H2O2 (1/1000) solution was added 100µL/well. Reaction was stopped with 50µL/well of H2SO4 (3N) and absorbance determined at 492nm in Multiskan™ GO Microplate Spectrophotometer (Fisher Scientific). Cut off was calculated as the uninfected group’s higher mean plus 3 times its standard deviation.
Parasite burden estimation
Quantization of Leishmania burdens in target organs was performed at the end of the experiment by Limiting Dilution Assay (LDA) as described by Castro et al. [Citation26]. Liver and spleen were freshly weighed and homogenized using a cell strainer 70 µm (Corning) in modified Schneider’s Drosophila medium (Lonza) supplemented with 20% heat-inactivated (56°C, 30 min) fetal bovine serum (Gibco), 1% L-glutamine (Lonza), 100 U/mL penicillin+100 mg/mL streptomycin (Lonza) and 2% sterile human urine. Organ homogenates were further diluted (10 mg/mL) and 0.2 mL of the suspension was placed in 96-well culture plates (Corning) for four-fold serial dilution. Two weeks after incubation at 27°C presence of parasites was checked by optical microscopy and the last positive dilution was recorded to calculate the number of parasites per organ gram following Buffet et al. [Citation27]. Cultures were performed in quadruplicate.
Biochemical markers
At the end point of the experiment alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), urea, and creatinine levels in hamsters’ serum were determined by IDEXX Laboratories (Spain).
Statistical analysis
Statistical analysis of the data at the end point (organs’ weight, parasite burden, blood biochemistry) was carried out by one-way ANOVA followed by multiple comparison test (Tukey’s test) using Graphpad Prism 6 software. Repeated measures (lw, antibody levels) were analyzed with a mixed model for repeated measures considering time and groups employing SAS/STAT ® 9.2 software and differences at each time point between groups were determined by one-way ANOVA. Differences were considered statistically significant when P < 0.05.
Ethical issues
The experiment was designed and performed following 3Rs principles. Protocols were approved by Regional authorities (PROEX 169/15) and all animals and procedures were supervised by qualified veterinary personal.
Results
Infection and follow-up
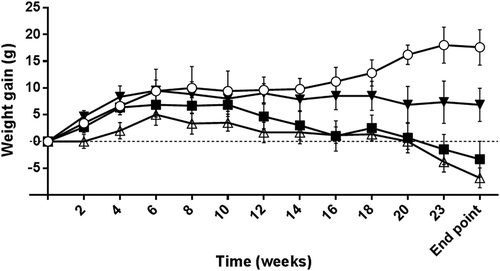
Inoculation of the animals did not elicit immediate adverse effects except for one animal from the IC group. Clinical evaluation showed that some animals (RO:1, IC:2, IP: 1) displayed cutaneous lesions including peri-orbital and nose alopecia and/or dry brittle hair in dorsal and abdominal areas. IC and RO inoculation with L.donovani elicited a reduction of live weight (lw) loss from week 7, and particularly from week 16 onwards (P < 0.05), whereas this pattern was not observed in animals inoculated by the IP route. At the end point of the experiment, IC and RO showed a significant lw loss (P < 0.05-P < 0.001) ().
Figure 1. Live weight gain of experimental hamsters along the experimental infection with 108 stationary phase promastigotes of Leishmania donovani (MHOM/SD/43/124). (○): uninfected control animals; (■): hamsters inoculated by the retro-orbital route; (▼): animals infected by intraperitoneal inoculation; (△): animal group infected by via intracardiac injection. Values are means ± standard error of the mean (SEM).
Antibody response
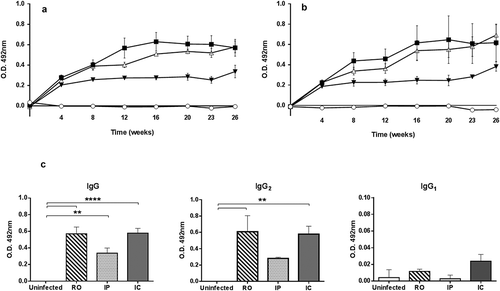
There was a relationship between specific IgG response and time elapsed after infection and the inoculation via. All infected groups showed significant levels of antibodies when compared to the uninfected control animals (). Infection elicited a rapid response after 4 weeks (P < 0.05) specific antibodies reaching the maximal values 16 weeks after inoculation. Hamsters subjected to IC and RO inoculation displayed comparable IgG patterns whereas animals IP infected showed lower average values. However, at the end point of the experiment (week 26), no significant differences between groups were found. Apparently, the total IgG response was mainly due to IgG2 () since their patterns along the experiment were comparable. Similar to that found in the IgG response, hamsters inoculated IP displayed lower IgG2 antibodies levels. There was high individual variation among animals and thus no significant intergroup differences were found except for the higher OD values of RO group compared to IP animals on weeks 8 and 12. Specific IgG1 response detected was low although hamsters IC inoculated showed higher OD values in all postinoculation samplings, and uninfected and animals inoculated IP displayed the lower values. shows the average IgG1 values of the experimental animals at the end point of the experiment.
Figure 2. Peripheral serum antibody response of hamsters inoculated with L.donovani by retro-orbital (RO), intracardiac (IC) and intraperitoneal (IP) routes along the experimental period estimated by ELISA. (a) Serum IgG response. (b) Serum IgG2 response. (○) uninfected, (■) RO, (▼) IP, and (△) IC. (c) Comparison of serum IgG, IgG1 and IgG2 levels of experimental groups of hamsters inoculated by different routes at the end point of the experiment. Values are mean ± SEM. **P < 0.01 and ****P < 0.0001: significant differences. Cut off was established at 0.167 for IgG, 0.027 for IgG1 and 0.049 for IgG2.
Gross pathology of target organs
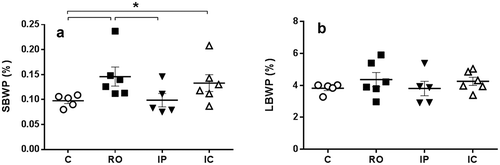
No significant macroscopic lesions were evident in internal organs of any of the animals except for the significant spleen enlargement in group RO hamsters compared to uninfected control animals and IP inoculated (P < 0.05) (). Hamster groups inoculated by the RO via and IC displayed the highest spleen weight although the group IC showed higher individual variability this precluding the significance. No weight increase of the liver was found. To rule out the possibility of the apparent spleen enlargement in infected animals being artifactual the relative weight of the target organs for Leishmania was considered (). Results confirmed the spleen enlargement of RO and IC animal groups compared to the IP-inoculated hamsters (P < 0.05).
Table 1. Spleen and liver weight of experimental animals (mean±SEM) at the end point of the experiment.
Liver and kidney functionality markers
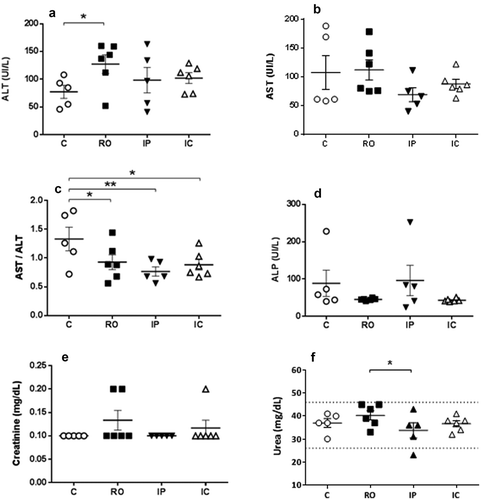
To evaluate the effect of the chronic Leishmania infection plasma levels of hepatic and renal markers were estimated at the end point of the experiment. AST and ALT were elevated in all infected hamsters although there were no significant differences among experimental groups (,)). However, the most sensitive De Ritis index (AST/ALT) showed that the value of the index was significantly lower in all animal groups inoculated with the parasite () (P < 0.05). No differences in ALP among groups were found () and the alterations of kidney functionality markers did not show any clear pattern (,)). Actually, the only significant difference (P < 0.05) was observed between urea of RO and IP groups (). Possibly this finding has scarce biological significance since no differences were found in BUN (blood urea nitrogen)/creatinine ratio (not shown).
Figure 4. Levels (mean±SEM) of hepatic and liver functionality markers of hamsters inoculated with L.donovani by different routes at the end point of the experiment. (a) alanine aminotransferase (ALT); (b) aspartate aminotransferase (AST); (c) AST/ALT; (d) alkaline phosphatase (ALP); (e) creatinine; (f): urea. *P < 0.05; **P < 0.01: significant differences.
Parasite burden in target organs
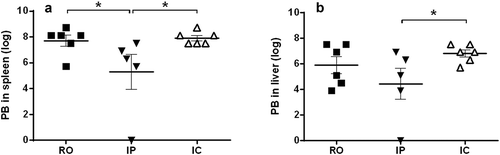
Parasite burden in spleen and liver was estimated by LDA at the end point, 26 weeks pi. All hamsters inoculated by IC and RO were infected in both organs whereas in the IP group the infection could not be detected in one of the animals. Since IgG and IgG2 values from this animal were over the cut off in the ELISA the Leishmania burden in this hamster was possibly low and below the detection level of the technique employed (LDA). Despite the high individual variability, the Leishmania burden of the IP group was significantly lower (P < 0.05) than that found in RO and IC inoculated animals (). shows the individual values of normalized parasite numbers (log x+1) in spleen and liver. Apparently, spleen was more heavily infected than the liver with all inoculation methods employed but the differences were not significant. No parasites were detected in any of the control animals.
Table 2. Leishmania parasite burden (PB, parasites/gram) (Mean ± SEM) in spleen and liver estimated by Limiting Dilution Assay (LDA) at the end of the experiment.
Discussion
Development of VL, including dissemination of parasites to target organs, is the result of a complex interaction of host, vector, and parasite factors eventually leading to a generalized infection [Citation28]. Value of surrogate laboratory models relates to their reliability to reproduce the events observed in natural infections including clinical signs and lesions, biochemical abnormalities, immune response elicited by the infection and parasite burden and lesions in target organs at the necropsy. Hamsters are considered a very useful model for human VL since, contrary to that observed in most mice models, this species lacks the major antileishmanial effector mechanism (iNOs production) to control the Leishmania infection this leading to a progressive VL in hamster [Citation14,Citation29].
Hamster infections with Leishmania species causing VL, L.donovani and L.infantum, can be initiated with both promastigotes and amastigotes using different inoculation methods, mainly IC [Citation13,Citation17,Citation19–Citation22,Citation30] and IP [Citation17–Citation19,Citation30]. Natural vector-transmitted infection has been considered a good method for immunological and pathogenicity studies [Citation15] although requires the availability of sand flies colonies. By its part, ID inoculation of hamsters did not produce parasite dissemination of L.infantum even after 9 months of incubation [Citation19]. IP and IC are the most common inoculations methods although comparative studies have yielded conflicting results. Wyllie and Fairlamb [Citation17] did not find any difference between both methods of infection with L.donovani, except for the longer incubation period after IP inoculation, whereas Moreira et al. [Citation19,Citation30] reported that IC injection of L.infantum was more efficient eliciting consistent high parasite burdens in target organs and associated lesions. Our present results showed a comparatively lower efficacy of IP inoculation although the differential virulence of the Leishmania species and strains, duration of the experiment, and host receptivity could be responsible for the reported inconsistencies [Citation10]. Drug discovery and development (DDD) requires a robust inoculation method to rapidly elicit VL. Insofar, the best available for this purpose is the IC route. However, this inoculation system has several drawbacks including fatalities.
Aim of our work was to explore the possibility of using retro-orbital (RO) injection of L.donovani to get VL infections in an advanced rodent model and to compare its efficacy with IP and IC inoculation routes. RO injection is being currently used in mice for different purposes and is technically less challenging than other inoculation routes [Citation23]. Moreover, this route has been recently used to inoculate in mice inoculated with L.infantum [Citation24] although, as far as we know, this infection method has not been tested before in hamster. Results obtained showed that, under our conditions and contrary to IP, both IC and RO were able to get consistent infections in all inoculated animals within the time frame of our experiment. IC and RO routes elicited cutaneous lesions compatible with leishmaniasis (alopecia, dry brittle hair) [Citation13,Citation30] at the end of the experiment (26 weeks postinoculation). Weight loss is a frequent finding in hamster VL irrespective of the infective dose and experimental design [Citation13–Citation15] and we found that there was a significant reduction of lw gain in RO and IC groups. Splenomegaly, characteristic of human and hamster VL [Citation13,Citation17,Citation30] was factual and the more accurate estimate index (spleen weight/live weight) was significantly higher in the animals inoculated by the RO via. Biopathological markers have been not extensively studied in hamster despite alteration of transaminases and kidney functionality markers being a frequent finding in humans. The De Ritis index (AST/ALT) was diminished in all infected animals this suggesting its value as unspecific infection marker in hamster VL. Considering the inoculation method the animals infected by the RO route displayed higher ALT serum levels. Absence of alterations in kidney functionality markers is consistent with the duration of the infection (26 weeks) and it suggests that no kidney lesion was present. VL is characterized by hyper gamma globulinemia and the pattern of specific antibody response is considered a good marker of Leishmania dissemination in hamster [Citation13,Citation14,Citation19,Citation21]. Our results showed that both specific IgG and IgG2 anti-Leishmania response displayed a steady increase along the experimental period in all infected groups without differences between RO and IC infected hamsters. Possibly the most accurate measure of inoculation efficacy is the parasite burden at end point of the experiment. Despite the difficulties of comparing our results with those obtained by other groups (inoculation route, experimental design, parasite strain) [Citation10,Citation17] all animals from the RO and IC groups were infected, with comparable parasite burdens, whereas one of the hamsters from the IP group (n = 5) did not show Leishmania amastigotes in the spleen or liver with the technique employed (LDA) and the parasite burden of this group was significantly lower in the spleen. It is possible that longer follow-up of IP-inoculated animals or more sensitive analytical techniques (e.g. PCR) would show the infected status of this group.
Considering all results together the RO inoculation method is useful for both mice [Citation24] and hamster. RO is at least as efficient as IC to induce VL in a hamster model based on the in vivo and postmortem lesions observed, biopathological alterations, specific antibody response and parasite burden in target organs. As any other inoculation method, RO injection could be harmful to experimental animals. However, it is easily mastered after supervised training, is less risky than IC inoculation, induces comparable VL progression and it is a good alternative inoculation via when reliable and efficient VL infections of hamster are needed.
Acknowledgments
L.donovani strain was supplied by Dr. M. Domínguez from the Instituto de Salud Carlos III (ISCIII), Madrid. We thank the help with the mixed model analysis from the Supporting Service of Statistics (UCM).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- World Health Organization (WHO). Working to overcome the global impact of neglected tropical diseases. First WHO report on neglected tropical diseases. WHO Geneva. 2010:184.
- Kedzierski L, Zhu Y, Handman E. Leishmania vaccines: progress and problems. Parasitology. 2006;133(SupplS2):S87–112.
- Grimaldi G, Teva A Jr, dos-Santos CB, et al. Field trial of efficacy of the Leish-tec ® vaccine against canine leishmaniasis caused by Leishmania infantum in an endemic area with high transmission rates. PLoS ONE. 2017;12:e0185438.
- Otranto D, Dantas-Torres F. The prevention of canine leishmaniasis and its impact on public health. Trends Parasitol. 2013;29(7):339–345.
- Costa DNCC, Codeço CT, Silva MA, et al. Culling dogs in scenarios of imperfect control: realistic impact on the prevalence of canine visceral Leishmaniasis. PLoS Negl Trop Dis. 2013;7:e2355.
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318.
- Chakravarty J, Sundar S. Drug resistance in Leishmaniasis. J Glob Infect Dis. 2010;2:167–176.
- Mohapatra S. Drug resistance in leishmaniasis: newer developments. Trop Parasitol. 2014;4:4–9.
- Nagle AS, Khare S, Kumar AB, et al. Recent developments in drug discovery for Leishmaniasis and human African trypanosomiasis. Chem Rev. 2014;114:11305−11347.
- Loeuillet C, Bañuls AL, Hide M. Study of Leishmania pathogenesis in mice: experimental considerations. Parasit Vectors. 2016;9:144.
- Loría-Cervera EN, Andrade-Narváez FJ. Animal models for the study of leishmaniasis immunology. Rev Inst Med Trop Sao Paulo. 2014;56(1):1–11.
- Garg R, Dube A. Animal models for vaccine studies for visceral leishmaniasis. Indian J Med Res. 2006;123:439–454.
- Requena JM, Soto M, Doria MD, et al. Immune and clinical parameters associated with Leishmania infantum infection in the golden hamster model. Vet Immunol Immunopathol. 2000;76:269–281.
- Melby PC, Chandrasekar B, Zhao W, et al. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol. 2001;166:1912–1920.
- Aslan H, Dey R, Meneses C, et al. A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies. J Inf Dis. 2013;207:1328–1338.
- Field KJ, Sibold AL. The laboratory hamster & Gerbil. Boca Raton: CRC Press LLC; 1999.
- Wyllie S, Fairlamb AH. Refinement of techniques for the propagation of Leishmania donovani in hamsters. Acta Trop. 2006;97:364–369.
- Corral MJ, Serrano DR, Moreno I, et al. Efficacy of low doses of amphotericin B plus allicin against experimental visceral leishmaniasis. J Antimicrob Chemother. 2014;69:3268–3274.
- Moreira MD, Vitoriano-Souza J, Mendes Roatt B, et al. Parasite burden in hamsters infected with two different strains of Leishmania (Leishmania) infantum: “Leishman Donovan units” versus Real-Time PCR. PLoS ONE. 2012;10:e47907.
- Rama-Íñiguez S, Dea-Ayuela MA, Sánchez-Brunete JA, et al. Real-Time reverse transcription–PCR quantification of cytokine mRNA expression in golden Syrian hamster infected with Leishmania infantum and treated with a new amphotericin B formulation. Antimicrob Agents Chemother. 2006;50:1195−1201.
- Dea-Ayuela MA, Rama-Íniguez S, Alunda JM, et al. Setting new immunobiological parameters in the hamster model of visceral leishmaniasis for in vivo testing of antileishmanial compounds. Vet Res Commun. 2007;31:703–717.
- Kushawaha PK, Gupta R, Sundar S, et al. Elongation factor-2, a Th1 stimulatory protein of Leishmania donovani, generates strong IFN-γ and IL-12 response in cured Leishmania-infected patients/hamsters and protects hamsters against Leishmania challenge. J Immunol. 2011;187:6417–6427.
- Yardeni T, Eckhaus M, Morris D, et al. Retro-orbital injections in mice. Lab Anim. 2011;40:155–160.
- Khalid KE, Lima Nascimento MS, Amorim Sacramento L, et al. T1/ST2 deficient mice display protection against Leishmania infantum experimental infection. Acta Trop. 2017;172:1–6.
- Picazo MG, Benito PJ, García-Olmo DC. Efficiency and safety of a technique for drawing blood from the hamster cranial vena cava. Lab Anim NY. 2009;38:211−216.
- Castro H, Teixeira F, Romao S, et al. Leishmania mitochondrial peroxiredoxin plays a crucial peroxidase-unrelated role during infection: insight into its novel chaperone activity. PLoS Pathog. 2011;7:e1002325.
- Buffet PA, Sulahian A, Garin YJF, et al. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob Agents Chemother. 1995;3:2167–2168.
- McCall LI, Zhang WW, Matlashewski G. Determinants for the development of visceral leishmaniasis disease. PLoS Pathog. 2013;9:e1003053.
- Pérez LE, Chandrasekar B, Saldarriaga OA, et al. Reduced nitric oxide synthase 2 (NOS2) promoter activity in the Syrian hamster renders the animal functionally deficient in NOS2 activity and unable to control an intracellular pathogen. J Immunol. 2006;176:5519−5528.
- Moreira MD, Vitoriano-Souza J, Mendes Roatt B, et al. Clinical, hematological and biochemical alterations in hamster (Mesocricetus auratus) experimentally infected with Leishmania infantum through different routes of inoculation. Parasit Vectors. 2016;9:181.