Abstract
Gamma delta (γδ) T cells are the all-rounders of our immune-system with their major histocompatibility complex-unrestricted cytotoxicity, capacity to secrete immunosti-mulatory cytokines and ability to promote the generation of tumor antigen-specific CD8+ and CD4+ T cell responses. Dendritic cell (DC)-based vaccine therapy has the prospective to harness these unique features of the γδ T cells in the fight against cancer. In this review, we will discuss our current knowledge on DC-mediated γδ T cell activation and related opportunities for tumor immunologists.
Abbreviations:
- γδ, gamma delta
- BCG, Bacillus Calmette-Guérin
- BrHPP, bromohydrin pyrophosphate
- CTL, cytotoxic T lymphocyte
- DC, dendritic cell
- GM-CSF, granulocyte-macrophage colony-stimulating factor
- HIV, human immunodeficiency virus
- HMBPP, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate
- iDC, immature DC
- IFN, interferon
- IL, interleukin
- IPP, isopentyl pyrophosphate
- mDC, myeloid dendritic cell
- MHC, major histocompatibility complex
- mo-DC, monocyte-derived dendritic cell
- pDC, plasmacytoid dendritic cell
- PD, programmed cell death protein
- TCR, T-cell receptor
- Th, T-helper cell
- TLR, toll-like receptor
- TNF, tumor necrosis factor
- Treg, regulatory T cell
Introduction
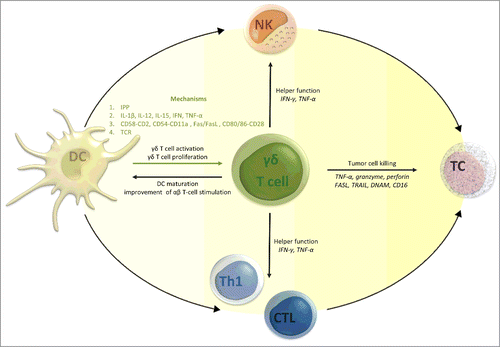
Since the discovery of γδ T cells in the late 1980s, insights have been gathered of this enigmatic and versatile T cell subtype, bridging innate and adaptive immunity.Citation1,2 Over the last decade, this component of cellular immunity received a growing body of interest from tumor immunologists, for the sake of their immunostimulatory properties and their ability to recognize and eradicate tumor cells. In particular, their ability to identify antigens out of the context of classical major histocompatibility complex (MHC) molecules, has raised expectations for their use in therapeutic anticancer applications.Citation3 Translational research efforts in the field of γδ T cell-based immunotherapy are currently focusing on two main approaches: (a) adoptive transfer of ex vivo activated and expanded γδ T cells, and (b) in vivo targeting of γδ T cells using therapeutic agents, such as IL-2, bisphosphonates or tumor-directed monoclonal antibodies (e.g. rituximab and trastuzumab) specifically aimed at the recruitment and expansion of γδ T cells (excellently reviewed elsewhereCitation4,5). Another path that could be explored in view of γδ T cell activation is DC vaccination. DCs are the orchestrators of our immune system. As sentinels, they recognize foreign invaders as well as stressed cells, after which they initiate an immune response appropriate to the hazard. Hence, DCs have caught the attention for their use as therapeutic agents for immunotherapy of cancerCitation6 and infectious diseases, such as human immunodeficiency virus (HIV).Citation7,8 Research on DC-based immunotherapy is currently focusing on the vaccine-mediating effects on the adaptive immune system, aiming at inducing (tumor)antigen-specific cytotoxic T lymphocytes (CTLs). Less extensively studied is the effect of DC-based immunotherapy on γδ T cells. Within this context, recent evidence has emerged that DCs can induce the activation and proliferation of γδ T cells, enhancing their cytotoxic and immunoregulatory functions.Citation9,10 γδ T cells, in turn, promote DC maturation and improve their capacity to stimulate adaptive αβ T-cell responses.Citation1 This review summarizes for the first time the current knowledge on DC-mediated γδ T cell activation, mechanisms behind the cell-to-cell interactions and its therapeutic potential for implementation in DC-based cancer immunotherapy ().
Figure 1. How γδ T cells can contribute to the antitumor efficacy of dendritic cell (DC)-based vaccination. It can be postulated that DC vaccination has the ability to activate γδ T cells and initiate their expansion. In turn, activated γδ T cells can (I) further stimulate vaccine and host DCs indirectly supporting sustained antitumor T-cell immunity and NK-DC crosstalk, (II) fulfil their immunomodulatory function through the secretion of pro-inflammatory cytokines regulating innate (natural killer cells) and adaptive (T cells) cellular immunity, and (III) directly kill tumor cells. Abbreviations: CTL, cytotoxic T lymphocyte; DC, dendritic cell; DNAM, DNAX accessory molecule; FASL, Fas ligand; IFN, interferon; IL, interleukin; IPP, isopentenyl pyrophosphate; NK, natural killer cells; TC, tumor cell; TCR, tumor cell; Th1, T-helper 1 cell; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand.
γδ T Cells and Cancer Immunity
Human γδ T cells are a group of unconventional T cells that can be subdivided based on their δ T-cell receptor (TCR) chain into Vδ1 and Vδ2 T cells. The majority of the tissue-associated γδ T cells bear the Vδ1 TCR, whereas the Vδ2 T cells represent the largest group in the blood, reaching up to 95%.Citation11 γδ T cells in blood represent 1–10% of the entire T-cell population,Citation11 but an impressive increase in their relative share is observed upon infection, detecting increments from as low as 1% to over 50% in a few days. The presence of activated γδ T cells is associated with resistance against infectious pathogens, such as Listeria monocytogenesCitation12 and the influenza virus,Citation13 underscoring their role in protecting the host against infectious disease.Citation14,15 Furthermore, γδ T cells are considered to be important players in cancer immune surveillance.Citation2 This is, among other things, substantiated by (I) their overrepresentation in reactive lymphatic regions associated with neoplasia,Citation16 (II) their potential to kill a variety of tumor cells including bladder cancer,Citation17 colon cancer,Citation18 glioblastoma multiforme,Citation19 hematological malignanciesCitation20 and multiple myeloma,Citation21 and (III) the observed trend between the presence of activated γδ T cells in cancer patients and a beneficial outcome.Citation22-24
γδ T cells dispose of a sensitive ability to distinguish “foreign” or transformed cells from healthy self-cells, using activating receptors and inhibitory killer Ig-like receptors (). γδ T cells are therefore not restricted to MHC priming, in contrast to the classic αβ T cells, which is a major asset in cancer cell recognition.Citation25,26 γδ T cells exert their cytotoxicity by releasing tumor necrosis factor (TNF)-α and granzymes in conjunction with perforins, and by binding Fas ligand, TNF-related apoptosis-inducing ligand and DNAX accessory molecule-1. Membrane-expression of CD16 leaves the possibility of antibody-dependent cellular cytotoxicity.Citation4,27,28 Another feature of γδ T cells is their immunoregulatory function. As being an important early source of pro-inflammatory cytokines such as interferon (IFN)-γ, TNF and interleukin (IL)-17, γδ T cells support DC, T cell, B cell and stromal cell functions.Citation1 γδ T cells can also develop properties of antigen-presenting cells, reminiscent of mature DCs, supporting the development of T-helper 1 (Th1) cells and cytotoxic T cells.Citation29 Both classic MHC I and MHC II loading pathways are therefore present in γδ T cells.Citation30 Even though γδ T cells predominantly exert innate-like responses, they are able to transform into memory cells. Thus naïve (CD45RA+CD27+) and central memory (CD45RA−CD27+) γδ T cells will home to secondary lymphoid organs, while effector memory (CD45RA−CD27−) and terminally differentiated (CD45RA+CD27−) γδ T cells show immediate effector functions on inflammatory sites.Citation28
Table 1. Main inhibitory and activating γδ T cell receptors and their ligands
Tumor cells, however, exploit different mechanisms to escape γδ T cell-mediated antitumor immunity, reviewed by others.Citation31,32 For example, tumor cells promote γδ T cells to adopt a regulatory T cell (Treg) phenotype. Such γδ Tregs damp antitumor immunityCitation33 and contribute to the immunosuppressive micro-environment that is characteristic of most tumor cells.Citation34 Deficient γδ T cell functions have already been observed in various malignancies, including hematological,Citation20 liver,Citation35 breastCitation35 and gastric cancers.Citation36 Re-establishment of γδ T cell antitumor activity is therefore valuable.
DC-mediated γδ T Cell Activation
Since the discovery by Ismaili et al.(2002) that phosphoantigen-activated γδ T cells are capable of inducing maturation of monocyte-derived DCs (mo-DCs), a plethora of research papers focused on the activation of DCs by γδ T cells.Citation37-40 It is only recently that γδ T cell activation by DCs and their crosstalk got its rightful attention. summarizes our current understanding of DC-mediated γδ T cell activation.
Table 2. Dendritic cell (DC)-mediated γδ T cell activation
DC-mediated γδ T cell proliferation
Seminal work by Takamizawa et al.Citation41 in the mid-1990s provided the first evidence that human DCs can mediate γδ T cell activation by inducing their proliferation, a finding that was later confirmed by Ye et al. in the early 2000s.Citation42 Although the nomenclature of the DC system is still expanding, two main in vivo DC subsets can be distinguished: plasmacytoid (pDCs) and myeloid DCs (mDCs). However, most DC studies, including those on the interaction between DCs and γδ T cells (), have relied on the use of ex vivo generated mo-DCs. To obtain mo-DCs, peripheral blood monocytes are cultured in vitro into immature (i)DCs in the presence of differentiation stimuli such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4. Immature DCs can in turn be converted into mature DCs by exposure to maturation stimuli such as cytokines and Toll-like receptor (TLR) ligands.Citation43 Neither immature mo-DCs or their mature counterparts are capable of inducing γδ T cell proliferation,Citation44 but they can acquire this capacity when stimulated by the bisphosphonate zoledronate.Citation44 Such zoledronate-treated mo-DCs were found to preferentially stimulate the proliferation of central memory and effector memory γδ T cells.Citation44 Recently, the ability to induce γδ T cell proliferation was also demonstrated for zoledronate-exposed CD56+ mDCs isolated from the blood of healthy volunteers.Citation10 The mechanism by which zoledronate-treated DCs mediate γδ T cell activation (Vγ9Vδ2 subset) relies on inhibition of the mevalonate pathway in DCs, leading to accumulation of mevalonate metabolites such as isopentenyl pyrophosphate (IPP).Citation45,46 Healthy somatic mammalian cells constitutively express the latter, but in a too low concentration for γδ T cell activation.Citation45 IPP and other phosphoantigens, such as (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), have been shown to promote Vγ9δ2 T cell proliferation, provided that there are MHC class II-positive cells (e.g., DCs) present.Citation47 However, this is not a property of the entire group of the phosphoantigens since another phosphoantigen, bromohydrin pyrophosphate (BrHPP), did not show any stimulatory effect on proliferation.Citation43
Similar to bisphosphonate and phosphoantigen stimulation, infection-related signals can also provide the necessary stimulus for DC-mediated γδ T cell proliferation (). For example, Bacillus Calmette-Guérin (BCG)- and Mycobacterium tuberculosis-infected DCs have been shown to induce proliferation of Vγ9δ2 T cells with a central memory phenotype, both in autologous and allogeneic settings.Citation48,49 In contrast, DCs infected with HIV are not able to induce Vγ9δ2 T cell proliferation,Citation50 indicating that DC-mediated γδ T cell proliferation following infection is dependent on the type of pathogen. In the context of infection-related signals, maturation of DCs with pathogen-associated molecular patterns (PAMPs; e.g. TLR-agonists) is promising.Citation9,51
Finally, cytokines, such as IL-15 and high-doses of IL-2, can provide the extra stimulus to enable mo-DCs to stimulate the proliferation of γδ T cells.Citation51 IL-15 is a pleiotropic cytokine, important for, among others, (γδ) T cell homeostasis.Citation52,53 Lack of IL-15 production by infected DCs results in the absence of γδ T cell effector functions, making the differentiation of central memory γδ T cells into effector memory cells difficult.Citation49 These data could support the implementation of IL-15 expressing DCs into clinical trials.Citation54-57
Impact of DCs on effector functions of γδ T cells
The two main effector functions of γδ T cells which would be desirable in the context of cancer immunotherapy are a direct cytotoxicity toward tumor cells and an immunomodulatory function through the secretion of pro-inflammatory cytokines including IFN-γ and TNF-α.
Immunomodulatory function
In line with the findings for γδ T cell proliferation, neither immature mo-DCsCitation48 or their cytokine matured counterpartsCitation9 are found to be capable of stimulating the cytokine secretion function of γδ T cells.
Likewise, pulsing DCs with zoledronate, which, as discussed above, results in production of phosphoantigens by DCs, enables their capacity to trigger the secretion of Th1 cytokines (e.g., IFN-γ, TNF-α and IL-1β) from γδ T cells.Citation10 A similar observation was made by Martino et al., who showed that zoledronate-treated DCs can stimulate the production of IFN-γ and TNF-α by Vγ9δ2 T cells.Citation48 DCs stimulated with the bisphosphonate pamidronateCitation43 or with ibandronateCitation51 have also been shown to trigger a Vγ9δ2 T-cell Th1 cytokine response, as defined by secretion of TNF-α and/or IFN-γ ().
An infection can also function as an additional signal γδ T cells need to exert their secretory function. Thus, IFN-γ and TNF-α secretion is observed with BCG-infected DCs.Citation48 Active infection is mandatory for Vγ9δ2 T cell triggering, as DCs infected with heat-killed BCG lose their incentive effect.Citation48 This ties in with the use of TLR ligand-based maturation cocktails in mo-DC generation protocols instead of the classic ‘Jonuleit cocktail’ (comprising TNF-α, IL-1β, IL-6 and PGE2).Citation58 TLR-matured DCs can namely potentiate the cytokine secretion function of γδ T cells.Citation9 Furthermore, lipoteichoic acids (TLR2 ligand),Citation59 poly(I:C) (TLR3 ligand), LPS (TLR4 ligand) and flagellin (TLR5 ligand)-mediated IFN-γ secretion of Vγ9δ2 T cells requires the presence of iDCs,Citation60 which was also previously observed with CD11c+ immature DC dependency of poly(I:C)-mediated activation of γδ T cells.Citation61 In line with the aforementioned, R848 (TLR7/8 ligand), CpG (TLR9 ligand) and vaccinal yellow fever virus strain (YF-17D)-triggered pDC induce Vγ9δ2 T cell IFN-γ production.Citation60 Generally, the secretory immune stimulatory effect is associated with an upregulation of activation markers on the γδ T cells like CD25 and CD69,Citation9,48 which could be interesting for immunomonitoring of DC vaccination.
In addition to Th1 cytokines, γδ T cells have been demonstrated to become major producers of IL-17 in reaction to IL-1β produced by DCs after encounter of immunogenic dying tumor cells. Although the role of IL-17 in antitumor defense is still unclear, there is some evidence that IL-17 secretion by γδ T cells, leading to invasion of tumor-reactive CTL, is required for optimal anticancer immune response to cytotoxic chemotherapeutics.Citation62 On the other hand, IL-17-producing γδ T cells support tumor growth by promoting angiogenesis.Citation63 This pro-tumor effect, associated with a poor clinical response, is more pronounced in solid tumors comparing with hematologic malignancies.Citation64 IL-17 in the tumor micro-environment remains therefore controversial.
Cytotoxicity of γδ T cells
In context of cancer immunotherapy, the cytotoxic activity is an important effector function of γδ T cells. However, irrespective of the positive effect of co-culture with DCs on proliferation and cytokine secretion of γδ T cells, DCs, whether or not pulsed with ibandronate, did not induce an increase in cytotoxic activity against myeloma cells.Citation51 This is in line with findings from Devilder et al. who reported a potentiating effect of DCs on Th1 and Th2 cytokine responses of BrHPP-stimulated Vγ9δ2 T cells, but not on their cytotoxicity.Citation43 The cause of this discrepancy is unknown and warrants further investigation, especially given the fact that under some circumstances DCs are capable to enhance Vγ9δ2 T cell killing of tumor cells, for example against the acute myeloid leukemia cell line THP-1.Citation48 Moreover, zoledronate-exposed DCs increase the expression of cytotoxic effector molecules such as NKG2D on γδ T cells.Citation48 Also, TLR-activated DCs induce the degranulation of granzyme B leading to an improved killing of Daudi cells, as compared to classic DCs. These effects, moreover, are maintained long-term.Citation9
γδ T cells influence the adaptive arm of the immune system
Initially, the use of DCs as vaccine is based on their T cell stimulatory ability. Tumor antigen-specific CD8+ CTLs, primed by DCs, are able to recognize tumor antigens expressed on malignant cells, leading to the killing of the latter. The question arises if γδ T cells can add to this effect. The majority of the reports state a valuable role for γδ T cell activation in the design of vaccine therapies for malignancy with regard to stimulation of the adaptive arm of the immune system. Indeed, Brucella-infected DCs cultured in the presence of Vγ9δ2 T cells induce the amplification of naive CD4+ T cells.Citation37 HLA-A2-positive CD56high+mo-DCs successfully induce Mart-1-derived modified peptide (A27L)-specific CD8+ T cells through preferential expansion of CD56+ Vγ9δ2 T cells in the presence of A27L, zoledronate, and IL-2.Citation65 In connection herewith, stimulating tumor antigen-pulsed (MART-1-modified peptide) mo-DCs with zoledronate alone leads first to the activation of Vγ9δ2 T cells which in turn results as well in an amplified activation and proliferation of tumor antigen-specific CD8+ T cells.Citation66 On the contrary, Traxlmayr et al. reported that γδ T-cells adversely regulate the proliferative capacity of CD4+ and CD8+ T cells after stimulation with IL-12 secreting mo-DCs.Citation67 Overall, simultaneous activation of γδ T cells and αβ T cells improves the antigen-specific cytotoxic responses mediated by the latter.Citation44 From this, it can be concluded that γδ T cells can indirectly contribute to the efficiency of DC based cancer vaccines by promoting the generation of tumor antigen-specific CD8+ T cell responses.
Mechanisms Behind DC-mediated γδ T Cell Activation
A first potential key mechanism behind DC-mediated Vγ9δ2 T cell activation is the production and secretion of IPP by DCs. This is the case for the improved γδ T cell proliferation by zoledronate-pretreated mo-DCs.Citation68 Extracellular concentrations of IPP were found to be 1000 times higher than the intracellular levels.Citation69 The importance of IPP was confirmed by the abrogation of the proliferative effect by adding simvastatin or mevastatin to the pretreatment of DCs.Citation44,68,69 Statins, a common group of cholesterol-lowering drugs, have an inhibiting effect on the mevalonate pathway by blocking the rate-limiting enzyme in the cholesterol synthesis, the HMG-CoA reductase. This results in a depletion of the starting products of IPP, impeding γδ T cell activation and the positive effect of the bisphosphonates.Citation70 Addition of mevastatin on the contrary has no effect on αβ T-cell counts.Citation44 It should however be pointed out that phosphoantigens do not solely influence DC-mediated γδ T cell activation.Citation48,49
Since phosphoantigens are not responsible for the entire effect, there must be other growth factors, cytokines, or contact-dependent factors contributing to this crosstalk.Citation69 Soluble mediators described to induce γδ T cell activation are as follows: IL-12Citation9,71,72, IL-15Citation48, IL-1β, TNF-αCitation72 and IFN-α/β.Citation60,61 IL-12 secretion abrogation by TLR-matured mo-DCs resulted in the almost complete absence of IFN-γ secretion by γδ T cells.Citation9 The importance of IL-12, along with IL-1β and TNF-α, secretion by BCG-DCs has been demonstrated by the increased production of IFN-γ, granzyme B, and the augmented cytotoxicity of Vγ9δ2 T cells against Daudi cells, pertaining to the IL-12-blocked conditions.Citation71,72 The IL-12 secretion by BCG blood DCs relies on IFN-γ production of memory CD4+ T cells, reveling a complex biology between cells of the innate and adaptive immune system.Citation71 IL-12 however does not seems to have an indisputable positive effect. IL-12 secreting DCs, in contrast to DCs that are not enabled for IL-12 secretion, give rise to immune suppression by γδ T cells.Citation67 In this context, the importance of the STAT3 pathway should be mentioned. The activating effect of STAT3 is at least in part mediated by increased IL-12 production and STAT3 silencing in mo-DCs enhances IFN-γ production of γδ T cells.Citation73 IFN-α/β, produced by CD11c+ DCs, contribute to the activation of γδ T cells by poly(I:C).Citation61 Type I IFNs play also an important role in TLR-matured DC-γδ T cell activation, shown by a ±80% reduction of the induced IFN-γ secretion by blocking IFNAR2.Citation60
Finally, transwell assays point out that both soluble and contact-dependent interactions play a role. The main activating and inhibitory γδ T cell receptors and their ligands are summarized in . Experiments with bacterial-infected IL-4 DCs, i.e. being pulsed with BCG,Citation48 Brucella,Citation37 B. burgdorferiCitation74 or M. tuberculosis,Citation49 indicate that γδ T cell activation and proliferation by these DCs requires direct cell-to-cell contact. The latter is based on the expression of the activation and maturation markers CD25 and CD69, and the production of IFN-γ. Separation of γδ T cells and pamidronate stimulated mo-DCs by a semipermeable membrane completely reverses the γδ T cell activation, which argues against the implication of soluble factors.Citation75 The cytokine secretion by phosphoantigen-activated Vγ9δ2 T cells enhanced by iDCs requires close cell-to-cell contact as well.Citation43 Contact-dependent interactions described in the literature include CD58-CD2, CD54-CD11aCitation41, Fas/FasLCitation74 and CD80/86-CD28.Citation41,75 Moreover, Takamizawa et al. discovered a possible role for interaction with HLA-DR.Citation41 Accordingly, the presence of the γδ TCR enables the recognition of peptidic and non-peptidic molecules too, whether or not in the context of MHC presentation.Citation3 Thus, the γδ TCR would play a vital role in recognizing a ligand, most likely a M. tuberculosis-derived phosphoantigen on M. tuberculosis-infected DCs, resulting in Vγ9δ2 T cell proliferation.Citation49 As evident from the current literature, DC-mediated γδ T cell activation is apparently based on combinatory effects, in turn, dependent on the experimental setting.
Conclusion and Perspectives
DCs have the capacity to harness γδ T cells for the development of antitumor immunity. Mechanisms directing DC-mediated γδ T cell activation are versatile and rely on combinatorial effects depending on the stimuli used for activation. In this context, DC vaccines hold potential to empower γδ T cells, assisting directly and indirectly the development of an antitumor immune response (). Given the fact that the presence of activated γδ T cells in cancer patients is associated with a beneficial outcomeCitation16,22-24, monitoring γδ T cells would be an interesting parameter for evaluation of DC vaccines.
Importantly, this strategy must be examined with scrutiny. To date, research is predominantly performed on γδ T cells of healthy donors. γδ T cells of healthy donors and patients, however, may respond differently. While a strong proliferation of γδ T cells was observed in the majority of healthy donors after single phosphoantigen stimulation ex vivo, no such amplification was achieved with γδ T cells of cancer patients.Citation68,69 This weak response could be overcome by co-culturing γδ T cells with zoledronate-pretreated mo-DCs.Citation68,69 Immunophenotyping of the amplified γδ T cells revealed the pro-inflammatory state of these cells, based on the expression of costimulatory molecules (HLA-DR, CD86, CD80) and the absence of inhibitory molecules (programmed cell death protein (PD)-1, PD-L1).Citation69
To get the full potential from DC vaccination for the treatment of cancer patients, some pitfalls should be overcome. Consideration should be given to the fact that conventional mo-DCs generally lack the ability to stimulate (patients') γδ T cells and that additional/alternative signals are required. Moreover, DCs also have the potential of inducing adverse effects on antitumor immunity, like induction of γδ Treg cells, induction of immunosuppressive IL-10Citation33,76 or dampening of CD4/8 T cell proliferation.Citation67 It has been determined that co-culturing γδ T cells with DCs matured with an α-type one polarizing cocktail, results in the consistent production of the anti-inflammatory cytokine IL-10.Citation9 Therefore, one should not forget to look at the negative impact on immunostimulatory capacity, together with the positive effects.
Although there is mounting evidence for DC-mediated γδ T cell activation in vitro, to date only two clinical studies have monitored γδ T cells following DC vaccination.Citation67,77 Traxlmayr et al. reported a γδ T cell-mediated negative regulatory feedback mechanism triggered by IL-12 secreting DCs, potentially hampering the clinical effect of DCs.Citation67 Kitawaki et al. assessed the numbers of γδ T cells in peripheral blood and bone marrow, as well as serum IFN-γ serum levels, after vaccination with zoledronate/Wilms' tumor 1-pulsed DCs, but could not detect any changes.Citation77
Based on our current knowledge, there is still no conclusive answer on how to generate a potent DC vaccine that harbors the ideal stimulatory triggers for γδ T cell antitumor activity. Bisphosphonate-pretreated mo-DCs respond to the fact that phosphoantigens are known activators of Vγ9δ2 T cells. The molecules, however, which are responsible for phosphoantigen presentation remain to date unknown.Citation11 Including infection-related signals in the protocol is a second way to optimize DC vaccines. Maturation cocktails with TLR-ligands have shown to induce γδ T cell cytokine secretion and granzyme degranulation.Citation9 As opposed to phosphoantigens, TLR-stimulation has the potential to stimulate both the Vδ1 and Vδ2 T cell subset.Citation78 Activation with TLR8 is of particular interest due to its potential to reverse immunosuppressive activity of γδ T cells. However, this effect has not been investigated so far through mo-DCs.Citation78 Finally, activating receptor-ligand interactions () and secreting/presenting cytokines by DCs (vide supra), are possible mechanisms to harness γδ T cells in the antitumor DC vaccine effect. Here, a special mention of IL-15 is in place.Citation48,51,79 The latter can lead to the implementation of IL-15 expressing DCs in the clinic.Citation54,55
To expand our knowledge on potentiating γδ T cell functions by DCs, evaluation of combinatorial vaccination strategies aiming at engagement of γδ T cells and inclusion of γδ T cell monitoring in DC-vaccination trials is warranted.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant FWO G.0399.14N from the Research Foundation Flanders (FWO), by grant #2012–193 of the Belgian Foundation against Cancer, the Belgian public utility foundation VOCATIO, a Methusalem grant of the University of Antwerp and the Belgian Hercules Foundation. HHVA is supported by a PhD grant of the Institute for the Promotion of Innovation through Science and Technology (IWT) (Belgium). SA is a former PhD fellow of the Research Foundation Flanders (FWO) and a former holder of an Emmanuel van der Schueren Fellowship granted by the Flemish League against Cancer. Part of this work was performed under the umbrella of the European COST TD1104 action (EP4Bio2MED; www.electroporation.net).
References
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol 2013; 13:88-100; PMID:23348415; http://dx.doi.org/10.1038/nri3384
- Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity 2009; 31:184-96; PMID:19699170; http://dx.doi.org/10.1016/j.immuni.2009.08.006
- Born WK, Kemal Aydintug M, O'Brien RL. Diversity of gammadelta T-cell antigens. Cell Mol Immunol 2013; 10:13-20; PMID:23085946; http://dx.doi.org/10.1038/cmi.2012.45
- Braza MS, Klein B. Anti-tumour immunotherapy with Vgamma9Vdelta2 T lymphocytes: from the bench to the bedside. Br J Haematol 2013; 160:123-32; PMID:23061882; http://dx.doi.org/10.1111/bjh.12090
- Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. gammadelta T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology 2014; 3:e27572; PMID:24734216; http://dx.doi.org/10.4161/onci.27572
- Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol 2014; 15:e257-e67; PMID:24872109; http://dx.doi.org/10.1016/S1470-2045(13)70585-0
- Fajardo-Moser M, Berzel S, Moll H. Mechanisms of dendritic cell-based vaccination against infection. Intl J Med Microbiol 2008; 298:11-20; PMID:17719274; http://dx.doi.org/10.1016/j.ijmm.2007.07.003
- Van Gulck E, Vlieghe E, Vekemans M, Van Tendeloo VF, Van De Velde A, Smits E, Anguille S, Cools N, Goossens H, Mertens L et al. mRNA-based dendritic cell vaccination induces potent antiviral T-cell responses in HIV-1-infected patients. AIDS 2012; 26:F1-12; PMID:22156965; http://dx.doi.org/10.1097/QAD.0b013e32834f33e8
- Massa C, Seliger B. Fast dendritic cells stimulated with alternative maturation mixtures induce polyfunctional and long-lasting activation of innate and adaptive effector cells with tumor-killing capabilities. J Immunol 2013; 190:3328-37; PMID:23447683; http://dx.doi.org/10.4049/jimmunol.1202024
- Gruenbacher G, Gander H, Rahm A, Nussbaumer W, Romani N, Thurnher M. CD56+ human blood dendritic cells effectively promote TH1-type gammadelta T-cell responses. Blood 2009; 114:4422-31; PMID:19762486; http://dx.doi.org/10.1182/blood-2009-06-227256
- Ferreira LM. Gammadelta T cells: innately adaptive immune cells? Int Rev Immunol 2013; 32:223-48; PMID:23617235; http://dx.doi.org/10.3109/08830185.2013.783831
- Sheridan BS, Romagnoli PA, Pham QM, Fu HH, Alonzo F, III, Schubert WD, Freitag NE, Lefrancois L. gammadelta T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity 2013; 39:184-95; PMID:23890071; http://dx.doi.org/10.1016/j.immuni.2013.06.015
- Jameson JM, Cruz J, Costanzo A, Terajima M, Ennis FA. A role for the mevalonate pathway in the induction of subtype cross-reactive immunity to influenza A virus by human gammadelta T lymphocytes. Cell Immunol 2010; 264:71-7; PMID:20483407; http://dx.doi.org/10.1016/j.cellimm.2010.04.013
- Zheng J, Liu Y, Lau YL, Tu W. gammadelta-T cells: an unpolished sword in human anti-infection immunity. Cell Mol Immunol 2013; 10:50-7; PMID:23064104; http://dx.doi.org/10.1038/cmi.2012.43
- Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev 2007; 215:59-76; PMID:17291279; http://dx.doi.org/10.1111/j.1600-065X.2006.00479.x
- Aswald JM, Wang XH, Aswald S, Lutynski A, Minden MD, Messner HA, Keating A. Flow cytometric assessment of autologous gammadelta T cells in patients with acute myeloid leukemia: potential effector cells for immunotherapy? Cytometry B Clin Cytom 2006; 70:379-90; PMID:16977635; http://dx.doi.org/10.1002/cyto.b.20115
- Yuasa T, Sato K, Ashihara E, Takeuchi M, Maita S, Tsuchiya N, Habuchi T, Maekawa T, Kimura S. Intravesical administration of gammadelta T cells successfully prevents the growth of bladder cancer in the murine model. Cancer Immunol Immunother 2009; 58:493-502; PMID:18682944; http://dx.doi.org/10.1007/s00262-008-0571-9
- Todaro M, D'Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol 2009; 182:7287-96; PMID:19454726; http://dx.doi.org/10.4049/jimmunol.0804288
- Bryant NL, Suarez-Cuervo C, Gillespie GY, Markert JM, Nabors LB, Meleth S, Lopez RD, Lamb LS Jr. Characterization and immunotherapeutic potential of gammadelta T-cells in patients with glioblastoma. Neuro Oncol 2009; 11:357-67; PMID:19211933; http://dx.doi.org/10.1215/15228517-2008-111
- Rey J, Veuillen C, Vey N, Bouabdallah R, Olive D. Natural killer and gammadelta T cells in haematological malignancies: enhancing the immune effectors. Trends Mol Med 2009; 15:275-84; PMID:19487160; http://dx.doi.org/10.1016/j.molmed.2009.04.005
- Knight A, Mackinnon S, Lowdell MW. Human Vdelta1 gamma-delta T cells exert potent specific cytotoxicity against primary multiple myeloma cells. Cytotherapy 2012; 14:1110-8; PMID:22800570; http://dx.doi.org/10.3109/14653249.2012.700766
- Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, La Mendola C, Guggino G, D'Asaro M, Orlando V et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol 2010; 161:290-7; PMID:20491785
- Naoe M, Ogawa Y, Takeshita K, Morita J, Shichijo T, Fuji K, Fukagai T, Iwamoto S, Terao S. Zoledronate stimulates gamma delta T cells in prostate cancer patients. Oncol Res 2010; 18:493-501; PMID:20681408; http://dx.doi.org/10.3727/096504010X12671222663638
- Godder KT, Henslee-Downey PJ, Mehta J, Park BS, Chiang KY, Abhyankar S, Lamb LS. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplan 2007; 39:751-7; PMID:17450185; http://dx.doi.org/10.1038/sj.bmt.1705650
- Kalyan S, Kabelitz D. Defining the nature of human gammadelta T cells: a biographical sketch of the highly empathetic. Cell Mol Immunol 2013; 10:21-9; PMID:23085947; http://dx.doi.org/10.1038/cmi.2012.44
- Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournie JJ, Scotet E, Bonneville M. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue? Immunol Rev 2007; 215:123-35; PMID:17291284; http://dx.doi.org/10.1111/j.1600-065X.2006.00468.x
- Toutirais O, Cabillic F, Le Friec G, Salot S, Loyer P, Le Gallo M, Desille M, de La Pintiere CT, Daniel P, Bouet F et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vgamma9Vdelta2 T cells. EurJ Immunol 2009; 39:1361-8; PMID:19404979; http://dx.doi.org/10.1002/eji.200838409
- Beetz S, Marischen L, Kabelitz D, Wesch D. Human gamma delta T cells: candidates for the development of immunotherapeutic strategies. Immunol Res 2007; 37:97-111; PMID:17695246; http://dx.doi.org/10.1007/BF02685893
- Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science 2005; 309:264-8; PMID:15933162; http://dx.doi.org/10.1126/science.1110267
- Moser B, Eberl M. gammadelta T-APCs: a novel tool for immunotherapy? Cell Mol Life Sci 2011; 68:2443-52; PMID:21573785; http://dx.doi.org/10.1007/s00018-011-0706-6
- Capietto AH, Martinet L, Fournie JJ. How tumors might withstand gammadelta T-cell attack. Cell Mol Life Sci 2011; 68:2433-42; PMID:21547501; http://dx.doi.org/10.1007/s00018-011-0705-7
- Martinet L, Poupot R, Fournie JJ. Pitfalls on the roadmap to gammadelta T cell-based cancer immunotherapies. Immunol Lett 2009; 124:1-8; PMID:19465238; http://dx.doi.org/10.1016/j.imlet.2009.03.011
- Ye J, Ma C, Hsueh EC, Eickhoff CS, Zhang Y, Varvares MA, Hoft DF, Peng G. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol 2013; 190:2403-14; PMID:23355732; http://dx.doi.org/10.4049/jimmunol.1202369
- Ye J, Ma C, Wang F, Hsueh EC, Toth K, Huang Y, Mo W, Liu S, Han B, Varvares MA et al. Specific recruitment of gammadelta regulatory T cells in human breast cancer. Cancer Res 2013; 73:6137-48; PMID:23959855; http://dx.doi.org/10.1158/0008-5472.CAN-13-0348
- Yi Y, He HW, Wang JX, Cai XY, Li YW, Zhou J, Cheng YF, Jin JJ, Fan J, Qiu SJ. The functional impairment of HCC-infiltrating gammadelta T cells, partially mediated by regulatory T cells in a TGFbeta- and IL-10-dependent manner. J Hepatol 2013; 58:977-83; PMID:23262246; http://dx.doi.org/10.1016/j.jhep.2012.12.015
- Gaafar A, Aljurf MD, Al-Sulaiman A, Iqniebi A, Manogaran PS, Mohamed GE, Al-Sayed A, Alzahrani H, Alsharif F, Mohareb F et al. Defective gammadelta T-cell function and granzyme B gene polymorphism in a cohort of newly diagnosed breast cancer patients. Exp Hematol 2009; 37:838-48; PMID:19446661; http://dx.doi.org/10.1016/j.exphem.2009.04.003
- Ni M, Martire D, Scotet E, Bonneville M, Sanchez F, Lafont V. Full restoration of Brucella-infected dendritic cell functionality through Vgamma9Vdelta2 T helper type 1 crosstalk. PloS One 2012; 7:e43613
- Dunne MR, Madrigal-Estebas L, Tobin LM, Doherty DG. (E)-4-hydroxy-3-methyl-but-2 enyl pyrophosphate-stimulated Vgamma9Vdelta2 T cells possess T helper type 1-promoting adjuvant activity for human monocyte-derived dendritic cells. Cancer Immunol Immunother 2010; 59:1109-20; PMID:20306041; http://dx.doi.org/10.1007/s00262-010-0839-8
- Martino A, Poccia F. Gamma delta T cells and dendritic cells: close partners and biological adjuvants for new therapies. Curr Mol Med 2007; 7:658-73; PMID:18045144; http://dx.doi.org/10.2174/156652407782564345
- Casetti R, Martino A. The plasticity of gamma delta T cells: innate immunity, antigen presentation and new immunotherapy. Cell Mol Immunol 2008; 5:161-70; PMID:18582397; http://dx.doi.org/10.1038/cmi.2008.20
- Takamizawa M, Fagnoni F, Mehta-Damani A, Rivas A, Engleman EG. Cellular and molecular basis of human gamma delta T cell activation. Role of accessory molecules in alloactivation. J Clin Invest 1995; 95:296-303; PMID:7814628; http://dx.doi.org/10.1172/JCI117654
- Ye Z, Haley S, Gee AP, Henslee-Downey PJ, Lamb LS, Jr. In vitro interactions between gamma deltaT cells, DC, and CD4+ T cells; implications for the immunotherapy of leukemia. Cytotherapy 2002; 4:293-304; PMID:12194726; http://dx.doi.org/10.1080/146532402320219817
- Devilder MC, Maillet S, Bouyge-Moreau I, Donnadieu E, Bonneville M, Scotet E. Potentiation of antigen-stimulated V gamma 9V delta 2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J Immunol 2006; 176:1386-93; PMID:16424165; http://dx.doi.org/10.4049/jimmunol.176.3.1386
- Fiore F, Castella B, Nuschak B, Bertieri R, Mariani S, Bruno B, Pantaleoni F, Foglietta M, Boccadoro M, Massaia M. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood 2007; 110:921-7; PMID:17403919; http://dx.doi.org/10.1182/blood-2006-09-044321
- Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 2003; 197:163-8; PMID:12538656; http://dx.doi.org/10.1084/jem.20021500
- Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000; 96:384-92; PMID:10887096
- Soriano-Sarabia N, Sandvold H, Jomaa H, Kubin T, Bein G, Hackstein H. Primary MHC-class II(+) cells are necessary to promote resting Vdelta2 cell expansion in response to (E)-4-hydroxy-3-methyl-but-2-enyl-pyrophosphate and isopentenyl pyrophosphate. J Immunol 2012; 189:5212-22; PMID:23105138; http://dx.doi.org/10.4049/jimmunol.1200093
- Martino A, Casetti R, Sacchi A, Poccia F. Central memory Vgamma9Vdelta2 T lymphocytes primed and expanded by bacillus Calmette-Guerin-infected dendritic cells kill mycobacterial-infected monocytes. J Immunol 2007; 179:3057-64; PMID:17709520; http://dx.doi.org/10.4049/jimmunol.179.5.3057
- Meraviglia S, Caccamo N, Salerno A, Sireci G, Dieli F. Partial and ineffective activation of V gamma 9V delta 2 T cells by Mycobacterium tuberculosis-infected dendritic cells. J Immunol 2010; 185:1770-6; PMID:20592281; http://dx.doi.org/10.4049/jimmunol.1000966
- Sacchi A, Rinaldi A, Tumino N, Casetti R, Agrati C, Turchi F, Bordoni V, Cimini E, Martini F. HIV infection of monocytes-derived dendritic cells inhibits Vgamma9Vdelta2 T cells functions. PloS One 2014; 9:e111095; PMID:25340508; http://dx.doi.org/10.1371/journal.pone.0111095
- von Lilienfeld-Toal M, Sievers E, Bodemuller V, Mihailescu C, Marten A, Gorschluter M, Schmidt-Wolf IG. Coculture with dendritic cells promotes proliferation but not cytotoxic activity of gamma/delta T cells. Immunol Lett 2005; 99:103-8; PMID:15894118; http://dx.doi.org/10.1016/j.imlet.2005.02.001
- Van den Bergh JM, Van Tendeloo VF, Smits EL. Interleukin-15: New kid on the block for antitumor combination therapy. Cytokine Growth Factor Rev 2014; 26(1):15-24; PMID:25306466; http://dx.doi.org/10.1016/j.cytogfr.2014.09.001
- Caccamo N, Meraviglia S, Ferlazzo V, Angelini D, Borsellino G, Poccia F, Battistini L, Dieli F, Salerno A. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vgamma9Vdelta2 naive, memory and effector T cell subsets. Eur J Immunol 2005; 35:1764-72; PMID:15915537; http://dx.doi.org/10.1002/eji.200525983
- Harris KM. Monocytes differentiated with GM-CSF and IL-15 initiate Th17 and Th1 responses that are contact-dependent and mediated by IL-15. J Leukocyte Biol 2011; 90:727-34; PMID:21724805; http://dx.doi.org/10.1189/jlb.0311132
- Anguille S, Lion E, Van den Bergh J, Van Acker HH, Willemen Y, Smits EL, Van Tendeloo VF, Berneman ZN. Interleukin-15 dendritic cells as vaccine candidates for cancer immunotherapy. Hum Vaccin Immunother 2013; 9:1956-61; PMID:23778748; http://dx.doi.org/10.4161/hv.25373
- Anguille S, Smits EL, Cools N, Goossens H, Berneman ZN, Van Tendeloo VF. Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties. J Transl Med 2009; 7:109; PMID:20021667; http://dx.doi.org/10.1186/1479-5876-7-109
- Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur J Immunol 2007; 37:1678-90; PMID:17492620; http://dx.doi.org/10.1002/eji.200636329
- Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 1997; 27:3135-42; PMID:9464798; http://dx.doi.org/10.1002/eji.1830271209
- Shrestha N, Ida JA, Lubinski AS, Pallin M, Kaplan G, Haslett PA. Regulation of acquired immunity by gamma delta T-cell/dendritic-cell interactions. Ann N Y Acad Sci 2005; 1062:79-94; PMID:16461791; http://dx.doi.org/10.1196/annals.1358.011
- Devilder MC, Allain S, Dousset C, Bonneville M, Scotet E. Early triggering of exclusive IFN-gamma responses of human Vgamma9Vdelta2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol 2009; 183:3625-33; PMID:19710464; http://dx.doi.org/10.4049/jimmunol.0901571
- Kunzmann V, Kretzschmar E, Herrmann T, Wilhelm M. Polyinosinic-polycytidylic acid-mediated stimulation of human gammadelta T cells via CD11c dendritic cell-derived type I interferons. Immunology 2004; 112:369-77; PMID:15196204; http://dx.doi.org/10.1111/j.1365-2567.2004.01908.x
- Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011; 208:491-503; PMID:21383056; http://dx.doi.org/10.1084/jem.20100269
- Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, Kitamura H, Nishimura T. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. EurJ Immunol 2010; 40:1927-37; PMID:20397212; http://dx.doi.org/10.1002/eji.200940157
- Kunzmann V, Smetak M, Kimmel B, Weigang-Koehler K, Goebeler M, Birkmann J, Becker J, Schmidt-Wolf IG, Einsele H, Wilhelm M. Tumor-promoting versus tumor-antagonizing roles of gammadelta T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother 2012; 35:205-13; PMID:22306909; http://dx.doi.org/10.1097/CJI.0b013e318245bb1e
- Nieda M, Terunuma H, Eiraku Y, Deng X, Nicol AJ. Effective induction of melanoma-antigen-specific CD8+ T cells via Vgamma9gammadeltaT cell-expansion by CD56 Interferon-alpha-induced dendritic cells. Exp Dermatol 2014; 24(1):35-41; PMID:25363560; http://dx.doi.org/10.1111/exd.12581
- Takahara M, Miyai M, Tomiyama M, Mutou M, Nicol AJ, Nieda M. Copulsing tumor antigen-pulsed dendritic cells with zoledronate efficiently enhance the expansion of tumor antigen-specific CD8+ T cells via Vgamma9gammadelta T cell activation. J Leukocyte Biol 2008; 83:742-54; PMID:18156189; http://dx.doi.org/10.1189/jlb.0307185
- Traxlmayr MW, Wesch D, Dohnal AM, Funovics P, Fischer MB, Kabelitz D, Felzmann T. Immune suppression by gammadelta T-cells as a potential regulatory mechanism after cancer vaccination with IL-12 secreting dendritic cells. J Immunother 2010; 33:40-52; PMID:19952957; http://dx.doi.org/10.1097/CJI.0b013e3181b51447
- Cabillic F, Toutirais O, Lavoue V, de La Pintiere CT, Daniel P, Rioux-Leclerc N, Turlin B, Monkkonen H, Monkkonen J, Boudjema K et al. Aminobisphosphonate-pretreated dendritic cells trigger successful Vgamma9Vdelta2 T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunol Immunother 2010; 59:1611-9; PMID:20582413; http://dx.doi.org/10.1007/s00262-010-0887-0
- Castella B, Riganti C, Fiore F, Pantaleoni F, Canepari ME, Peola S, Foglietta M, Palumbo A, Bosia A, Coscia M et al. Immune modulation by zoledronic acid in human myeloma: an advantageous cross-talk between Vgamma9Vdelta2 T cells, alphabeta CD8+ T cells, regulatory T cells, and dendritic cells. J Immunol 2011; 187:1578-90; PMID:21753152; http://dx.doi.org/10.4049/jimmunol.1002514
- Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced gamma,delta-T-cell proliferation and activation in vitro. J Bone Miner Res 2004; 19:278-88; PMID:14969398; http://dx.doi.org/10.1359/JBMR.0301230
- Fowler DW, Copier J, Dalgleish AG, Bodman-Smith MD. Tripartite immune cell co-operation in the Bacillus Calmette Guerin-induced activation of gammadelta T cells. Immunol Cell Biol 2013; 91:461-8; PMID:23797069; http://dx.doi.org/10.1038/icb.2013.30
- Fowler DW, Copier J, Wilson N, Dalgleish AG, Bodman-Smith MD. Mycobacteria activate gammadelta T-cell anti-tumour responses via cytokines from type 1 myeloid dendritic cells: a mechanism of action for cancer immunotherapy. Cancer Immunol Immunother 2012; 61:535-47; PMID:22002242; http://dx.doi.org/10.1007/s00262-011-1121-4
- Sanseverino I, Purificato C, Varano B, Conti L, Gessani S, Gauzzi MC. STAT3-silenced human dendritic cells have an enhanced ability to prime IFNgamma production by both alphabeta and gammadelta T lymphocytes. Immunobiology 2014; 219:503-11; PMID:24674241; http://dx.doi.org/10.1016/j.imbio.2014.02.012
- Collins C, Wolfe J, Roessner K, Shi C, Sigal LH, Budd RC. Lyme arthritis synovial gammadelta T cells instruct dendritic cells via fas ligand. J Immunol 2005; 175:5656-65; PMID:16237055; http://dx.doi.org/10.4049/jimmunol.175.9.5656
- Conti L, Casetti R, Cardone M, Varano B, Martino A, Belardelli F, Poccia F, Gessani S. Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol 2005; 174:252-60; PMID:15611247; http://dx.doi.org/10.4049/jimmunol.174.1.252
- Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol 2014; 5:7; PMID:24550907; http://dx.doi.org/10.3389/fimmu.2014.00007
- Kitawaki T, Kadowaki N, Fukunaga K, Kasai Y, Maekawa T, Ohmori K, Kondo T, Maekawa R, Takahara M, Nieda M et al. A phase I/IIa clinical trial of immunotherapy for elderly patients with acute myeloid leukaemia using dendritic cells co-pulsed with WT1 peptide and zoledronate. Brit J Haematol 2011; 153:796-9; PMID:21477159; http://dx.doi.org/10.1111/j.1365-2141.2010.08490.x
- Dar AA, Patil RS, Chiplunkar SV. Insights into the Relationship between Toll Like Receptors and Gamma Delta T Cell Responses. Front Immunol 2014; 5:366; PMID:25132835; http://dx.doi.org/10.3389/fimmu.2014.00366
- Ribot JC, Ribeiro ST, Correia DV, Sousa AE, Silva-Santos B. Human gammadelta thymocytes are functionally immature and differentiate into cytotoxic type 1 effector T cells upon IL-2/IL-15 signaling. J Immunol 2014; 192:2237-43; PMID:24489097; http://dx.doi.org/10.4049/jimmunol.1303119
