ABSTRACT
In order for entomopathogenic fungi to colonize an insect host, they must first attach to, and penetrate, the cuticle layers of the integument. Herein, we explored the interactions between the fungal pathogen Metarhizium brunneum ARSEF 4556 and two immunologically distinct morphs, melanic (M) and non-melanic (NM), of the greater wax moth Galleria mellonella. We first interrogated the cuticular compositions of both insect morphs to reveal substantial differences in their physiochemical properties. Enhanced melanin accumulation, fewer hydrocarbons, and higher L-dihydroxyphenylalanine (DOPA) decarboxylase activity were evident in the cuticle of the M larvae. This “hostile” terrain proved challenging for M. brunneum – reflected in poor conidial attachment and germination, and elevated expression of stress-associated genes (e.g., Hsp30, Hsp70). Lack of adherence to the cuticle impacted negatively on the speed of kill and overall host mortality; a dose of 107 conidia killed ~30% of M larvae over a 12-day period, whereas a 100-fold lower dose (105 conidia) achieved a similar result for NM larvae. Candidate gene expression patterns between the insect morphs indicated that M larvae are primed to “switch-on” immunity-associated genes (e.g., phenoloxidase) within 6–12 h of conidia exposure and can sustain a “defense” response. Critically, M. brunneum responds to the distinct physiochemical cues of both hosts and adjusts the expression of pathogenicity-related genes accordingly (e.g., Pr2, Mad1, Mad2). We reveal previously uncharacterized mechanisms of attack and defence in fungal-insect antibiosis.
Background
The sclerotized integument, or exoskeleton, of an insect provides the first line of defence against opportunistic and obligate pathogens. This robust, multi-layered, physiochemical barrier is composed of biocidal epicuticular fatty acids and a protein-chitin procuticle reinforced with melanic polymers [Citation1,Citation2]. Hypocrealean entomopathogenic fungi (EPF) such as Metarhizium brunneum and Beauveria bassiana have evolved adhesion factors, hydrolytic enzymes, and specialized infection structures to invade the cuticle directly [Citation3,Citation4]. These unique features contribute invariably to their success as biocontrol agents – providing an environmentally friendly alternative to chemical pesticides that have been withdrawn from the market or to which pests have developed resistance [Citation5].
The physiochemical properties of the cuticle can interfere with different facets of the fungal infection process which, in turn, impacts specificity and virulence [Citation3]. For example, the surface chemistry can influence the adhesion of inocula due to weak adhesion forces [Citation6,Citation7] or fungistasis [Citation3,Citation8]. Sequestration of fungistatic plant allelochemicals by the epicuticular waxes can also impede infection [Citation3]. Delaying penetration of the cuticle predisposes the fungal inoculum to other biotic and abiotic factors that are deleterious to the pathogen. Such factors include low or fluctuating humidity, rainfall (which washes off spores), and UV radiation [Citation9–Citation11]. Thus, successful EPF strains are not only able to cope with the physical environment of the host surface but also the preformed innate immune defences of the insect. Beneath the cuticle, the epidermis synthesizes antimicrobial peptides and activates the stress management apparatus [Citation12,Citation13]. These front line defenses are augmented with the humoral and cellular responses within the hemolymph, some of which follow the gradient of cues emanating from the fungus or infection site [Citation14,Citation15]. The innate immune responses within the hemocoel (body cavity) of the host have received comparatively more attention than the cuticle, in spite of the latter being the primary and most important barrier to disease-causing agents.
Larvae of the greater wax moth, Galleria mellonella, are used frequently as in vivo models for assessing the virulence of disease-causing agents, e.g., entomopathogens [Citation16,Citation17], and identifying the biological targets of toxins [Citation18]. In 2013, a distinct melanic (darker) morph of G. mellonella was discovered, which demonstrated enhanced resistance to the EPF B. bassiana relative to the “normal” non-melanic morph [Citation12]. Having access to both tolerant (melanic; M) and susceptible (non-melanic; NM) morphs of G. mellonella further enhances its usefulness as an experimental tool for deciphering the molecular mechanisms that underpin the attack and counterattack strategies of entomopathogens and insects, respectively. The overall aim of this study was to characterize the interactions between a virulent strain of M. brunneum and the distinct morphs of G. mellonella during the initial, critical stages of infection. To achieve this, we first assessed the preformed defenses, i.e., immune gene expression and physiochemical (melanization, hydrocarbon, fatty acid) contents, of naive M and NM morphs. Second, we exposed larvae to M. brunneum and recorded conidial attachment to, and germination on, the cuticle of each morph as well as differential expression of insect genes encoding immune factors (e.g., apolipophorin III), stress management (e.g., HSP90) and detoxification (e.g., glutathione-S-transferase). Third, we surveyed fungal gene expression across key stages of insect colonization: adhesion to the cuticle (Mad1, Mad2), cuticle degradation (Pr1, Pr2), stress management (Hsp30, Hsp70), differentiation of infection structures (cag8), and nutrient assimilation (nrr1).
Results
Biochemical and biophysical properties of melanic versus non-melanic integuments
The integument of melanic (M) G. mellonella are visibly darker in colour compared to the non-melanic (NM) morph (), and ~1.5-fold thicker (); p < 0.001, t = 15.18 df = 348). This distinct colouration is due to the M insects having ~1.8-fold more surface covered in melanic spots (p < 0.001, t = 6.058, df = 18), with each spot ~14.7 times darker than their NM counterparts (p < 0.001, t = 79.72, df = 18). Additionally, the non-melanized distance between each spot measures 4.66 ± 0.15 µm and 7.25 ± 0.31 µm on M and NM larvae, respectively (p < 0.001, t = 7.485, d f = 18; ,).
Figure 1. Integument properties and candidate gene expression of Galleria mellonella morphs. Appearance (a), cuticle thickness (n = 170, *** = p < 0.001), and patterns of melanin deposition (c, d) of melanic and non-melanic morphs. Enhanced expression (mRNA levels) of genes encoding immune factors (gloverin, gallerimycin, apolipophorin III, IMPI, DDC, PPO), stress management (HSP90), detoxification, and cell proliferation of naive melanic (M) larvae compared to non-melanic (NM) larvae (e).
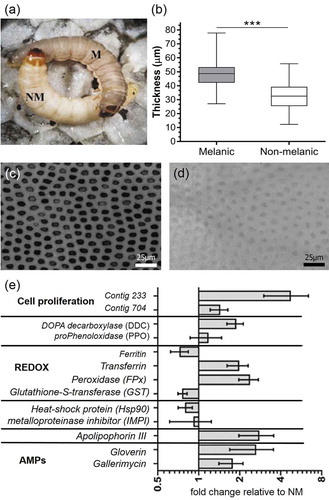
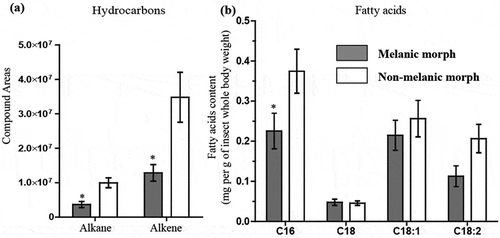
An initial survey of candidate gene expression between un-stimulated (non-infected) M and NM insects revealed key differences in “immune-vigor”. Levels of mRNA for antimicrobial peptides (gallerimycin, gloverin) and the multi-functional immune-factor apolipophorin III were two- to threefold higher in M larvae (). Several genes linked to reduction/oxidation (REDOX) management (e.g., peroxidase), eumelanin biogenesis (e.g., DOPA-decarboxylase), and growth factors (1.5-fold for contig704 and fivefold for contig233) in the integument were elevated in M larvae (). Using a preliminary annotation of the recently published G. mellonella genome [Citation19], we identified contig704 to be a pleiotrophin-like protein precursor. Phenotypic differences between insect morphs were reflected in the biochemical compositions of the integuments. Epicuticular extracts of M larvae contained 2.7–2.9 fold fewer alkanes/alkenes (p < 0.05, t = 3.745, df = 4) and hydrocarbons (p < 0.05, t = 2.877, df = 4) compared to their NM counterparts (). Melanic larvae also had significantly reduced numbers of C16 fatty acids (p < 0.05, t = 2.74) (). The C18, C18:1 and C18:2 contents appeared similar between the two insect morphs.
Figure 2. Biochemical profiles of Galleria mellonella morphs. Quantities and compositions of epicuticular hydrocarbons (a) and fatty acids (b) from un-stimulated (i.e., naive) melanic and non-melanic wax moth larvae. Unpaired t-tests were used to assess differences between each insect morph (* = p < 0.05).
Differential susceptibility of Galleria Mellonella morphs to Metarhizium brunneum
Topical exposure of G. mellonella morphs to two doses of M. brunneum conidia (1 × 105 and 1 × 107) led to significant decreases in survival over a 12-day period (); X2(5) = 108.5, p < 0.0001). The highest mortality of 67% was observed for NM larvae exposed to 1 × 107 conidia of M. brunneum. Moreover, NM larvae were significantly more susceptible to either fungal dose (p = 0.0083 (1 × 105), p < 0.0001 (1 × 107)). The lower dose (1 × 105) of conidia was sufficient to kill ~36% of NM larvae, whereas the higher dose (1 × 107) was required to kill ~33% of the M larvae (). A resistance ratio (RR) calculation indicated M larvae are 31-fold less sensitive to M. brunneum. Mortalities for uninfected (control) insects were ≤3.3% for either insect morph.
Figure 3. Development of mycosis (Metarhizium brunneum) in melanic (M) and non-melanic (NM) morphs of Galleria mellonella larvae after topical inoculation. The survival of each insect morph was recorded over 12 days following exposure to two doses of M. brunneum conidia. LС30 values demonstrate similar mortality levels of M and NM larvae in the presence of 107 conidia (p < 0.01, **) and 105 conidia (p < 0.001, ***), respectively (n = 60). The numbers of conidia per 0.25 mm2 cuticle were counted on M and NM morphs during infection with M. brunneum (107) (b), and the percentage of germinating conidia (c) that successfully adhered (*** = p < 0.001; n = 22). Penetration of the integument by germinating conidia led to the formation of melanotic lesions (white arrows) on NM larvae – representing de novo synthesis of melanin (d). Conversely, the pattern of melanin deposition on M larvae remained unchanged (e), indicating the melanic-defenses are preformed (images were taken 24 h.p.i. with 107 conidia).
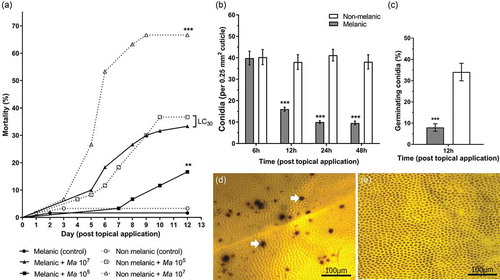
Adhesion of M. brunneum conidia to the integuments of M and NM larvae were almost identical (~40%) within 6-h post-topical application (). Over the subsequent 12–48 h, ~75% of the conidia dropped-off the M larvae, and <8% of those remaining showed signs of germination (, p < 0.001). Germination of conidia on the living insects following fungal application were fourfold higher at 12-h post-infection on the NM larvae (); t = 14.71, p < 0.001). At 24 and 48 h, conidial germination on M larvae were 37% and 71%, respectively, compared to 100% on NM larvae (t = 14.03 and t = 6.53, p < 0.001; Supplementary Figure 1). The formation of melanic lesions caused by multiple penetrating fungi were clearly visible on the integument of NM larvae (). Conversely, no clear signs of additional melanin deposition were seen on M larvae in response to the pathogen over the same experimental period ().
Expression of insect immunity/stress-related genes in the integument
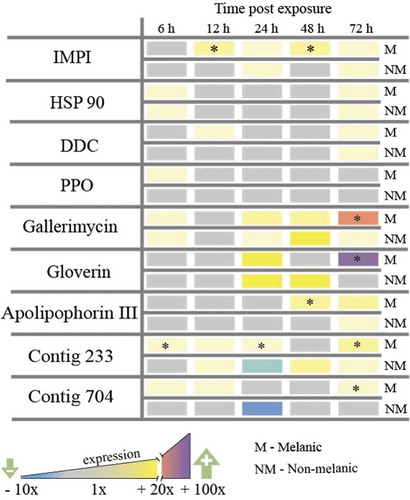
Exposure of M larvae to M. brunneum via topical application stimulated the rapid (6 – 12 h) up-regulation of several key genes encoding anti-infective factors in the integument: fivefold increases in the insect metalloproteinase inhibitor protein (IMPI [Citation20]), the antifungal peptide gallerimycin, and the melanin-generating enzymes DOPA-decarboxylase and proPhenoloxidase (). Elevated expression of genes encoding two putative growth factors, contig233 and contig704, and the stress-associated protein chaperone, heat shock protein 90 (Hsp90), were observed also in M larvae within 12 h. Conversely, NM larvae that were exposed to the same dose of M. brunneum contained increased mRNA levels for Hsp90 and gallerimycin only (at 12 h). By 72 h post-infection, M larvae increased the expression of gallerimycin and the gene encoding the antibacterial peptide gloverin by 38-fold and 24-fold, respectively (). Regarding the multifunctional β-glucan-binding protein apolipophorin III, M larvae switched this gene on earlier at 48 h compared to 72 h for NM larvae, and overall, produced sixfold more mRNA.
Figure 4. Gene expression (mRNA levels) of Galleria mellonella integumental tissues exposed to Metarhizium brunneum conidia (LС30) relative to uninfected (control) insects. Insect metalloproteinase inhibitor (IMPI), heat-shock protein (HSP 90), DOPA decarboxylase (DDC), prophenoloxidase (PPO), antifungal peptide gallerimycin, antibacterial peptide gloverin, β-glucan binding protein apolipophorin III, growth factors contig 233 (growth-blocking peptide) and contig 704 (pleiotrophin-like protein). (* = p < 0.05; melanic versus non-melanic at the respective time point).
Messenger RNA levels of the antioxidant enzymes, peroxidase (FPx) and glutathione-S-transferase (GST), increased in a dose-dependent manner across 72 h in the integuments of both M and NM larvae (). Differential expression patterns were higher in the NM, and were perhaps linked to the de novo synthesis of melanin at the sites of fungal penetration (). Expression of the siderophore transferrin also increased with inoculation dose in the NM, but this was not the case for the M larvae ().
Figure 5. Detoxification-associated gene expression in Galleria mellonella morphs exposed to Metarhizium brunneum. Antioxidant enzymes, peroxidase (FPx) (a), glutathione-S-transferase (GST) (b) and transferrin, (c) in the integuments of M and NM larvae at 72 h post fungal infection. The asterisk [*] indicates a significant difference (p < 0.05) between NM (107) and M (105). The hashtag [#] indicates a significant difference (p < 0.01) between NM (107) and NM (105).
![Figure 5. Detoxification-associated gene expression in Galleria mellonella morphs exposed to Metarhizium brunneum. Antioxidant enzymes, peroxidase (FPx) (a), glutathione-S-transferase (GST) (b) and transferrin, (c) in the integuments of M and NM larvae at 72 h post fungal infection. The asterisk [*] indicates a significant difference (p < 0.05) between NM (107) and M (105). The hashtag [#] indicates a significant difference (p < 0.01) between NM (107) and NM (105).](/cms/asset/1aa4bddc-c3c4-422f-99ba-49593fd76cd6/kvir_a_1693230_f0005_b.gif)
Expression of Metarhizium brunneum virulence/stress-related genes on the insect integument
Metarhizium brunneum gene expression on the integument indicated a host-dependent response, with adhesion (Mad2), stress management (Hsp30, Hsp70), differentiation of infection structures (cag8), nutrient assimilation (nrr1), and cuticle degradation (Pr2) genes all up-regulated significantly (5x to 25x) after 12 h on M larvae (,). Fungi infecting the NM larvae switched-on the insect-specific adhesion factor Mad1 (sixfold), whereas, those infecting the M larvae expressed the generalist (plant-specific) Mad2 (20-fold; ), ). Moreover, increased expression of protease Pr2 ranged from 2- to 10-fold between 12 and 48 h, with cag 8 and nitrogen regulator (nrr1) mRNA increasing from 2- to 6-fold between 12 and 72 h ().
Figure 6. Gene expression (mRNA levels) of Metarhizium brunneum during the infection cycle on melanic and non-melanic morphs of Galleria mellonella. Scheme representing the broad stages of host colonization (a). Subtilisin-like proteases (Pr1a, Pr1b, Pr2), heat-shock proteins (HSP30, HSP70), adhesin-like proteins (MAD1 and MAD2), conidiation – associated gene (cag 8) and nitrogen regulator response (nrr) expression on the insect integuments from 6 to 72-h post-exposure.
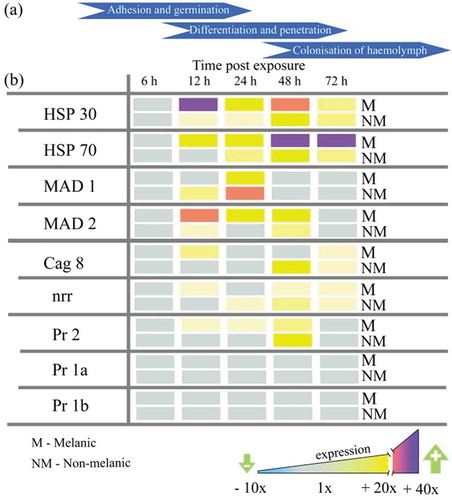
Figure 7. Relative expression patterns of Metarhizium brunneum genes on the integuments of melanic larvae compared directly to non-melanic larvae. Fungal protease Pr2 (a), adhesin-like proteins (MAD1 and MAD2) (b), heat-shock proteins 30/70 (HSP30/70, c/e), conidiation-associated gene (cag 8, d) and nitrogen regulator response (nrr, f).
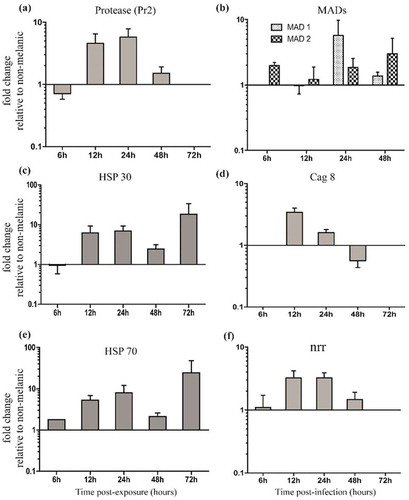
The expression of fungal virulence/stress management genes on M and NM integuments using the same lethal concentration (LC30; ) showed that the protease gene Pr2 was upregulated 4.5 and 5.7-fold at 12 h and 24 h (). Surprisingly, differential expression of the other subtilisin-like (cuticle-degrading) protease genes Pr1a and Pr1b was not detected on either M or NM larvae across the 72 h period (). Levels of M. brunneum heat-shock proteins (Hsp 30 and Hsp 70) were 5 to 25-fold higher on M integuments compared to NM at each time point from 12 h onwards (). Expression of the fungal adhesin Mad1 was sixfold higher in NM larvae at 24 h. Mad2 expression in M larvae was consistently higher. The Cag8 and nitrogen regulator (nrr) mRNA levels were threefold higher in integuments of M insects at 12 h and 24 h pi compared to NM insects (,).
Discussion
In this study, two morphs (melanic (M) and non-melanic (NM)) of G. mellonella were used successfully to interrogate M. brunneum-insect antibiosis at the cuticle interface. Firstly, our data strengthen earlier findings that the two insect variants are indeed immunologically discrete [Citation12]. The cuticle of the M morph is thicker, replete with melanic deposits that limit colonizable area, and contains significantly fewer hydrocarbons and fatty acids. These features describe a rather inhospitable surface to which M. brunneum could adhere to, thereby resulting in prolonged exposure of the fungus to environmental stress (e.g., UV) and nutrient limitations. In contrast to NM larvae, and in the absence of infection, the M cuticle has higher basal levels of specific antifungal factors (apolipophorin III, gallerimycin, IMPI), antibacterial factors (gloverin) and general defenses (DOPA-decarboxylase, transferrin). Therefore, the M larvae are primed/front-loaded to tackle diverse disease-causing agents in contrast to their NM counterparts. This observation is supported by the retarded infection process displayed by M. brunneum on the M larvae when compared to the NM; fewer conidia adhere to and germinate on the cuticle, distinct up-regulation of stress genes (Hsp30, Hsp70), and it takes 100-times more fungi to reduce larval survival by 30%.
Delaying the fungus at the surface of the cuticle provides the M larvae with more time to mobilize hemolymph defenses should the fungus eventually gain entry into the hemocoel (body cavity). Cuticular penetration of M larvae by the entomopathogen, B. bassiana, was subdued by rapid cellular (hemocyte) encapsulation [Citation12]. This was not the case when B. bassiana was applied to M larvae using intrahemocoelic injection – indicating that signals from the cuticle are relayed to the hemolymph. Mukherjee and Vilcinskas [Citation14] recently discovered that Metarhizium robertsii could sense distinct antimicrobial peptides and protease inhibitors derived from insects (e.g., G. mellonella), and respond by producing specific chymotrypsin-like and metalloproteinases to neutralize the host molecules. The intensity of this response can be attributed to the virulence of each fungal strain, and is regulated epigenetically during infection. A “good” entomopathogen colonizes and then compromises the host without killing (i.e., immune-suppression and/or immune-evasion) – thereby monopolizing the insect resources. In turn, the insect unleashes a battery of immune effectors to recognize and immobilize the fungal threat (e.g., apolipophorin III [Citation21];). Fungi-stasis can be achieved by weaponizing melanin through phenoloxidase activity [Citation22] and is accompanied by fungicidal compounds (e.g., gallerimycin). Insects also release proteinase inhibitors, e.g., IMPI [Citation20], siderophores and detoxifying agents (e.g., transferrin, glutathione-s-transferase [Citation23]) in order to thwart the fungus. The co-evolution of these attack and defense strategies has led to an intimate gene-for-gene relationship between certain EPF and insects, in addition to episodes of molecular diversification to broaden host ranges [Citation24]. Herein, our data support both observations that insects and their would-be colonizers communicate at the cuticle interface, and activate antagonistic (targeted) gene products throughout the various stages of infection.
The quantity and distribution pattern of melanic deposits in M larvae appeared to interfere with fungal adhesion and germination. As insect mortality is dependent on the conidial dose [Citation9], this could account for the differences in susceptibility for M and NM larvae. Initially, high numbers of conidia attached to both M and NM insects. This suggests that the first, passive adhesion step entailing hydrophobic interactions was unaffected. However, the second step, which involves the production of enzymes and mucilage to consolidate adhesion, was disrupted since the number of conidia adhering 12 h post-inoculation declined dramatically on M insects. Most often, a decline is attributed to fungistatic compounds in/on the cuticle, as reported for the flea beetle Psylliodes chrysocephala [Citation3,Citation8]. However, other aspects of the pathogen-cuticle interaction cannot be ignored, especially those influencing the secretion of cuticle-degrading enzymes such as ambient pH and cuticle peptide digest products [Citation3, Citation25–Citation27]. The numbers of conidia on M cuticle at 24 h and 48 h remained intact, indicating they had consolidated attachment and represent the population of conidia that landed on the more conducive, non-melanized, regions. We posit that melanization interfered with M. brunneum conidia attachment but there are many examples of EPF adhering to and infecting insects with highly melanized cuticles [Citation3]. However, differences in conidia adhesion could also be linked to the alternate expression of adhesins, Mad1 and Mad2. The insect cuticle-specific Mad1 adhesin was expressed sooner on NM than M, peaking at 24 h with significantly higher levels of transcripts being observed on NM. In contrast, Mad2, which is linked with conidial attachment to plants [Citation26], was highly expressed at 12 h on M insects and produced for longer when compared to the NM hosts. Barelli et al. (2011) [Citation27] found that Mad2 was up-regulated in M. robertsii by nutrient starvation but not oxidative or osmotic stress. In contrast, Hsp30 and Hsp70 were up-regulated by oxidative stress even under nutrient-rich conditions [Citation28]. The genes associated with the development of infection structures (cag8) and nutrient assimilation (nrr) were highly expressed on M larvae at 12 h pi, demonstrating that M. brunneum ARSEF 4556 was responding to different cues and presumably implementing different infection strategies [Citation28,Citation29].
Besides poor adhesion, infection of M larvae would be slower because melanized cuticles are harder to degrade [Citation30]. Melanic polymers protect against enzyme degradation and liberation of nutrients (i.e., steric hindrance). The cuticle-degrading proteases Pr1 and Pr2 release melanin from cuticles by hydrolysis of the associated protein [Citation31]. Pr1 is a major cuticle degrading enzyme expressed by EPF in response to cuticle and nutritional cues with the Pr1a being the dominant of eleven (a-k) isoforms [Citation32,Citation33]. Surprisingly, Pr1a and Pr1b expression were not detected here but Pr2 levels were elevated on both M and NM cuticles. It is possible that M. brunneum ARSEF 4556 used here lacks the genes encoding Pr1, as previous work has characterized the presence of spontaneous Pr1a/b deficient mutants within wild-type Metarhizum spore populations [Citation34]. We presume that the expression of Pr2 is intended to compensate for Pr1 deficiencies during the first critical 48 h. However, we cannot rule out the likely possibility that other virulence factors, such as chymotrypsin-like proteinases and metalloproteinases, compensate for Pr1 absence (especially as we detected the elevated expression of IMPI on infected G. mellonella). The M cuticle did induce elevated Pr2 levels earlier than the NM cuticle demonstrating the ability of the fungus to implement different attack strategies in response to surface chemistry cues. The capacity for M. brunneum to compensate for a decline in Pr1 was reported for a Pr1-deficient mutant [Citation34]. This mutant, although less virulent overall than the wild type, was more virulent for wax moth larvae than the more melanized mealworm larvae [Citation34]. Rosas-Garcia et al. (2014) [Citation35] reported variations in the expression of Pr1 and Pr2 and suggested that these enzymes are pathogenicity (not virulence) determinants. They found the most virulent strain of M. anisopliae for Spodoptera exigua was one that produced the most Pr2. Similarly, Golo et al. (2015) [Citation36] could not establish a relationship between Pr1 and virulence of M. anisopliae against ticks. These findings are contrary to those of other workers who found Pr1 being an important virulence determinant [Citation37]. Altogether, these observations suggest that strains of EPF have diverged as to which enzymes to deploy when infecting different hosts.
Alkane, alkene and fatty acid hydrocarbons at the surface of the insect cuticle can influence spore attachment, germination, and viability [Citation3,Citation38]. Crespo et al. (2002) [Citation39] found that some alkanes enhanced fungal virulence, while Jarrold et al. (2007) [Citation40] suggest that simple polar compounds at the cuticle surface may be required to stimulate germination before the entomopathogenic fungus can utilize more complex blends of nonpolar lipids. Herein, the relatively low quantities of hydrocarbons at the surface of the M cuticle may represent a nutrient-restricted environment. This would require less investment of energy than insects dependent on the secretion of copious amounts of antifungal compounds. Interestingly, an earlier study showed that the G. mellonella cuticle lacked fungistatic fatty acids [Citation41].
Heat shock proteins 30 and 70 play pivotal roles in stress management during insect, plant and vertebrate mycosis [Citation6,Citation42,Citation43]. Both heat shock proteins were expressed in M. brunneum ARSEF 4556 from 12 h onwards with levels being significantly higher on M larvae. The expression coincides with the second, active phase of conidia adhesion (12 h pi) with another burst of activity during penetration and colonization (72 h pi). It is likely that the fungus was responding to different stresses. The initial peak, especially Hsp30, is likely due to nutritional and oxidative stress [Citation27]. High levels of reactive oxygen species are produced by the host, which triggers a concomitant antioxidant response by the fungal pathogen [Citation44,Citation45]. Heat shock proteins can limit damage and may explain why they were elevated in both M and NM larvae. The enhanced expression of Hsp70 by M. brunneum at 12 h on M larvae during the critical penetration and colonization phase likely reflects the prolonged attempt to overcome the reinforced cuticle of the M morph as outlined above.
Generally, the up-regulation of immune genes was faster in M than NM larvae demonstrating that signalling is not impaired by either cuticle thickness or degree of melanization. However, differences in the temporal expression of immune and stress management genes likely reflect differences in the “translation” of these signals. For example, DOPA-decarboxylase activity was twofold higher in M larvae and partially accounts for the eumelanin load observed. In insects, L-dihydroxyphenylalanine (L-DOPA) is an endogenous substrate of phenoloxidase enzymes in the hemolymph, however, DOPA-decarboxylase converts L-DOPA into dopamine, which tends to be re-directed to the insect cuticle and oxidized by laccases (para-diphenoloxidase) into melanic polymers [Citation22]. Dopamine itself can be a marker of stress during fungal infections with “bursts” of dopamine fuelling the development of local defense reactions of NM larvae [Citation46]. When M. brunneum started to penetrate the cuticle of our M larvae there was no evidence of further release or de novo synthesis of the pigment. It is possible that M larvae either pre-allocate the maximum melanogenesis-associated resources into the cuticle, or, the toxic by-products of melanin precursors would exceed the tolerance levels of the host. Excessive levels of melanin and its precursor quinones are not only harmful to the invading pathogen but can prove lethal for the insect if they are not modulated [Citation47–Citation49].
If conidia manages to overcome the front-loaded melanin-associated defenses, they face higher levels of the anti-fungal peptide gallerimycin, and the multi-functional β-glucan binding protein apolipophorin III [Citation21]. Antibacterial peptides such as gloverin would have a limited impact on EPF but deter the establishment of opportunistic saprophytic microbes [Citation3,Citation14]. Apolipophorin III enables G. mellonella larvae to discriminate between pathogens and mount an adequate cellular immune response [Citation50].
During the hemocoel colonization phase (72 hpi), M. brunneum elicited a strong antioxidant (FPx, GST) response in wax moth larvae with activity being dose-dependent. The response was greater in NM insects than M larvae due to more rapid and higher infection – resulting in greater numbers of colonizing hyphal bodies. In contrast, transferrin levels increased with dose in the NM but not M larvae, reflecting a divergence in the regulation of this siderophore. As new regions of the NM cuticle became melanized during fungal invasion, it would be sensible to employ detoxification machinery to avoid collateral damage – an approach that has been recently reported in G. mellonella during hemocyte encapsulation [Citation23].
The M morph of G. mellonella is evolutionarily primed to withstand EPF and can survive doses of M. brunneum and B. bassiana that are lethal to NM larvae. Such immune-vigor does have trade-offs. In our previous study, we characterized reduced fecundity in M larvae compared to NM, but markedly higher resistance to B. bassiana [Citation12]. Selective breeding of the M morph of G. mellonella over 25 generations led to enhanced resistance against B. bassiana – characterized by higher PO activities and expression of defense genes in the integument [Citation13]. High levels of contigs 233 and 704 suggest that cell proliferation and regeneration in M insects could help repair the sites of fungal penetration faster than the average NM larva. Therefore, the M larvae are quick to respond to microbial attack and quick to heal/repair.
Concluding remarks
Our work has taken advantage of a previously reported distinct (melanic) morph of G. mellonella, and examined whether melanization plays a vital role in anti-infective defense of insects and how parasitic fungi cope with hosts differing in susceptibility to microbial attack. We provide strong evidence that the entomopathogenic fungus, M. brunneum ARSEF 4556, can distinguish between the cuticular properties of these phenotypically distinct morphs of G. mellonella, melanic (tolerant) and non-melanic (susceptible). Upon encountering the NM larvae, M. brunneum activated an insect-specific attack strategy and proceeded with colonizing, penetrating and killing the host. Conversely, M. brunneum initiated a broader approach, e.g., plant-specific adhesion gene Mad2, when exposed to the tolerant M larvae, and demonstrated a reduced capacity to overcome the hosts’ preformed defenses. We contest that the cuticle surface of the M larvae is a nutrient poor, “stressful” environment leading to fewer fungi establishing themselves.
Collectively, these data enhance our understanding of insect-pathogen interactions, particularly with regards the intimate molecular traffic that make-up these “skirmishes on the cuticle” and versatile strategies used to outcompete each other. In response to distinct morphs of G. mellonella, M. brunneum demonstrates plasticity in gene expression and putative host range, which could be exploited for biocontrol efforts.
Materials and methods
Insects
Two separate geographic populations of the greater wax moth, Galleria mellonella, were used: the melanic (M) morph, and a non-melanic (NM) (). All insects were maintained at 28°C on an artificial medium (AM) as described in Dubovskiy et al., (2013) [Citation12]. Wax moth larvae were photographed (in triplicate) under magnification (400x) with a Zeis Axio Imager A1 (Carl Zeiss, Germany). ImageJ 1.45 (National Institute of Health, USA) was used to quantify the extent of melanization and the size of melanic deposits on each cuticle (n = 10 per morph). The average cuticle thickness was calculated from the eighth sternite region of uninfected M (n = 35) and NM (n = 35) final instar larvae. Further details are provided in the Supplementary Materials S1.2.
Fungal infections
Metarhizium brunneum ARSEF 4556 was used for all experiments. Unless otherwise stated, insects infected by topical application were final instar larvae raised in the same cohort, and sampled at 6, 12, 24 and 48-h post-infection (pi). Three topical doses of M. brunneum (1 × 104, 1 × 105 and 1 × 107 conidia/ml) were used to determine the susceptibility of M and NM larvae to fungal infection over 12 d. To determine the resistance ratio (RR) of M and NM larvae, the LC50 of the M line was divided by the LC50 of the NM line. StatPlus 2009 (AnalystSoft Inc.) was used to conduct Probit analysis of the dose-mortality data to determine the LC50 value (i.e. concentration to kill 50% larvae), 95% fiducial limits of the LC50, the slope of the dose-mortality line, and the standard error of the slope. LC50 values were considered significantly different if their 95% fiducial limits did not overlap, which is a conservative criterion.
Topical exposure of M and NM G. mellonella larvae with two doses of M. brunneum conidia (1 × 105 and 1 × 107) were used to investigate insect and fungal gene expression (see the section below). The experimental sampling times reflected the optimal intervals to observe the acute stages of mycosis (when germination and penetration should be peaking in susceptible insects) and concomitant insect defences. Further details of fungus culture and inoculation methods are provided in the Supplementary Materials S1.3
Conidial adhesion and germination
Conidial adhesion and germination on the surface of the M and NM larval cuticles were assessed using methods adapted from Ment et al. (2010) [Citation51]. Further details are provided in the Supplementary Materials S1.4.
Fatty acid and hydrocarbon compositions of G. mellonella epicuticular waxes
The fatty acid composition of the wax layer was determined using whole larvae before infection. Briefly, 3 independent groups of 20 larvae were immersed in 20 ml 99% dichloromethane and incubated in a rotary shaker (130 rpm) at 22°C for 5 min. Samples were dried under a stream of nitrogen before examination by GC-MS. Fatty acid methyl esters (FAMEs) were analyzed by an Agilent 6890GC interfaced directly to an Agilent 5975 mass spectrometer (split/splitless injection, 80:1 split ratio; 70eV, EI). The separation was achieved by a HP-INNOWAX (Agilent, USA) capillary column (30 m x 0.32 mm; film thickness 0.25 μm). The oven temperature program was held at 120°C for 9 min, and ramped at 20°C min−1 to 230°C where it was held for 10 min. The carrier gas was helium. Identification was achieved using the National Institute of Standards and Technology (NIST) spectral library and compared with published spectra. Quantification was achieved using a calibration curve of known amounts of C17 FAME using the same conditions as the samples. Peak areas were used for quantifying fatty acid and hydrocarbon compositions.
Quantitative RT-PCR analysis of insect immunity-related and fungal virulence gene expression
To identify resistance factors, a comparison of the expression of genes was made in the integuments of M and NM larvae under native conditions (uninfected) and during fungal infection. Thirteen genes previously attributed to the immune response, repair, regeneration and stress regulation in G. mellonella were investigated [Citation12,Citation13]: antimicrobial peptides (gallerimycin, gloverin), the multi-functional protein apolipophorin III, siderophores (transferrin and ferritin), the insect metalloproteinase inhibitor (IMPI), heat-shock protein 90 (HSP-90), oxidative stress (peroxidase (contig 17,373) and glutathione-S-transferase (GST)); cell proliferation (Contigs 704 and 233), and two enzymes involved in melanin synthesis (prophenoloxidase (PPO) and DOPA-decarboxylase (DCC)) .
Comparisons of fungal virulence and stress management gene expression were made across both M and NM larvae after topical exposure (i.e., the presence of fungi on the integument). Eight genes previously detected as a part of virulence and stress regulation in Metarhizium sp. were investigated [Citation12,Citation13]. Such genes code for Subtilisin-like proteases (Pr1a/b and Pr2), heat-shock proteins (HSP30 and HSP70), adhesin-like proteins (MAD1, MAD2), one multifactorial transcription factor cag 8 (responsible for hydrophobin synthesis and mycelial growth), and one nitrogen regulator (nrr) [Citation3].
Gene expression (mRNA) was measured by quantitative, reverse transcriptase PCR using normalized cDNA samples with an CFX96 Touch™ Real-Time PCR Detection System (Bio Rad, USA) relative to reference genes, Elongation Factor 1-alpha (EF1; AF423811) for insects and Translation elongation factor 1-alpha (tEF; XM_014686196.1) for fungi [Citation52]. Reactions were prepared by following the manufacturer’s protocol for the Rotor-Gene SYBR Green PCR mix (Qiagen). Further details are provided in Supplementary Materials S1.6 and S1.7, SI Table 1 and SI Table 2.
Data analyses
Data were analyzed using GraphPad Prism v7.0 (GraphPad Software Inc, USA). Data were checked for normality (Gaussian) using the D’Agostino-Pearson omnibus test, and if non-normally distributed, a more conservative non-parametric analysis was applied. For qRT-PCR data with a Gaussian distribution, Grubbs’ extreme studentized deviate (ESD) test was used to exclude extreme outliers. Triplicate samples comprising integuments from five insects were used for genes expression (qRT-PCR analysis). Adhesion of conidia, cuticular thickness, melanization and size of melanic spots, epicuticular hydrocarbons and fatty acids comparisons between M and NM morphs were made using an unpaired t-test. Germination and the number of conidia detected on cuticles were compared using two-way ANOVA (with Bonferroni post hoc tests). Individual gene comparisons were made with non-parametric one-way ANOVA (Kruskal–Wallis with Dunn’s post hoc test). Cox’s proportional hazards and survival regression was used to assess differences in mortality rates after fungal infections between M and NM larvae.
Author contributions
CC, ID, and TB conceived and designed the experiments, and provided resources. EG and ID performed the experiments. EG, ID, and CC collated and analysed the data. CC, ID, and TB wrote the manuscript. CC revised the manuscript.
Datasets are available on request
The raw data supporting the conclusions of this manuscript will be made available by the authors (ID and EG), without undue reservation, to any qualified researcher.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental Material
Download MS Word (1.6 MB)Acknowledgments
We thank Dr Christopher Cunningham (Swansea University) for access to the (unpublished) preliminary annotation of the G. mellonella genome. We thank Dr. Olga Polenogova and Dr. Vadim Kryukov for help with pilot experiments of wax moth infection, and Carolyn Greig (Swansea University) for some technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
supplemental data for this article can be accessed here.
Additional information
Funding
References
- Liao C, Upadhyay A, Liang J, et al. 3, 4-Dihydroxyphenylacetaldehyde synthase and cuticle formation in insects. Dev Comp Immunol. 2018;83:pp.44–50.
- Vincent JF, Wegst UG. Design and mechanical properties of insect cuticle. Arthropod Struct Dev. 2004;33(3):pp.187–199.
- Butt TM, Coates CJ, Dubovskiy IM, et al. Entomopathogenic fungi: new insights into host–pathogen interactions. In: Advances in genetics. Vol. 94. Academic Press; 2016. p. 307–364.
- Ortiz-Urquiza A, Keyhani NO. Action on the Surface: entomopathogenic Fungi versus the Insect Cuticle. Insects. 2013;4(3):357–374.
- de Faria MR, Wraight SP. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control. 2007;43(3):237–256.
- Alkhaibari AM, Lord AM, Maffeis T, et al. Highly specific host-pathogen interactions influence Metarhizium brunneum blastospore virulence against Culex quinquefasciatus larvae. Virulence. 2018;9(1):pp.1449–1467.
- Greenfield BP, Lord AM, Dudley E, et al. Conidia of the insect pathogenic fungus, Metarhizium anisopliae, fail to adhere to mosquito larval cuticle. R Soc Open Sci. 2014;1(2):140193.
- Butt TM, Ibrahim L, Clark SJ, et al. The germination behaviour of Metarhizium anisopliae on the surface of aphid and flea beetle cuticles. Mycol Res. 1995;99(8):945–950.
- Butt TM, Goettel MS. Bioassays of entomogenous fungi. In: Navon A, Ascher KRS, Editor. Bioassays of entomopathogenic microbes and nematodes. 2000. p. 141–195.
- Inglis GD, Goettel MS, Butt TM, et al. Use of hyphomycetous fungi for managing insect pests. In: Fungi as biocontrol agents. 2001. p. 23–69.
- Lovett B, St Leger RJ. Stress is the rule rather than the exception for Metarhizium. Curr Genet. 2015;61(3):253.
- Dubovskiy IM, Whitten MMA, Kryukov VY, et al. More than a colour change: insect melanism, disease resistance and fecundity. Proc R Soc B. 2013a;280(1763):p.20130584.
- Dubovskiy IM, Whitten MMA, Yaroslavtseva ON, et al. Can Insects Develop Resistance to Insect Pathogenic Fungi? Plos One. 2013b;8(4):60248.
- Mukherjee K, Vilcinskas A. The entomopathogenic fungus Metarhizium robertsii communicates with the insect host Galleria mellonella during infection. Virulence. 2018;9(1):402–413.
- Wojda I. Immunity of the greater wax moth Galleria mellonella. Insect Sci. 2017;24(3):342–357.
- Mukherjee K, Grizanova E, Chertkova E, et al. Experimental evolution of resistance against Bacillus thuringiensis in the insect model host Galleria mellonella results in epigenetic modifications. Virulence. 2017;8(8):1618–1630.
- Lim J, Coates CJ, Seoane PI, et al. Characterizing the mechanisms of nonopsonic uptake of Cryptococci by macrophages. J Immunol. 2018;200(10):3539–3546.
- Champion OL, Wagley S, Titball RW. “Galleria mellonella as a model host for microbiological and toxin research.”. Virulence. 2016;7(7):840–845.
- Lange A, Beier S, Huson DH, et al. Genome Sequence of Galleria mellonella (Greater Wax Moth). Genome Announc. 2018;6(2). DOI:10.1128/genomeA.01220-17
- Wedde M, Weise C, Nuck R, et al. The insect metalloproteinase inhibitor gene of the lepidopteran Galleria mellonella encodes two distinct inhibitors. Biol Chem. 2007;388(1):119–127.
- Whitten MMA, Tew IF, Lee BL, et al. A novel role for an insect apolipoprotein (Apolipophorin III) in beta-1,3-glucan pattern recognition and cellular encapsulation reactions. J Iimmunol. 2004;172(4):2177–2185.
- Whitten MMA, Coates CJ. Re-evaluation of insect melanogenesis research: views from the dark side. Pigment Cell Melanoma Res. 2017;30(4):386–401.
- Grizanova EV, Semenova AD, Komarov DA, et al. Maintenance of redox balance by antioxidants in hemolymph of the greater wax moth Galleria mellonella larvae during encapsulation response. Arch Insect Biochem Physiol. 2018;98(4): e21460.
- Wang C, Wang S. Insect pathogenic fungi: genomics, molecular interactions, and genetic improvements. Annu Rev Entomol. 2017;62:73–90.
- Paterson IC, Charnley AK, Cooper RM, et al. Partial characterization of specific inducers of a cuticle-degrading protease from the insect pathogenic fungus Metarhizium anisopliae. Microbiology. 1994;140(11):3153–3159.
- Wyrebek M, Bidochka MJ. Variability in the insect and plant adhesins, Mad1 and Mad2, within the fungal genus Metarhizium suggest plant adaptation as an evolutionary force. PloS One. 2013;8(3):e59357.
- Barelli L, Padilla-Guerrero IE, Bidochka MJ. Differential expression of insect and plant specific adhesin genes, Mad1 and Mad2, in Metarhizium robertsii. Fungal Biol. 2011;115(11):1174–1185.
- Fang W, Pei Y, Bidochka MJ. A regulator of a G protein signalling (RGS) gene, cag8, from the insect-pathogenic fungus Metarhizium anisopliae is involved in conidiation, virulence and hydrophobin synthesis. Microbiology. 2007;153(Pt 4):1017–1025.
- Screen S, Bailey A, Charnley K, et al. Isolation of a nitrogen response regulator gene (nrr1) from Metarhizium anisopliae. Gene. 1998;221(1):17–24.
- St. Leger R, Cooper RM, Charnley AK. The effect of melanization of Manduca sexta cuticle on growth and infection by Metarhizium anisopliae. J Invertebr Pathol. 1988;52(3):459–470.
- St. Leger RJ, Joshi L, Roberts D. Ambient pH is a major determinant in the expression of cuticle-degrading enzymes and hydrophobin by Metarhizium anisopliae. Appl Environ Microbiol. 1998;64(2):709–713.
- Bagga S, Hu G, Screen SE, et al. Reconstructing the diversification of subtilisins in the pathogenic fungus Metarhizium anisopliae. Gene. 2004;324:159–169.
- Andreis FC, Schrank A, Thompson CE. Molecular evolution of Pr1 proteases depicts ongoing diversification in Metarhizium spp. Mol Genet Genomics. 2019;1–17.
- Wang C, Typas MA, Butt TM. Detection and characterisation of pr1 virulent gene deficiencies in the insect pathogenic fungus Metarhizium anisopliae. FEMS Microbiol Lett. 2002;213(2):251–255.
- Rosas-García NM, Ávalos-de-León O, Villegas-Mendoza JM, et al. Correlation between pr1 and pr2 Gene Content and Virulence in Metarhizium anisopliae Strains. J Microbiol Biotechnol. 2014;24(11):1495–1502.
- Golo PS, Santos HA, Perinotto WM, et al. The influence of conidial Pr1 protease on pathogenicity potential of Metarhizium anisopliae senso latu to ticks. Parasitol Res. 2015;114(6):2309–2315.
- Shah FA, Wang CS, Butt TM. Nutrition influences growth and virulence of the insect-pathogenic fungus Metarhizium anisopliae. FEMS Microbiol Lett. 2005;251(2):259–266.
- Lin L, Fang W, Liao X, et al. The MrCYP52 cytochrome P450 monoxygenase gene of Metarhizium robertsii is important for utilizing insect epicuticular hydrocarbons. Plos One. 2011;6(12):e28984.
- Crespo R, Juarez MP, Dal Bello GM, et al. Increased mortality of Acanthoscelides obtectus by alkane-grown Beauveria bassiana. Biocontrol. 2002;47:685–696.
- Jarrold SL, Moore D, Potter U, et al. The contribution of surface waxes to pre-penetration growth of an entomopathogenic fungus on host cuticle. Mycol Res. 2007;111(2):240–249.
- Kryukov VY, Yaroslavtseva ON, Whitten MM, et al. Fungal infection dynamics in response to temperature in the lepidopteran insect Galleria mellonella. Insect Sci. 2018;25(3):454–466.
- Ghosh A. Small heat shock proteins (HSP12, HSP20 and HSP30) play a role in Ustilago maydis pathogenesis. FEMS Microbiol Lett. 2014;361(1):17–24.
- Bailao AM, Schrank A, Borges CL, et al. Differential gene expression by Paracoccidioides brasiliensis in host interaction conditions: representational difference analysis identifies candidate genes associated with fungal pathogenesis. Microbes Infect. 2006;8(12-13):2686–2697.
- Huarte-Bonnet C, Juárez MP, Pedrini N. Oxidative stress in entomopathogenic fungi grown on insect-like hydrocarbons. Curr Genet. 2015;61(3):289–297.
- Zhang LB, Feng MG. Antioxidant enzymes and their contributions to biological control potential of fungal insect pathogens. Appl Microbiol Biotechnol. 2018;102:4995–5004.
- Chertkova EA, Grizanova EV, Dubovskiy IM. Bacterial and fungal infections induce bursts of dopamine in the haemolymph of the Colorado potato beetle Leptinotarsa decemlineata and greater wax moth Galleria mellonella. J Invertebr Pathol. 2018;153:203–206.
- St Leger RJ, Joshi L, Bidochka MJ, et al. Construction of an improved mycoinsecticide overexpressing a toxic protease. Proc Nat Acad Sci. 1996;93(13):6349–6354.
- Cerenius L, Lee BL, Kenneth S. “The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29(6):263–271.
- Coates CJ, Lim J, Harman K, et al. The insect, Galleria mellonella, is a compatible model for evaluating the toxicology of okadaic acid. Cell Biol Toxicol. 2019;35:219–232.
- Stączek S, Zdybicka-Barabas A, Mak P, et al. Studies on localization and protein ligands of Galleria mellonella apolipophorin III during immune response against different pathogens. J Insect Physiol. 2018;105:18–27.
- Ment D, Gindin G, Rot A, et al. Novel technique for quantifying adhesion of Metarhizium anisopliae conidia to the tick cuticle. Appl Environ Microbiol. 2010;76(11):3521–3528.
- Fang W, Bidochka MJ. Expression of genes involved in germination, conidiogenesis and pathogenesis in Metarhizium anisopliae using quantitative real-time RT-PCR. Mycol Res. 2006;110(10):1165–1171.
