ABSTRACT
Candida albicans, which can cause superficial and life-threatening systemic infections, is the most common opportunistic fungal pathogen in the human microbiome. The two-component system is one of the most important C. albicans signal transduction pathways, regulating the response to oxidative and osmotic stresses, adhesion, morphogenesis, cell wall synthesis, virulence, drug resistance, and the host–pathogen interactions. Notably, some components of this signaling pathway have not been found in the human genome, indicating that the two-component system of C. albicans can be a potential target for new antifungal agents. Here, we summarize the composition, signal transduction, and regulation of the two-component system of C. albicans to emphasize its essential roles in the pathogenesis of C. albicans and the new therapeutic target for antifungal drugs.
Introduction
The infection and mortality rate of candidiasis have significantly increased in recent years due to tumor chemoradiotherapy, the widespread use of antibiotics, and the increase in the number of immunocompromised patients, such as those with HIV infection [Citation1,Citation2]. Candida albicans is the major pathogenic agent for candidiasis and one of the most common conditionally pathogenic polymorphic fungi from the human microbiome. It colonizes multiple ecological niches, including the oral cavity, reproductive mucosa, and the respiratory and gastrointestinal tract of healthy individuals. C. albicans can also cause cutaneous and mucosal infections such as thrush, vaginal infections, and life-threatening invasive infections [Citation2,Citation3]. Among the 20,788 isolates of invasive Candida collected from around the world for 20 years (1997–2016) in the SENTRY Antifungal Surveillance Program, 46.9% were C. albicans [Citation4]. The incidence of C. albicans-induced candidaemia in China is 40.1% and up to 69.8% in Norway [Citation5]. The proportion of C. albicans in ventilator-associated pulmonary candidiasis in ICU patients is as high as 46.36% [Citation6]. C. albicans is even one of the most common coinfection fungi in COVID-19 patients [Citation7].
The C. albicans colonization of different host niches depends on the capability to sense multiple environmental signals and then regulate its adaptation and switch between colonization and pathogenesis. C. albicans can transform reversibly between yeast, pseudohyphae, and hyphae forms, adapting to the stresses at different host niches and infected tissues under different conditions, including nutrition, pH value, temperature, oxidation, and immune status. C. albicans possesses a powerful signal transduction network, “the two-component system,” to continuously monitor the external environment and regulate its colonization and pathogenesis [Citation8–11]. In the two-component system, the signal is introduced by the histidine protein kinase, and transferred through a series of phosphorylation events, finally phosphorylating the response regulator protein. Compared with the one-step transduction in the two-component system of prokaryotes, eukaryotes have a more complex multi-step phosphate transduction system. The two-component system in C. albicans regulates morphogenesis, responses to oxidative and osmotic stresses, quorum sensing, virulence regulation, etc. Here we summarize and discuss the structure and function of the two-component system in C. albicans, highlighting its role in pathogenesis and as a therapeutic target for new antifungal agents.
The structure and signal transduction of the two-component system
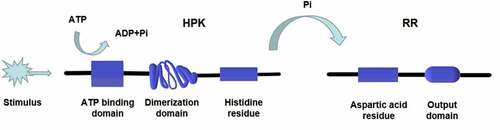
Both prokaryotes and eukaryotes, including fungi, myxomycetes, and plants, contain the two-component signaling system [Citation12–17], which can be divided into one-step and multi-step transduction modes. A typical two-component signaling system consists of a membrane-associated histidine protein kinase (HPK) and a response regulatory (RR) protein. The HPK is a dimer composed of two subunits, each containing an ATP binding domain, a dimerization domain, and a kinase domain (phosphorylation site). When the input domain of HPK is appropriately stimulated, the dimerization domain of one subunit approaches the kinase domain of the other subunit to promote phosphorylation [Citation18] (). The phosphorylation level of HPK affects the phosphorylation rate of the RR. Multiple HPKs might regulate one RR, or one HPK might regulate multiple RRs [Citation19]. RR consists of a receiving module and an output domain. The receiving module regulates the output domain activity through the phosphorylation of aspartic acid residues (Asp). The output structure might be a transcription factor regulating gene expression or a protein activity regulator [Citation18]. The two-component system was originally discovered by Ninfa and Magasanik et al. [Citation20] in the nitrogen regulatory protein system of Escherichia coli. It is a typical one-step two-component system as an HPK is autophosphorylated on a histidine residue, and the signal is subsequently transferred to an RR on an aspartate residue (). This nitrogen regulatory protein system of E. coli contains two proteins, NtrB (an HPK protein) and NtrC (an RR protein). NtrB catalyzes the transfer of a phosphate group to the aspartic acid of NtrC under nitrogen limitation conditions. The phosphorylated NtrC activates the transcription of nitrogen metabolism genes [Citation12]. On the contrary, when the concentration of amine is too high, NtrB is regulated by upstream GlnD and PII proteins to promote the dephosphorylation and inactivation of NtrC, turn off the expression of genes encoding nitrogen metabolism-related enzymes, and stop the bacteria from absorbing nitrogen from the environment [Citation21,Citation22]. In the one-step two-component signal transduction system, the phosphate group is directly transferred from the HPK to the RR (His-Asp) [Citation18].
Figure 1. Structure and phosphorylation of HPK. The HPK is a dimer composed of two subunits. Each subunit contains an ATP binding domain, a dimerization domain, and a kinase domain (phosphorylation site). When the input domain of HPK is appropriately stimulated, the dimerization domain of one subunit will approach to the kinase domain of the other subunit to promote the phosphorylation
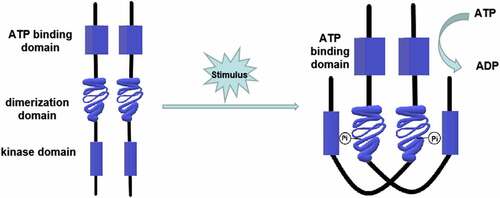
Figure 2. One-step phosphorylation of His-Asp in prokaryotes. A HPK is autophosphorylated on a histidine residue and the signal is subsequently transferred to a RR on an aspartate residue. The phosphorylated RR acts as a transcription factor regulating gene expression or a protein activity regulator. The transfer of phosphate acid from HPK to RR takes only one step (His-Asp)
In most eukaryotes, the two-component system is a multi-step phosphate transduction system [Citation23–33] (), usually consisting of a hybrid HPK, an intermediate transfer protein, and an RR. The structure and conduction pathway of the two-component system are different in various fungi. For example, C. albicans contains three HPKs [Citation34], Cryptococcus neoformans has seven HPKs [Citation32], and Neurospora crassa expresses eleven HPKs [Citation35]. The transmission mechanism is as follows. ATP is used as the donor to phosphorylate a conserved His residue called H-box after the HPK detects the stimulus signal. Subsequently, the phosphate group is transferred to the Asp residue of the same HPK receptor domain, followed by being transferred to the Asp residue of the RR receptor domain through the His residue of intermediate transfer protein. Four phosphorylation events occur sequentially, forming the four-step phosphate transfer (His-Asp-His-Asp) system (). The output components and processes of eukaryotic systems are more complex and diverse. For example, the two-component system and the downstream Hog1-MAPK pathway participate in signal transduction in C. albicans and other fungi [Citation15,Citation36], regulating the responses to oxidative and osmotic stresses, adhesion, cell wall synthesis, morphogenesis, and virulence [Citation37–48] ().
Figure 3. The two-component systems and the downstream pathways in different fungi. The two-component system in most eukaryotes is a multistep phosphate transduction model. The structure and conduction pathway of the two-component system are different in various fungi. For example, S. cerevisiae expresses only one HPK, C. albicans contains 3 HPKs, and C. neoformans has 7 HPKs. The phosphorylation level of HPK affects the phosphorylation rate of RR. Multiple HPKs may regulate one RR, while one HPK may also regulate multiple RRs
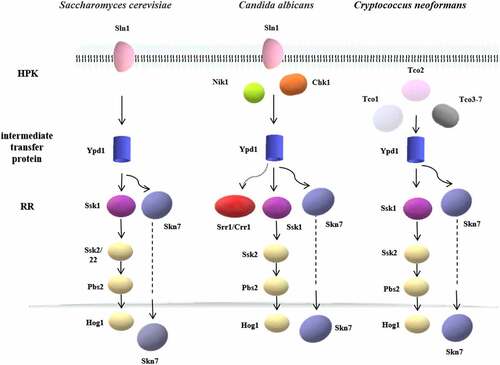
Figure 4. Multistep phosphorylation of His-Asp in fungi. After the HPK detects the stimulus signal, ATP is used as the donor to phosphorylate a conserved his residue. Subsequently, the phosphate group is transferred to the Asp residue of the same HPK receptor domain and then transferred to the Asp residue of the RR receptor domain through the his residue of intermediate transfer protein. Four phosphorylation events occur in sequence, forming the four-step phosphate transfer (His-Asp-His-Asp)
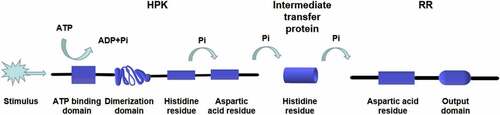
Figure 5. Two-component system of C. albicans and its downstream pathways. Seven proteins of the two-component system in C. albicans are shown, including three hybrid HPKs (Sln1p, Nik1p/Cos1p, Chk1p), three RRs (Ssk1p, Skn7p, Crr1p/Srr1p), and one intermediate transfer protein (Ypd1p). The downstream responses of two-component system are complex and diverse, which is highly related to morphogenesis, oxidative and osmotic stress, quorum sensing, virulence regulation and so on
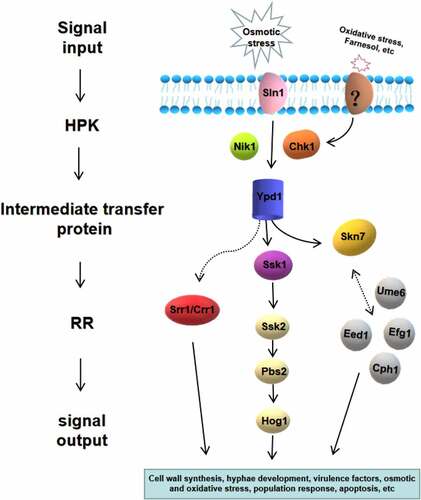
Composition of the two-component system of C. albicans
Currently, seven proteins have been identified in the two-component system in C. albicans (), including three hybrid HPKs (Sln1p, Nik1p/Cos1p, and Chk1p), three RRs (Ssk1p, Skn7p, and Crr1p/Srr1p), and one intermediate transfer protein (Ypd1p) [Citation49,Citation50]. Ssk1p, Skn7p, and Crr1p/Srr1p are located within the cytoplasm, nucleus, and mitochondria, respectively [Citation51–53].
Table 1. Components and functions of the two-component system of C. albicans.
HPKs of C. albicans (Sln1p, Nik1p/Cos1p, and Chk1p): Sln1p, a homolog of Saccharomyces cerevisiae Sln1, was initially identified in C. albicans by Nagahashi et al. [Citation43]. Sln1p consists of 1373 amino acids, including a histidine kinase domain and a C-terminal receptor domain [Citation54], with two transmembrane helices and can rescue the function in S. cerevisiae SLN1 null strain, indicating that the function of SLN1 is similar to ScSLN1. Nik1p/Cos1p contains 1081 amino acids and is an apparent ortholog of group III histidine kinases [Citation43,Citation54–57]. The NcNik1/Os1p (N. crassa), BcBos1p (Botrytis cinerea), AbNik1p (Alternaria Brassicicola), NcNik1p (Parastagonospora nodorum), and ChNik1p (Cochliobolus heterostrophus) also belong to this class [Citation57–60]. Nik1p has two H-box domains (H1 and H2) and is considered a cytoplasmic enzyme as it lacks a transmembrane domain [Citation43,Citation61]. The N-terminus of Nik1p contains 9 HAMP (histidine kinases, adenylylcyclases, methyl accepting chemotaxis proteins and phosphatases) domains, where mutations lead to the most severe osmosensitivity and dicarboximide resistance phenotypes [Citation57,Citation62,Citation63]. Although the structure of Nik1p is similar in these different fungi, the roles of orthologous proteins are not identical. For example, CaNik1p has no apparent effect on osmotolerance but is necessary for normal serum-induced hyphal growth [Citation44,Citation55]. The absence of Nik1p resulted in a near-complete loss of virulence in A. brassicicola [Citation60]. P. nodorum NIK1 deletion reduced asexual sporulation in vitro [Citation59]. Chk1p is composed of 2471 amino acid residues and contains a specific serine/threonine kinase and GAF domains (cGMP-phosphodiesterase, adenylyl cyclase and a formate hydrogen lyase transcriptional activator) [Citation61]. Chk1p might be a soluble protein as it has neither any trans-membrane hydrophobic domain nor localization signal domain. Currently, the mode of its sensory stimulation is unclear [Citation64].
Intermediate transfer protein of C. albicans (Ypd1p): Ypd1p serves as an intermediate transfer protein to transfer phosphate groups from HPK to RR and YPD1 can complement the S. cerevisiae YPD1 mutation defected functions [Citation65,Citation66]. Ypd1p is localized in both the nucleus and cytoplasm [Citation67] and encodes a protein of 184 amino acids and may regulate the phosphorylation of Ssk1p (cytoplasm) and Skn7p (nucleus) RRs [Citation45,Citation67–69], but the specific mechanism is not fully understood. YPD1 is the central molecule of the two-component system, and a decrease in YPD1 activity is expected to compromise fungal fitness, virulence, and viability [Citation70]. YPD1 inhibition is fatal to S. cerevisiae and C. neoformans [Citation49,Citation70–72]. However, C. albicans can adapt to the continuous activation of Hog1-MAPK triggered by YPD1 deletion, actively reducing the level of phosphorylated Hog1 [Citation49], indicating that the function of YPD1 seems to be different among fungal species.
RRs of C. albicans (Ssk1p, Skn7p, and Crr1p/Srr1p): Ssk1p is a structural homolog of both S. cerevisiae Ssk1p and Schizosaccharomyces pombe Mcs4p [Citation52]. Ssk1p is located downstream of the Sln1p-Ypd1p pathway and plays a vital role in cell wall biosynthesis, virulence factor regulation, polymorphonuclear neutrophils (PMNs) immune evasion, osmotic stress response, and antioxidative stresses of C. albicans [Citation40–42,Citation73,Citation74]. Skn7p is a heat-shock transcription factor of fungi, initially found in S. cerevisiae. When cells receive thermal or oxidative stimulation, the signal is transmitted along Sln1p-Ypd1p, eventually phosphorylating Skn7p to regulate gene expression [Citation45,Citation53,Citation68,Citation75–77]. In C. albicans, Skn7p plays an essential role in oxidative stress and morphogenesis, but it has less effect upon the maintenance of the cell wall and the osmotic stress response [Citation53,Citation78–80]. CRR1/SRR1 is a newly discovered RR in the CUG branch of Candida [Citation51,Citation81–83]. Bruce et al. [Citation82] reported that it was located in the cytoplasm and nucleus, with little virulence effect, while Mavrianos et al. [Citation51] showed that Srr1p is located within the mitochondria of C. albicans and plays an important role in virulence, morphogenesis, apoptosis, osmotic and oxidative stress, etc. [Citation51,Citation83], indicating that the localization and function of Crr1p/Srr1p needs further investigation.
Functions of C. albicans two-component system
Cell wall integrity
The cell wall is the main organelle of fungi, which determines its viability, cell shape, and interactions with the environment, especially in mediating adhesion and host immune response [Citation84,Citation85]. The differences in cell wall mannan and mannoprotein compositions between yeast and hyphal phases lead to marked differences in the cytokine profiles exhibited by different types of C. albicans cells [Citation86]. The RR (Ssk1p) and each type of HPK in the two-component signaling system are critical for cell wall assembly in C. albicans [Citation10,Citation40,Citation42–44,Citation55,Citation56,Citation64,Citation87–89]. There are numerous changes in the cell wall structure of CHK1 mutants, including the truncation of mannan oligosaccharide and β-1,3-glucan (shortened by about 50%) and β-1,6-glucan (increased about four-fold) levels [Citation61,Citation90]. Interestingly, these two glucans are also indirectly regulated by the hyphal-specific gene (RIM101) under different pH conditions [Citation8,Citation91,Citation92]. The killing efficiency of neutrophils to Candida was lower when cell wall mannan was added, suggesting that the changes of glucan and mannan in CHK1 mutants might lead to enhanced PMN response [Citation93,Citation94]. The adhesion and invasion of SSK1 and CHK1 mutants to the reconstituted human esophageal tissue (RHE) was lower than that of wild-type strains [Citation38,Citation42], which might be attributed to the changes in cell wall components of CHK1 mutants and the down-regulation of Als1p [Citation95] of SSK1 mutants [Citation41,Citation42,Citation96]. Besides, both SLN1 and NIK1 mutants altered the transcription levels of some N- and O-mannosyltransferases, suggesting their role in cell wall assembly and maintenance [Citation61].
Hyphal forms and virulence
Many factors are believed to be related to the virulence of C. albicans, including the expression of adhesion molecules (adhesins and extracellular enzymes), immune escape (cell wall mannoprotein and phagocytosis interference), and the morphological transformation of yeast to pseudohyphae/hyphae [Citation9,Citation42,Citation89,Citation97,Citation98]. A complex transcriptional regulatory network controls the morphological transformation of C. albicans [Citation8]. Many environmental factors can initiate or inhibit the morphogenetic switch, such as pH, temperature, serum, presence or lack of specific nutrients, etc [Citation80,Citation89,Citation99–101]. Hypha-specific genes (HSGs) have been classified into at least three groups, including transcription factors (CPH1 and EFG1), genes encoding the mitogen-activated protein (MAP) kinase signaling pathway (MEK1 and CST20), and genes expressed only during hyphal growth: hyphal cell wall protein (HWP1) and candidalysin (ECE1). A basic correlation has been established between hyphal growth defects and virulence [Citation8]. The virulence of C. albicans with SLN1 or NIK1 deletion is decreased, while the deletion of CHK1 resulted in loss of virulence, in line with the hyphal defect [Citation44]. The yeast to hyphae transition depends not only on induction conditions but also on the physical state of the medium (solid or liquid) [Citation40,Citation102–106]. The hyphal forms of the NIK1 mutant cultured in 30°C liquid media could not be distinguished from that of the wild-type strain, while the hypha formation of NIK1 mutants was defective on a solid agar plate at 37°C [Citation44,Citation55,Citation56,Citation87]. CHK1 and SSK1 mutants had a hypha-forming defect on medium 199 (pH = 7.5), Spider medium, and serum-mediated solid medium. However, they developed hyphae and flocculate extensively in liquid media, possibly due to the false expression of proteins on the cell surface [Citation18,Citation40,Citation44,Citation89,Citation107]. CHK1 mutants can also form hyphae similar to the wild type strain but down-regulate the expression of virulence factors in liquid media when co-cultured with oral epithelial cells, indicating its critical role in oral candidiasis [Citation107]. Interestingly, cell aggregation occurs not only in liquid media, but also on solid media. The CHK1 mutant formed smooth colonies on solid media probably because the cells aggregated in the colonies and could not grow normally to form the same fuzzy colonies as the wild-type strains [Citation89,Citation90].
The SSK1 mutants were defective in hypha development on the nitrogen-rich solid media; however, they formed many hyphae and invaded the solid agar on nitrogen-limited solid media. Therefore, SSK1 might not be required to develop hyphae but might play a role in hypha regulation [Citation40].The TPK1-encoded protein kinase A (PKA) plays a critical role in regulating morphogenesis of C. albicans [Citation80,Citation108]. The hyphal formation defect of TPK1 mutants on solid media was similar to that of SKN7 mutants [Citation53,Citation80,Citation108], suggesting that SKN7 may be related to TPK1 [Citation80], but further evidence is needed to reveal the interaction between them. SKN7 was also closely correlated with hyphal-specific genes such as CPH1, EED1, EFG1 and UME6 [Citation80]. Overexpressing SKN7 in wild type strains were formed wrinkled colonies and contained filamentous cells. However, the overexpression of SKN7 in CPH1, EED1 or EFG1 mutants did not appear wrinkled colonies and only yeast cells were found, while overexpression of SKN7 in UME6 mutants resulted in slightly wrinkled colonies with yeast cells and pseudohyphae [Citation80]. These results suggest that CPH1, EED1, EFG1 and UME6 are essential for SKN7 function in morphogenesis regulation. SKN7 was closely correlated with hyphal-specific genes such as CPH1, EED1, EFG1, and UME6 [Citation80] (). The hypha formation is also related to the accumulation of reactive oxygen species (ROS) [Citation109]. When C. albicans are exposed to hypha-inducing solid media, SKN7 is required to limit the accumulation of ROS [Citation80]. A limited number of studies have evaluated intermediate transfer proteins, but it has been reported that the deletion of Ypd1p increased the hypha formation and flocculation of C. albicans in liquid media [Citation67]. However, it seems that decreased virulence was not due to the absence of hyphae in vivo [Citation110]. The virulence of NIK1 mutants is significantly lower than that of the wild-type strain; however, they still form extensive pseudohyphae in tissues [Citation110]. Hypha formation of SKN7 mutants is defective, but the virulence does not decrease [Citation53,Citation96]. In the downstream MAPK pathway of the two-component system, both PBS2 and HOG1 mutants attenuated virulence in a mouse model of disease [Citation111], indicating that PBS2 and HOG1 positively regulate the virulence of C. albicans. Currently, the regulation of SSK2 on virulence has not been reported.
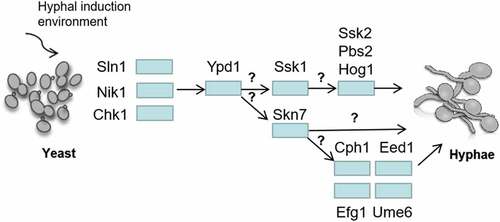
Figure 6. Regulation of C. albicans hyphal development by two-component system. Sln1p, Nik1p and Chk1p transfer the regulation signals to RR through Ypd1p. It is still unknown how to distinguish and transmit signals to the downstream RR (Ssk1p and Skn7p). The hyphal forms of NIK1 mutant cultured in 30°C liquid media is similar with the wild-type strain, while it was defective on a solid agar at 37°C. CHK1 mutants and SSK1 mutants have a hyphal formation defect on solid medium, but they can develop hyphae and flocculate extensively in liquid media. The deletion of YPD1 increased the hyphae formation and flocculation in liquid media. Overexpressing SKN7 in EED1, EFG1, CPH1 and UME6 mutants did not show the similar wrinkled and contained filamentous cells compared to that in wild type strain, suggesting that EED1, CPH1, UME6 and EFG1 are essential for SKN7 function in morphogenesis, but the mechanisms are still unknown
The virulence and immune system evasion of C. albicans seem to be tissue-specific [Citation42,Citation112]. CHK1 and SSK1 mutants are both nontoxic in the disseminated murine model of candidiasis; however, CHK1 mutants are toxic in the rat model of vaginitis [Citation40,Citation73,Citation87,Citation113]. This could be associated with the difference in pH value between the surface of vaginal mucosa and blood (acid vs neutral) resulting in differential C. albicans gene expression at these two sites. Increased production of neutrophil-dependent lactic acid induces cell wall remodeling, masking critical pathogen-associated molecular patterns (PAMPs), such as glucans, blocking immune recognition, and allowing C. albicans to colonize and invade the host [Citation114]. PMNs are essential for the host’s resistance to invasive candidiasis, but they are not observed in cell infiltrations of vaginitis in rats [Citation115–117]. Although PMNs are recruited in the vagina, they do not impact the clearance of C. albicans [Citation112]. Compared with parental strains, SSK1 and CHK1 mutants are more susceptible to growth inhibition and killing efficacy of PMNs [Citation74,Citation116]. The sensitivity of SSK1 mutants to human neutrophil defensin-1 (HNP-1) was higher than that of wild-type strains [Citation74].
It is noteworthy that inhibiting the expression of YPD1 increases C. albicans virulence and its ability to kill macrophages, which might be related to the phenotype of increased hyphae [Citation49]. The SKN7 mutants were significantly less susceptible to the killing by PMNs than the SSK1 mutants, and their virulence in the disseminated murine candidiasis model was only mild or not weakened [Citation53]. By knocking out the HPK gene and constructing combinations of single and double mutants, the CHK1 and SLN1, and CHK1 and NIK1 double mutants were survivable, while the SLN1 and NIK1 double mutants could not be constructed, indicating that the pairing loss of these kinases was a fatal event [Citation44]. Moreover, C. albicans lack of Chk1p was nontoxic in the disseminated murine candidiasis model; however, if CHK1 mutation was accompanied by SLN1 or NIK1 deletion, both hyphal development and virulence of the mutant were enhanced [Citation44]. Deletion of both SSK1 and HOG1 can negatively regulate the expression of CHK1 [Citation41,Citation96], suggesting that a complex HPK interaction regulates the development of hyphae and virulence [Citation18] (). In addition, the SSK1 mutant also down-regulated the expression of the following hypha regulation and virulence factors: HYR1, HWP1, ECE1, MIG1, GCN4, RFG1 (ROX1), RBF1, RIM101, HAC1, HAP5, TUP1, NRG1, EFG1, and CPH1 [Citation73].
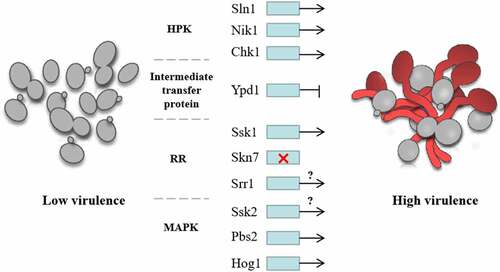
Figure 7. Regulation of C. albicans virulence by two-component system. The virulence of C. albicans with SLN1 or NIK1 deletion is decreased. CHK1 and SSK1 mutants are both nontoxic in the disseminated murine model of candidiasis, suggesting that HPKs and SSK1 positively regulate the virulence of C. albicans. The inhibition of the expression of YPD1 increased the virulence of C. albicans, indicating Intermediate transfer protein negatively regulated the virulence. SKN7 had little effects on the virulence of C. albicans, while the regulation of SRR1 on virulence is unclear. In the downstream MAPK pathway, both PBS2 and HOG1 mutants attenuated virulence in a mouse model, indicating that PBS2 and HOG1 positively regulate the virulence of C. albicans, while the regulation of SSK2 on virulence is still unknown
Osmotic stress sensitivity
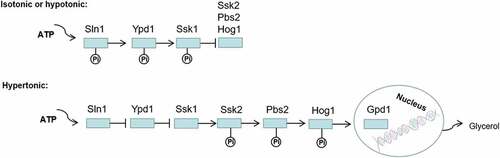
Sln1p serves as an osmotic sensor protein and regulates the Hog1-MAPK signal transduction system in S. cerevisiae and C. albicans. When the cells are in an iso-osmotic or hypo-osmotic environment, Sln1p first phosphorylates histidine residues in its kinase region and transfers phosphate groups to its aspartic acid residues, phosphorylating the downstream proteins Ypd1p (His) and Ssk1p (Asp). The phosphorylated Ssk1p cannot activate Ssk2p, leading to the shutdown of the Hog1-MAPK cascade. Another situation is that increased extracellular osmolarity deactivates Sln1p without the above-mentioned phosphate transport. A non-phosphorylated form of Ssk1p initiates the downstream signal system and continuously activates Ssk2p/Ssk22p (MAPKKK), Pbs2p (MAPKK), and Hog1p (MAPK). Finally, the phosphorylated Hog1p is transferred to the nucleus, activating transcription factors to induce the GPD1 expression, which increases the intracellular glycerol content to adapt to hyperosmotic stress (). In SLN1 or YPD1 mutants of S. cerevisiae, constitutive activation of the Hog1-MAPK pathway results in glycerol overproduction and cell death [Citation15,Citation36,Citation69,Citation118–122]. Ssk2p and Ssk22p are redundant proteins in S. cerevisiae, while Ssk2 is required for the stress-induced phosphorylation and nuclear accumulation of Hog1 in C. albicans [Citation88,Citation123]. There is another SHO1 upstream branch in the Hog1-MAPK pathway [Citation124]; however, the SHO1 branch does not significantly affect the activation of the Hog1 pathway in C. albicans [Citation125,Citation126].
Figure 8. Regulation of osmotic stress response by two-component system. Sln1p acts as an osmotic sensor protein to regulate the Hog1-MAPK signal transduction system in C. albicans. When the cells are in an isoosmotic or hypoosmotic environment, phosphorylation of Ssk1p inhibits activation of the Hog1-MAPK cascade, but in hyperosmotic cells, unphosphorylated Ssk1p activates the Ssk2/22 MAPKKK and subsequent phosphorylation of Pbs2p and Hog1p. Finally, the phosphorylated Hog1p is transferred to the nucleus, which activates transcription factors to induce the expression of GPD1, increasing the intracellular glycerol content to adapt to hyperosmotic stress
C. albicans can tolerate higher levels of osmotic stresses than many other fungi [Citation127]. Although the absence of Sln1p makes the strain, slightly to moderately, sensitive to osmotic stresses, this mutation is not fatal [Citation18,Citation43]. However, the NIK1 mutant is not sensitive to osmotic stresses, and its growth is not significantly affected by hypertonic conditions [Citation18,Citation55]. Many phenotypes of YPD1 mutants depend on the overactivation of HOG1, including increased virulence, hyphae, flocculation development, and reduced antioxidant activity [Citation49,Citation67]. It is noteworthy that S. cerevisiae Ypd1p can stabilize the Asp phosphorylation of Ssk1p and mediate the retrograde transfer of phosphate from Ssk1p to Sln1p [Citation18,Citation66,Citation69,Citation128], reducing the constitutive lethal activation of the Hog1-MAPK kinase cascade.
Oxidative stress sensitivity
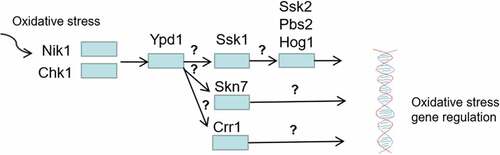
Two-component system proteins play an essential role in the oxidative stress response of C. albicans (). Of the three HPKs, the CHK1 mutant was the most sensitive to H2O2, followed by the NIK1 mutant; the SLN1 mutant was similar to the wild-type strain [Citation96]. Three RRs (SSK1, SKN7, and CRR1) are necessary for C. albicans to resist oxidative stresses [Citation41,Citation53,Citation80,Citation82]. Cells lacking SSK1 and SKN7 are more sensitive to a series of oxidants, including H2O2 and t-BOOH in vitro [Citation41,Citation53,Citation74,Citation80]. The three RRs seem to transmit oxidative stimulation signals to different downstream proteins because the phosphorylation of Hog1p under oxidative stress requires Ssk1p, which is independent of SKN7 and CRR1 [Citation41,Citation53,Citation69,Citation80,Citation82]. SKN7 activation by oxidative stresses requires functional mitochondria in S. cerevisiae [Citation41]. The YPD1 mutant of C. albicans was highly resistance to H2O2 and t-BOOH, but it was very sensitive to sodium arsenite [Citation49,Citation67].
Figure 9. Regulation of oxidative stress response by two-component system. Among the three HPKs Nik1 and Chk1 are required for activation of Ypd1 in response to oxidative stress, then the three RRs (Ssk1, Skn7, Crr1) are activated to regulate oxidative stress by transmitting oxidative stimulation signals to different downstream proteins
Quorum sensing
The relationship between cell density and hypha formation of C. albicans is similar to the quorum sensing system of some bacterial species [Citation129]. Under the same conditions, C. albicans exist in the form of yeast when the cell density is more than 106 cells/ml, while hyphae are formed when the cell density is less than 106 cells/ml [Citation130–132]. Farnesol is an important quorum-sensing molecule of C. albicans, which might inhibit biofilm formation by regulating hyphal morphogenesis [Citation132–136]. Chk1p might be the receptor of the farnesol quorum-sensing pathway of C. albicans as SLN1, NIK1, and SSK1 mutants respond to farnesol similar to the wild-type strains, while CHK1 mutants can still form biofilms when farnesol is added [Citation130]; however, the specific transmission mechanism is not clear. Farnesol may be sensed by proteins upstream of Chk1p and activate pathways containing Chk1p [Citation130], as Chk1p is a cytoplasmic protein.
Antifungal agents
Currently, classic antifungal drugs mainly include echinocandins, polyenes, and azoles [Citation137]. Due to the significant similarity between fungi and human cells in their genome, cell structure, and signal transduction pathways, the side effects and the development of drug resistance limit the application of antifungal agents [Citation4,Citation138]. The polyene antifungal drugs, such as amphotericin B, have serious hepatorenal toxicity, and azole drugs inhibit the p450-dependent enzymes of mammals, causing common adverse drug reactions such as rash, headache, gastrointestinal reactions, and hepatic injury [Citation139]. The biofilm formed by Candida on the surface of mucous membranes, dentures, central venous catheters and other medical devices can serve as physical barriers to drug or molecular penetration, making Candida inherently resistant to traditional antifungal drugs and host immune responses [Citation140–142]. Therefore, it is imperative to develop new, safe, and effective antifungal agents. The two-component system is important for the virulence and growth of bacteria and fungi, and, importantly, this signal system has not been found in the human genome sequence. Therefore, the new drug developed for the two-component system can effectively fight against fungi without damaging the host cells, making it an ideal antifungal drug target [Citation15,Citation69,Citation87,Citation143–146]. Shivarathri et al. [Citation147] reported that SSK1 and HOG1 mutations can restore the susceptibility of clinical strains of the emerging Candida species Candida auris to amphotericin B and caspofungin. The SSK1 and CHK1 mutants of C. albicans were highly sensitive to fluconazole and voriconazole, and the sensitivity of SSK1 mutants to fluconazole was 30 times higher than the CHK1 mutants, while the sensitivity of SLN1 and SKN7 mutants were slightly higher or equal to the wild-type strains [Citation148]. Both rivanol and niclosamide inhibited the two-component signal system of C. albicans and caused cell wall defects by inhibiting the hypha formation and growth. They also significantly enhanced antifungal effects when combined with fluconazole [Citation149]. The deletion of all HAMP domains of Nik1p expressed in S. cerevisiae could activate Hog1p in the absence of external stimuli, similar to the effect of bactericide treatment [Citation63], suggesting that it might also be a target for antifungal drug development. The deletion of YPD1 is not fatal to C. albicans and even enhances its virulence [Citation49]. These findings question the efficacy of YPD1 as a broad-spectrum antifungal target.
Conclusion and prospect
The two-component system plays a vital role in the activities and pathogenicity of C. albicans, and significant progress has been made in the past two decades; however, there are still many aspects to be further explored. For example, how are signals distinguished and transmitted to the downstream different RRs after the intermediate transfer protein received stimulation from different HPKs? What other functions of the Crr1p/Srr1p have not been identified? If Crr1p/Srr1p is present in mitochondria, can the HPK bypass Ypd1 in the nucleus and cytoplasm and directly transmit the signal to the RR? The different regulatory functions of the same two-component system under different conditions and the interaction of the system with other transcription factors or signaling proteins are also needs to be investigated. The components of the signal pathway have not been found in the human genome. The development of new drugs against the two-component system can effectively target fungi without damaging the host cells, to greatly reduce the toxic or side effects of antifungal drugs. The two-component system of C. albicans therefore provides the ideal therapeutic target of for new antifungal drugs against C. albicans.
Acknowledgments
This work was supported by the National Natural Science Foundation of China grant: 81870778 (BR), 81600858 (BR), 81870759 (LC); Applied Basic Research Programs of Sichuan Province, 2020YJ0227 (BR); the Youth Grant of the Science and Technology Department of Sichuan Province, China, 2017JQ0028 (LC); Innovative Research Team Program of Sichuan Province (LC); Fund of State Key Laboratory of Oral Diseases (SKLOD201913) (BR).
Disclosure statement
The authors declare no competing interests.
Data availability statement
Data sharing does not apply to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Fu J, Ding Y, Wei B, et al. Epidemiology of Candida albicans and non-C.albicans of neonatal candidemia at a tertiary care hospital in Western China. BMC Infect Dis. 2017;17(1). DOI:10.1186/s12879-017-2423-8.
- Nur Y. Epidemiology and risk factors for invasive candidiasis. Therapeutics & Clinical Risk Management. 2014; 95.
- Dadar M, Tiwari R, Karthik K, et al. Candida albicans-Biology, molecular characterization, pathogenicity, and advances in diagnosis and control-An update. Microb Pathog.
- Pfaller MA, Diekema DJ, Turnidge JD, et al. Twenty years of the SENTRY antifungal surveillance program: results for Candida species from 1997–2016. Open Forum Infect Dis.
- Quindós G, Marcos-Arias C, San-Millán R, et al. The continuous changes in the aetiology and epidemiology of invasive candidiasis: from familiar Candida albicans to multiresistant Candida auris. Int Microbiol.
- Xiao G, Liao W, Zhang Y, et al. Ventilator associated pulmonary candida infection in intensive care units in the Meizhou region of China: species distribution and resistance and the risk factors for patient mortality. Chinese Journal of Zoonoses. 2019;35(7):613–619.
- Chen X, Liao B, Cheng L, et al. The microbial coinfection in COVID-19. Appl Microbiol Biotechnol. 2020;104(18):7777–7785.
- Chen H, Zhou X, Ren B, et al. The regulation of hyphae growth in Candida albicans. Virulence. 2020;11(1):337–348.
- Calderone R, Suzuki S, Cannon R. Candida albicans : adherence, signaling and virulence. Med Mycol.
- Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9(7):327–335.
- Alves R, Barata-Antunes C, Casal M, et al. Adapting to survive: how Candida overcomes host-imposed constraints during human colonization[EB/OL]. (2020-05-01)[5].
- Nixon BT, Ronson CW, Ausubel FM. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci U S A. 1986;83(20):20.
- Thomason P, Kay R. Eukaryotic signal transduction via histidine-aspartate phosphorelay. J Cell Sci. 2000;113(Pt 18):3141–3150.
- Chang C, Stewart RC. The two-component system. Regulation of diverse signaling pathways in prokaryotes and eukaryotes. Plant Physiol. 1998;117(3):723–731.
- Wurgler-Murphy SM, Saito H. Two-component signal transducers and MAPK cascades. Trends Biochem Sci. 1997;22(5):172–176.
- Stock AM, Robinson VL, Goudreau PN, et al. Two-Component Signal Transduction. Annu.rev.biochem. 2000;69(1):183–215.
- Schaller GE. Histidine kinases and the role of two-component systems in plants. Advances in Botanical Research, 2000,32: 109–148.
- Saito H. Histidine phosphorylation and two-component signaling in Eukaryotic cells.
- Quan-Sheng Q. Two-component system: a sensor for the perception of osmotic signal in cells. Prog Biochem Biophys. 2000;27(6):593–596.
- Ninfa AJ, Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986;83(16):5909–5913.
- Pioszak AA, Ninfa AJ. Mutations altering the N-terminal receiver domain of NRI (NtrC) that prevent dephosphorylation by the NRII-PII complex in Escherichia coli. J Bacteriol. 2004;186(17):5730–5740.
- Merrick MJ, Edwards RA. Nitrogen control in bacteria. Microbiol Rev. 1995;59(4):604–622.
- Appleby JL, Parkinson JS, Bourret RB. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86(6):845–848.
- Perraud AL, Weiss V, Gross R. Signalling pathways in two-component phosphorelay systems. Trends Microbiol.
- Furukawa K, Hohmann S. A fungicide-responsive kinase as a tool for synthetic cell fate regulation. Nucleic Acids Res.
- Salas-Delgado G, Ongay-Larios L, Kawasaki-Watanabe L, et al. The yeasts phosphorelay systems: a comparative view. World J Microbiol Biotechnol.
- Day AM, Quinn J. Stress-Activated protein kinases in human fungal pathogens. Front Cell Infect Microbiol. 2019;9:261.
- Ikner A, Shiozaki K. Yeast signalling pathways in oxidative stress response. Mutat Res. 2005;569(1–2):13–27.
- He Y, Chen Y, Song W, et al. A Pap1-Oxs1 signaling pathway for disulfide stress in Schizosaccharomyces pombe. Nucleic Acids Res. 2017;45(1):106–114.
- Quinn J, Malakasi P, Smith D, et al. Two-Component mediated peroxide sensing and signal transduction in fission yeast. Antioxid Redox Signal. 2010;15(1):153–165.
- Rutherford JC, Bahn Y, Van Den Berg B, et al. Nutrient and stress sensing in pathogenic yeasts. Front Microbiol. 2019;10:442.
- Bahn YS, Kojima K, Cox GM, et al. A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol Biol Cell. 2006;17(7):3122–3135.
- Maliehe M, Ntoi MA, Lahiri S, et al. Environmental factors that contribute to the maintenance of Cryptococcus neoformans pathogenesis. Microorganisms. 2020;8(2):180.
- Kruppa M, Calderone R. Two-component signal transduction in human fungal pathogens. FEMS Yeast Res. 2006;6(2):149–159.
- Galagan J, Calvo S, Borkovich K, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422(6934):859–868.
- Posas F, Saito H. Activation of the yeast Ssk2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J. 1998;17(5):1385–1394.
- Alonso-Monge R, Navarro-Garcia F, Roman E, et al. The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryot Cell. 2003;2(2):351–361.
- Bernhardt J, Herman D, Sheridan M, et al. Adherence and invasion studies of Candida albicans Strains, using in vitro models of Esophageal Candidiasis. J Infect Dis.
- Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66(2):300–372.
- Calera JA, Zhao XJ, Calderone R. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect Immun. 2000;68(2):518–525.
- Chauhan N, Inglis D, Roman E, et al. Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryot Cell. 2003;2(5):1018–1024.
- Li D, Bernhardt J, Calderone R. Temporal expression of the Candida albicans genes CHK1 and CSSK1, adherence, and morphogenesis in a model of reconstituted human esophageal epithelial candidiasis. Infect Immun. 2002;70(3):1558–1565.
- Nagahashi S, Mio T, Ono N, et al. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology (Reading). 1998;144(Pt 2):425–432.
- Yamada-Okabe T, Mio T, Ono N, et al. Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J Bacteriol. 1999;181(23):7243–7247.
- Li S, Ault A, Malone CL, et al. The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p]. EMBO J. 1998;17(23):6952–6962.
- Morgan BA, Bouquin N, Johnston LH. Two-component signal-transduction systems in budding yeast MAP a different pathway?. Trends Cell Biol. 1995;5(12):453–457.
- San JC, Monge RA, Pérez-Díaz R, et al. The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J Bacteriol. 1996;178(19):5850–5852.
- Arana DM, Nombela C, Alonso-Monge R, et al. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology. 2005;151(4):1033–1049.
- Day A, Smith D, Ikeh M, et al. Blocking two-component signalling enhances Candida albicans virulence and reveals adaptive mechanisms that counteract sustained SAPK activation. PLoS Pathog. 2017;13(1):e1006131.
- Shor E, Chauhan N. A case for two-component signaling systems as antifungal drug targets. PLoS Pathog. 2015;11(2):e1004632.
- Mavrianos J, Berkow EL, Desai C, et al. Mitochondrial Two-Component Signaling Systems in Candida albicans. Eukaryot Cell. 2013;12(6):913–922.
- Calera J, Calderone R. Identification of a putative response regulator two-component phosphorelay gene (CaSSK1) from Candida albicans. Yeast. 1999;15(12):1243–1254.
- Singh P, Chauhan N, Ghosh A, et al. SKN7 of Candida albicans: mutant Construction and Phenotype Analysis. Infect Immun. 2004;72(4):2390–2394.
- Salas-Delgado G, Ongay-Larios L, Kawasaki-Watanabe L, et al. The yeasts phosphorelay systems: a comparative view. World J Microbiol Biotechnol. 2017;33(6):111.
- Alex L, Korch C, Selitrennikoff C, et al. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc Natl Acad Sci U S A. 1998;95(12):7069–7073.
- Srikantha T, Tsai L, Daniels K, et al. The two-component hybrid kinase regulator CaNIKl of Candida albicans. Microbiology. 1998;144(10):2715–2729.
- Catlett NL, Yoder OC, Turgeon BG. Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot Cell. 2003;2(6):1151–1161.
- Fillinger S, Ajouz S, Nicot PC, et al. Functional and structural comparison of pyrrolnitrin- and iprodione-induced modifications in the class III histidine-kinase Bos1 of Botrytis cinerea. PLoS One. 2012;7(8):e42520.
- John E, Lopez-Ruiz F, Rybak K, et al. Dissecting the role of histidine kinase and HOG1 mitogen-activated protein kinase signalling in stress tolerance and pathogenicity of Parastagonospora nodorum on wheat. Microbiology (Reading). 2016;162(6):1023–1036.
- Cho Y, Kim KH, La Rota M, et al. Identification of novel virulence factors associated with signal transduction pathways in Alternaria brassicicola. Mol Microbiol. 2009;72(6):1316–1333.
- Kruppa M, Jabra-Rizk MA, Meiller TF, et al. The histidine kinases of Candida albicans: regulation of cell wall mannan biosynthesis. FEMS Yeast Res.
- Avenot H, Simoneau P, Iacomi-Vasilescu B, et al. Characterization of mutations in the two-component histidine kinase gene AbNIK1 from Alternaria brassicicola that confer high dicarboximide and phenylpyrrole resistance. Curr Genet. 2005;47(4):234–243.
- El-Mowafy M, Bahgat M, Bilitewski U. Deletion of the HAMP domains from the histidine kinase CaNik1p of Candida albicans or treatment with fungicides activates the MAP kinase Hog1p in S. cerevisiae transformants. BMC Microbiol. 2013;13(1):209.
- Calera JA, Choi GH, Calderone RA. Identification of a putative histidine kinase two-component phosphorelay gene (CaHK1) in Candida albicans. Yeast. 1998;14(7):665–674.
- Calera J, Herman D, Calderone R. Identification of YPD1, a gene of Candida albicans which encodes a two-component phosphohistidine intermediate protein. Yeast. 2000;16(11):1053–1059.
- Janiak-SPens F, Sparling D, West A. Novel role for an HPt domain in stabilizing the phosphorylated state of a response regulator domain. J Bacteriol. 2001;182(23):6673–6678.
- Mavrianos J, Desai C, Chauhan N. Two-Component Histidine Phosphotransfer protein Ypd1 is not essential for viability in Candida albicans. Eukaryot Cell. 2014;13(4):452–460.
- Ketela T, Brown JL, Stewart RC, et al. Yeast Skn7p activity is modulated by the Sln1p-Ypd1p osmosensor and contributes to regulation of the HOG pathway. Molecular & General Genetics Mgg. 1998;259(4):372–378.
- Santos JL, Shiozaki K. Fungal Histidine Kinases. Sci Stke.
- Fassler JS, West AH. Histidine phosphotransfer proteins in fungal two-component signal transduction pathways. Eukaryot Cell. 2013;12(8):1052–1060.
- Lee JW, Ko YJ, Kim SY, et al. Multiple roles of Ypd1 phosphotransfer protein in viability, stress response, and virulence factor regulation in Cryptococcus neoformans. Eukaryot Cell. 2011;10(7):998–1002.
- Porter SW, West AH. A common docking site for response regulators on the yeast phosphorelay protein Ypd1. Biochim Biophys Acta. 2005;1748(2):138–145.
- Menon V, De Bernardis F, Calderone R, et al. Transcriptional profiling of the Candida albicans Ssk1p receiver domain point mutants and their virulence. FEMS Yeast Res.
- Du C, Calderone R, Richert J, et al. Deletion of the SSK1 response regulator gene in Candida albicans contributes to enhanced killing by human polymorphonuclear neutrophils. Infect Immun. 2005;73(2):865–871.
- Charizanis C, Juhnke H, Krems B, et al. The oxidative stress response via Pos9/Skn7 is negatively regulated by the Ras/PKA pathway in Saccharomyces cerevisiae. Mol Gen Genet. 1999;261(4–5):740–752.
- Lee J, Godon C, Lagniel G, et al. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J Biol Chem. 1999;274(23):16040–16046.
- Raitt D, Johnson A, Erkine A, et al. The Skn7 response regulator of Saccharomyces cerevisiae Interacts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative Stress. Mol Biol Cell. 2000;11(7):2335–2347.
- Murielle C, Audrey N, Vitor C, et al. A versatile overexpression strategy in the pathogenic yeast Candida albicans: identification of regulators of morphogenesis and fitness. Plos One. 2012;7(9):e45912.
- Homann O, Dea J, Noble S. et al. A phenotypic profile of the Candida albicans regulatory network. PLoS Genet. 12. 2009; (5)e1000783. doi: 10.1371/journal.pgen.1000783.
- Basso V, Znaidi S, Lagage V, et al. The two-component response regulator Skn7 belongs to a network of transcription factors regulating morphogenesis in Candida albicans and independently limits morphogenesis-induced ROS accumulation. Mol Microbiol. 2017;106(1):157–182.
- Butler G, Rasmussen M, Lin M, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459(7247):657–662.
- Bruce CR, Smith DA, Rodgers D, et al. Identification of a novel response regulator, Crr1, that is required for hydrogen peroxide resistance in Candida albicans. Plos One. 2011;6(12):e27979.
- Desai C, Mavrianos J, Chauhan N. Candida albicans SRR1, a Putative Two-Component response regulator gene, is required for stress adaptation, morphogenesis, and virulence. Eukaryot Cell. 2011;10(10):1370–1374.
- Chaffin WL. Candida albicans cell wall proteins. Microbiol Mol Biol Rev. 2008;72(3):495–544.
- Gow N, Latge JP, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr. 2017;5(3). DOI:10.1128/microbiolspec.FUNK-0035-2016
- Erwig LP, Gow NA. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol. 2016;14(3):163–176.
- Calera JA, Calderone R. Histidine kinase, two-component signal transduction proteins of Candida albicans and the pathogenesis of candidosis. Mycoses. 1999;42 Suppl 2:49-53.
- Kruppa M, Calderone R. Two-component signal transduction in human fungal pathogens. FEMS Yeast Res.
- Calera J, Calderone R. Flocculation of hyphae is associated with a deletion in the putative CaHK1 two-component histidine kinase gene from Candida albicans. Microbiology (Reading). 1999;145(Pt 6):1431–1442.
- Kruppa M, Goins T, Cutler JE, et al. The role of the Candida albicans histidine kinase (CHK1) gene in the regulation of cell wall mannan and glucan biosynthesis. FEMS Yeast Res. 2003;3(3):289–299.
- Davis D, Wilson RB, Mitchell AP. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol Cell Biol.
- Fonzi WA. PHR1 and PHR2 of Candida albicans Encode Putative Glycosidases required for proper Cross-Linking of β-1,3- and β-1,6-Glucans. J Bacteriol. 1999;181(22):7070–7079.
- Cleary JA, Kelly GE, Husband AJ. The effect of molecular weight and β-1,6-linkages on priming of macrophage function in mice by (1,3)-β-D-glucan. Immunol Cell Biol. 1999;77(5):395–403.
- Wright CD, Bowie JU, Gray GR, et al. Candidacidal activity of myeloperoxidase: mechanisms of inhibitory influence of soluble cell wall mannan. Infect Immun. 1983;42(1):76–80.
- Hoyer LL. The ALS gene family of Candida albicans. Trends Microbiol. 2001;9(4):176–180.
- Li D, Gurkovska V, Sheridan M, et al. Studies on the regulation of the two-component histidine kinase gene CHK1 in Candida albicans using the heterologous lacZ reporter gene. Microbiology. 2004;150(Pt 10):3305–3313.
- Nasution AI. Virulence Factor and Pathogenicity of Candida albicans in Oral Candidiasis. World Journal of Dentistry. 2013;4(4):267–271.
- Lengeler KB, Davidson RC, D’Souza C, et al. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev.
- Shapiro RS, Robbins N, Cowen LE. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev. 2011;75:213–267.
- Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9(10):737–748.
- Ernst JF. Transcription factors in Candida albicans – environmental control of morphogenesis. Microbiology(Reading). 2000;146 (Pt 8):1763-1774.
- Köhler JR, Fink GR. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc Natl Acad Sci U S A. 1996;93(23):13223–13228.
- Leberer E, Harcus D, Broadbent ID, et al. Signal transduction through Homologs of the Ste20p and Ste7p Protein Kinases can Trigger Hyphal formation in the pathogenic fungus Candida albicans. Proc Natl Acad Sci U S A. 1996;93(23):13217–13222.
- Liu H, Kohler J, Fink G. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266(5191):1723–1726.
- Csank C, Makris C, Meloche S, et al. Derepressed Hyphal Growth and Reduced Virulence in a VH1 Family-related Protein Phosphatase Mutant of the Human Pathogen Candida albicans. Mol Biol Cell. 1998;8(12):2539–2551.
- Sonneborn A, Bockmühl DP, Gerads M, et al. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol Microbiol.
- Zhou Y, Cheng L, Liao B, et al. Candida albicans CHK1 gene from two-component system is essential for its pathogenicity in oral candidiasis. Appl Microbiol Biotechnol. 2021;105(6):2485–2496.
- Cao C, Wu M, Bing J, et al. Global regulatory roles of the cAMP/PKA pathway revealed by phenotypic, transcriptomic and phosphoproteomic analyses in a null mutant of the PKA catalytic subunit in Candida albicans. Mol Microbiol. 2017;105(1):46–64.
- Schröter C, Hipler U, Wilmer A, et al. Generation of reactive oxygen species by Candida albicans in relation to morphogenesis. Arch Dermatol Res. 2000;292(5):260–264.
- Selitrennikoff CP, Alex L, Miller TK, et al. COS-1, a putative two-component histidine kinase of Candida albicans, is an in vivo virulence factor. Med Mycol. 2001;39(1):69–74.
- Cheetham J, MacCallum DM, Doris KS, et al. MAPKKK-independent regulation of the Hog1 stress-activated protein kinase in Candida albicans. J Biol Chem. 2011;286(49):42002–42016.
- Black CA, Eyers FM, Russell A, et al. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect Immun. 1998;66(3):1273–1275.
- Calera JA, Zhao XJ, De Bernardis F, et al. Avirulence of Candida albicans CaHK1 Mutants in a murine model of hematogenously disseminated Candidiasis. Infect Immun. 1999;67(8):4280–4284.
- Cottier F, Hall RA. Face/Off: the Interchangeable Side of Candida Albicans. Front Cell Infect Microbiol. 2019;9:471.
- De Bernardis F, Boccanera M, Adriani D, et al. Intravaginal and Intranasal Immunizations are equally effective in Inducing vaginal antibodies and conferring protection against vaginal Candidiasis. Infect Immun. 2002;70(5):2725–2729.
- Torosantucci A, Chiani P, De Bernardis F, et al. Deletion of the Two-Component Histidine Kinase Gene (CHK1) of Candida albicans contributes to enhanced growth inhibition and killing by human neutrophils in Vitro. Infect Immun. 2002;70(2):985–987.
- Fidel P, Luo W, Steele C, et al. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect Immun. 1999;67(6):3135–3140.
- Maeda T, S M W-M, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369(6477):242–245.
- Posas F, Wurgler-Murphy SM, Maeda T, et al. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component”Osmosensor. Cell. 1996;86(6):865–875.
- Maeda T, Takekawa M, Saito H. Activation of yeast Pbs2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science.
- Reiser V, Raitt DC, Saito H. Yeast osmosensor Sln1 and plant cytokinin receptor Cre1 respond to changes in turgor pressure. J Cell Biol. 2003;161(6):1035–1040.
- Singh KK. The Saccharomyces cerevisiae Sln1p-Ssk1p two-component system mediates response to oxidative stress and in an oxidant-specific fashion. Free Radic Biol Med. 2000;29(10):1043–1050.
- Cheetham J, Smith DA, Da SDA, et al. A single MAPKKK regulates the Hog1 MAPK pathway in the pathogenic fungus Candida albicans. Mol Biol Cell. 2007;18(11):4603–4614.
- Takayama T, Yamamoto K, Saito H, et al. Interaction between the transmembrane domains of Sho1 and Opy2 enhances the signaling efficiency of the Hog1 MAP kinase cascade in Saccharomyces cerevisiae. PloS One. 2019;14(1):e211380.
- Román E, Nombela C, Pla J. The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal Pathogen Candida albicans. Mol Cell Biol. 2005;25(23):10611–10627.
- Bahn Y, Xue C, Idnurm A, et al. Sensing the environment: lessons from fungi. Nat Rev Microbiol. 2007;5(1):57–69.
- Nikolaou E, Agrafioti I, Stumpf M, et al. Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol Biol. 2009;9:44.
- Janiak-Spens F, Sparling JM, Gurfinkel M, et al. Differential stabilities of phosphorylated response regulator domains reflect functional roles of the yeast osmoregulatory Sln1 and Ssk1 proteins. J Bacteriol.
- Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55(1):165.
- Kruppa M, Krom BP, Chauhan N, et al. The two-component signal transduction protein Chk1p regulates quorum sensing in Candida albicans. Eukaryot Cell. 2004;3(4):1062–1065.
- Calderone, RA. Candida and candidiasis. Washington, DC: ASM; 2002.
- Hornby JM, Jensen EC, Lisec AD, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol. 2001;67(7):2982–2992.
- Chen H, Fujita M, Feng Q, et al. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc Natl Acad Sci U S A.
- Alem MA, Oteef MD, Flowers TH, et al. Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development. Eukaryot Cell. 2006;5(10):1770–1779.
- Ramage G, Saville SP, Wickes BL, et al. Inhibition of Candida albicans Biofilm Formation by Farnesol, a Quorum-Sensing Molecule. Appl Environ Microbiol. 2002;68(11):5459.
- Cao YY, Cao YB, Xu Z, et al. cDNA microarray analysis of differential Gene Expression in Candida albicans Biofilm Exposed to Farnesol. Antimicrob Agents Chemother. 2005;49(2):584–589.
- Kai C, Ren-yi L, Lan Y. Research progress of pathogenic factors and antifungal drugs of Candida albicans. Chinese Journal of Mycology. 2017;12(3):180–183.
- Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17(12):e383.
- Yuan Z , Chen A J , Shen Y W, et al. Advances in clinical application of triazole antifungal agents. Pharmaceutical and Clinical Research. 2018;26(2):125–129.
- Nobile CJ, Johnson AD. Candida albicans biofilms and human disease. Annu Rev Microbiol. 2015;69(1):71–92.
- Soll DR, Daniels KJ. Plasticity of Candida albicans Biofilms. Microbiol Mol Biol Rev. 2016;80(3):565–595.
- Taff HT, Mitchell KF, Edward JA, et al. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013;8(10):1325–1337.
- Barrett JF, Hoch JA. Two-component signal transduction as a target for microbial anti-infective therapy. Antimicrob Agents Chemother. 1998;42(7):1529–1536.
- Koretke KK, Lupas AN, Warren PV, et al. Evolution of two-component signal transduction. Mol Biol Evol.
- Venter JC, Adams MD, Myers EW. The sequence of the human genome. Science. 2001;291(5507):1304–1351.
- Wolanin PM, Thomason PA, Stock JB. Histidine protein kinases: key signal transducers outside the animal kingdom. Genome Biol. 2002;3(10):s3011–s3013.
- Shivarathri R, Jenull S, Stoiber A, et al. The Two-Component response regulator Ssk1 and the Mitogen-Activated Protein Kinase Hog1 control antifungal drug resistance and cell wall architecture of Candida auris. mSphere. 2020;5(5). doi:10.1128/mSphere.00973-20.
- Chauhan N, Kruppa M, Calderone R. The Ssk1p response regulator and Chk1p histidine kinase mutants of Candida albicans are hypersensitive to fluconazole and voriconazole. Antimicrob Agents Chemother.
- Chen W. Study on the activity of bicomponent signal transduction system inhibitor against Candida albicans in vitro[D]. Fujian Medical University, 2011.
