ABSTRACT
Cilia are microtubule-based organelles extending from a basal body at the surface of eukaryotic cells. Cilia regulate cell and fluid motility, sensation and developmental signaling, and ciliary defects cause human diseases (ciliopathies) affecting the formation and function of many tissues and organs. Over the past decade, various Rab and Rab-like membrane trafficking proteins have been shown to regulate cilia-related processes such as basal body maturation, ciliary axoneme extension, intraflagellar transport and ciliary signaling. In this review, we provide a comprehensive overview of Rab protein ciliary associations, drawing on findings from multiple model systems, including mammalian cell culture, mice, zebrafish, C. elegans, trypanosomes, and green algae. We also discuss several emerging mechanistic themes related to ciliary Rab cascades and functional redundancy.
Introduction
The compartmentalisation of the endomembrane system into spatially segregated organelles of distinct composition and identity depends on vesicular membrane trafficking processes such as secretion and endocytosis that transport distinct lipids and proteins from donor to target membranes. Rab GTPases are critical regulators of endomembrane organization, controlling many aspects of vesicle identity and transport such as fusion, fission, budding, tethering and motility.Citation1-4 Rabs comprise an ancient conserved subfamily of the small Ras-like GTPases, with at least 20 members in the last eukaryotic common ancestor and more than 60 proteins in mammals.Citation5,6 Rabs share the basic Ras-like structure of a 6-stranded β sheet, flanked by 5 α helices, and are defined by Rab family (RabF)-specific motifs as well as C-terminal prenyl groups for membrane targeting (geranyl-geranyl or farnesyl).Citation7
Rabs function as molecular switches, alternating between cytosolic GDP-bound ‘inactive’ and membrane-associated GTP-bound ‘active’ states. GTP binding induces conformational changes in the switch regions, causing exposure of binding motifs for downstream effector proteins such as molecular motors, vesicle tethering factors and membrane fusion mediators. GTP to GDP exchange is regulated by the Rab's intrinsic GTPase activity, assisted by GTPase-activating proteins (GAPs), whereas GDP to GTP exchange requires GTP exchange factors (GEF).Citation8 Additional regulation is provided by GDIs (guanine nucleotide dissociation inhibitors) and GDFs (GDI dissociation factors) that regulate Rab cycling between active membrane-bound and inactive cytosolic states.Citation9 Because Rab GEFs, GDFs and effectors can localize at distinct membranes, these proteins also provide Rabs with endomembrane-specific functions by trapping their cognate Rab at a target membrane.Citation9 Furthermore, multiple Rabs can function within the same pathway, sometimes as part of a Rab cascade; for example, the effector of an upstream Rab can be a GEF of a downstream Rab.Citation10,11
Despite progress, the functions of a large number of Rabs remain unknown or are poorly understood, and in many cases there is scant knowledge of the target effectors, as well as the cognate GEF/GAP/GDI/GDF regulators, some of which are shared with other Rabs. Among the myriad of additional questions, little is known about feedback control within many Rab-driven pathways, nor is there a clear picture of in vivo functional redundancy within the Rab family.
In recent years, various Rab and Rab-like proteins have been linked to cilia, which are motile and non-motile microtubule-based organelles extending from eukaryotic cell surfaces (). Like the Rab superfamily, the cilium is ancestral, present in the last eukaryotic common ancestor (LECA), and subsequently lost in some eukaryote branches.Citation5,6,12 The canonical cilium consists of a cylinder of 9 doublet microtubules extending from a mother centriole-derived basal body, enveloped by a specialized patch of plasma membrane called the ciliary membraneCitation13 (). In some cells, the basal body lies at the apex of a deep depression in the plasma membrane termed the ciliary pocket.Citation14 Cilia serve a wide number of functions related to motility and the transduction of physical (light, temperature, touch) and chemical (odorants, taste compounds, biochemical ligands) signalsCitation13 (). Cilia also coordinate extrinsic signaling pathways required for tissue development and homeostasis (e.g., Shh, Wnt, PDGFα).Citation15 For example, sonic hedgehog (Shh) signal transduction from the Patched receptor (Ptch) via Smoothened (Smo) to the Gli transcriptional factors occurs within the cilium and requires dynamic transport of signaling intermediates and regulators into (e.g., Smo) and out of (e.g., Ptch) the organelle.Citation16-22 Defects in cilia are associated with more than 20 genetically inherited (mostly recessive) diseases, termed ciliopathies, affecting many tissues and organsCitation23,24 (). Examples include polycystic kidney disease, retinitis pigmentosa, Bardet-Biedl syndrome (BBS) and Meckel Gruber syndrome, which are characterized by cystic kidneys, blindness, bone abnormalities, organ patterning defects, brain abnormalities, infertility and other symptoms. Presently, more than 150 ciliopathy genes are known.
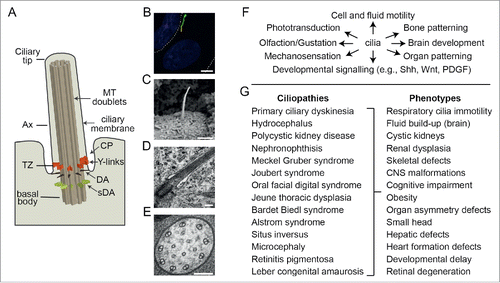
Figure 1. Overview of cilium structure, function and disease associations. (A) Schematic of cilium structure. TZ; transition zone, Ax; axoneme, DA; distal appendages, sDA; subdistal appendages; MT; microtubules, CP; ciliary pocket. (B) Epifluorescence image of a hTERT-RPE1 cell expressing an ARL13B-GFP reporter that stains the ciliary membrane (green). Nucleus stained blue (DAPI). Dotted line denotes the plasma membrane. Scale bar; 5 μm. (C) SEM image of a kidney epithelial cell cilium. Image provided by K. Phelps (Electron Microscopy Unit, UT Southwestern Medical Center). Scale bar; 1 μm. (D) TEM image of the proximal end of a primary cilium on a hTERT-RPE1 cell. Scale bar; 200 nm. (E) TEM cross section of a C. elegans sensory cilium showing the 9 doublet microtubules (plus additional inner singlet microtubules). Scale bar; 100 nm. (F) Selection of ciliary-based functions. (G) Ciliopathies and associated phenotypes.
Cilia form when cells exit the cell cycle and 2 cell type-specific pathways of mammalian ciliogenesis have been described based on seminal electron microscopy work by Sorokin in the 1960s.Citation25-27 In the extracellular pathway, the mother centriole migrates to the cell periphery, docks directly with the plasma membrane via its distal appendages, followed by ciliary axoneme and membrane elongation. In the intracellular pathway, the migrating mother centriole first associates with a presumptive Golgi-derived (ciliary) vesicle; this is followed by partial extension of the ciliary axoneme (i.e., intracellular extension) before the elongated ciliary vesicle fuses with the plasma membrane to complete basal body docking.
Many of the key steps in ciliogenesis such as basal body formation and maturation, as well as ciliary microtubule and membrane biogenesis and elongation, are regulated by intracellular transport pathways. The most extensively studied is intraflagellar transport (IFT), which is a bidirectional motility along the ciliary axoneme that traffics protein cargos such as tubulin subunits and signaling molecules into and out of the organelle. Sandwiched between the microtubules and membrane, IFT trains consist of kinesin-2 and cytoplasmic dynein motors, together with IFT-A, IFT-B and BBSome complexes that serve as cargo adaptors.Citation28-31 These adaptor complexes are large macromolecular assemblies, consisting of at least 6 IFT-A, 8 BBSome and 16 IFT-B proteins; furthermore, the IFT-B complex is subdivided into 2 biochemically distinct IFT-B1 (10 proteins) and IFT-B2 (6 proteins) subcomplexes.Citation32-36
Multiple small GTPases of the Arl and Rab families, together with membrane remodelling factors such as EHD1/3, are also critical for ciliogenesis, serving important roles in ciliary vesicle formation, centriole uncapping, ciliary membrane elongation, and IFT regulation.Citation35,37-44 Post-ciliogenic maintenance and regulation of cilium composition is also driven by IFT as well as polarized delivery of post-Golgi vesicles and associated cargo to the periciliary membrane, together with endocytic events at the ciliary base ().Citation45-48 Finally, the formation of a compartmentalised cilium of defined composition depends on ‘gating’ mechanisms at the ciliary base. Specifically, basal body distal appendages and the Y-link connectors in the proximal-most part of the ciliary axoneme (transition zone) physically inhibit vesicles from crossing into the cilium, and membrane and cytosolic TZ diffusion barriers regulate the ciliary entry and exit of at least some proteins.Citation49-51
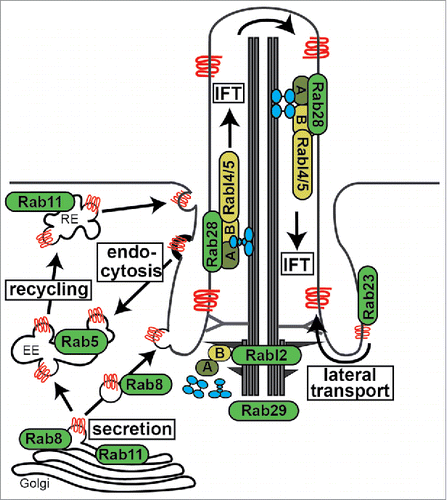
Figure 2. Overview of cilia-related transport functions for Rab proteins. Shown are Rab and Rab-like proteins mapped to major routes of ciliary membrane protein (red) trafficking and IFT (intraflagellar transport) machinery. A; IFT-A complex, B; IFT-B complex, RE; recycling endosome, EE; early endosome. IFT motors (kinesin-2 and IFT-dynein) shown in blue.
In this review, we examine the roles for Rab (Rab5/8/11/23/28/29) and Rab-like (Rabl2/4/5), proteins in establishing and maintaining the ciliary compartment (see for overview of Rab ciliary associations). Since Rab ciliary associations are also outlined in several excellent review articles,Citation52-54 we focus here on more recent findings, and how this new data reflects on models of Rab function related to ciliary formation, function, transport and signaling. We also consider the conservation of Rab protein function in different cell and animal systems and outline some of the important questions that remain unanswered.
Rab8 and Rab11
Rab8‘s’ role during ciliogenesis
Rab8, a crucial regulator of exocytosis, was first linked to cilia from studies of the retina, where Rab8 was shown to regulate the transport and entry of rhodopsin to the specialized cilium (outer segment) of photorecepter cells.Citation54-56 In addition to ciliary transport functions (discussed below), various experimental approaches have linked Rab8 to cilium formation. In human hTERT-RPE1 cells, overexpression of a Rab8 GAP (XM_037557) or Rab8(GDP) blocks cilium formation.Citation37 Similarly, depletion of a Rab8 GEF (Rabin8) abolishes ciliogenesis in hTERT-RPE1 cells, while RAB8(GTP) overexpression drives ciliary membrane extension.Citation35 In further support of a ciliogenic role, siRNA and morpholino depletion of Rab8 reduces ciliation in cultured mammalian cells and causes a BBS-like phenotype in zebrafish.Citation35,37,41,44 In addition, RAB-8(WT) or RAB-8(GTP) overexpression in a subset of C. elegans sensory neurons induces cilium length and integrity defects, although a rab-8 null mutant appears normal.Citation57,58 However, somewhat surprisingly, Rab8a and Rab8a/Rab8b (double) knockout mice do not display ciliopathy-like phenotypes or cilia defects (at least for those cilia that were analyzed), suggesting that there may be other Rab GTPases working redundantly with Rab8 in vivo.Citation59,60 One candidate is Rab10, which is a paralogue of Rab8 previously shown to localize to the ciliary axoneme and base.Citation61 Indeed, Rab10 depletion in fibroblasts cultured from Rab8a/b double knockout mice leads to a significant reduction of ciliation.Citation60
Multiple studies have shown that Rab8 associates with ciliary membranes. In early studies, GFP-tagged Rab8a was found to localize to the primary cilia of hTERT-RPE1 cells.Citation35,37 Subsequent studies revealed that GFP-Rab8a localizes to the ciliary base and the growing axoneme but it is lost from the mature cilium.Citation41 GFP-Rab8a is also recruited to the basal body before axoneme extension suggesting a role for Rab8 at the ciliary vesicle, and several studies used this dynamic localization to analyze early ciliogenesis steps.Citation62-64 A recent study from the Westlake group using live cell imaging and ultrastructural analyses found that Rab8 is recruited to ciliary vesicles after the recruitment of other proteins linked to ciliary vesicle formation such as the endocytic transport regulator EHD1, the ciliary transition zone protein B9D2 and Golgi-associated IFT20.Citation44 The latter study also found that Rab8 is dispensable for initial docking of ciliary vesicles to the mother centriole, indicating that this GTPase may function at the slightly later step of ciliary vesicle membrane elongation ().Citation44
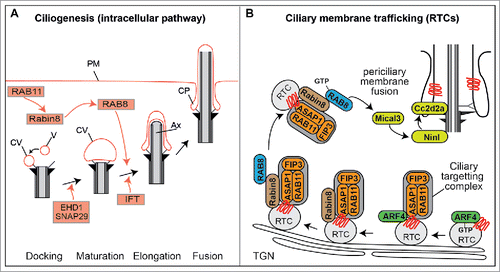
Figure 3. RAB cascades during ciliogenesis and protein transport. (A) Proposed model of RAB11-RAB8 cascade during intracellular ciliogenesis. Shortly after induction of ciliogenesis, Rab11 activates and recruits Rabin8 to the pericentriolar compartment and vesicles (V) dock to the distal appendages of the mother centriole forming small ciliary vesicles (CV). EHD1 together with SNAP29 promotes the fusion of these small vesicles resulting in the maturation of a large CV that caps the distal end of the mother centriole. Rab8, which is recruited and activated by Rabin8, subsequently drives CV membrane extension, and IFT elongates the ciliary axoneme (Ax). This is followed by CV fusion with the plasma membrane (PM), often resulting in the formation of a ciliary pocket (CP). (B) ARF4-RAB11-RAB8 cascade that sorts and delivers rhodopsin transport carriers (RTC) to the periciliary membrane of photoreceptor cells. TGN; trans-Golgi network.
Taken together, the various localization and functional data described above supports a role for Rab8 in cilium formation, at least in cultured mammalian cells. Indeed, this scenario is further supported by the growing number of ciliogenic proteins that physically interact with Rab8 and/or its GEF Rabin8, and regulate Rab8 ciliary localisations in some cases. These include the BBSome complex, several centriolar satellite proteins (HOOK2, PCM1, Talpid, Cep290), and several mother centriole/basal body proteins (Cep164, Chibby, ODF2/cenexin).Citation35,37,43,65-69 Consistent with a role for Rab8 during early steps of ciliogenesis, some of these Rab8 interactors function during the formation and fusion of ciliary vesicles (Cep164, HOOK2, PCM1, Chibby), and there are several reports showing that overexpression of Rab8(WT), Rab8(GTP) or Rabin8 (but not Rab8(GDP)) rescues cilia loss observed in cells depleted of Cep164, HOOK2, TALPID3, or Cep290.Citation43,66,67
Ciliogenesis regulation via a Rab8/Rab11 cascade
Targeting and activation of Rab8 at the ciliary base is thought to be achieved by a Rab cascade involving Rab8, Rabin8 (Rab8 GEF) and the recycling endosome-associated GTPase, Rab11.Citation41,70 In this model, Rab11 stimulates the GEF activity and mother centriole recruitment of Rabin8, which in turn leads to recruitment and activation of Rab8Citation41,71 (). Recent studies report that the upstream recruitment of activated Rab11 at the mother centriole depends on the appendage protein ODF2/cenexin and a phosphatidylinositol 3-kinase (PI3K-C2) that generates a pool of phospholipid phosphatidylinositol 3-phosphate (PtdIns3P) at the ciliary base.Citation72,73 This PtdIns3P pool appears necessary for Rab11 activation and the ciliary Rab cascade because PI3K-C2a mouse knockout cells display Rab8 mislocalization as well as cilium length and Shh signaling defects, and these phenotypes are rescued via overexpression of Rab11(GTP) but not Rab11(WT).Citation73 Downstream of Rab11, the transfer of Rabin8 to the mother centriole is regulated by the NDR2 kinase that phosphorylates Rabin8 at Ser-272; specifically, phosphorylation decreases Rabin8 affinity for phosphatidylserine on Rab11 positive vesicles and increases Rabin8 affinity for the centriolar exocyst component Sec15.Citation74 Finally, the amount of free Rab8(GDP) available for Rabin8 activation is negatively controlled by binding with the GDI2 inhibitor and positively controlled by the GDF protein, Dzip1, which stimulates release of Rab8 from GDI2 at the pericentriolar region.Citation75 The finding that Dzip1 itself is positively regulated via phosphorylation by GSK3β, a kinase active in growth arrested cells, also serves to link Rab8 activation and ciliogenesis onset with cell cycle exit.
Rab8 and Rab11-mediated regulation of ciliary protein trafficking
From studies in mammalian cells, and especially the photoreceptor cell, the Rab11-Rabin8-Rab8 cascade has also been linked to ciliary membrane trafficking processes that maintain the mature cilium (). The photoreceptor is a well-established model for ciliary protein transport and findings predominantly from the Deretic laboratory have implicated an ordered Arf-Rab cascade that regulates the formation and delivery of rhodopsin transport carriers (RTC) from initial sorting at Golgi membranes to end-stage fusion at the periciliary membrane. Since this topic is extensively covered in several excellent reviews, we present here a summary of some of the main conclusions.Citation47,54,76 Briefly, sorting of Rhodopsin into Golgi/TGN transport carriers in initiated via binding of activated Arf4(GTP) to the Rhodopsin ciliary targeting sequence. This is followed by the Golgi recruitment of the Arf-GAP ASAP1, which also binds Rhodopsin, and forms part of a ciliary targeting complex with Rab11 and FIP3 (Arf-Rab11 effector). Following inactivation and dissociation of Arf4, ASAP1-Rab11-FIP3 recruits Rabin8 and Rab8, after which RTCs bud from the TGN and eventually fuse with the periciliary membrane in a Rab8 and exocyst complex-dependent manner.Citation54-56,76-80 In this model, ASAP1 acts as a scaffold to link Arf GTPases with the Rab11-Rabin8-Rab8 cascade and provides a Rab8 positive ciliary sorting address for Golgi/TGN-derived RTCsCitation54 (). With regard to FIP3, it has been shown that this protein facilitates the orderly assembly and activation of the Rab cascade by disrupting Rhodopsin interaction with the Arf4-ASAP1 ternary complex and enhancing ASAP1-Rab11 binding to Rabin8.Citation80
Data from zebrafish photoreceptors has advanced our understanding of how Rab8 facilitates RTC docking at the ciliary base region. Specifically, the ciliary transition zone protein CC2D2A, mutated in Joubert Syndrome, links the transport and fusion of Rab8-positive carrier vesicles at the ciliary base (). Loss of CC2D2A leads to an accumulation of vesicles in the photoreceptor and mislocalization of Rab8.Citation81 Further mechanistic insight was obtained from proteomics and genetic interaction studies that identified the centrosome and basal body protein NINL as an interactor of CC2D2A as well as MICAL3.Citation82 MICAL3 is a known interactor of Rab8 and was previously reported to be involved in exocytotic vesicle fusion.Citation83 Together, these zebrafish data suggest a model whereby NINL and CC2D2A provide a docking platform for incoming Rab8- and MICAL- positive vesicles at the cilia base ().
In addition to rhodopsin in photoreceptor cells, Rab8 is also implicated in transport of other ciliary proteins. Overexpression of Rab8(GDP) decreases the ciliary entry kinetics of the membrane proteins SmoA1 and Kim1 as well as the soluble protein EB1.Citation84 Furthermore, Rab8 was shown to interact with the ciliary targeting sequence of fibrocystin and polycystin1, and regulate their transport to the ciliary membrane.Citation85,86 Mammalian Rab8 also regulates the ciliary trafficking of polycystin 2;Citation87 indeed, a similar finding was very recently reported for Rab11,Citation88 which could suggest that that a Rab11-Rab8 cascade may deliver polycystin 2 to cilia. Interestingly, a recent study reported that Rab8 together with transportin 1 (Importinβ2) regulates the lateral diffusion of membrane proteins from the plasma membrane to the ciliary membrane.Citation89 In this model, Rab8(GDP) and ciliary membrane proteins (fibrocystin, rhodopsin and others) form ternary complexes with transportin1, which facilitates the translocation to the ciliary compartment across the diffusion barrier at the ciliary base. Once in the cilium, Rab8 is activated by its ciliary GEF leading to dismantling of the ternary complex and release of the ciliary membrane proteins. Thus, Rab8 appears to regulate vesicular and non-vesicular modes of membrane protein trafficking into the cilium. Also, the recent Rab8-transportin 1 study supports the notion that nucleocytoplasmic transport regulators such as transportin 1 facilitate trafficking of proteins into cilia, across the permeability barrier at the ciliary base, and that Rab proteins may partake in this process (see also Rab23 section below).Citation90,91
Rab29
In a recent study in cultured mouse cells, GFP-Rab29 was found to localize near the ciliary base, and Rab29 depletion resulted in shorter and fewer cilia, as well as a defect in targeting a Smoothened GFP reporter to the ciliary membrane.Citation92 It was also found that Rab29 biochemically interacts with Rab8, Rab11 and IFT20 in ciliated cells.Citation92 Interestingly, Rab29 also regulates the assembly of the immune synapse, which is a specialized cell junction between T-cells and antigen presenting cells.Citation92 Indeed, the immune synapse and cilium share structural and regulatory features underpinning their biogenesis and function such as a plasma membrane-docked centriole and Rab8-Rab11-IFT20/52/57/88 protein transport machinery.Citation93 As a ‘frustrated cilium,Citation94 discoveries in the context of immune synapse can inform the biology of the cilium and the specific functions of cilia-related Rabs such as Rab8, Rab11 and Rab29.
Rab23
Rab23 was first described as a regulator of sonic hedgehog signaling in the mouse dorsal neural tube.Citation95 Since then, multiple studies show Rab23 to be an inhibitor of cilium-based Shh signaling, acting at a similar step to IFT, downstream of Smoothened and upstream of the Gli transcription factors.Citation16,84,96-99 Rab23 function is also implicated in autophagy, planar cell polarity, nodal signaling and cancer cell invasion.Citation98,100-102
A number of studies have found that Rab23 reporters localize to primary cilia and flagella, and in mammalian cells, this localization depends on GTP binding and the phosphorylated state of the GTPase.Citation84,99,103,104 Thus, in addition to previously suggested transport functions at the plasma membrane, Rab23 may regulate transport events within the cilium itself.Citation105 It is unlikely, however, that these events include direct regulation of IFT because Rab23 depletion or loss does not affect cilia/flagella formation and length in various cultured mammalian cells lines, zebrafish (Kuppfers vesicle), trypanosomes, and null mice (nodal cilia).Citation98,99,103,104 Despite these observations, overexpression of mammalian Rab23(GDP) in cultured cells reduces ciliation and cilium length, whereas overexpression of Rab23(GTP) increases cilium length.Citation37,99 In agreement with these observations, overexpression of a Rab23-specific GAP (EVI5like) in hTERT-RPE1 cells also reduces ciliation.Citation37 Although caution must be excised when interpreting overexpression phenotypes, these data raise the possibility that Rab23 serves a ciliogenesis-related function, perhaps in a redundant capacity with other ciliary Rabs.
There is now mounting evidence that Rab23 regulates the transport of ciliary proteins. Data from 2 studies suggest that Rab23 negatively regulates the ciliary levels and transport of Smoothened, which correlates with Rab23s role as a Shh signaling inhibitor. In the first study (MDCK cells), Rab23 depletion or Rab23(GDP) overexpression was found to increase SmoA1 entry kinetics into cilia, whereas the opposite occurs in cells expressing Rab23(WT).Citation84 In the second study (NIH3T3 cells), Rab23 depletion caused a modest elevation of steady-state ciliary SmoA1 levels.Citation99 This study also found that Rab23 promotes the ciliary entry and tip accumulation of the KIF17 IFT-associated motor, and facilitates the formation of a ternary complex with KIF17 and its proposed ciliary import regulator, importinβ2.Citation90,99 On the basis of epistasis data indicating independent functions for Rab23 and Ran, it was suggested that Rab23 regulates KIF17 transport toward the cilium, whereas the ciliary/cytoplasmic Ran gradient subsequently delivers KIF17 into the cilium.Citation90,99 Thus, while Rab23 restricts ciliary targeting of Smo, the opposite is true for KIF17. How these 2 functions relate to each other in the context of Shh signaling remains to be determined. Also, since Rab23 and Rab8 are both linked to importinβ2-regulated ciliary trafficking, it will interesting to determine whether these GTPases function in common ciliary import pathways with nucleocytoplasmic proteins.Citation89,99
RAB23 was also recently reported to traffic the D1 dopaminergic receptor (D1R) and the somatostatin receptor 3 (SSTR3) to mammalian cilia.Citation104 In this study, photoactivatable reporters revealed that D1R laterally diffuses from the plasma membrane into the ciliary membrane via a mechanism involving its C-terminal tail, together with Rab23, KIF17 and IFT complex B.Citation104 As further evidence of its ciliary targeting capacity, fusions of Rab23(GTP) and non-ciliary receptors are ectopically targeted to cilia, as are Rab23 fusions with a normally mislocalised D1R variant (Δ381–395).Citation104 Furthermore, Rab23(GTP) fused to D1R(Δ381–395) bypassed, at least in part, the ciliary targeting requirement of KIF17 and IFT-B.Citation104 From this work, the authors conclude that Rab23 functions in an integrated pathway with KIF17 and IFT-B to target D1R from the plasma membrane to the ciliary membrane.
In summary, Rab23 is required for transport-based regulation of ciliary signaling, but is mostly dispensable for ciliogenesis (). Ciliary roles for Rab23 are further supported by phylogenetic analyses showing Rab23 loss in some organisms lacking cilia, including most organisms lacking motile flagella.Citation6,103 In addition, Rab23 is mutated in Carpenter syndrome, which features ciliopathy-related symptoms such as craniofacial defects, cardiac abnormalities, obesity, digit and limb defects, hydrocephalus and mental retardation.Citation106-109 Despite progress, there are many unanswered questions related to: (1) the nature of the Rab23 GEF/GAP regulators and effectors in the ciliary context, (2) the precise mechanistic relationship between Rab23, KIF17, Rab8 and Importinβ2/Ran transport pathways, and (3) how Rab23 functionally interacts with known ciliary transport pathways to inhibit Shh signaling.
Rab5
RAB5 is a central regulator of early endosome formation and associated protein sorting, driving key processes such as membrane tethering, fusion and motility.Citation110 Despite these critical functions, surprisingly little is known about the role of Rab5 in ciliary processes. However, as outlined below, insight is now beginning to emerge from various studies.
A number of observations indicate that Rab5 functionally associates with cilia-related membranes. In C. elegans sensory neurons, GFP::RAB-5-marked early endosomes and additional endocytic regulators localize at the ciliary base, within the so-called periciliary membrane compartment (PCMC). An additional minor pool of RAB-5 is also found within at least some ciliary axonemes.Citation111-113 In support of RAB-5 functions near the ciliary base, disruption of a C. elegans p38 MAPK ortholog (PMK-3) that extracts inactive RAB5 from membranes causes elevated PCMC accumulations of RAB-5.Citation113,114 PCMC-associated RAB-5 also colocalises with OSTA-1, which is an organic solute transporter α homolog that regulates cilium morphology, ciliary membrane protein localisations, and the dendritic trafficking of RAB-5–associated endosomes.Citation115 In addition, C. elegans RAB-5 colocalises at the ciliary base with ESCRT protein orthologues (STAM1, HRS) required for the ciliary localization and signaling functions of TRP channel polycystin 1/2 orthologues (LOV-1, PKD-2).Citation111 Consistent with the nematode findings, mammalian Rab5 is found on internalized cilium-derived early endosomes together with the ciliogenesis regulator, inositol 5-phosphatase (OCRL1).Citation116,117 Furthermore, mammalian Rab5(GDP) overexpression slows the ciliary recovery of the apical receptor Kim1, indicating that Kim1 ciliary targeting involves Rab5-mediated recycling from apical membranes.Citation84 Together, these data provide good evidence that endosomal Rab5 functions at or near the ciliary base where many ciliogenic and ciliary signaling factors reside. Such findings are also consistent with the ciliary pocket being a unique site of endocytosis, enriched for clathrin coated pits, endocytic vesicles and endosomal compartments.Citation118-120
Although RAB5 appears to participate in ciliary endomembrane processes, its requirement for ciliogenesis remains unclear. In cultured mammalian cells, overexpression of Rab5(GTP) or Rabaptin5 (Rab5 GEF co-activation factor) reduces ciliation, whereas this does not occur in cells overexpressing Rab5(GDP) or depleted for Rabaptin5.Citation121,122 These observations suggest that Rab5 is an inhibitor of ciliogenesis in cultured cells, and this notion is further supported by the very recent finding that RAB5(GDP) overexpression blocks cilium disassembly.Citation122 In contrast, overexpression of RAB-5(WT), RAB-5(GDP) or RAB-5(GTP) does not affect the integrity and length of most examined cilia in C. elegans.Citation112,113 The one exception is the AWB cilium, which possesses branching and membrane expansion defects in worms overexpressing RAB-5(GTP) or RAB-5(GDP).Citation112 Consistent with a mostly non-ciliogenic role for nematode RAB-5, cilium structures appear normal in worms depleted of RAB-5 (RNAi) and in worms with disrupted RAB-5 GEFs (RABX-5 or RME-6).Citation112,113 Interestingly, loss-of-function mutations in these RAB-5 GEFs suppress the cilium structure defects of G-protein α-disrupted worms,Citation113 which could indicate that RAB-5 negatively regulates ciliogenesis in this context, similar to what is described above for mammalian RAB5. Taken together, the mammalian and C. elegans data indicate that RAB5 is largely dispensable for ciliogenesis, although it serves as a negative regulator of cilium formation and resorption in some contexts.
Consistent with the above model, disruption of genes involved in the earliest steps of clathrin dependent endocytosis (CDE), upstream of Rab5, cause only modest or no effects on cilium structure. In AP2 (clathrin adaptor complex 2) or clathrin heavy chain-depleted mammalian cells, cilium formation is normal (IMCD3 cells) or only modestly affected (RPE1 cells).Citation118,123 Similarly, cilium structure defects are only observed for a subset of cilia in C. elegans AP2 subunit gene mutants.Citation112 Thus, like RAB5, regulators of early CDE events are not globally required for ciliogenesis. However, and again like RAB5, the earliest steps of CDE are required for ciliary protein localization and signaling functions. For example, in AP2-disrupted worms, the ciliary localisations of multiple proteins (e.g., GPCRs and IFT components) are partially disrupted, IFT rates are abnormal, and AP2 subunit genes interact with known ciliary transport genes such as bbs-8 to regulate ciliary membrane volume and homeostasis.Citation112 In mammalian cells, several studies show that CDE events at the ciliary base or pocket regulate Shh and TGF-B ciliary signaling.Citation120,123-125 Together, the data discussed here for upstream components of presumptive RAB-5 pathways is consistent with the model that C. elegans RAB-5 is not a major regulator of ciliogenesis, but rather a regulator of ciliary protein trafficking and ciliary membrane homeostasis.
Rabl4 (IFT27)
Rabl4 belongs to the Rab-like (Rabl) clade of proteins distinguished from the canonical Rab family based on distinct sequence features such as the lack of a C-terminal prenylation motif.Citation5,126,127 Although Rabl4 is not a classical Rab, it nonetheless retains all 5 Rab-specific consensus sequences (F1-F5) and it has been suggested that Rabl4 was part of the ancestral Rab family in LECA.Citation6,127 Until the recent discovery of ciliary associations, nothing was known about Rabl4 functions in any cellular context.
In 2005, Lucker and colleagues identified a 27kDa protein, termed IFT27, that biochemically associates with a salt stable IFT-B complex core in Chlamydomonas.Citation34 IFT27 was subsequently identified as the algal ortholog of Rabl4 and shown to biochemically interact with IFT-B, localize to cilia and undergo IFT in multiple systems.Citation128-134 In agreement with its lack of a prenylation motif, flagellar IFT27 biochemically partitions with the aqueous phase in Chlamydomonas, indicating that IFT27 function may not depend on ciliary membrane associations.Citation128 However, the ciliary localization, IFT movement and IFT-B association of IFT27 is dependent on GTP binding, as well as nucleotide-independent heterodimer formation with the IFT25 phosphoprotein.Citation129,130,132-138 IFT25/27 is thought to associate with the IFT-B1 core module via a surface-exposed patch adjacent to the GTP-binding site that interacts with the C-terminal coiled coil region of the IFT81/74 complex.Citation130,139-141 Thus, RabL4 is a conserved bona fide IFT protein that forms a stable complex with IFT25, tethered to the IFT-B1 module. Consistent with its ciliary association, RabL4 is lost from the genomes of organisms that lack cilia.Citation6,127
Despite a conserved biochemical association with the IFT-B particle, IFT27 is dispensable for cilium formation in some cell types but not others. In Chlamydomonas and trypanosomes, IFT27 depletion or overexpression of putative GDP- or GTP-locked variants (trypanosomes) results in truncated flagella and cell division/growth defects.Citation128,133 In IFT27–depleted trypanosomes, the phenotype resembles a retrograde IFT defect, consisting of flagellar accumulation of IFT-B proteins and flagellar loss of IFT-A and IFT dynein proteins.Citation133 This led to the model that IFT27 controls the flagellar import of retrograde IFT machinery by regulating its docking to IFT trains.Citation133 In contrast, most ciliary structures are normal in IFT27 (and IFT25) knockout mice, with normal ciliary levels of IFT-A, IFT-B and IFT dynein, despite a neonatal-lethality phenotype.Citation132,134,138,142 Interestingly, the one exception is the mouse sperm flagellum, whose formation relies on an intact IFT25/27 complex.Citation143 The ciliogenesis and neonatal lethality phenotype of IFT25 and IFT27 null mice contrasts with the strong ciliogenesis defects and mid-gestation lethality phenotype of mice with mutations in other IFT-B genes.Citation144,145 Thus, in higher organisms, the IFT25/27 heterodimer serves as a functionally distinct submodule of IFT-B1 that is not required for ciliogenesis and IFT in somatic cells.
Although most cilium structures are normal, IFT27 null mice display cilium-associated developmental defects in digit formation, bone, left-right asymmetry, neural tube patterning and cardiac formation.Citation132 Similar abnormalities occur in IFT25 knockout mice, although the phenotypic severity and penetrance is somewhat reduced.Citation138 Associated with these tissue level phenotypes are defects in cilium-associated sonic hedgehog signaling.Citation132,138,142 Specifically, IFT27−/− fibroblasts treated with SAG (Shh agonist) possess a reduced capacity to upregulate Gli1/Ptch1 expression and redistribute the Gli2 transcription factor to the ciliary tip; also, Smo/Ptch1/Gpr161 abnormally accumulate in the cilia of untreated IFT27−/− cells.Citation132,134,142 Furthermore, expression of a constitutively active Smo variant (SmoM2) causes Gli2 to localize all along IFT27-disrupted cilia, rather than just the tip, indicating that IFT27 is not required for Gli2 ciliary entry but rather its transport to, or association with, the ciliary tip.Citation132 Thus, IFT27 regulates the ciliary distribution and removal of Shh signal mediators.
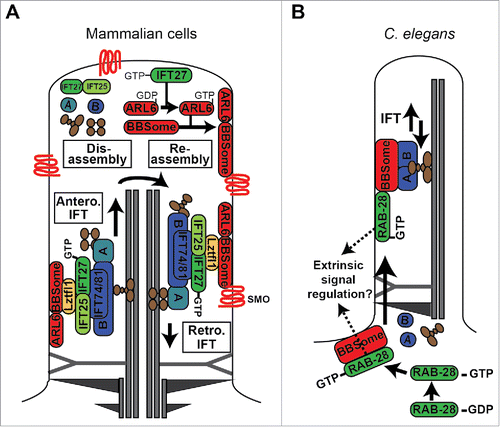
Mechanistically, these mammalian IFT27 functions appear to be linked to the BBSome and associated recruitment factors (Lztfl1 and ARL6) based on: (1) BBSome-associated proteins abnormally accumulate in IFT27 (and IFT25) disrupted cilia, (2) Shh signaling mediators (Smo, GPR161) accumulate within BBSome and ARL6 disrupted cilia, (3) ARL6, and specifically the nucleotide free form, biochemically interacts with IFT27 and not other IFT proteins, and (4) BBSome ciliary exit kinetics are retarded in IFT27-disrupted cells.Citation132,134,146 In one model, Lztfl1 couples IFT-B-associated IFT25/27 with the BBSome, which in turn interacts with Smo at the ciliary membrane to facilitate its removal from the organelle via retrograde IFT.Citation132 In a second complementary model, IFT25/27 released from IFT-B at the ciliary tip serves as a GEF to activate ARL6, which in turn drives assembly and recruitment of the BBSome (and associated cargo) at the ciliary tip membrane, followed by BBSome association with retrograde IFT trains. These models (shown in ) are consistent with a role for the Chlamydomonas BBSome in flagellar export of signaling molecules, the observation that a portion of IFT25/27 is biochemically separated from IFT-B, and the pathogenic association of IFT27 with Bardet-Biedl syndrome.Citation129,131,147,148 In relation to the second model, it is important to note that although the IFT25/27 complex stabilises nucleotide-free ARL6, its ability to induce GDP release from ARL6 is very limited. While this could be explained by a requirement of other factors to reconstitute full GDP-release activity,Citation134 it remains to be shown whether IFT27 is a true ARL6 GEF.
Figure 4. Models of RABL4 (IFT27) and RAB28 association with IFT and the BBSome cargo adaptor. (A) IFT27 forms a submodule of IFT-B that associates with the BBSome via Ltzfl1. At the ciliary tip, where IFT trains are remodelled for retrograde transport, IFT27 acts as a GEF to activate ARL6, which in turn directs the BBSome to the ciliary membrane for assembly into retrograde IFT trains that remove signaling proteins such as Smoothened (Smo) from the cilium. IFT complexes (A and B) shown in blue. IFT motors (kinesin-II and cytoplasmic dynein) shown in brown. (B) In C. elegans, activated RAB-28 is targeted to the periciliary membrane via the BBSome where it incorporates into IFT trains as cargo. RAB-28 is proposed to serve cell non-autonomous functions by regulating extrinsic signaling to a nearby glial cell.Citation164 Note that it remains to be shown if RAB-28 directly interacts with the BBSome. For simplicity, only one microtubule doublet is shown in the cartoon.
Taken together, the available data establishes IFT- and BBSome- associated roles for Rabl4 (IFT27) in regulating cilium structural integrity (protists), function and developmental signaling. Such functions are consistent with the loss of Rabl4 from the genomes of non-ciliated organisms.Citation6 Of great interest for future work will be to identify the mechanisms regulating the activity of Rabl4 itself. Similar to other small ciliary GTPases like Arl13b, Rabl4 possesses a poorly conserved switch II catalytic glutamine, which might explain why stabilised IFT27 (and Arl13b) possesses very low intrinsic GTPase activities.Citation39,130,149 Low GTPase activity also indicates that IFT27 GTP hydrolysis probably requires a GAP regulator that remains to be identified.Citation130 Also, it is noteworthy that stabilised IFT27 has relatively weak micromolar affinity for GDP, indicating that GDP to GTP exchange may not require a GEF.Citation130
Rabl5 (IFT22)
RAB-like 5 (Rabl5) displays a high degree of sequence divergence from canonical Rab proteins, lacking, for example, several the Rab consensus sequences (F4, F5).Citation5,6,126,127,150 In fact, multiple studies place Rabl5 closer to Ras/Rho/Ran family members than Rabs.Citation5,6,127 Like Rabl4, the cellular functions of Rabl5 were completely unknown before ciliary associations were uncovered.
The first functional association with the cilium was uncovered by Schafer and colleagues who found that the sole Rabl5 ortholog (termed IFT-associated protein 2 or IFTA-2) in C. elegans is expressed exclusively in ciliated cells and undergoes IFT.Citation150 This study also found that a predicted IFTA-2(GDP) variant is excluded from the cilium, indicating that GTP-binding regulates the GTPase's association with IFT trains.Citation150 Consistent with these findings, Rabl5 is also found in the ciliary and flagellar axonemes of mammalian cells and protists (Chlamydomonas, trypanosomes); also, trypanosome Rabl5 undergoes IFT and accumulates at the basal body and axoneme of IFT-B and IFT-A gene depleted cells, respectively.Citation37,126,150,151 Thus, Rabl5 is a conserved IFT-associated protein, similar to Rabl4.
Work in Chlamydomonas has revealed that Rabl5 biochemically associates with IFT complexes. Specifically, the 22kDa Rabl5 ortholog (called FAP9 or IFT22) co-sediments with IFT81 (but not IFT139), indicating association with IFT-B, but not IFT-A, complexes.Citation151 This conclusion is further supported from immunoprecipitation and IFT-B1 reconstitution experiments showing that Chlamydomonas IFT22 binds to the IFT81/74 coiled-coil region, similar to the interaction observed for IFT25/27.Citation140 However, despite a common binding region, IFT22 does not directly interact with IFT25/27, nor is IFT22 required for the formation of a stable IFT81/74/27/25 tetrameric complex.Citation140,152 Therefore, although IFT27 and IFT22 both associate with IFT-B1, they likely occupy distinct functional submodules of the assembly.
Despite the low overall sequence homology between Rabl5 with Rabl4, the shared association with IFT-B implicates related functions for these GTPases. Indeed, like Rabl4, Rabl5 is conserved in ciliated organisms versus non ciliated organisms, lacks C-terminal prenylation and switch II glutamine residues, and biochemically partitions with the aqueous (rather than the membrane) flagellar fraction in Chlamydomonas.Citation126,150,151,153 Furthermore, and again similar to Rabl4, trypanosome Rabl5 depletion causes a retrograde IFT-like phenotype, comprised of a short bulbous flagellum with accumulation of electron dense material that includes IFT172.Citation126 Consistent with this finding, Chlamydomonas IFT22 depletion causes IFT-A/B protein redistribution to the flagellum, although flagellum length remains normal.Citation151 Thus, like Rabl4, protist Rabl5 appears to regulate retrograde IFT, as well as flagellum structural integrity in trypanosomes.
Rabl5 function is different in metazoans. In C. elegans, a predicted null mutant of the worm ortholog (called IFTA-2) displays normal ciliary localisations and IFT movement of IFT-A/B and an IFT-dynein light intermediate chain protein, as well as normal cilium structures.Citation150 Despite these observations, IFTA-2 loss causes defects in cilium-associated signaling pathways related to lifespan and the formation of the alternative ‘dauer’ developmental stage.Citation150 Therefore, like mammalian Rabl4, Rabl5 appears dispensable for cilium formation in C. elegans, but necessary for cilium-mediated signaling.Citation132,134,150 Interestingly, the ciliary localisations of 2 key lifespan and dauer formation signaling molecules (DAF-2, AGE-1) are not affected in IFTA-2-disrupted worms;Citation150 thus, unlike mammalian Rabl4, there is no evidence at this point that nematode Rabl5 regulates the ciliary levels of signaling molecules.
Rab28
Rab28 is considered a peripheral member of the Rab superfamily, possibly forming an atypical Rab subfamily (Group VI) with its closest homolog, Rabl4.Citation5,6,127,154 Rab28 possesses several unusual features including divergent switch 1 sequences and the ability to undergo an abnormally large nucleotide-dependent conformational change.Citation155 Although relatively unstudied compared with other Rabs, Rab28 is reported to control Glut4 trafficking, NF-kB nuclear transport, endosomal sorting (trypanosomes), and plant germination.Citation156-159
The first ciliary connection was reported by Roosing and colleagues in 2013. In this study, Rab28 was found to localize at the ciliary basal body and rootlet of rat photoreceptor cells, and nonsense mutations (R137X, E189X) in RAB28 were identified in 2 families with the non-syndromic retinal ciliopathy, cone-rod dystrophy (CRD).Citation160,161 Subsequent studies identified additional CRD families with homozygous splice donor site (exon 2) and missense (C217W) RAB28 mutationsCitation162 and additional Rab28 localisations to the mouse photoreceptor outer segment (modified cilium).Citation163
Consistent with the mammalian localization data, our laboratory in collaboration with the Leroux group recently reported that the sole ortholog in C. elegans is expressed almost exclusively in ciliated cells, accumulates at the periciliary membrane and ciliary axoneme, and undergoes IFT.Citation164 We also found that RAB-28s ciliary membrane and IFT associations depend on GTP binding and the BBSome IFT cargo adaptor.Citation164 Consistent with RAB-28 being an IFT cargo, rather than a core component of the IFT machinery, rab-28 null mutants display apparently normal cilium structures and sensory functions, as well as normal localisations and transport behaviors of IFT proteins and the BBSome.Citation164 Together, these findings establish RAB-28 as the third IFT-associated Rab alongside Rabl4 and Rabl5, and suggest that nematode RAB-28 functions as an IFT cargo, downstream of the BBSome, to regulate a discrete set of ciliary pathways (). Although mammalian Rab28 ciliary functions are not yet described, the nematode findings could indicate that Rab28 and Rabl4 function at distinct steps of a common BBS-related pathway, upstream (Rabl4) and downstream (Rab28) of the BBSome.Citation132,134,164 This scenario is consistent with the relatively severe BBS phenotype of patients with RABL4 (IFT27) mutations compared with the restricted CRD phenotype of patients with likely null mutations in RAB28.Citation148,160,161
An additional interesting finding from nematode studies is that RAB-28 may serve cell non-autonomous functions in neighboring glial cells (). In worms overexpressing RAB-28(GDP) or RAB-28(GTP), defects occur in the non-ciliated glial (sheath) cell that forms the supporting channel for the amphid ciliary bundle. Specifically, RAB-28(GTP) overexpression dramatically enlarges the channel, whereas RAB-28(GDP) overexpression slightly reduces channel size and causes large matrix filled vesicles (MFVs) to accumulate in the sheath cell.Citation164 These phenotypes appear to be cell non-autonomous effects because RAB-28 is not expressed in the sheath.Citation164 In one model, based on seminal work from the Shaham group,Citation165-167 MFV delivery to the sheath cell membrane, and therefore the expansion of the amphid pore, is regulated by RAB-28.Citation164 Thus, excessive RAB-28 activation would enhance MFV delivery to cause an abnormally large channel, whereas the opposite occurs when RAB-28 is inactivated. Although we don't know how ciliary RAB-28 might signal to glia, one possibility is that the GTPase regulates release of ciliary ectosomes carrying glial cell morphogenic factors. In support of this idea, the ciliary base region (where activated RAB-28 localizes) has been proposed as one potential site of nematode extracellular vesicle (EV) release and RAB-28 expression is upregulated in EV-releasing cells.Citation168-170
Rabl2
Rabl2 sequences are significantly diverged from most Rab proteins, with some studies considering it to be more ‘Ran-like’ than ‘Rab-like’.Citation6,127 However, Rabl2 retains all 5 Rab consensus motifs and therefore Rab-like functions for RABL2 cannot be fully excluded.Citation5,171 Although very little is known about its cellular functions, there is evidence that Rabl2 serves cilia-related roles.
In 2012, it was reported that mice carrying a hypomorphic mutation (Mot) in Rabl2 are sterile, possessing modestly truncated sperm tails and reduced sperm motility.Citation171 Consistent with a ciliary role, Rabl2 localizes to the sperm tail mid-piece (proximal segment of flagellum) and immunoprecipitates IFT-B1 proteins (IFT27/81/172).Citation171 In addition, the study identified 5 candidate Rabl2 effectors, which localize at the sperm tail principal piece and the more proximal mid piece.Citation171 These localisations are reduced in Rabl2(Mot) sperm, which led to the proposal that Rabl2 delivers effector proteins into the sperm flagellum up to the point of the annulus (distal-most end of mid-piece).Citation171 Together, these data implicate ciliary and IFT-associated functions for Rabl2 and agree with a recent phylogenetic analysis indicating that Rabl2 is an ancestral ciliary component.Citation172 Furthermore, the suggested IFT association reflects a role for Rabl2 during flagellum formation, since IFT is absent in mature mouse sperm.Citation173
In a recent follow-up study, the O'Bryan group reported that Rabl2 knockout mice develop adult onset obesity and fatty livers (steatosis), as well as impaired glucose and lipid metabolism.Citation174 Although reminiscent of a ciliopathy pathology, these mice lack hallmarks of cilia-related obesity such as hyperphagia and leptin resistance, which lead to the conclusion that Rabl2 is not a global ciliary regulator.Citation174 Indeed, this study found that Rabl2 loss alters hepatic mitochondrial fatty acid oxidation and mitochondrial transport along microtubules, suggesting that Rabl2 serves a non-ciliary function in addition to the previously reported role in sperm flagella.Citation171,174 Interestingly, Rabl2 colocalises with specific IFT proteins (IFT172) along hepatic cytoplasmic microtubules, indicating that a cytosolic Rabl2 function may involve interaction with a distinct IFT complex.Citation174
Very recent studies from the Jackson and Nakayama groups now reconcile and extend the previous findings in mice.Citation175,176 Specifically, these works show that RABL2 localizes at the base of the Chlamydomonas flagellum and human RPE1 cell primary cilia, using a recruitment mechanism that involves CEP19, FOP and CEP350.Citation175,176 These studies also show that activated RABL2B binds the IFT-B complex via the IFT74/81 heterodimer, regulates its entry into the cilium, and that RABL2 disruption affects cilium and flagella formation.Citation175,176 Furthermore, Rabl2 knockout mice display retinal degeneration and digit abnormalities, which together with the described previously obesity and sperm defects, suggests that Rabl2 may in fact serve broad cilia-related functions in mammals.Citation171,174,175 Together, all the various data supports a conserved role for RABL2 at the base of motile and primary cilia in regulating IFT-B complex entry into cilia.
Concluding remarks
In this review, we outline the cilium-associated functions of various Rab and related Rab-like proteins. From the available data, we make the following general points.
First, the cilia-related Rabs are not globally required for ciliogenesis in the manner observed for other ciliary trafficking modalities such as IFT. For example, despite ciliogenic requirements for Rab8 in cultured mammalian cells and zebrafish, and Rabl2/4/5 in protists, loss of ciliary Rab function does not affect ciliary structures in worms (Rab8, Rab28, Rabl5), zebrafish (Rab23), and mice (Rab8, Rabl4/5). Instead, the ciliary Rabs could regulate ciliogenesis in specific subsets of metazoan cells not yet analyzed; alternatively, as shown for Rab8 and Rab10 in mice, the ciliary Rabs may function redundantly with other Rabs to regulate ciliogenesis by sharing common effectors, for example.
Second, like other Rabs, the ciliary Rabs can function within GTPase cascades to control early ciliogenesis events in cultured mammalian cells (Rab11-Rab-8), post-ciliogenic sorting and delivery of RTCs in photoreceptors (ARF4-Rab11-Rab8), and turnaround of the BBSome at the ciliary tip (Rabl4-Arl6). These exciting findings raise important questions with regards to the conservation of such cascades in vivo, and the tissue-specific relevance of the ARF-Rab TGN sorting cascade for ciliary membrane proteins other than Rhodopsin. Indeed, a very recent study in rat kidney cells (NRK1) showed that overexpression of RAB11(GTP) or RAB11(GDP) does not affect cilium formation, suggesting that ciliogenesis is not under the strict control of a Rab11-Rab8 cascade.Citation88 Furthermore, regulation of mammalian fibrocystin ciliary steady-state levels appears to require Rab8, but not Arf4.Citation85,177 Similarly, mammalian Arf4 was reported in a recent study to be dispensable for polycystin 1 (PC1) ciliary localization, despite previous findings that the ciliary targeting of a PC1-chimera is dependent on Rab8 and Arf4.Citation86,178 The in vivo significance of the ARF-Rab cascade is also brought into light by another recent study, which shows that Arf4 deletion in the retina does not cause rhodopsin trafficking or photoreceptor degeneration.Citation179 It also remains to be determined if Rab11 functions with Rab8 to regulate PC1 and fibrocystin ciliary targeting.
Third, it is interesting that 4 atypical Rabs, Rabl2/4/5 and Rab28, are all linked to IFT-related processes, perhaps forming distinct cargo adaptor submodules of IFT-BBSome assemblies, or in the case of Rabl2, providing a mechanism for IFT-B entry into cilia. Indeed, these GTPases appear to have co-evolved with the cilium, and therefore IFT, and are typically lost in the genomes of organisms lacking cilia. An additional interesting feature of Rabl2/4/5 is their lack of a prenylation modification motif in the C-terminus, indicating ciliary functions not directly related to the ciliary membrane.
Fourth, we know very little about the regulatory proteins (e.g., GEFs, GAPs, GDIs, GDFs) that control Rab function in the ciliary context. Also, how the ciliary Rabs collaborate with other ciliary transport and ciliopathy modules remains poorly understood, although some progress has been made.Citation175,176,180
Fifth, 3 of the 8 known Rab/Rabl ciliary proteins (Rab23, Rabl4, Rab28) are already known to cause ciliopathy or ciliopathy-related (Carpenters syndrome) diseases. Based on their phylogenetic restriction to ciliated organisms, together with their apparent non-essential requirement for ciliogenesis, Rabl2 and Rabl5 represent excellent candidates for mutation analysis in ciliopathy patient studies.
Sixth, it is necessary to address the essential nature of some of the ciliary Rabs (Rab5, Rab11). To determine their cilium-specific functions, whole organism, tissue and/or cell specific knockouts (e.g., via CRISPR) will need to be used to derive specific Rab alleles (e.g., GDP- and GTP- locking mutations). It will also be necessary to use knock-in Rab reporters (e.g., GFP tagged) to overcome the potential pitfalls of Rab reporter overexpression.
Finally, the cilium represents a new paradigm for uncovering novel Rab functions, mechanisms of action and disease associations. Indeed, findings for Rabs in the ciliary context can inform the functions of Rabs in other subcellular contexts, and vice versa, and therefore facilitate a greater overall understanding of Rab-mediated membrane trafficking.
Disclosure of potential conflicts of interest
No conflicts of interest were disclosed.
Acknowledgments
SEM image in kindly provided by K. Phelps (Electron Microscopy Core Facility, UT Southwestern Medical Center), funded via NIH grant S10 OD020103–01.
Funding
OEB acknowledges principal investigator funding from Science Foundation Ireland (11/PI/1037).
References
- Brown FC, Pfeffer SR. An update on transport vesicle tethering. Mol Membr Biol. 2010;27:457-61. doi:10.3109/09687688.2010.501765. PMID:21067454
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119-49. doi:10.1152/physrev.00059.2009. PMID:21248164
- Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol. 2014;6:a022616. doi:10.1101/cshperspect.a022616. PMID:25341920
- Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci. 2015;128:3171-6. doi:10.1242/jcs.166074. PMID:26272922
- Elias M, Brighouse A, Gabernet-Castello C, Field MC, Dacks JB. Sculpting the endomembrane system in deep time: High resolution phylogenetics of Rab GTPases. J Cell Sci. 2012;125:2500-8. doi:10.1242/jcs.101378. PMID:22366452
- Klopper TH, Kienle N, Fasshauer D, Munro S. Untangling the evolution of Rab G proteins: Implications of a comprehensive genomic analysis. BMC Biol. 2012;10:71. doi:10.1186/1741-7007-10-71. PMID:22873208
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299-304. doi:10.1126/science.1062023. PMID:11701921
- Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269-309. doi:10.1152/physrev.00003.2012. PMID:23303910
- Pfeffer SR. Rab GTPase localization and Rab cascades in Golgi transport. Biochem Soc Trans. 2012;40:1373-7. doi:10.1042/BST20120168. PMID:23176483
- Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637-59. doi:10.1146/annurev-biochem-052810-093700. PMID:22463690
- Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25:414-9. doi:10.1016/j.ceb.2013.04.002. PMID:23639309
- Mitchell DR. Evolution of cilia. Cold Spring Harb Perspect Biol. 2017;9:a028290. doi:10.1101/cshperspect.a028290. PMID:27663773
- Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499-503. doi:10.1242/jcs.050377. PMID:20144997
- Benmerah A. The ciliary pocket. Curr Opin Cell Biol. 2013;25:78-84. doi:10.1016/j.ceb.2012.10.011. PMID:23153502
- Goetz S, Anderson K. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331-44. doi:10.1038/nrg2774. PMID:20395968
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83-7. doi:10.1038/nature02061. PMID:14603322
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018-21. doi:10.1038/nature04117. PMID:16136078
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi:10.1371/journal.pgen.0010053. PMID:16254602
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372-6. doi:10.1126/science.1139740. PMID:17641202
- Liem KF Jr, Ashe A, He M, Satir P, Moran J, Beier D, Wicking C, Anderson KV. The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. J Cell Biol. 2012;197:789-800. doi:10.1083/jcb.201110049. PMID:22689656
- Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210-23. doi:10.1016/j.cell.2012.12.026. PMID:23332756
- He M, Agbu S, Anderson KV. Microtubule motors drive hedgehog signaling in primary cilia. Trends Cell Biol. 2016;27(2):110-25. PMID:27765513
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533-43. doi:10.1056/NEJMra1010172. PMID:21506742
- Waters AM, Beales PL. Ciliopathies: An expanding disease spectrum. Pediatr Nephrol. 2011;26:1039-56. doi:10.1007/s00467-010-1731-7. PMID:21210154
- Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363-77. doi:10.1083/jcb.15.2.363. PMID:13978319
- Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207-30. PMID:5661997
- Ghossoub R, Molla-Herman A, Bastin P, Benmerah A. The ciliary pocket: A once-forgotten membrane domain at the base of cilia. Biol Cell. 2011;103:131-44. doi:10.1042/BC20100128. PMID:21275905
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519-23. doi:10.1073/pnas.90.12.5519. PMID:8516294
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev. 2002;3:813-25. doi:10.1038/nrm952. PMID:12415299
- Ishikawa H, Marshall W. Ciliogenesis: Building the cell's antenna. Nat Rev Mol Cell Biol. 2011;12:222-56. doi:10.1038/nrm3085. PMID:21427764
- Taschner M, Lorentzen E. The intraflagellar transport machinery. Cold Spring Harb Perspect Biol. 2016;8:a028092. doi:10.1101/cshperspect.a028092. PMID:27352625
- Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc Natl Acad Sci U S A. 1997;94:4457-62. doi:10.1073/pnas.94.9.4457. PMID:9114011
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993-1008. doi:10.1083/jcb.141.4.993. PMID:9585417
- Lucker BF, Behal RH, Qin H, Siron LC, Taggart WD, Rosenbaum JL, Cole DG. Characterization of the intraflagellar transport complex B core: Direct interaction of the IFT81 and IFT74/72 subunits. J Biol Chem. 2005;280:27688-96. doi:10.1074/jbc.M505062200. PMID:15955805
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201-13. doi:10.1016/j.cell.2007.03.053. PMID:17574030
- Taschner M, Weber K, Mourao A, Vetter M, Awasthi M, Stiegler M, Bhogaraju S, Lorentzen E. Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin-binding IFT-B2 complex. EMBO J. 2016;35:773-90. doi:10.15252/embj.201593164. PMID:26912722
- Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363-9. doi:10.1083/jcb.200703047. PMID:17646400
- Tsang WY, Bossard C, Khanna H, Peranen J, Swaroop A, Malhotra V, Dynlacht BD. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15:187-97. doi:10.1016/j.devcel.2008.07.004. PMID:18694559
- Cevik S, Hori Y, Kaplan OI, Kida K, Toivenon T, Foley-Fisher C, Cottell D, Katada T, Kontani K, Blacque OE. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J Cell Biol. 2010;188:953-69. doi:10.1083/jcb.200908133. PMID:20231383
- Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol. 2010;189:1039-51. doi:10.1083/jcb.200912001. PMID:20530210
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108:2759-64. doi:10.1073/pnas.1018823108. PMID:21273506
- Li Y, Ling K, Hu J. The emerging role of Arf/Arl small GTPases in cilia and ciliopathies. J Cell Biochem. 2012;113:2201-7. doi:10.1002/jcb.24116. PMID:22389062
- Schmidt KN, Kuhns S, Neuner A, Hub B, Zentgraf H, Pereira G. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol. 2012;199:1083-101. doi:10.1083/jcb.201202126. PMID:23253480
- Lu Q, Insinna C, Ott C, Stauffer J, Pintado PA, Rahajeng J, Baxa U, Walia V, Cuenca A, Hwang YS, et al. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol. 2015;17:228-40. doi:10.1038/ncb3109. PMID:25686250
- Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: How to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59-87. doi:10.1146/annurev.cellbio.042308.113337. PMID:19575670
- Hsiao Y-C, Tuz K, Ferland R. Trafficking in and to the primary cilium. Cilia. 2012;1:4. doi:10.1186/2046-2530-1-4. PMID:23351793
- Deretic D. Crosstalk of Arf and Rab GTPases en route to cilia. Small GTPases. 2013;4:70-7. doi:10.4161/sgtp.24396. PMID:23567335
- Malicki J, Avidor-Reiss T. From the cytoplasm into the cilium: Bon voyage. Organogenesis. 2014;10:138-57. doi:10.4161/org.29055. PMID:24786986
- Reiter J, Blacque O, Leroux M. The base of the cilium: Roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13:608-18. doi:10.1038/embor.2012.73. PMID:22653444
- Szymanska K, Johnson CA. The transition zone: An essential functional compartment of cilia. Cilia. 2012;1:10. doi:10.1186/2046-2530-1-10. PMID:23352055
- Garcia-Gonzalo FR, Reiter JF. Open sesame: How transition fibers and the transition zone control ciliary composition. Cold Spring Harb Perspect Biol. 2017;9:a028134. doi:10.1101/cshperspect.a028134. PMID:27770015
- Li Y, Hu J. Small GTPases and cilia. Protein Cell. 2011;2:13-25. doi:10.1007/s13238-011-1004-7. PMID:21337006
- Lim YS, Chua CE, Tang BL. Rabs and other small GTPases in ciliary transport. Biol Cell. 2011;103:209-21. doi:10.1042/BC20100150. PMID:21488838
- Wang J, Deretic D. Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res. 2014;38:1-19. doi:10.1016/j.preteyeres.2013.08.004. PMID:24135424
- Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108 (Pt 1):215-24. PMID:7738098
- Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12:2341-51. doi:10.1091/mbc.12.8.2341. PMID:11514620
- Mukhopadhyay S, Lu Y, Shaham S, Sengupta P. Sensory signaling-dependent remodeling of olfactory cilia architecture in C. elegans. Dev Cell. 2008;14:762-74. doi:10.1016/j.devcel.2008.03.002. PMID:18477458
- Kaplan OI, Molla-Herman A, Cevik S, Ghossoub R, Kida K, Kimura Y, Jenkins P, Martens JR, Setou M, Benmerah A, et al. The AP-1 clathrin adaptor facilitates cilium formation and functions with RAB-8 in C. elegans ciliary membrane transport. J Cell Sci. 2010;123:3966-77. doi:10.1242/jcs.073908. PMID:20980383
- Sato T, Mushiake S, Kato Y, Sato K, Sato M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji A, et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448:366-9. doi:10.1038/nature05929. PMID:17597763
- Sato T, Iwano T, Kunii M, Matsuda S, Mizuguchi R, Jung Y, Hagiwara H, Yoshihara Y, Yuzaki M, Harada R, et al. Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J Cell Sci. 2014;127:422-31. doi:10.1242/jcs.136903. PMID:24213529
- Babbey CM, Bacallao RL, Dunn KW. Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am J Physiol. 2010;299:F495-506
- Kuhns S, Schmidt KN, Reymann J, Gilbert DF, Neuner A, Hub B, Carvalho R, Wiedemann P, Zentgraf H, Erfle H, et al. The microtubule affinity regulating kinase MARK4 promotes axoneme extension during early ciliogenesis. J Cell Biol. 2013;200:505-22. doi:10.1083/jcb.201206013. PMID:23400999
- Kurtulmus B, Wang W, Ruppert T, Neuner A, Cerikan B, Viol L, Dueñas-Sánchez R, Gruss OJ, Pereira G. WDR8 is a centriolar satellite and centriole-associated protein that promotes ciliary vesicle docking during ciliogenesis. J Cell Sci. 2016;129:621-36. doi:10.1242/jcs.179713. PMID:26675238
- Wang L, Lee K, Malonis R, Sanchez I, Dynlacht BD. Tethering of an E3 ligase by PCM1 regulates the abundance of centrosomal KIAA0586/Talpid3 and promotes ciliogenesis. Elife. 2016;5:e12950. doi:10.7554/eLife.12950. PMID:27146717
- Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17:3796-805. doi:10.1093/hmg/ddn277. PMID:18772192
- Baron Gaillard CL, Pallesi-Pocachard E, Massey-Harroche D, Richard F, Arsanto JP, Chauvin JP, Lecine P, Krämer H, Borg JP, Le Bivic A. Hook2 is involved in the morphogenesis of the primary cilium. Mol Biol Cell. 2011;22:4549-62. doi:10.1091/mbc.E11-05-0405. PMID:21998199
- Kobayashi T, Kim S, Lin YC, Inoue T, Dynlacht BD. The CP110-interacting proteins Talpid3 and Cep290 play overlapping and distinct roles in cilia assembly. J Cell Biol. 2014;204:215-29. doi:10.1083/jcb.201304153. PMID:24421332
- Barbelanne M, Hossain D, Chan DP, Peranen J, Tsang WY. Nephrocystin proteins NPHP5 and Cep290 regulate BBSome integrity, ciliary trafficking and cargo delivery. Hum Mol Genet. 2015;24:2185-200. doi:10.1093/hmg/ddu738. PMID:25552655
- Tollenaere MA, Mailand N, Bekker-Jensen S. Centriolar satellites: Key mediators of centrosome functions. Cell Mol Life Sci. 2015;72:11-23. doi:10.1007/s00018-014-1711-3. PMID:25173771
- Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010;107:6346-51. doi:10.1073/pnas.1002401107. PMID:20308558
- Feng S, Knodler A, Ren J, Zhang J, Zhang X, Hong Y, Huang S, Peränen J, Guo W. A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem. 2012;287:15602-9. doi:10.1074/jbc.M111.333245. PMID:22433857
- Hehnly H, Chen CT, Powers CM, Liu HL, Doxsey S. The centrosome regulates the Rab11- dependent recycling endosome pathway at appendages of the mother centriole. Curr Biol. 2012;22:1944-50. doi:10.1016/j.cub.2012.08.022. PMID:22981775
- Franco I, Gulluni F, Campa CC, Costa C, Margaria JP, Ciraolo E, Martini M, Monteyne D, De Luca E, Germena G, et al. PI3K class II alpha controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev Cell. 2014;28:647-58. doi:10.1016/j.devcel.2014.01.022. PMID:24697898
- Chiba S, Amagai Y, Homma Y, Fukuda M, Mizuno K. NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. EMBO J. 2013;32:874-85. doi:10.1038/emboj.2013.32. PMID:23435566
- Zhang B, Zhang T, Wang G, Wang G, Chi W, Jiang Q, Zhang C. GSK3beta-Dzip1-Rab8 cascade regulates ciliogenesis after mitosis. PLoS Biol. 2015;13:e1002129. doi:10.1371/journal.pbio.1002129. PMID:25860027
- Vetter M, Wang J, Lorentzen E, Deretic D. Novel topography of the Rab11-effector interaction network within a ciliary membrane targeting complex. Small GTPases. 2015;6:165-73. doi:10.1080/21541248.2015.1091539. PMID:26399276
- Deretic D, Puleo-Scheppke B, Trippe C. Cytoplasmic domain of rhodopsin is essential for post-Golgi vesicle formation in a retinal cell-free system. J Biol Chem. 1996;271:2279-86. doi:10.1074/jbc.271.4.2279. PMID:8567690
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 2009;28:183-92. doi:10.1038/emboj.2008.267. PMID:19153612
- Wang J, Morita Y, Mazelova J, Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J. 2012;31:4057-71. doi:10.1038/emboj.2012.253. PMID:22983554
- Wang J, Deretic D. The Arf and Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting. J Cell Sci. 2015;128:1375-85. doi:10.1242/jcs.162925. PMID:25673879
- Bachmann-Gagescu R, Phelps IG, Stearns G, Link BA, Brockerhoff SE, Moens CB, Doherty D. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum Mol Genet. 2011;20:4041-55. doi:10.1093/hmg/ddr332. PMID:21816947
- Bachmann-Gagescu R, Dona M, Hetterschijt L, Tonnaer E, Peters T, de Vrieze E, Mans DA, van Beersum SE, Phelps IG, Arts HH, et al. The ciliopathy protein CC2D2A associates with NINL and functions in RAB8-MICAL3-regulated vesicle trafficking. PLoS Genet. 2015;11:e1005575. doi:10.1371/journal.pgen.1005575. PMID:26485645
- Grigoriev I, Yu KL, Martinez-Sanchez E, Serra-Marques A, Smal I, Meijering E, Demmers J, Peränen J, Pasterkamp RJ, van der Sluijs P, et al. Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr Biol. 2011;21:967-74. doi:10.1016/j.cub.2011.04.030. PMID:21596566
- Boehlke C, Bashkurov M, Buescher A, Krick T, John AK, Nitschke R, Walz G, Kuehn EW. Differential role of Rab proteins in ciliary trafficking: Rab23 regulates smoothened levels. J Cell Sci. 2010;123:1460-7. doi:10.1242/jcs.058883. PMID:20375059
- Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol. 2010;188:21-8. doi:10.1083/jcb.200910096. PMID:20048263
- Ward HH, Brown-Glaberman U, Wang J, Morita Y, Alper SL, Bedrick EJ, Gattone VH 2nd, Deretic D, Wandinger-Ness A. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell. 2011;22:3289-305. doi:10.1091/mbc.E11-01-0082. PMID:21775626
- Hoffmeister H, Babinger K, Gurster S, Cedzich A, Meese C, Schadendorf K, Osten L, de Vries U, Rascle A, Witzgall R. Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. J Cell Biol. 2011;192:631-45. doi:10.1083/jcb.201007050. PMID:21321097
- Monis WJ, Faundez V, Pazour GJ. BLOC-1 is required for selective membrane protein trafficking from endosomes to primary cilia. J Cell Biol. 2017;216(7):2131-50. doi:10.1083/jcb.201611138. PMID:28576874
- Madugula V, Lu L. A ternary complex comprising transportin1, Rab8 and the ciliary targeting signal directs proteins to ciliary membranes. J Cell Sci. 2016;129:3922-34. doi:10.1242/jcs.194019. PMID:27633000
- Dishinger JF, Kee HL, Jenkins PM, Fan S, Hurd TW, Hammond JW, Truong YN, Margolis B, Martens JR, Verhey KJ. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat Cell Biol. 2010;12:703-10. doi:10.1038/ncb2073. PMID:20526328
- Takao D, Verhey KJ. Gated entry into the ciliary compartment. Cell Mol Life Sci. 2016;73:119-27. doi:10.1007/s00018-015-2058-0. PMID:26472341
- Onnis A, Finetti F, Patrussi L, Gottardo M, Cassioli C, Spano S, Baldari CT. The small GTPase Rab29 is a common regulator of immune synapse assembly and ciliogenesis. Cell Death Differ. 2015;22:1687-99. doi:10.1038/cdd.2015.17. PMID:26021297
- Finetti F, Onnis A, Baldari CT. Regulation of vesicular traffic at the T cell immune synapse: Lessons from the primary cilium. Traffic. 2015;16:241-9. doi:10.1111/tra.12241. PMID:25393976
- Angus KL, Griffiths GM. Cell polarisation and the immunological synapse. Curr Opin Cell Biol. 2013;25:85-91. doi:10.1016/j.ceb.2012.08.013. PMID:22990072
- Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194-8. doi:10.1038/35084089. PMID:11449277
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325-30. doi:10.1073/pnas.0505328102. PMID:16061793
- Eggenschwiler JT, Bulgakov OV, Qin J, Li T, Anderson KV. Mouse Rab23 regulates hedgehog signaling from smoothened to Gli proteins. Dev Biol. 2006;290:1-12. doi:10.1016/j.ydbio.2005.09.022. PMID:16364285
- Fuller K, O'Connell JT, Gordon J, Mauti O, Eggenschwiler J. Rab23 regulates Nodal signaling in vertebrate left-right patterning independently of the Hedgehog pathway. Dev Biol. 2014;391:182-95. doi:10.1016/j.ydbio.2014.04.012. PMID:24780629
- Lim YS, Tang BL. A role for Rab23 in the trafficking of Kif17 to the primary cilium. J Cell Sci. 2015;128:2996-3008. doi:10.1242/jcs.163964. PMID:26136363
- Pataki C, Matusek T, Kurucz E, Ando I, Jenny A, Mihaly J. Drosophila Rab23 is involved in the regulation of the number and planar polarization of the adult cuticular hairs. Genetics. 2010;184:1051-65. doi:10.1534/genetics.109.112060. PMID:20124028
- Nozawa T, Aikawa C, Goda A, Maruyama F, Hamada S, Nakagawa I. The small GTPases Rab9A and Rab23 function at distinct steps in autophagy during Group A Streptococcus infection. Cell Microbiol. 2012;14:1149-65. doi:10.1111/j.1462-5822.2012.01792.x. PMID:22452336
- Chen Y, Ng F, Tang BL. Rab23 activities and human cancer-emerging connections and mechanisms. Tumour Biol. 2016;37:12959-67. doi:10.1007/s13277-016-5207-7. PMID:27449041
- Lumb JH, Field MC. Rab23 is a flagellar protein in Trypanosoma brucei. BMC Res Notes. 2011;4:190. doi:10.1186/1756-0500-4-190. PMID:21676215
- Leaf A, Von Zastrow M. Dopamine receptors reveal an essential role of IFT-B, KIF17, and Rab23 in delivering specific receptors to primary cilia. Elife. 2015;4. doi:10.7554/eLife.06996. PMID:26182404
- Evans TM, Ferguson C, Wainwright BJ, Parton RG, Wicking C. Rab23, a negative regulator of hedgehog signaling, localizes to the plasma membrane and the endocytic pathway. Traffic. 2003;4:869-84. doi:10.1046/j.1600-0854.2003.00141.x. PMID:14617350
- Jenkins D, Seelow D, Jehee FS, Perlyn CA, Alonso LG, Bueno DF, Donnai D, Josifova D, Mathijssen IM, Morton JE, et al. RAB23 mutations in Carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am J Hum Genet. 2007;80:1162-70. doi:10.1086/518047. PMID:17503333
- Alessandri JL, Dagoneau N, Laville JM, Baruteau J, Hebert JC, Cormier-Daire V. RAB23 mutation in a large family from Comoros Islands with Carpenter syndrome. Am J Med Genet A. 2010;152A:982-6. doi:10.1002/ajmg.a.33327. PMID:20358613
- Ben-Salem S, Begum MA, Ali BR, Al-Gazali L. A novel aberrant splice site mutation in RAB23 leads to an eight nucleotide deletion in the mRNA and is responsible for carpenter syndrome in a consanguineous emirati family. Mol Syndromol. 2013;3:255-61. PMID:23599695
- Haye D, Collet C, Sembely-Taveau C, Haddad G, Denis C, Soule N, Suc AL, Listrat A, Toutain A. Prenatal findings in carpenter syndrome and a novel mutation in RAB23. Am J Med Genet A. 2014;164A:2926-30. doi:10.1002/ajmg.a.36726. PMID:25168863
- Zeigerer A, Gilleron J, Bogorad RL, Marsico G, Nonaka H, Seifert S, Epstein-Barash H, Kuchimanchi S, Peng CG, Ruda VM, et al. Rab5 is necessary for the biogenesis of the endolysosomal system in vivo. Nature. 2012;485:465-70. doi:10.1038/nature11133. PMID:22622570
- Hu J, Wittekind SG, Barr MM. STAM and Hrs down-regulate ciliary TRP receptors. Mol Biol Cell. 2007;18:3277-89. doi:10.1091/mbc.E07-03-0239. PMID:17581863
- Kaplan OI, Doroquez DB, Cevik S, Bowie RV, Clarke L, Sanders AA, Kida K, Rappoport JZ, Sengupta P, Blacque OE. Endocytosis genes facilitate protein and membrane transport in C. elegans sensory cilia. Curr Biol. 2012;22:451-60. doi:10.1016/j.cub.2012.01.060. PMID:22342749
- van der Vaart A, Rademakers S, Jansen G. DLK-1/p38 MAP kinase signaling controls cilium length by regulating RAB-5 mediated endocytosis in caenorhabditis elegans. PLoS Genet. 2015;11:e1005733. doi:10.1371/journal.pgen.1005733. PMID:26657059
- Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell. 2001;7:421-32. doi:10.1016/S1097-2765(01)00189-7. PMID:11239470
- Olivier-Mason A, Wojtyniak M, Bowie RV, Nechipurenko IV, Blacque OE, Sengupta P. Transmembrane protein OSTA-1 shapes sensory cilia morphology via regulation of intracellular membrane trafficking in C. elegans. Development. 2013;140:1560-72. doi:10.1242/dev.086249. PMID:23482491
- Coon BG, Hernandez V, Madhivanan K, Mukherjee D, Hanna CB, Barinaga-Rementeria Ramirez I, Lowe M, Beales PL, Aguilar RC. The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Hum Mol Genet. 2012;21:1835-47. doi:10.1093/hmg/ddr615. PMID:22228094
- Luo N, West CC, Murga-Zamalloa CA, Sun L, Anderson RM, Wells CD, Weinreb RN, Travers JB, Khanna H, Sun Y. OCRL localizes to the primary cilium: A new role for cilia in Lowe syndrome. Hum Mol Genet. 2012;21:3333-44. doi:10.1093/hmg/dds163. PMID:22543976
- Molla-Herman A, Ghossoub R, Blisnick T, Meunier A, Serres C, Silbermann F, Emmerson C, Romeo K, Bourdoncle P, Schmitt A, et al. The ciliary pocket: An endocytic membrane domain at the base of primary and motile cilia. J Cell Sci. 2010;123:1785-95. doi:10.1242/jcs.059519. PMID:20427320
- Ghossoub R, Lindbaek L, Molla-Herman A, Schmitt A, Christensen ST, Benmerah A. Morphological and functional characterization of the ciliary pocket by electron and fluorescence microscopy. Methods Mol Biol. 2016;1454:35-51. doi:10.1007/978-1-4939-3789-9_3. PMID:27514914
- Pedersen LB, Mogensen JB, Christensen ST. Endocytic control of cellular signaling at the primary cilium. Trends Biochem Sci. 2016;41:784-97. doi:10.1016/j.tibs.2016.06.002. PMID:27364476
- Troilo A, Alexander I, Muehl S, Jaramillo D, Knobeloch KP, Krek W. HIF1alpha deubiquitination by USP8 is essential for ciliogenesis in normoxia. EMBO Rep. 2014;15:77-85. doi:10.1002/embr.201337688. PMID:24378640
- Saito M, Otsu W, Hsu KS, Chuang JZ, Yanagisawa T, Shieh V, Kaitsuka T, Wei FY, Tomizawa K, Sung CH. Tctex-1 controls ciliary resorption by regulating branched actin polymerization and endocytosis. EMBO Rep. 2017;18:1460–1472. doi:10.15252/embr.201744204. PMID:28607034
- Pal K, Hwang SH, Somatilaka B, Badgandi H, Jackson PK, DeFea K, Mukhopadhyay S. Smoothened determines beta-arrestin-mediated removal of the G protein-coupled receptor Gpr161 from the primary cilium. J Cell Biol. 2016;212:861-75. doi:10.1083/jcb.201506132. PMID:27002170
- Christ A, Christa A, Kur E, Lioubinski O, Bachmann S, Willnow TE, Hammes A. LRP2 is an auxiliary SHH receptor required to condition the forebrain ventral midline for inductive signals. Dev Cell. 2012;22:268-78. doi:10.1016/j.devcel.2011.11.023. PMID:22340494
- Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, Henriques de Jesus MP, Pedersen LB, Benmerah A, Andersen CY, Larsen LA, et al. TGF-beta signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep. 2013;3:1806-14. doi:10.1016/j.celrep.2013.05.020. PMID:23746451
- Adhiambo C, Blisnick T, Toutirais G, Delannoy E, Bastin P. A novel function for the atypical small G protein Rab-like 5 in the assembly of the trypanosome flagellum. J Cell Sci. 2009;122:834-75. doi:10.1242/jcs.040444. PMID:19240117
- Diekmann Y, Seixas E, Gouw M, Tavares-Cadete F, Seabra MC, Pereira-Leal JB. Thousands of rab GTPases for the cell biologist. PLoS Comput Biol. 2011;7:e1002217. doi:10.1371/journal.pcbi.1002217. PMID:22022256
- Qin H, Wang Z, Diener D, Rosenbaum J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr Biol. 2007;17:193-202. doi:10.1016/j.cub.2006.12.040. PMID:17276912
- Wang Z, Fan ZC, Williamson SM, Qin H. Intraflagellar transport (IFT) protein IFT25 is a phosphoprotein component of IFT complex B and physically interacts with IFT27 in Chlamydomonas. PloS One. 2009;4:e5384. doi:10.1371/journal.pone.0005384. PMID:19412537
- Bhogaraju S, Taschner M, Morawetz M, Basquin C, Lorentzen E. Crystal structure of the intraflagellar transport complex 25/27. EMBO J. 2011;30:1907-18. doi:10.1038/emboj.2011.110. PMID:21505417
- Richey EA, Qin H. Dissecting the sequential assembly and localization of intraflagellar transport particle complex B in Chlamydomonas. PloS One. 2012;7:e43118. doi:10.1371/journal.pone.0043118. PMID:22900094
- Eguether T, San Agustin JT, Keady BT, Jonassen JA, Liang Y, Francis R, Tobita K, Johnson CA, Abdelhamed ZA, Lo CW, et al. IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev Cell. 2014;31:279-90. doi:10.1016/j.devcel.2014.09.011. PMID:25446516
- Huet D, Blisnick T, Perrot S, Bastin P. The GTPase IFT27 is involved in both anterograde and retrograde intraflagellar transport. Elife. 2014;3:e02419. doi:10.7554/eLife.02419. PMID:24843028
- Liew GM, Ye F, Nager AR, Murphy JP, Lee JS, Aguiar M, Breslow DK, Gygi SP, Nachury MV. The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev Cell. 2014;31:265-78. doi:10.1016/j.devcel.2014.09.004. PMID:25443296
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173-8. doi:10.1038/nature04209. PMID:16189514
- Follit J, Xu F, Keady B, Pazour G. Characterization of mouse IFT complex B. Cell Motil Cytoskeleton. 2009;66:457-68. doi:10.1002/cm.20346. PMID:19253336
- Lechtreck KF, Luro S, Awata J, Witman GB. HA-tagging of putative flagellar proteins in Chlamydomonas reinhardtii identifies a novel protein of intraflagellar transport complex B. Cell Motil Cytoskeleton. 2009;66:469-82. doi:10.1002/cm.20369. PMID:19382199
- Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ. IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev Cell. 2012;22:940-51. doi:10.1016/j.devcel.2012.04.009. PMID:22595669
- Lucker BF, Miller MS, Dziedzic SA, Blackmarr PT, Cole DG. Direct interactions of intraflagellar transport complex B proteins IFT88, IFT52, and IFT46. J Biol Chem. 2010;285:21508-18. doi:10.1074/jbc.M110.106997. PMID:20435895
- Taschner M, Kotsis F, Braeuer P, Kuehn EW, Lorentzen E. Crystal structures of IFT70/52 and IFT52/46 provide insight into intraflagellar transport B core complex assembly. J Cell Biol. 2014;207:269-82. doi:10.1083/jcb.201408002. PMID:25349261
- Mourao A, Christensen ST, Lorentzen E. The intraflagellar transport machinery in ciliary signaling. Curr Opin Struct Biol. 2016;41:98-108. doi:10.1016/j.sbi.2016.06.009. PMID:27393972
- Yang N, Li L, Eguether T, Sundberg JP, Pazour GJ, Chen J. Intraflagellar transport 27 is essential for hedgehog signaling but dispensable for ciliogenesis during hair follicle morphogenesis. Development. 2015;142:2194-202. doi:10.1242/dev.115261. PMID:26023097
- Liu H, Li W, Zhang Y, Zhang Z, Shang X, Zhang L, Zhang S, Li Y, Somoza AV, Delpi B, et al. IFT25, an intraflagellar transporter protein dispensable for ciliogenesis in somatic cells, is essential for sperm flagella formation. Biol Reprod. 2017;96:993–1006. doi:10.1093/biolre/iox029. PMID:28430876
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347-55. PMID:10804177
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709-18. doi:10.1083/jcb.151.3.709. PMID:11062270
- Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, Nachury MV. Proteomics of primary cilia by proximity labeling. Dev Cell. 2015;35:497-512. doi:10.1016/j.devcel.2015.10.015. PMID:26585297
- Lechtreck K-F, Johnson E, Sakai T, Cochran D, Ballif B, Rush J, Pazour GJ, Ikebe M, Witman GB. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187:1117-49. doi:10.1083/jcb.200909183. PMID:20038682
- Aldahmesh MA, Li Y, Alhashem A, Anazi S, Alkuraya H, Hashem M, Awaji AA, Sogaty S, Alkharashi A, Alzahrani S, et al. IFT27, encoding a small GTPase component of IFT particles, is mutated in a consanguineous family with Bardet-Biedl syndrome. Hum Mol Genet. 2014;23:3307-15. doi:10.1093/hmg/ddu044. PMID:24488770
- Miertzschke M, Koerner C, Spoerner M, Wittinghofer A. Structural insights into the small G-protein Arl13B and implications for Joubert syndrome. Biochem J. 2014;457:301-11. doi:10.1042/BJ20131097. PMID:24168557
- Schafer JC, Winkelbauer ME, Williams CL, Haycraft CJ, Desmond RA, Yoder BK. IFTA-2 is a conserved cilia protein involved in pathways regulating longevity and dauer formation in Caenorhabditis elegans. J Cell Sci. 2006;119:4088-100. doi:10.1242/jcs.03187. PMID:16968739
- Silva DA, Huang X, Behal RH, Cole DG, Qin H. The RABL5 homolog IFT22 regulates the cellular pool size and the amount of IFT particles partitioned to the flagellar compartment in Chlamydomonas reinhardtii. Cytoskeleton. 2012;69:33-48. doi:10.1002/cm.20546. PMID:22076686
- Taschner M, Bhogaraju S, Vetter M, Morawetz M, Lorentzen E. Biochemical mapping of interactions within the intraflagellar transport (IFT) B core complex: IFT52 binds directly to four other IFT-B subunits. J Biol Chem. 2011;286:26344-52. doi:10.1074/jbc.M111.254920. PMID:21642430
- Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541-52. doi:10.1016/S0092-8674(04)00450-7. PMID:15137946
- Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. PMID:15367757
- Lee SH, Baek K, Dominguez R. Large nucleotide-dependent conformational change in Rab28. FEBS Lett. 2008;582:4107-11. doi:10.1016/j.febslet.2008.11.008. PMID:19026641
- Borrell A, Cutanda MC, Lumbreras V, Pujal J, Goday A, Culianez-Macia FA, Pagès M. Arabidopsis thaliana atrab28: A nuclear targeted protein related to germination and toxic cation tolerance. Plant Mol Biol. 2002;50:249-59. doi:10.1023/A:1016084615014. PMID:12175017
- Lumb JH, Leung KF, Dubois KN, Field MC. Rab28 function in trypanosomes: Interactions with retromer and ESCRT pathways. J Cell Sci. 2011;124:3771-83. doi:10.1242/jcs.079178. PMID:22100919
- Jiang J, Qi YX, Zhang P, Gu WT, Yan ZQ, Shen BR, Yao QP, Kong H, Chien S, Jiang ZL. Involvement of Rab28 in NF-kappaB nuclear transport in endothelial cells. PloS One. 2013;8:e56076. doi:10.1371/journal.pone.0056076. PMID:23457503
- Zhou Z, Menzel F, Benninghoff T, Chadt A, Du C, Holman GD, Al-Hasani H. Rab28 is a TBC1D1/TBC1D4 substrate involved in GLUT4 trafficking. FEBS Lett. 2017;591:88-96. doi:10.1002/1873-3468.12509. PMID:27929607
- Estrada-Cuzcano A, Roepman R, Cremers FP, den Hollander AI, Mans DA. Non-syndromic retinal ciliopathies: Translating gene discovery into therapy. Hum Mol Genet. 2012;21:R111-24. doi:10.1093/hmg/dds298. PMID:22843501
- Roosing S, Rohrschneider K, Beryozkin A, Sharon D, Weisschuh N, Staller J, Kohl S, Zelinger L, Peters TA, Neveling K, et al. Mutations in RAB28, encoding a farnesylated small GTPase, are associated with autosomal-recessive cone-rod dystrophy. Am J Hum Genet. 2013;93:110-7. doi:10.1016/j.ajhg.2013.05.005. PMID:23746546
- Riveiro-Alvarez R, Xie YA, Lopez-Martinez MA, Gambin T, Perez-Carro R, Avila-Fernandez A, López-Molina MI, Zernant J, Jhangiani S, Muzny D, et al. New mutations in the RAB28 gene in 2 Spanish families with cone-rod dystrophy. JAMA Ophthalmol. 2015;133:133-9. doi:10.1001/jamaophthalmol.2014.4266. PMID:25356532
- Hanke-Gogokhia C, Wu Z, Gerstner CD, Frederick JM, Zhang H, Baehr W. Arf-like protein 3 (ARL3) regulates protein trafficking and ciliogenesis in mouse photoreceptors. J Biol Chem. 2016;291:7142-55. doi:10.1074/jbc.M115.710954. PMID:26814127
- Jensen VL, Carter S, Sanders AA, Li C, Kennedy J, Timbers TA, Cai J, Scheidel N, Kennedy BN, Morin RD, et al. Whole-organism developmental expression profiling identifies RAB-28 as a novel ciliary GTPase associated with the BBSome and intraflagellar transport. PLoS Genet. 2016;12:e1006469. doi:10.1371/journal.pgen.1006469. PMID:27930654
- Perens EA, Shaham S. C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell. 2005;8:893-906. doi:10.1016/j.devcel.2005.03.009. PMID:15935778
- Heiman MG, Shaham S. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell. 2009;137:344-55. doi:10.1016/j.cell.2009.01.057. PMID:19344940
- Oikonomou G, Perens EA, Lu Y, Watanabe S, Jorgensen EM, Shaham S. Opposing activities of LIT-1/NLK and DAF-6/patched-related direct sensory compartment morphogenesis in C. elegans. PLoS Biol. 2011;9:e1001121. doi:10.1371/journal.pbio.1001121. PMID:21857800
- Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH, Barr MM. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol. 2014;24:519-25. doi:10.1016/j.cub.2014.01.002. PMID:24530063
- Wang J, Kaletsky R, Silva M, Williams A, Haas LA, Androwski RJ, Landis JN, Patrick C, Rashid A, Santiago-Martinez D, et al. Cell-specific transcriptional profiling of ciliated sensory neurons reveals regulators of behavior and extracellular vesicle biogenesis. Curr Biol. 2015;25:3232-8. doi:10.1016/j.cub.2015.10.057. PMID:26687621
- Wang J, Barr MM. Ciliary extracellular vesicles: Txt msg organelles. Cell Mol Neurobiol. 2016;36:449-57. doi:10.1007/s10571-016-0345-4. PMID:26983828
- Lo JC, Jamsai D, O'Connor AE, Borg C, Clark BJ, Whisstock JC, Field MC, Adams V, Ishikawa T, Aitken RJ, et al. RAB-like 2 has an essential role in male fertility, sperm intra-flagellar transport, and tail assembly. PLoS Genet. 2012;8:e1002969. doi:10.1371/journal.pgen.1002969. PMID:23055941
- Elias M, Klimes V, Derelle R, Petrzelkova R, Tachezy J. A paneukaryotic genomic analysis of the small GTPase RABL2 underscores the significance of recurrent gene loss in eukaryote evolution. Biol Direct. 2016;11:5. doi:10.1186/s13062-016-0107-8. PMID:26832778
- San Agustin JT, Pazour GJ, Witman GB. Intraflagellar transport is essential for mammalian spermiogenesis but is absent in mature sperm. Mol Biol Cell. 2015;26:4358-72. doi:10.1091/mbc.E15-08-0578. PMID:26424803
- Yi Lo JC, O'Connor AE, Andrews ZB, Lo C, Tiganis T, Watt MJ, O'Bryan MK. RABL2 is required for hepatic fatty acid homeostasis and its dysfunction leads to steatosis and a diabetes-like state. Endocrinology. 2016;157:4732-43. doi:10.1210/en.2016-1487. PMID:27732084
- Kanie T, Abbott KL, Mooney NA, Plowey ED, Demeter J, Jackson PK. The CEP19-RABL2 GTPase complex binds IFT-B to initiate intraflagellar transport at the ciliary base. Dev Cell. 2017;42(1):22-36.e12. doi:10.1016/j.devcel.2017.05.016. PMID:28625565
- Nishijima Y, Hagiya Y, Kubo T, Takei R, Katoh Y, Nakayama K. RABL2 interacts with the intraflagellar transport-B complex and CEP19 and participates in ciliary assembly. Mol Biol Cell. 2017;28:1652-66. doi:10.1091/mbc.E17-01-0017. PMID:28428259
- Follit JA, San Agustin JT, Jonassen JA, Huang T, Rivera-Perez JA, Tremblay KD, Pazour GJ. Arf4 is required for Mammalian development but dispensable for ciliary assembly. PLoS Genet. 2014;10:e1004170. doi:10.1371/journal.pgen.1004170. PMID:24586199
- Su X, Wu M, Yao G, El-Jouni W, Luo C, Tabari A, Zhou J. Regulation of polycystin-1 ciliary trafficking by motifs at its C-terminus and polycystin-2 but not by cleavage at the GPS site. J Cell Sci. 2015;128:4063-73. doi:10.1242/jcs.160556. PMID:26430213
- Pearring JN, San Agustin JT, Lobanova ES, Gabriel CJ, Lieu EC, Monis WJ, Stuck MWStrittmatter LJaber SMArshavsky VY, et al. Loss of Arf4 causes severe degeneration of the exocrine pancreas but not cystic kidney disease or retinal degeneration. PLoS Genet. 2017;13:e1006740. doi:10.1371/journal.pgen.1006740. PMID:28410364
- Omori Y, Zhao C, Saras A, Mukhopadhyay S, Kim W, Furukawa T, Sengupta P, Veraksa A, Malicki J. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437-44. doi:10.1038/ncb1706. PMID:18364699
