ABSTRACT
The amount of ion channels and transporters present at the plasma membrane is a crucial component of the overall regulation of ion transport. The number of channels present result from an intricate network of proteins that controls the late events of channel trafficking, such as endocytosis, recycling and targeting to lysosomal degradation. Small GTPases of the Rab family are key players in these processes thus contributing to regulation of fluid secretion and ion homeostasis. In epithelia, this involves mainly the balance between the chloride channel CFTR and the sodium channel ENaC, whose misfunction is a hallmark of cystic fibrosis - the commonest recessive disorder in Caucasians. Here, we review the role of GTPases in regulating trafficking of ion channels and transporters, comparing what is known for CFTR and ENaC with other types of channels. We also discuss how feasible would be to target the Rab machinery to handle a disorder such as CF.
Trafficking and Rab proteins
Regulation of ion and solute transport at the membrane of cells depends on the balance between the function of each channel or transporter and on the number of molecules present.Citation1 The latter is regulated by the protein trafficking machinery, which controls the process through which a membrane protein is delivered from its place of synthesis – the membrane of the endoplasmic reticulum – to its final destination – the plasma membrane (PM). Besides these anterograde trafficking pathways, channels and transporters also undergo endocytosis followed by either recycling to the PM or targeting to the lysosomal compartment. These late trafficking events are controlled by several protein partners, that include protein kinases, myosins and small GTPases, among which Rab proteins.Citation1
Rab GTPases form the biggest subfamily of the Ras superfamily of small GTPases. As most members of this superfamily, they function as molecular switches alternating between 2 conformational status – a GTP-bound active form and a GDP-bound inactive form.Citation2,3 The human subfamily contains more than 60 members that reversibly associate with different intracellular membranes, due to their post-translational modification with geranylgeranyl groups.Citation4 This association with membranes promotes interactions with other components of the trafficking machinery, such as coat components, motor proteins and SNAREs.Citation2 These characteristics make them relevant players in the control of membrane identity, in vesicle formation, motility and function, regulating the trafficking of many different classes of proteins, among which channels and transporters.Citation5
Epithelia homeostasis, channels and cystic fibrosis
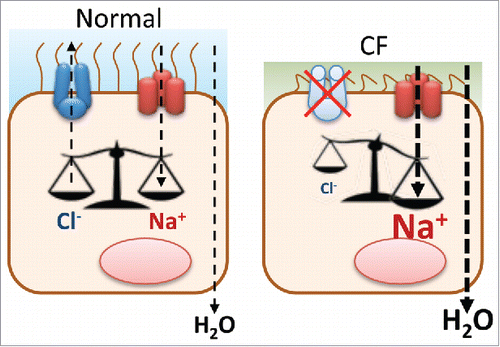
Epithelial cells - that line the internal and external body surfaces in animals - transport ions and small molecules from one to the other side of the epithelium. Channels and transporters enable epithelial cells to do this transport in a polarized way, due to their localization either in the apical or the basolateral membrane.Citation6 Among the different channels that are involved in such transport, the cystic fibrosis transmembrane conductance regulator (CFTR) and the epithelial sodium channel (ENaC) have an essential role in regulating the overall fluid homeostasis in epithelia. Dysfunction of such molecular entities leads to several human pathologies – among them is cystic fibrosis, the most common lethal autosomal recessive disorder among Caucasians, caused by mutations in the gene that encodes CFTR.Citation7 CFTR functions as a cAMP and protein kinase A (PKA) regulated Cl− channel in the apical membrane of epithelial cells, and belongs to the ABC transporters superfamily.Citation8 Lung disease is the major cause of mortality in CF - airway obstruction by thick mucus and chronic infection by different pathogens, most of the time accompanied by severe inflammation.Citation9 Despite the fact that there are more than two thousand mutations described, the most common mutation found in CF patients is a single deletion of a phenylalanine residue at position 508 (F508del), found in 70% of all CF chromosomes, which leads to the retention of CFTR in the endoplasmic reticulum (ER).Citation10 Regulation of both CFTR and ENaC trafficking is a key issue in this disorder, as there is an interplay between the 2 channels, with lack of CFTR resulting in increased activity of ENaC, an event that is responsible for many of the phenotypic characteristics of CF () (reviewed previously in Citationref. 5, 11, 12).
Figure 1. Imbalance of CFTR and ENaC in cystic fibrosis. In wt cells, CFTR is active at the PM where it secretes Cl− and regulates ENaC-mediated Na+ absorption. In CF cells, there is no functional CFTR leading to an increased Na+ absorption due to ENaC hyperactivity. These changes result in increased water uptake from the extracellular medium (resulting in the CF-characteristic viscous mucus).
Regulation of CFTR membrane trafficking by Rab GTPases
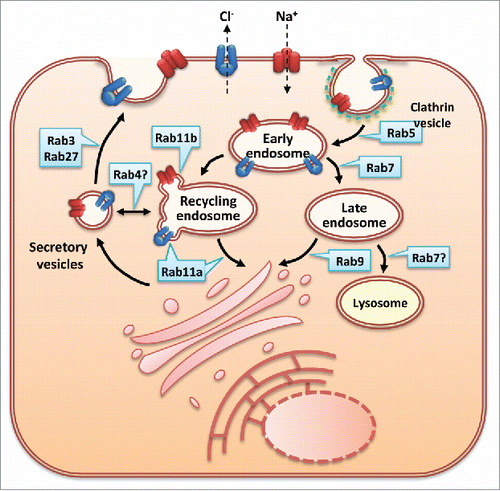
Regulation of CFTR at the PM is a complex process that involves on one side the modulation of CFTR Cl− channel activity and on the other the amount of the channel that resides at the PM at each moment. The later is regulated by endocytosis in clathrin-coated vesicles, followed by either recycling to the PM or targeting for lysosomal degradation. These trafficking events are controlled by several protein partners - including Rab GTPases (), Rme-1 and myosins as well as some protein kinases.Citation1
Figure 2. Rab proteins as regulators of CFTR and ENaC membrane trafficking CFTR (blue cartoon) and ENaC (red cartoon) are endocytosed from the PM in clathrin-coated vesicles. Several members of the RabGTPase family have been shown to modulate the trafficking of these proteins. Rab5 promotes their endocytosis of CFTR. Exit of CFTR from early endosomes is mediated by Rab7 which increases CFTR degradation mediated by delivery to late endosomes and lysosomes. Rab9 is involved in CFTR trafficking from late endosomes to the TGN. Rab11 mediates CFTR and ENaC trafficking from recycling endosomes to the PM. Rab3 and Rab27 have also been implicated in CFTR and ENaC trafficking.
The first report assessing the role of Rab GTPases in CFTR PM trafficking was done using external epitope-tagged CFTR constructs and aimed at dissecting possible differences between wt- and F508del-CFTR when expressed in BHK cells.Citation13 Besides identifying a stability defect for mutant CFTR when rescued to the PM by low temperature incubation, this study identified a role for different Rab proteins in CFTR endocytosis/recycling in BHK cells. PM CFTR is endocytosed in Rab5a-dependent step, with overexpression of a dominant negative (DN) Rab5a drastically inhibiting endocytosis and increasing CFTR PM amounts. After this initial step, Rab7 was shown to have a role in promoting early-to-late endosome and late endosome-to-lysosome transport, as the use of DN Rab7 greatly increases the intracellular pool of the protein and the overexpression of wt Rab7 decreases the amount of PM and intracellular CFTR, suggesting an increase of these 2 steps. Interestingly, it was also reported that Rab9 promotes transit to the trans-Golgi network. The same study also reported a role for Rab4 and Rab11 in CFTR recycling to the PM.Citation13 These 2 proteins had been previously shown to mediate different recycling steps (using other model proteins) – Rab4 involved in a direct recycling from early endosomes to the PM and Rab11 in a later step from recycling endosomes to the PM.Citation14,15 Gentzsch and coworkers reported that overexpression of Rab11 moves endocytosed CFTR to the PM and a minor effect for Rab4, suggesting that the Rab11 route, rather than the Rab4 one, is dominant in CFTR recycling. This study also reported that blocking of endocytosis with DN Rab5a and enhancement of recycling with overexpression of Rab11 is able to promote accumulation of rescued F508del-CFTR at the PM – one of the first studies to report that the late steps of trafficking are amenable to therapeutic modulation to promote mutant protein stability (reviewed in Citationref. 16).
The role of Rab11a in the recycling of CFTR was soon after confirmed in polarized human airway epithelial cells. Swiatecka-Urban and colleagues described a reduced apical membrane half-life for rescued F508del-CFTR when compared with wt-CFTR,Citation17 in what appeared to be an intrinsic problem of the mutant protein and not on the endocytosis machinery or on the possible upregulation of Rab5a (observed in other bronchial cell linesCitation18). A detailed characterization of the mechanism for the decreased stability of F508del-CFTR confirmed an interaction between Rab11a and either wt- or F508del-CFTR in epithelial cells and reported that the siRNA knock-down of Rab11a decreases PM expression of CFTR.Citation17 Additionally, expression of DN Rab11a decreases PM expression of F508del-CFTR whereas overexpression of a constitutively active (CA) Rab11a variant increases these levels, consistent with the view that Rab11a facilitates the endocytic recycling of wt-CFTR and rescued F508del-CFTR in polarized human airway epithelial cells.Citation17 This process involves the motor protein Myosin Vb which is thought to be recruited to the recycling endosomes through interaction with the GTP-bound form of Rab11a. In fact, myosin Vb has been shown to facilitate CFTR recycling, but not endocytosis, in human airway cells.Citation19
The role of Rab11 in CFTR trafficking was also assessed in colonic epithelial cells. In this cellular model, the 2 Rab11 isoforms – Rab11a and Rab11b – colocalize with CFTR. However, conversely to airway cells, modulation of Rab11a quantity or activity (by usage of siRNA knock-down or DN overexpression) does not affect CFTR recycling, whereas expression of DN Rab11b in polarized cells leads to inhibition of forskolin-stimulated transepithelial anion secretion and decrease in apical membrane CFTR.Citation20 Rab11a and Rab11b share 89% amino acid sequence homology, and whereas the first is expressed ubiquitously, the later is enriched in some tissues. The observed differences between these 2 studies may account for a tissue specific mechanism – that is dependent on a specific interaction partner, with each of them performing the same function in each cell type.Citation21
Recent data have also reported that the Rab11 machinery is involved in the recycling of corrector-rescued F508del-CFTR, confirming that chemical correction redirects F508del trafficking from the degradation pathway to regulated recycling – with the proteins that are involved in the process being obvious targets for the improvement of the available correctors.Citation22 Interestingly, this study reports an improvement in mutant CFTR stability due to decreased lysosomal accumulation/targeting when correctors are added acutely, suggesting that i) the compounds may also act on peripheral quality control and ii) that temperature/corrector-promoted F508del-CFTR escape from the ER does not correspond to its full correction in terms of misfolding.Citation22
Rab4 was found initially to have only a minor role in CFTR direct recycling to the PM from early endosomes in BHK cells.Citation13 Later studies reported that overexpression of Rab4 inhibits CFTR-mediated current in the colonic epithelial cell line HT29 and that, reversely, Rab4 inhibition (by overexpression of an anti-Rab4 antibody) reverses the observed inhibition of CFTR.Citation23 This reduction in CFTR function is explained by a decrease in the amount of CFTR at the apical membrane, suggesting that in Rab4 transfected cells CFTR is retained in an intracellular pool as its total levels remain unchanged.Citation23 Although no further mechanistical characterization is available, this observation suggests that, in contrast to BHK cells, Rab4 seems to have a relevant role in modulation of CFTR in, at least some, epithelial cells.
Rab 27a, that mediates various types of regulated exocytic events, has also been implicated in CFTR trafficking.Citation24 Overexpression of this GTPase decreases CFTR-mediated currents in colonic cells, an effect that does not depend on the GDP/GTP-bound status as both the GDP- and GTP-locked variants promoted the same degree of inhibition. Downregulation of Rab27a conversely leads to an increase in CFTR function and these regulation events rely on Rab27a interaction with CFTR, as assessed by immunoprecipitation. When this interaction is promoted, there is a decrease in CFTR levels at the PM and the correspondent increase of the internal/cytosolic pool of the protein. The interaction between Rab27a and CFTR is mediated by the Rab27a-specific partners Munc13–4 and synaptogamin like protein 5 (SLP5), as the overexpression of these 2 proteins partially reverses CFTR inhibition by Rab27a.Citation24
Interestingly, Rab27a role may contribute to the elucidation of some particular phenotypic manifestations of cystic fibrosis. One of the still open questions in the field is whether neutrophil dysfunction in CF results from the genetic defect or is secondary to infection and inflammation. A recent study by Pohl and colleagues shows that neutrophil dysfunction in CF patients, with either F508del or the severe gating mutation G551D, is related to an impaired degranulation of antimicrobial peptides.Citation25 In fact, CF neutrophils were observed to release less secondary and tertiary granule components and this decrease is linked to a defective activation of Rab27a. A decrease in magnesium concentration in vivo leads to a decrease in the amount of GTP-bound (thus active) Rab27a, a defect that can be reversed in G551D-bearing patients with the EMA/FDA-approved potentiator ivacaftor/Kalydeco.Citation25
Regulation of ENaC density at the cell surface by Rab GTPases
The amiloride-sensitive epithelial sodium channel (ENaC) is composed of 3 homologous subunits (α, β and γ) and maintains body salt and water homeostasis by allowing the flow of Na+ ions across high resistance absorptive epithelia, including the conducting airways, alveolar airspaces, the distal colon, the sweat duct and the distal nephron of the kidney.Citation26
Evidence of interplay between the mechanisms regulating of CFTR and ENaC surface density has arisen from data showing the concomitant and reciprocal regulation of these channels' activities (reviewed in Citationref. 27). In most epithelial tissues, CFTR activity downregulates ENaC and ENaC activation promotes CFTR activity. This can, at least partially, explain how normal airway cells switch from ENaC-mediated absorption to CFTR-mediated secretion, and why ENaC is hyperactive in CFCitation28 (). Sweat duct cells, however, are an exception since in these CFTR activity promotes ENaC activation, prerequisite for the reabsorption of sodium and chloride that results in a hypotonic sweat and retention of salt in the body during sweating.Citation29 Loss of this synergy is the cause of the hypertonic sweat characteristic of CF patients. The uneven interaction between CFTR and ENaC activities is particularly relevant in the lung of CF patients because the unbalance between CFTR-mediated Cl− secretion and ENaC-mediated Na+ reabsorption dysregulates the net amount of salt and water in the airway surface liquid (ASL), and thereby causes the thickening of airway secretions and impairs the clearance of bacteria and other contaminants from the lungs.
The importance of ENaC in the in vivo pathogenesis of CF lung disease was further highlighted by a series of studies in mice overexpressing ENaC in the airways (reviewed in Citationref. 30). To mimic increased ENaC activity observed in CF airways and prevent unrelated pathologies in other organs, the Clara cell secretory protein (CCSP) promoter element was used to target expression of ENaC to mouse airways. Since Clara cells are the most abundant cell type in mouse airways (50% - 80% of the epithelial cell content) the CCSP promoter enables tissue-specific transgene expression throughout the conducting airways of mice. Transgenic mice with airway-specific overexpression of the 3 individual ENaC subunits (α, β and γ) were engineered, but measurements of bioelectric properties demonstrated that overexpression of β-ENaC alone was sufficient to produce an up to 3-fold increase in amiloride-sensitive Na+ transport in the airways of these mice, while maintaining Cl− secretion unchanged.Citation31 Subsequent studies with these βENaC-Tg mice, demonstrated that increased ENaC-mediated sodium absorption causes ASL depletion, hyperconcentrated mucus and impaired mucus clearance, and that these defects trigger CF-like lung disease with airway mucus plugging, spontaneous infection and inflammation, and structural lung damage in vivo.Citation30 The lung phenotype exhibited by the β-ENaC ovexpressing mouse was in fact more prominent that the one exhibited by CF-knockout mice that in general fail to recapitulate this characteristic manifestation of CF.
The tight interplay between CFTR and ENaC in producing the CF airway phenotype would suggest a crosstalk between the regulatory elements of their trafficking machinery. It is therefore not surprising that several Rab proteins that have been shown to regulate CFTR trafficking also affect ENaC delivery to the PM, internalization and recycling ().
Studies in the colonic epithelial cell line HT29 showed that, in contrast to what was observed for CFTR, overexpression of Rab3 produced a considerable reduction in ENaC currents whereas the overexpression of Rab5 failed to elicit any statistically significant effect on amiloride-sensitive currents,Citation32 suggesting independent regulatory pathways for each channel. Surface protein biotinylation studies revealed that downregulation of ENaC function by Rab3 is due to a reduced apical abundance of the channel; possibly resulting from an impaired trafficking to the apical membrane, since no decreased was observed in its steady-state levels.Citation32 In colonic epithelia, Rab4, which affects CFTR activity only when inhibited, leads to an augmentation of amiloride-sensitive ENaC currents when expressed in low amounts and inhibits them when expressed at high levels.Citation33 Importantly, subcellular distribution analysis revealed that the degree of ENaC functional modulation by differential Rab4 activity correlates with reciprocal changes in levels of the channel at the cell surface and in the intracellular pool. This differential effect of Rab4 was suggested to relate to either interference with ENaC sequestration to early endosomes (at low levels of activity) or the inhibition of ENaC exocytosis (at high levels).Citation33
The stimulation of Rab27a, however, appears to produce similar effects in both CFTR and ENaC. In colonic HT29 cells, overexpression of wild type Rab27a or expression of a CA mutant (Q78L) of this Rab isoform inhibits amiloride-sensitive currents, whereas the DN mutant T23N showed no effect.Citation23,24,32,34 Moreover, at least in colonic cells, the molecular mechanism of this regulation also seems to be common since the effects of Rab27a on ENaC were also reversed by the Rab27a-binding SLP-5 and Munc13–4 accessory proteins,Citation35 similarly to what was shown for CFTR.Citation24 Rab27a appears to impair ENaC expression at the cell surface by trapping it in intracellular compartments and, as with CFTR, the presence of Munc13–4 and SLP-5 proteins interferes with Rab27a availability thus preventing its hindering of ENaC trafficking.Citation24,35 This observation diminished Rab27a as a potential target for cystic fibrosis therapy since its targeting might also lead to an increased destabilization of modulator-rescued CFTR channels.
Rab11 is another Rab protein whose activity produces similar regulatory consequences in both CFTR and ENaC. However, there might be either a Rab11 isoform or a cell type selectivity in the regulation of the 2 channels. Although, CFTR was found to localize to both Rab11a- and Rab11b- associated vesicles only interference with Rab11b, but not with Rab11a, affected CFTR-mediated anion conductance and apical recycling in polarized T84 colonic epithelial cells.Citation36 In contrast, in murine cortical collecting duct (CCD) mpkCCDc14 cells, and also in CHO and Cos-7 cells, ENaC was found colocalizing to Rab11a-associated endosomes at both the cytosol and submembrane fractions and the overexpression of wild-type Rab11a promotes ENaC trafficking toward the membrane increasing its abundance at the cell surface and nearly doubling ENaC-dependent currents.Citation34 This effect is additive with the downregulation of dynamin, indicating that Rab11a acts by actively promoting ENaC recycling back to the PM.
Studies in aldosterone-induced increases in ENaC density and function at the apical membrane of CCD cells identified the Rab GTPase-activating protein AS160 [Akt substrate of 160 kDa, also known as TBC1 domain family member 4 (TBC1D4)] as a potential regulator of ENaC trafficking.Citation37 AS160 is a known regulator of GLUT4 glucose transporter translocation to the PM upon insulin stimulation of fat and muscle cells.Citation38 Insulin stimulation triggers Akt activation which phosphorylates AS160 leading to its inactivation and binding to 14–3–3 proteins and the consequent activation of Rab 8A and 13 in myocytes, and Rab10 in adipocytes, which then facilitate the targeting of GLUT4-containing vesicles to the cell surface. In CCD cells, Na+ transport involves the increased expression of 14–3–3 proteins and their association with the ubiquitin E3 ligase Nedd4–2, a substrate of serum- and glucocorticoid-induced kinase (SGK1).Citation37 Nedd4–2 phosphorylation by SGK1 inhibits ENaC internalization via clathrin adaptor proteins that contain ubiquitin interacting motifs, such as epsin, which increases ENaC apical density in Xenopus oocytes.Citation39 SGK1 also phosphorylates AS160 downstream of aldosterone stimulation and, by doing so, promotes its interaction with 14–3–3 proteins that increases the apical trafficking of ENaC and amiloride-sensitive currents in CCD epithelia.Citation40 Whether Rab8, 10 or 13 are involved in this remains to be determined, but these findings indicate that phosphorylation of the AS160 Rab-GAP, by either Akt or SGK1, enables ENaC forward trafficking to the apical membrane increasing Na+ absorption, which could be an additional mechanism contributing to CF phenotype. Nevertheless, in a recent in vivo study using AS160-deficient mice, Loffing and coworkers described no significant alteration in the renal control of sodium and water homeostasis compared with wild type mice, although a significant reduction in GLUT4-mediated basolateral glucose uptake was observed in distal tubules.Citation41
Rab proteins as putative therapeutic targets
As aforementioned, Rab GTPases function as key regulators of membrane traffic, organelle biogenesis and maturation, and related cellular processes in all eukaryotic organisms. They have distinctive yet overlapping subcellular distributions that allow these proteins to coordinate vesicle traffic within the exocytic, endocytic and recycling pathways.Citation2 As with most Ras-related GTPases, Rab proteins have an extremely high affinity pocket for guanosine nucleotides binding and a sleek topology, lacking suitable grooves for small molecule targeting. Coupled with their fundamental cellular roles and their mostly ubiquitous cellular expression, these make the pharmacological targeting of Rab proteins a significant challenge.Citation42 Notwithstanding, a few examples are available where preclinical studies have identified promising molecules targeting specific Rab proteins, with potential therapeutic implications. One interesting example came from investigation of the successful use of the intestinal whipworm Trichuris suis in clinical trials, to reduce inflammation in immune disorders such as inflammatory bowel disease and multiple sclerosis. It was demonstrated that the effect of T. suis soluble products (SPs) on human dendritic cells was to induce Rab7b, a negative regulator of Toll-like receptor-4 (TLR4) signaling, that interfered with TLR4 trafficking and reduced its surface expression, explaining the decreased inflammatory response.Citation43 There are also examples of rational drug design targeting specific Rab proteins. For instance, using a virtual screening approach, Kang and colleagues identified several ((3,4-dihydroxy benzylidene)-hydrazinyl)pyridine-3-sulfonamide analogs that selectively target Rab27a.Citation44 Rab27a has been proven necessary for epithelial tumor invasion and metastasis, due to its essential role in exosome secretion,Citation45-47 and these molecules were shown to have significant anti-metastatic effects in breast cancer (MDA-MB231) and melanoma (A375) cell lines.Citation44 Given the effect of its inhibition on CFTR density at the cell surface, Rab27a was one of the first Rab proteins to be proposed as a putative target in CF disease.Citation24 Thus, it is tempting to suggest that it might be interesting to test whether ((3,4-dihydroxy benzylidene)-hydrazinyl)pyridine-3-sulfonamide analogs could have an application in CF treatment. Nevertheless, the discovery that the manipulation of Rab27a activity produced similar effects on ENaC abundance and function at the cell surface may hinder the therapeutic relevance of targeting Rab27a in CF.Citation35 Another caveat would be the role of Rab27a in regulating the trafficking of other channels – for instance, the voltage dependent calcium channel Cav1.3, whose current density is controlled by Rab27a to regulate the secretion of vascular endothelial growth factor A (VEGF-A) in the retinal pigmental epithelium.Citation48
The pharmacological or temperature rescue of F508del-CFTR has been shown to result in its targeting to the endosomal recycling compartment (ERC), colocalizing with Rab11 and EHD1 ERC markers.Citation22 Moreover, corrector-rescued F508del-CFTR in the ERC exhibited subsequent PKA-stimulated trafficking to the PM, indicating that corrector treatment redirects F508del trafficking from a degradative pathway to a regulated recycling route. This suggested that the proteins that mediate this process, namely Rab11, could become potential targets for improving the efficacy of current and future CFTR modulators. The apparent Rab11 isoform-specificity, at least in the colon, in the regulation of CFTR and ENaC recyclingCitation20,34 would support the targeting of Rab11a to decrease ENaC surface abundance while promoting the trafficking of rescued F508del via Rab11b-associated compartments. However, the role of Rab11a in regulating CFTR trafficking in the airwaysCitation17 would only advise for this isoform specific modulation in a tissue-specific manner. Furthermore, Rab11 is also known to play a crucial role in maintaining the proper distribution of the subapical Aquaporin 2 (AQP2) water channels and regulating the trafficking of AQP2. AQPs are membrane proteins serving in the transfer of water and small solutes across cellular membranes. Among them, AQP2 plays a critical role in water reabsorption. AQP2 is stored in Rab11-positive storage vesicles until AQP2 is phosphorylated by protein kinase A, leading to its translocation to the apical plasma membrane where it promotes the influx of water.Citation49 AQP2 is retrieved to early endosomes, and then transferred back to the Rab11-positive storage compartment. Thus, interference with Rab11 function might have unforeseen consequences, perturbing airway hindrance due to off-target effects on channels like AQP2 or potassium channels such as the potassium-voltage-gated subfamily Q member 1 (KCNQ1)Citation50 or the voltage-dependent Kv1.551 whose recycling is also mediated by Rab11. Interestingly, a recent study described the cholesterol-lowering drugs statins, as potential potentiators of vasopressin-independent trafficking of AQP2 channels in the kidney. The lack of functional V2 vasopressin receptors in congenital X-linked nephrogenic diabetes insipidus, prevents vasopressin-induced shuttling of AQP2 water channels to the apical plasma membrane of kidney collecting duct principal cells, thus promoting water reabsorption from urine to the interstitium.Citation52 Fluvastatin was shown to increase AQP2 membrane expression in the kidney collecting ducts, in a vasopressin-independent fashion, and significantly increase water reabsorption in mouse models of the disease. Fluvastatin reduced the isoprenylation of RhoA and Rab5 GTPases, resulting in decreased membrane tethering these proteins and the accumulation of AQP2 at the PM.Citation53 Because Rab5a was found upregulated in CF bronchial cell lines,Citation18 it might be useful to ascertain whether statins administration would benefit CF patients bearing mutations that decrease CFTR stability at the apical surface of airway cells.
The targeting of Rab4 could also be envisioned to reduce ENaC activity in CF airways. However Rab4 downregulation could also hinder the surface stability of pharmacologically rescued CFTR channels.Citation23 Even considering an ENaC-directed therapeutic regimen, Rab4 is known to participate in the regulation of density at the plasma membrane of hERG (ether-a-go-go-related), the pore-forming subunit of the rapidly activating delayed rectifier potassium channel (IKr).Citation54 A reduction in the hERG current causes long QT syndrome, which predisposes affected individuals to ventricular arrhythmias and sudden death. Consequently, any interference with Rab4 should always be monitored for its short and long-term effects in the heart.
In fact, while the most life-threatening manifestations of CF disease occur in the lungs, offering the possibility of limiting eventual systemic side-effects by using aerosols for drug delivery, past experiences with lung-targeted compounds have shown that off-target effects shouldn't be considered lightly. For example, early clinical trial with the ENaC selective inhibitor Amiloride did not significantly improve lung function in CF patients, probably due to its limited potency and short half-life on airway surfaces.Citation55 This led to the development of innovative high throughput screens to identify more effective ENaC blockers.Citation56,57 Similar to amiloride, however, the clinical development of inhalation therapy with some of these novel ENaC blockers has been hampered by their systemic absorption, leading to hyperkalemia resulting from inhibition of ENaC in the kidney.Citation58
Despite challenging, it is nonetheless tempting to speculate that the development of strategies to modulate the activity of one or more of these Rab proteins may prove relevant to CF therapy, particularly in combination with current and future ENaC blockers and small-molecule modulators of mutant CFTR trafficking and function. In fact, the latest evidence with this latter class of molecules agrees with the concept that the treatment of a pathology as complex as CF will require the combination several therapeutic approaches directed at the several biochemical and physiologic processes that are compromised in CF disease.Citation16
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Work supported by center grant (to BioISI, Centre Reference: UID/MULTI/04046/2013), grants IF2012 (FCT/MCTES, Portugal) and PGG-055–2014 (Gilead Genése Portugal) to P.M. and grant PGG-039–2014 (Gilead Genése Portugal) and Romain Pauwels Research Award to C.M.F. The funders had no role in the preparation of the manuscript.
References
- Farinha CM, Swiatecka-Urban A, Brautigan DL, Jordan P. Regulatory Crosstalk by Protein Kinases on CFTR Trafficking and Activity. Front Chem 2016; 4:1; PMID:26835446; https://doi.org/10.3389/fchem.2016.00001
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; https://doi.org/10.1038/nrm2728
- Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci 2007; 120:3905-10; PMID:17989088; https://doi.org/10.1242/jcs.015909
- Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol 2001; 313:889-901; PMID:11697911; https://doi.org/10.1006/jmbi.2001.5072
- Saxena SK, Kaur S. Regulation of epithelial ion channels by Rab GTPases. Biochem Biophys Res Commun 2006; 351:582-7; PMID:17084813; https://doi.org/10.1016/j.bbrc.2006.10.087
- Kunzelmann K. The cystic fibrosis transmembrane conductance regulator and its function in epithelial transport. RevPhysiol Biochem Pharmacol 1999; 137:1-70.
- Frizzell RA. Functions of the cystic fibrosis transmembrane conductance regulator protein. Am J Respir CritCare Med 1995; 151:S54-S8; https://doi.org/10.1164/ajrccm/151.3_Pt_2.S54
- Riordan JR. The cystic fibrosis transmembrane conductance regulator. Annu Rev Physiol 1993; 55:609-30; PMID:7682047; https://doi.org/10.1146/annurev.ph.55.030193.003141
- Amaral MD, Kunzelmann K. Molecular targeting of CFTR as a therapeutic approach to cystic fibrosis. Trends Pharmacol Sci 2007; 28:334-41; PMID:17573123; https://doi.org/10.1016/j.tips.2007.05.004
- Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem 2008; 77:701-26; PMID:18304008; https://doi.org/10.1146/annurev.biochem.75.103004.142532
- Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol 2006; 7:426-36; PMID:16723978; https://doi.org/10.1038/nrm1949
- Farinha CM, Matos P, Amaral MD. Control of CFTR membrane trafficking: not just from the ER to the Golgi. FEBS J 2013; 280:4396-406; PMID:23773658; https://doi.org/10.1111/febs.12392
- Gentzsch M, Chang XB, Cui L, Wu Y, Ozols VV, Choudhury A, Pagano RE, Riordan JR. Endocytic trafficking routes of wild type and DeltaF508 cystic fibrosis transmembrane conductance regulator. Mol Biol Cell 2004; 15:2684-96; PMID:15075371; https://doi.org/10.1091/mbc.E04-03-0176
- van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 1992; 70:729-40; PMID:1516131; https://doi.org/10.1016/0092-8674(92)90307-X
- Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol 1999; 145:123-39; PMID:10189373; https://doi.org/10.1083/jcb.145.1.123
- Farinha CM, Matos P. Repairing the basic defect in cystic fibrosis - one approach is not enough. FEBS J 2016; 283:246-64; PMID:26416076; https://doi.org/10.1111/febs.13531
- Swiatecka-Urban A, Brown A, Moreau-Marquis S, Renuka J, Coutermarsh B, Barnaby R, Karlson KH, Flotte TR, Fukuda M, Langford GM, et al. The short apical membrane half-life of rescued {Delta}F508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of {Delta}F508-CFTR in polarized human airway epithelial cells. J Biol Chem 2005; 280:36762-72; PMID:16131493; https://doi.org/10.1074/jbc.M508944200
- Virella-Lowell I, Herlihy JD, Liu B, Lopez C, Cruz P, Muller C, Baker HV, Flotte TR. Effects of CFTR, interleukin-10, and Pseudomonas aeruginosa on gene expression profiles in a CF bronchial epithelial cell Line. Mol Ther 2004; 10:562-73; PMID:15336656; https://doi.org/10.1016/j.ymthe.2004.06.215
- Swiatecka-Urban A, Talebian L, Kanno E, Moreau-Marquis S, Coutermarsh B, Hansen K, Karlson KH, Barnaby R, Cheney RE, Langford GM, et al. Myosin Vb is required for trafficking of the cystic fibrosis transmembrane conductance regulator in Rab11a-specific apical recycling endosomes in polarized human airway epithelial cells. J Biol Chem 2007; 282:23725-36; PMID:17462998; https://doi.org/10.1074/jbc.M608531200
- Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, Bradbury NA. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell 2009; 20:2337-50; PMID:19244346; https://doi.org/10.1091/mbc.E08-01-0084
- Lai F, Stubbs L, Artzt K. Molecular analysis of mouse Rab11b: a new type of mammalian YPT/Rab protein. Genomics 1994; 22:610-6; PMID:8001972; https://doi.org/10.1006/geno.1994.1434
- Holleran JP, Zeng J, Frizzell RA, Watkins SC. Regulated recycling of mutant CFTR is partially restored by pharmacological treatment. J Cell Sci 2013; 126:2692-703; PMID:23572510; https://doi.org/10.1242/jcs.120196
- Saxena SK, Kaur S, George C. Rab4GTPase modulates CFTR function by impairing channel expression at plasma membrane. Biochem Biophys Res Commun 2006; 341:184-91; PMID:16413502; https://doi.org/10.1016/j.bbrc.2005.12.170
- Saxena SK, Kaur S. Rab27a negatively regulates CFTR chloride channel function in colonic epithelia: involvement of the effector proteins in the regulatory mechanism. Biochem Biophys Res Commun 2006; 346:259-67; PMID:16762324; https://doi.org/10.1016/j.bbrc.2006.05.102
- Pohl K, Hayes E, Keenan J, Henry M, Meleady P, Molloy K, Jundi B, Bergin DA, McCarthy C, McElvaney OJ, et al. A neutrophil intrinsic impairment affecting Rab27a and degranulation in cystic fibrosis is corrected by CFTR potentiator therapy. Blood 2014; 124:999-1009; PMID:24934256; https://doi.org/10.1182/blood-2014-02-555268
- Hanukoglu I, Hanukoglu A. Epithelial sodium channel (ENaC) family: Phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 2016; 579:95-132; PMID:26772908; https://doi.org/10.1016/j.gene.2015.12.061
- Mall MA, Galietta LJ. Targeting ion channels in cystic fibrosis. J Cyst Fibros 2015; 14:561-70; PMID:26115565; https://doi.org/10.1016/j.jcf.2015.06.002
- Mall MA. Role of the amiloride-sensitive epithelial Na+ channel in the pathogenesis and as a therapeutic target for cystic fibrosis lung disease. Exp Physiol 2009; 94:171-4; PMID:19060118; https://doi.org/10.1113/expphysiol.2008.042994
- Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl- channel function. Nature 1999; 402:301-4; PMID:10580502; https://doi.org/10.1038/46297
- Zhou Z, Duerr J, Johannesson B, Schubert SC, Treis D, Harm M, Graeber SY, Dalpke A, Schultz C, Mall MA. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J Cyst Fibros 2011; 10 Suppl 2:S172-82; PMID:21658636; https://doi.org/10.1016/S1569-1993(11)60021-0
- Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 2004; 10:487-93; PMID:15077107; https://doi.org/10.1038/nm1028
- Saxena S, Singh M, Engisch K, Fukuda M, Kaur S. Rab proteins regulate epithelial sodium channel activity in colonic epithelial HT-29 cells. Biochem Biophys Res Commun 2005; 337:1219-23; PMID:16236259; https://doi.org/10.1016/j.bbrc.2005.09.186
- Saxena SK, Singh M, Shibata H, Kaur S, George C. Rab4 GTP/GDP modulates amiloride-sensitive sodium channel (ENaC) function in colonic epithelia. Biochem Biophys Res Commun 2006; 340:726-33; PMID:16389071; https://doi.org/10.1016/j.bbrc.2005.12.036
- Karpushev AV, Levchenko V, Pavlov TS, Lam VY, Vinnakota KC, Vandewalle A, Wakatsuki T, Staruschenko A. Regulation of ENaC expression at the cell surface by Rab11. Biochem Biophys Res Commun 2008; 377:521-5; PMID:18926797; https://doi.org/10.1016/j.bbrc.2008.10.014
- Saxena SK, Horiuchi H, Fukuda M. Rab27a regulates epithelial sodium channel (ENaC) activity through synaptotagmin-like protein (SLP-5) and Munc13-4 effector mechanism. Biochem Biophys Res Commun 2006; 344:651-7; PMID:16630545; https://doi.org/10.1016/j.bbrc.2006.03.160
- Silvis MR, Bertrand CA, Ameen N, Golin-Bisello F, Butterworth MB, Frizzell RA, Bradbury NA. Rab11b regulates the apical recycling of the cystic fibrosis transmembrane conductance regulator in polarized intestinal epithelial cells. Mol Biol Cell 2009; 20:2337-50; PMID:19244346; https://doi.org/10.1091/mbc.E08-01-0084
- Bhalla V, Daidie D, Li H, Pao AC, LaGrange LP, Wang J, Vandewalle A, Stockand JD, Staub O, Pearce D. Serum- and glucocorticoid-regulated kinase 1 regulates ubiquitin ligase neural precursor cell-expressed, developmentally down-regulated protein 4-2 by inducing interaction with 14-3-3. Mol Endocrinol 2005; 19:3073-84; PMID:16099816; https://doi.org/10.1210/me.2005-0193
- Klip A, Sun Y, Chiu TT, Foley KP. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am J Physiol Cell Physiol 2014; 306:C879-86; PMID:24598362; https://doi.org/10.1152/ajpcell.00069.2014
- Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, Perry CJ, Kester L, Butterworth MB, Peters KW, et al. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem 2006; 281:14129-35; PMID:16574660; https://doi.org/10.1074/jbc.M512511200
- Liang X, Butterworth MB, Peters KW, Frizzell RA. AS160 modulates aldosterone-stimulated epithelial sodium channel forward trafficking. Mol Biol Cell 2010; 21:2024-33; PMID:20410134; https://doi.org/10.1091/mbc.E10-01-0042
- Di Chiara M, Glaudemans B, Loffing-Cueni D, Odermatt A, Al-Hasani H, Devuyst O, Faresse N, Loffing J. Rab-GAP TBC1D4 (AS160) is dispensable for the renal control of sodium and water homeostasis but regulates GLUT4 in mouse kidney. Am J Physiol Renal Physiol 2015; 309:F779-90; PMID:26336159; https://doi.org/10.1152/ajprenal.00139.2015
- Zhao W, Jamshidiha M, Lanyon-Hogg T, Recchi C, Cota E, Tate EW. Direct targeting of the Ras GTPase Superfamily through structure- based design. Curr Top Med Chem 2017; 17:16-29; PMID:27530972; https://doi.org/10.2174/1568026616666160719165633
- Klaver EJ, van der Pouw Kraan TC, Laan LC, Kringel H, Cummings RD, Bouma G, Kraal G, van Die I. Trichuris suis soluble products induce Rab7b expression and limit TLR4 responses in human dendritic cells. Genes Immun 2015; 16:378-87; PMID:25996526; https://doi.org/10.1038/gene.2015.18
- Kang SM, Nam KY, Jung SY, Song KH, Kho S, No KT, Choi HK, Song JY. Inhibition of cancer cell invasion by new ((3,4-dihydroxy benzylidene)hydrazinyl)pyridine-3-sulfonamide analogs. Bioorg Med Chem Lett 2016; 26:1322-8; PMID:26810259; https://doi.org/10.1016/j.bmcl.2015.12.093
- Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012; 72:4920-30; PMID:22865453; https://doi.org/10.1158/0008-5472.CAN-12-0925
- Li W, Mu D, Tian F, Hu Y, Jiang T, Han Y, Chen J, Han G, Li X. Exosomes derived from Rab27aoverexpressing tumor cells elicit efficient induction of antitumor immunity. Mol Med Rep 2013; 8:1876-82; PMID:24146068
- Hendrix A, Hume AN. Exosome signaling in mammary gland development and cancer. Int J Dev Biol 2011; 55:879-87; PMID:22161843; https://doi.org/10.1387/ijdb.113391ah
- Reichhart N, Markowski M, Ishiyama S, Wagner A, Crespo-Garcia S, Schorb T, Ramalho JS, Milenkovic VM, Föckler R, Seabra MC, et al. Rab27a GTPase modulates L-type Ca2+ channel function via interaction with the II-III linker of CaV1.3 subunit. Cell Signal 2015; 27:2231-40; PMID:26235199; https://doi.org/10.1016/j.cellsig.2015.07.023
- Takata K, Matsuzaki T, Tajika Y, Ablimit A, Hasegawa T. Localization and trafficking of aquaporin 2 in the kidney. Histochem Cell Biol 2008; 130:197-209; PMID:18566824; https://doi.org/10.1007/s00418-008-0457-0
- Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, Baltaev R, Mack AF, Korniychuk G, Choudhury A, et al. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res 2007; 100:686-92; PMID:17293474; https://doi.org/10.1161/01.RES.0000260250.83824.8f
- Balse E, El-Haou S, Dillanian G, Dauphin A, Eldstrom J, Fedida D, Coulombe A, Hatem SN. Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes. Proc Natl Acad Sci U S A 2009; 106:14681-6; PMID:19706553; https://doi.org/10.1073/pnas.0902809106
- Bichet DG. Vasopressin receptor mutations in nephrogenic diabetes insipidus. Semin Nephrol 2008; 28:245-51; PMID:18519085; https://doi.org/10.1016/j.semnephrol.2008.03.005
- Procino G, Barbieri C, Carmosino M, Tamma G, Milano S, De Benedictis L, Mola MG, Lazo-Fernandez Y, Valenti G, Svelto M. Fluvastatin modulates renal water reabsorption in vivo through increased AQP2 availability at the apical plasma membrane of collecting duct cells. Pflugers Arch 2011; 462:753-66; PMID:21858457; https://doi.org/10.1007/s00424-011-1007-5
- Cui Z, Zhang S. Regulation of the human ether-a-go-go-related gene (hERG) channel by Rab4 protein through neural precursor cell-expressed developmentally down-regulated protein 4-2 (Nedd4-2). J Biol Chem 2013; 288:21876-86; PMID:23792956; https://doi.org/10.1074/jbc.M113.461715
- Pons G, Marchand MC, d'Athis P, Sauvage E, Foucard C, Chaumet-Riffaud P, Sautegeau A, Navarro J, Lenoir G. French multicenter randomized double-blind placebo-controlled trial on nebulized amiloride in cystic fibrosis patients. The Amiloride-AFLM Collaborative Study Group. Pediatr Pulmonol 2000; 30:25-31; PMID:10862159; https://doi.org/10.1002/1099-0496(200007)30:1%3c25::AID-PPUL5%3e3.0.CO;2-C
- Schoenberger M, Althaus M. Novel small molecule epithelial sodium channel inhibitors as potential therapeutics in cystic fibrosis - a patent evaluation. Expert Opin Ther Pat 2013; 23:1383-9; PMID:23957246; https://doi.org/10.1517/13543776.2013.829454
- Almaca J, Faria D, Sousa M, Uliyakina I, Conrad C, Sirianant L, Clarke LA, Martins JP, Santos M, Heriché JK, et al. High-content siRNA screen reveals global ENaC regulators and potential cystic fibrosis therapy targets. Cell 2013; 154:1390-400; PMID:24034256; https://doi.org/10.1016/j.cell.2013.08.045
- O'Riordan TG, Donn KH, Hodsman P, Ansede JH, Newcomb T, Lewis SA, Flitter WD, White VS, Johnson MR, Montgomery AB, et al. Acute hyperkalemia associated with inhalation of a potent ENaC antagonist: Phase 1 trial of GS-9411. J Aerosol Med Pulm Drug Deliv 2014; 27:200-8; PMID:23905576; https://doi.org/10.1089/jamp.2013.1037
