ABSTRACT
Thymic stromal lymphopoietin (TSLP) is an interleukin (IL)-7-like cytokine expressed by epithelial cells during allergic inflammation, and activating dendritic cells (DC). Its expression and functional role in cancer remain controversial. We conducted retrospective (n = 89), and prospective studies including patients with untreated primary head and neck squamous cell carcinoma (HNSCC). We found that TSLP was overexpressed by HNSCC tumor cells, and associated with a highly differentiated status. However, no significant difference in overall and recurrence-free survival was found between patients bearing a tumor with high and low TSLP levels, respectively. Surprisingly, there was no significant association between the levels of TSLP expression, and the number of tumor-infiltrating mature DCLAMP+ DC. In order to explain the apparent lack of TSLP-induced DC activation, we performed phenotypic and functional experiments on freshly resected tumors. Tumor-infiltrating immune cells, including DC, did not express the TSLP receptor heterodimer (TSLPR chain, IL-7Ralpha chain). Furthermore, freshly sorted blood CD11c+ DC from healthy donors cultured with tumor-conditioned supernatant exhibited an activated profile, but this was not affected by an anti-TSLP blocking antibody, suggesting a DC activation pathway independent of tumor-derived TSLP. Overall, our results demonstrate that TSLP is overexpressed in HNSCC but its function is hampered by the lack of TSLPR-expressing cells in the tumor microenvironment. Such a dissociated ligand–receptor expression may impact intercellular communication in other immune activation pathways, and tumor types.
Abbreviations
| DC | = | dendritic cells |
| HNSCC | = | head and neck squamous cell carcinoma |
| TSLP | = | thymic stromal lymphopoeitin |
Introduction
Thymic stromal lymphopoietin (TSLP) is an interleukin (IL)-7-like cytokine expressed by epithelial cells in skin or mucosae during allergic reactions, such as asthma and atopic dermatitis,Citation1-3 epidermal barrier disruption,Citation4 skin psoriasis,Citation5 and keratinocyte activation with pro-inflammatory cytokines.Citation2 Human dendritic cells (DC) are able to express the TSLP receptor heterodimer, constituted by the TSLP receptor chain (TSLPR) and the IL-7 receptor α chain (IL-7Ralpha), and are the main target cell for TSLP in humans.Citation6-8 TSLP directly induces maturation and activation of DC,Citation2,9 which subsequently promote an inflammatory T helper type 2 (Th2) response.Citation8,10-12
The hypothesis of an implication of TSLP in the tumor microenvironment stemmed from earlier studies in breast and pancreatic cancer, both in humans and in mice.Citation13-16 TSLP expression was associated in these studies with the presence of a Th2-biased inflammatory response, characterized by the expression in T cells of the transcription factor GATA-3, and the production of IL-4, IL-5, IL-13, and TNF. Because a Th2 response has been associated with a poor prognosis,Citation17,18 TSLP was proposed to be associated with tumor progression and to indicate a bad prognosis of the tumor. However, no survival analysis was performed in representative patient cohorts to directly assess the prognostic impact of high expression levels of TSLP. Furthermore, several studies highlighted a potential tumor-protective role for TSLP in mouse cutaneous skin cancer models,Citation19,20 and mouse early stage breast cancer models.Citation21 Hence, whether TSLP is a tumor suppressive factor or have tumor-promoting effects remains controversial.
Since TSLP is produced by epithelial cells, and in particular epidermal keratinocytes, we hypothesized that it could be expressed in head and neck squamous cell carcinoma (HNSCC), a tumor type with strong epidermoid differentiation. HNSCC is a severe disease with a dismal prognosis despite surgery, radiotherapy, and chemotherapy. Indeed, 35–55% of the patients relapse within 2 y, and the overall survival rate is less than 20% at 10 y.Citation22 Treatment decisions currently rely only on histopathological criteria of the tumor (TNM stage and lymph node capsular effraction).
Here, we show that HNSCC expressed high levels of TSLP, which was associated with a highly differentiated state of the tumor. Surprisingly, TSLP was not associated to DC activation in situ and ex vivo. We found no impact on disease prognosis, which we could attribute to a lack of TSLPR-expressing cells in the tumor microenvironment.
Results
TSLP is overexpressed in highly differentiated HNSCC tumors
To assess TSLP expression in HNSCC, we designed a retrospective study including 89 patients with untreated primary tumor (see patient characteristics in Material and Methods section and in Table S1). Tissue specimens were 41.6% from oral cavity, 24.7% from oropharynx, 19% from larynx, and 14.6% from hypopharynx. The cohort presented similar number of patients with tumors in stage T1/T2/T3 and T4, and with or without pathologic lymph node involvement. All the patients were exempt of metastasis at diagnosis. The male to female ratio was 3:1. Median age was 57 y (range 37–88 y). The main risk factors were tobacco (85%) and alcohol consumption (65.5%), whereas HPV infection represented 8% of the cases. TSLP expression was analyzed by immunohistology on frozen sections of both tumor and juxta-tumor (adjacent to the tumor and exempt of malignant tumor cells) tissues. The specificity of the anti-TSLP mAb staining was previously validated on human skin and tonsillar sections.Citation5 Normal and atopic dermatitis skin sections were used for TSLP expression as negative and positive controls, respectively.Citation2 As expected, TSLP was found to be highly expressed in keratinocytes of skin lesions obtained from atopic dermatitis patients (). High levels of TSLP were detected in epithelial tumor cells as compared to normal epithelial cells in the juxta-tumor counterpart (). The majority of HNSCC were positive for TSLP (90%), while only 40% of the corresponding juxta-tumor expressed TSLP (). The percentage of epithelial cells positive for TSLP ranged from 1 to 100% across TSLP-positive tumor samples (). Although a bimodal distribution of TSLP levels was observed in tumors (range from 50–100% positivity), very few juxta-tumor samples were highly positive (). The results showed a trend for higher TSLP levels in HNSCC of the oral cavity, as compared to HNSCC of the oropharynx (). Difference in TSLP expression levels between tumors and juxta-tumors was statistically significant independent of the anatomical location (oral cavity, hypopharynx, larynx, and oropharynx) (). No TSLP staining was detected in the stroma surrounding the tumor cells ().
Figure 1. TSLP is highly expressed by epithelial cancer cells in HNSCC. Frozen section of (A) skin lesions of atopic dermatitis and (B) tumor or juxta-tumor samples from 89 patients with primary HNSCC by means of immunohistology for TSLP (brown staining). (C) Percentage of TSLP-positive samples. (D–F) Percentages of TSLP staining in epithelial cells are shown for all patients. (D) Percent of epithelial cells positive for TSLP. Each symbol represents a different sample. (E) Percent of epithelial cancer cells positive for TSLP according to the location of the tumor. (F) Percent of epithelial cells positive for TSLP between tumors and juxta-tumors in each location. Horizontal bars indicate the median. n = 89 tumors and n = 52 corresponding juxta-tumors. p value is significant if p < 0.05.
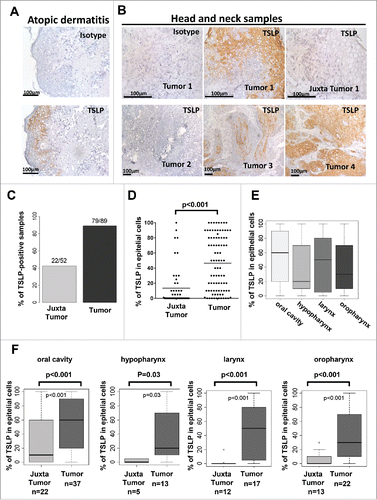
Since TSLP expression was highly variable, we questioned whether TSLP levels in the tumor were associated with clinical or histological characteristics. Neither the gender, age, alcohol consumption, HPV status, nor stages T, N, and lymph node capsular effraction, were associated with TSLP levels in the tumor (). TSLP expression levels were higher in non-smoking as compared to smoking patients (p = 0.01, ). Interestingly, highly differentiated tumors expressed higher level of TSLP (p = 0.04, and ). Immunofluorescence experiments (), revealed a co-localization of TSLP and cytokeratin (KL-1), further suggesting that TSLP expression is a feature of epidermoid differentiation.
Figure 2. TSLP expression is associated with high differentiation status of HNSCC tumors. (A) Frozen sections of tumors or juxta-tumors samples were stained for DAPI, cytokeratin (KL1), and TSLP by immunofluorescence. (B) Bar plot representation of the percentage of TSLP expression, quantified by immunohistology, in epithelial cancer cells according to the differentiation status of the tumors. Horizontal bars indicate the median. p value is significant if p < 0.05.
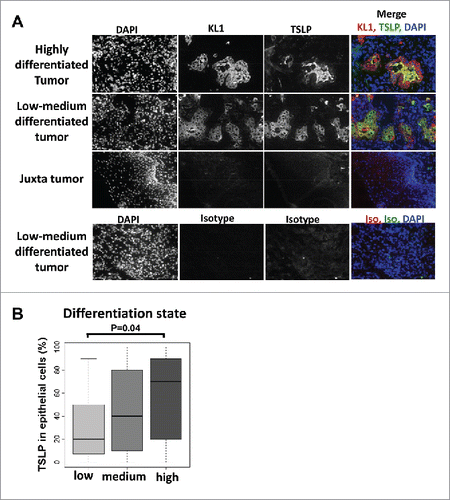
Table 1. Correlation between tumor-TSLP expression and clinical characteristics.
Overexpression of TSLP has no impact on clinical outcome
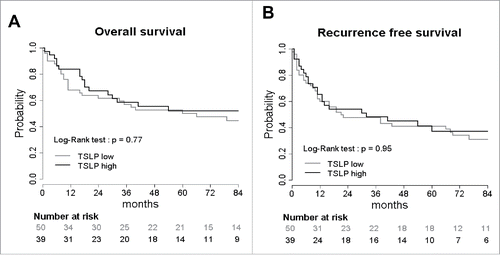
Next, we questioned whether TSLP expression was associated with clinical outcome of the patients. The follow-up of this cohort ranged from 1 to 171 mo, with a median of 102 mo. Based on Kaplan–Meier estimates (Log-rank test), the overall survival and recurrence-free survival were first compared according to clinical characteristic groups (). For patients with no lymph node involvement (5 y survival rate = 0.67) a significantly longer overall (p = 0.001) and recurrence free survival was observed compared to patients with lymph node involvement (5 y survival rate = 0.36). Lymph node capsular effraction was significantly associated with shorter overall (p = 0.001) and recurrence-free survival (p = 0.02). For all the other epidemiological parameters (age, gender, alcohol, tobacco) and pathological parameters (tumor location, T stage, differentiation, nuclear atypia, mitotic index), no significant statistical difference in clinical outcome was detected ().
Table 2. Correlation between TSLP and clinical characteristics, and clinical outcome. Log-Rank test was calculated. p value is significant if p < 0.05. RR = relative risk.
Patients were separated into two groups according to the level of TSLP expression in the tumor: TSLPhigh, Tumor TSLP level ≥ 50%; TSLPlow, Tumor TSLP level < 50%. Our results showed that TSLP did not influence clinical outcome of HNSCC patients since no statistical difference was observed for overall survival and recurrence-free survival between patients with a TSLPhigh tumor compared to patients with a TSLPlow tumor ( and ). Other cut-offs selected to discriminate low or high levels of TSLP did not change the absence of correlation between TSLP expression and prognosis (data not shown).
Figure 3. TSLP overexpression does not influence clinical outcome of HNSCC patients. Kaplan–Meier graph of (A) overall survival and (B) recurrence free survival according to TSLP expression by the tumors. Tumors were separated into two groups: TSLPhigh (>50% positivity) and TSLPlow (<50% positivity). Log-Rank test was calculated. p value is significant if p < 0.05.
In situ infiltrating DC are not associated with TSLP expression and do not express TSLPR
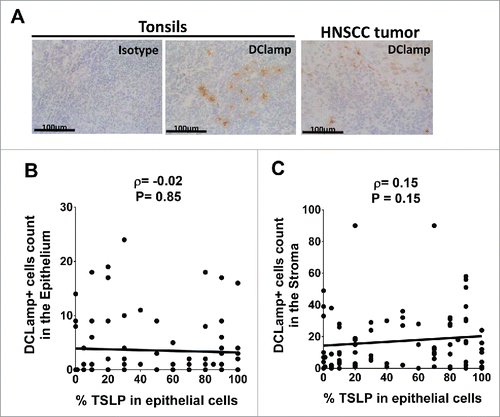
Since the main target cells for TSLP in humans are DC, we further quantified infiltration of mature DC in situ within tumors by immunohistology staining of DCLAMP (). DCLAMP+ DCs were found to infiltrate HNSCC, both in the epithelium and in the stroma (). However, statistical analysis revealed no association between the levels of TSLP expression and the number of DCLAMP+ DC infiltrating the tumor epithelium or in the surrounding stroma ().
Figure 4. Mature DCLAMP+ DC infiltrate HNSCC tumors whatever the tumor-TSLP expression level Frozen section of (A) Tonsils and HNSCC tumor by means of immunohistology for DCLAMP (brown staining). DCLAMP+-cells were counted both (B) in the epithelium and (C) in the stroma of tumor tissues and compared with corresponding percentage of TSLP expression. Each symbol represents a different sample. Pearson correlation was calculated. Linear regression was added (black lines).
To explain why TSLP is neither associated with a differential mature DC infiltration nor a differential clinical outcome, we questioned whether tumor-infiltrating immune cells in this inflammatory microenvironment expressed the receptor for TSLP (IL-7Rα chain and TSLPR chain). We used the Ba/F3 cell line retrovirally transfected to express both chains of the receptor Citation8,23 as positive control. In order to validate that enzymatic digestion used to treat all the tissues did not alter surface expression of TSLPR and IL-7Rα, Ba/F3 cells were also treated with enzymatic digestion cocktail before analyzed. shows that ∼70% of the Ba/F3 cells co-expressed the IL-7Rα and TSLPR, demonstrating that digestion did not suppress TSLPR or IL-7Rα expression. As previously described,Citation24 ∼5% of DC in human healthy tonsils co-expressed the IL-7Rα and TSLPR chains (). IL-7Rα chain was expressed by ∼16% of tumor and juxta-tumor infiltrating DCs, although at a lower level as compared to tonsil DCs (). Surprisingly, we found 0.6 % of DC co-expressing the IL-7Rα and TSLPR chains in only one tumor out of four studied, the three others being negative for the co-expression of the two chains (). Other immune (CD45+) and non-immune tumor-infiltrating cells also lacked expression of the TSLPR heterodimer.
Figure 5. Rare tumor-infiltrating DC express TSLPR heterodimer and soluble tumor microenvironment activates DC independently of TSLP. (A) Representative IL-7Rα and TSLPR chains flow cytometry analysis of cell suspensions from digested healthy tonsils or tumor tissues or Ba/F3 cell lines. Isotype control antibodies, gray curves. Staining antibodies, black curves. (B) Specific mean fluorescence intensity (MFI) for each chain and percentage of TSLPR+/IL7Ralpha+ cells. Each symbol represents a different sample. Horizontal bars indicate the median. n = 4 independent experiments at least. (C–E) Freshly isolated blood CD11c+-DC from healthy donors were cultured 24 h without or with soluble tumor microenvironment or with exogenous rhTSLP. (C) Representative CD86 expression assessed by flow cytometry. Isotype control antibodies, gray curves. Staining antiboby, black curves. (D) CD86 MFI level. n = 3 independent experiments with six different blood DC donors and six different soluble tumor supernatants. (E) Percentage of viable (DAPI-negative) cells, specific MFI of CD80 and CD86. Specific MFI (SpeMFI) was measured on DAPI− cells, by subtracting the MFI of isotype staining from the MFI of Ab staining. n = 4 independent experiments with at least eight different blood DC donors and eight different soluble tumor supernatants. Horizontal bars indicate the median. Statistical significance is indicated for pairwise comparison of culture condition groups. Paired Student t test. *p < .05; **p < 0.01. Ns = non-significant.
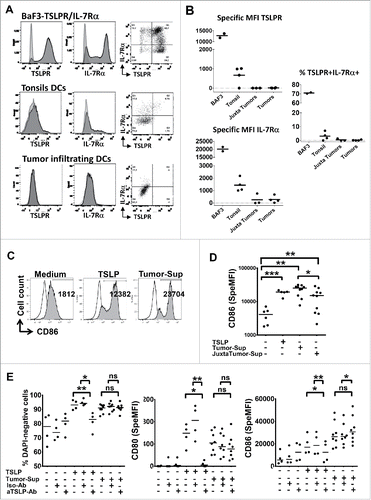
Soluble tumor microenvironment activates DC independently of TSLP
Even if very few DCs were found to express TSLPR ex vivo, it did not formally rule out a biological response to TSLP. We turned to functional assays in order to evaluate the capacity of the soluble tumor microenvironment to activate DC. Freshly isolated blood CD11c+ DC from healthy donors were used since such DC spontaneously upregulate TSLPR heterodimer after 3–6 h of culture in medium alone, and respond to TSLP.Citation24 Blood CD11c+ DC were cultured in the presence of soluble tumor microenvironment and the expression of co-stimulatory molecules was analyzed. Our results showed that both tumor and juxta-tumor supernatants activated DC and increased their viability, as assessed by reduced DAPI+ dead cells, and upregulation of costimulatory molecules CD80 and CD86 (). Supernatants from cultured freshly resected tumor samples were used to mimic soluble tumor microenvironment. First, we measured TSLP levels in those supernatants, even if dissociation between in situ expression and secretion in tissue-derived supernatants was previously observed.Citation5 We could not detect significant TSLP levels within the majority of the tested tumor-derived supernatants (Fig. S1A). In addition, TSLP titration activity on DC maturation showed that 5pg, corresponding to TSLP detection threshold by ELISA, are not sufficient to activate DC (Fig. S1B). However, the possibility remained that lower levels of TSLP will not activate DC on their own but would synergize with other components in tumor microenvironment. To formally rule out this hypothesis, we performed TSLP-blocking experiments with a blocking mAbs. The culture condition with exogenous addition of TSLP was used as positive control to test TSLP blocking. As expected, TSLP-induced DC activation was inhibited by the addition of TSLP-blocking mAb, as compared to Isotype control mAb (). However, DC activation induced by soluble tumor microenvironment was not affected by the TSLP-blocking mAbs (). Last, we performed experiments to further determine Th secretion profile induced using tumor or juxta-tumor derived supernatant primed DC. No difference was observed between Th profile generate by DC activated with tumor supernatant versus DC activated with juxta-tumor supernatant, which is concordant with absence of TSLP functional activity in this context (Figs. S2A–C). In particular, no Th2 biased toward Th2 (IL-4, IL-5, IL-13) was observed regardless of TSLP expression level in the tumors.
Overall, lack of TSLP activity together with an absence of TSLPR-expressing cells indicates that the TSLP pathway was not functional within the HNSCC microenvironment.
Discussion
For the first time, we analyzed the expression of TSLP in HNSCC and found that epithelial tumor cells highly expressed TSLP. In line with the fact that TSLP is mainly produced by keratinocytes,Citation26 high TSLP expression levels were associated with a high epidermoid differentiation. TSLP expression was restricted to epithelial cells since no other cell type was found, within tumor microenvironment, to express TSLP. These observations are in contrast to what was described in pancreatic cancer where TSLP was only found to be expressed in cancer-associated fibroblasts.Citation13 However, RT-PCR was used to assess the expression of TSLP in pancreatic cancer.Citation13 In our study, we used in situ immunohistology to detect TSLP at the protein level. Indeed, we have previously shown that in situ immunohistology was the most reliable technique to analyze TSLP protein expression as soon as TSLP is not always detected in supernatants of cultured tumor tissues or lysates from tumor tissues with protein detection assays.Citation5
Healthy oral epithelial mucosae was not described to express TSLP. However, we found few juxta-tumors positive for TSLP. Since, we know that mechanical disruption may induce TSLP production,Citation4 we cannot exclude that this expression was due to the manipulation of the tissues during surgery and experimentation. The alternative possibility could be that adjacent tissue is exposed to an overflow of local inflammation that promoted TSLP expression. In line with this last hypothesis, it has been recently shown that TSLP is overexpressed in oral lichen planus, a chronic inflammatory disease of oral mucosa.Citation27 Interestingly, higher levels of TSLP were found in oral cavity cancers compared to oropharynx, larynx, and hypopharynx ones. This suggests that the anatomical localization and exposure to external microenvironment may play a critical role in TSLP induction. One of our initial hypotheses was that TSLP could be induced by HPV infection, because of previous studies linking TSLP to viral infection.Citation28,29 However, the level of TSLP expression was comparable HPV-positive and HPV-negative HNSCC. Cigarette smoke extract induced TSLP expression in the mouse lung in an oxidative stress-dependent and TNF-α receptor I-dependent manner.Citation30 Tobacco consumption being a main risk factor for HNSCC, TSLP could be induced by cigarette smoke extract. However, in our study, lower levels of TSLP were found in smokers compared to non-smokers.
TSLP was not associated to DC activation and had no impact on disease prognosis, which we could mainly attribute to a lack of TSLPR-expressing cells in the tumor microenvironment. Such lack of TSLPR expression may be due to insufficient DC activating signals in situ, or to the presence of factor(s) inhibiting TSLPR expression by DCs, which remain to be identified. This is in contrast to previous reports finding TSLPR mainly expressed by CD11c+ cells in pancreatic cancer.Citation13 In this study, TSLPR was observed in CD11c+ DC in draining lymph nodes but not in the tumor. This raises the question of the functionality of TSLP protein expressed by cancer-associated fibroblasts and the putative target cells in the lymph node. In another study of TSLP in breast cancer, TSLPR expression in situ or ex vivo was not assessed.Citation15 In terms of prognosis, it was proposed that TSLP may have a negative impact in breast cancer, based on a mouse tumor model.Citation15 In our study, we have performed survival analysis using a large cohort with sufficient follow-up (median over 8 y) to provide statistical power to analyze prognostic impact in patients.
Functional experiments showed that soluble tumor microenvironment induces DC maturation. Extensive study of the soluble composition in cytokine and growth factors of the generated HNSCC supernatants should be performed in order to identify these stimulatory factor(s). Our preliminary data are showing increased presence in HNSCC supernatants of inflammatory analytes, such as GM-CSF, TNF-α, and IL-1, all being able to influence DC maturation. The subsequent T cell response induced by such activated DC should also be deeply dissected in order to identify a possible impact on Th profile responses even if our data showed no differences between DC activated with tumor supernatants versus DC activated with juxta-tumor supernatants.
Overall our work underscores the importance of studying cytokine pathways from the source to the expression of the receptor on target cells. The dissociation between cytokine and receptor expression that we have described for TSLP in HNSCC may very well be observed for other immune pathways in various tumor types. Our results suggest that the effect of TSLP in cancer may depend on the context, and on the tissue origin. Further studies are needed to elucidate the role of TSLP in distinct cancer types, including thorough biological and prognostic evaluation, in order to best guide clinical applications.
Materials and methods
Human samples and patient characteristics
Samples were obtained from primary tumor and juxta-tumor tissue (adjacent to the tumor and exempt of malignant tumor cells) of untreated HNSCC patients, from the Department of Pathology (Institut Curie, Paris). Patients signed an informed consent after approval of the study. This study was approved by the Internal Review Board and Clinical Research Committee of the Institut Curie. Retrospectively collected frozen samples, as well as freshly resected samples were used. For the retrospective study (1998–2009), 89 patients were included. Patient characteristics are summarized in Table S1. Each patient's disease was staged according to the 7th edition of the Union for International Cancer Control (UICC, 2009). Treatment modalities consisted of exclusive chemo-radiotherapy, or surgery combined or not to adjuvant radiotherapy and chemotherapy. Fresh healthy tonsils were obtained from children undergoing tonsillar resection (Necker Hospital, Paris) after informed consent of the parents. All the tissues were transported in CO2-independant medium (Gibco) and processed rapidly (<3 h) after resection.
Immunohistology, immunofluorescence, and scoring procedure
Tissues were frozen dry and/or embedded in OCT embedding matrix (Cell Path, UK) and stored at −80°C until used. Acetone-fixed cryosections of 4 μm thick were stained using the antibodies described in Table S2. The sections were counterstained with hematoxylin and mounted with Perthex mounting media (Histolab). The scoring was performed by experienced pathologists, blinded of the clinical outcome. Each marker was counted for all the samples by the same pathologist. For TSLP, the percentage of positivity was evaluated in epithelial cells. For DCLAMP, the absolute number of cells was counted in 10 fields at 40X (microscope Olympus BH2 ocular SWHK 10X L, Splan 120382) and in the more positive field, both within the epithelium and within the stoma. For immunoflurorescence, images were generated using Nikon Multicolors and Fluorescent Microscope and treated using ImageJ software.
Preparation of tissue single-cell suspensions and culture
After being cut in small pieces, fresh tissues were digested in RPMI 1640 Glutamax (Gibco) supplemented by 5%FBS (Hyclone) and with 2 mg/mL collagenase I (C0130, Sigma), 2 mg/mL hyaluronidase (H3506, Sigma) and 25 µg/mL DNAse (Roche) by three round of 15 min incubation in agitation at 37°C. The samples were filtered on a cell strainer 40 µm (Fischer Scientific) and diluted in PBS 1X (Gibco) supplemented with 1% decomplemented human serum (BioWest), and EDTA 2 mM (Gibco). After centrifugation, cells were resuspended in the same medium and counted before being assessed by flow cytometry.
Soluble tumor microenvironment
Tumor-derived soluble factors were obtained after tissue culture. Briefly, ∼20 mg tissue pieces were cultured at 37°C in 250 µL of complete medium: RPMI 1640 Glutamax (Gibco), FBS 10% (Hyclone), 1% Sodium pyruvate, non-essential amino acids, Penicilin/streptomycine (Gibco). Supernatants were collected after 24 h, filtered and frozen at −80°C until used for functional assays.
Pro B cell line Ba/F3 culture
The pro B cell line Ba/F3, previously infected with retroviral constructs encoding the receptor chain TSLPR and IL-7Ra,Citation8,23 were maintained in RPMI 1640 (Glutamax, Gibco) supplemented with 10% FBS (Hyclone) and 10 ng/mL of recombinant mouse IL-3 (Myltenyi Biotec).
Blood DC purification and culture
CD11c+ DC were purified to greater than 99% by means of FACSorting from buffy coats from healthy donors (Saint Antoine-Crozatier Blood Bank, Paris, France), as previously described.Citation31 Freshly sorted CD11c+ DCs were cultured in complete medium. Cells were seeded at 1.106 /mL in flat-bottom 96-well plates with human recombinant TSLP (50 ng/mL) or tumor supernatant (see soluble tumor microenvironment paragraph, diluted 1/10). CD11c+ DCs were harvested after 24 h of culture and analyzed by flow cytometry.
Flow cytometry
The monoclonal antibodies (mAbs) used to stain the cells were mouse anti-human CD3-FITC, CD16-FITC, CD11c-PE-Cy5, CD4+-APC, CD80-PE or CD80-FITC, CD40-FITC, CD45-APC-Cy7, (BD Biosciences), CD14-FITC, CD19-FITC (Miltenyi Biotec), CD123-PE-Cy7, HLADR-Alexa700, TSLPR-APC (Biolegend), IL-7Rα-PE, CD86-PE-Cy5 (eBiosciences); and matched isotype control mAb. The acquisition was performed with a LSRII flow cytometer (Becton Dickinson) and data analysis was performed using FlowJo software version 9.4.7. Dead cells were excluded based on side and forward scatter characteristics and DAPI (Life technologies) positive staining.
Statistical analysis
To describe the population, qualitative data were presented as a number and percentage of sample size and the continuous variables were described using mean and standard deviation. To compare the percentage of TLSP between clinical groups, we used a student test with Welch correction or a Kruskal–Wallis rank sum test. The Pearson's correlation test was used to determine the correlation between TSLP and DC markers. Overall survival was defined as the time from tumor resection until the occurrence of death or until last follow-up (right censored data). Recurrence-free survival was defined as the time from tumor resection until recurrent disease at the primary site or in the cervical lymph nodes or until death or until last follow-up (right censored data). The overall and the recurrence-free survival were estimated using the Kaplan–Meier method and survival curves were compared using a log-rank test. A difference was considered statistically significant when p value was p ≤ 0.05. All the analyses were performed using R software (version 2.13.2) (http://cran.r-project.org). Flow cytometry results from DCs cultured in different conditions were compared by using a paired two-tailed Student t test.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author Contributions
M.G-D, L.G., and V.S. designed research; M.G-D, L.G., A.V., P.M., P.S., and L.P. performed research; M.G-D and F.B. performed statistical analysis; M.G-D and V.S wrote the manuscript; V.M. and N.L-P contributed to statistical analysis; J.R., T.J., J.K, and X.S. contributed to patient sample recruitment for HNSCC samples; S.H., A.V., and M-P.S. contributed to clinical data management; C.B., A.N., M.H, O.C-M., and F.Z-K scored in situ immunostaining; E.L. performed HPV genotyping; A. S-D., X.S., C.L-T, and E.T. contributed with discussion and advice for the design and analysis.
KONI_A_1179414_s02.zip
Download Zip (546.9 KB)Acknowledgments
The authors greatly acknowledge the Nikon Imaging Center at Institut Curie-CNRS, the cytometry platform at Institut Curie in Paris, and the Center de resources biologiques (CRB) at Institut Curie in Paris. We thank Marie-Hélène Donnadieu for immunohistology.
Funding
This work was supported by funding from Institut National de la Santé et de la Recherche Médicale (BIO2012-02 and BIO2014-08), Fondation pour la Recherche Médicale, Association de la Recherche Contre le Cancer (PJA 20131200436), INCA (2011-1-PL BIO-12-IC-1 and 2012-1-GYN-04-IC-1), ANR-13-BSV1-0024-02, ANR-10-IDEX-0001-02 PSL* and ANR-11-LABX-0043, CIC IGR-Curie 1428, the European Research Council (ERC IT-DC 281987). M.G-D was supported by a grant from La Ligue Contre le Cancer.
References
- Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, Shelley M, Abbas AR, Austin CD, Jackman J et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol 2012; 129:104-11.e1–9; PMID:22664159; http://dx.doi.org/10.1016/j.jaci.2011.08.031
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002; 3:673-80; PMID:12055625; http://dx.doi.org/10.1038/nrm910
- Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol Baltim Md 1950 2005; 174:8183-90; PMID:15944327; http://dx.doi.org/10.4049/jimmunol.174.12.8183
- Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol 2010; 126:976-84, 984.e1-5; PMID:21050944; http://dx.doi.org/10.1016/j.jaci.2010.08.041
- Volpe E, Pattarini L, Martinez-Cingolani C, Meller S, Donnadieu M-H, Bogiatzi SI, Fernandez MI, Touzot M, Bichet J-C, Reyal F et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J Allergy Clin Immunol 2014; 134:373-81; PMID:24910175; http://dx.doi.org/10.1016/j.jaci.2014.04.022
- Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol 2000; 1:59-64; PMID:10881176; http://dx.doi.org/10.1038/76923
- Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med 2000; 192:659-70; PMID:10974032; http://dx.doi.org/10.1084/jem.192.5.659
- Reche PA, Soumelis V, Gorman DM, Clifford T, Liu Mr null, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol Baltim Md 1950 2001; 167:336-43; PMID:11418668; http://dx.doi.org/10.4049/jimmunol.167.1.336
- Gilliet M, Soumelis V, Watanabe N, Hanabuchi S, Antonenko S, de Waal-Malefyt R, Liu Y-J. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med 2003; 197:1059-63; PMID:12707303; http://dx.doi.org/10.1084/jem.20030240
- Liu Y-J, Soumelis V, Watanabe N, Ito T, Wang Y-H, Malefyt R de W, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol 2007; 25:193-219; PMID:17129180; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141718
- Soumelis V, Liu Y-J. Human thymic stromal lymphopoietin: a novel epithelial cell-derived cytokine and a potential key player in the induction of allergic inflammation. Springer Semin Immunopathol 2004; 25:325-33; PMID:14999427; http://dx.doi.org/10.1007/s00281-003-0152-0
- Ziegler SF, Liu Y-J. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol 2006; 7:709-14; PMID:16785889; http://dx.doi.org/10.1038/ni1360
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 2011; 208:469-78; PMID:21339327; http://dx.doi.org/10.1084/jem.20101876
- Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, Gress RE, Hesdorffer C, Leonard WJ, Biragyn A. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol 2011; 186:5656-62; PMID:21490155; http://dx.doi.org/10.4049/jimmunol.1100463
- Pedroza-Gonzalez A, Xu K, Wu T-C, Aspord C, Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med 2011; 208:479-90; PMID:21339324; http://dx.doi.org/10.1084/jem.20102131
- Wu T-C, Xu K, Banchereau R, Marches F, Yu CI, Martinek J, Anguiano E, Pedroza-Gonzalez A, Snipes GJ, O'Shaughnessy J et al. Reprogramming tumor-infiltrating dendritic cells for CD103+ CD8+ mucosal T-cell differentiation and breast cancer rejection. Cancer Immunol Res 2014; 2:487-500; PMID:24795361; http://dx.doi.org/10.1158/2326-6066.CIR-13-0217
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009; 16:91-102; PMID:19647220; http://dx.doi.org/10.1016/j.ccr.2009.06.018
- Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM, Mueller-Berghaus J, Kirkwood JM, Kwok WW et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med 2002; 196:619-28; PMID:12208877; http://dx.doi.org/10.1084/jem.20012142
- Di Piazza M, Nowell CS, Koch U, Durham A-D, Radtke F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell 2012; 22:479-93; PMID:23079658; http://dx.doi.org/10.1016/j.ccr.2012.08.016
- Demehri S, Turkoz A, Manivasagam S, Yockey LJ, Turkoz M, Kopan R. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell 2012; 22:494-505; PMID:23079659; http://dx.doi.org/10.1016/j.ccr.2012.08.017
- Demehri S, Cunningham TJ, Manivasagam S, Ngo KH, Moradi Tuchayi S, Reddy R, Meyers MA, DeNardo DG, Yokoyama WM. Thymic stromal lymphopoietin blocks early stages of breast carcinogenesis. J Clin Invest 2016; 126:1458-70; PMID:26927668; http://dx.doi.org/10.1172/JCI83724
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277-300; PMID:20610543; http://dx.doi.org/10.3322/caac.20073
- Zhong J, Pandey A. Site-directed mutagenesis reveals a unique requirement for tyrosine residues in IL-7Ralpha and TSLPR cytoplasmic domains in TSLP-dependent cell proliferation. BMC Immunol 2010; 11:5; PMID:20144186; http://dx.doi.org/10.1186/1471-2172-11-5
- Lu N, Wang Y-H, Wang Y-H, Arima K, Hanabuchi S, Liu Y-J. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J Exp Med 2009; 206:2111-9; PMID:19770269; http://dx.doi.org/10.1084/jem.20090153
- Volpe E, Pattarini L, Martinez-Cingolani C, Meller S, Donnadieu M-H, Bogiatzi SI, Fernandez MI, Touzot M, Bichet J-C, Reyal F et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell–derived IL-23 in patients with psoriasis. J Allergy Clin Immunol 2014; 134:373-81.e4; PMID:24910175; http://dx.doi.org/10.1016/j.jaci.2014.04.022
- Liu Y-J. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med 2006; 203:269-73; PMID:16432252; http://dx.doi.org/10.1084/jem.20051745
- Sun M, Tan W, Liu S, Liu G, Zhang X, Wang N, Qu X, Wei F. In situ expression and serum level of thymic stromal lymphopoietin in oral lichen planus. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol 2014; 43(10):740-5; PMID:24931732; http://dx.doi.org/10.1111/jop.12196
- Lee H-C, Headley MB, Loo Y-M, Berlin A, Gale M, Debley JS, Lukacs NW, Ziegler SF. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol 2012; 130:1187-96.e5; PMID:22981788; http://dx.doi.org/10.1016/j.jaci.2012.07.031
- Feng Q, Wei H, Morihara J, Stern J, Yu M, Kiviat N, Hellstrom I, Hellstrom KE. Th2 type inflammation promotes the gradual progression of HPV-infected cervical cells to cervical carcinoma. Gynecol Oncol 2012; 127:412-9; PMID:22828962; http://dx.doi.org/10.1016/j.ygyno.2012.07.098
- Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, Shimokawa N, Ohnuma Y, Katoh R, Ogawa H et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol 2008; 122:1208-14; PMID:18926564; http://dx.doi.org/10.1016/j.jaci.2008.09.022
- Bogiatzi SI, Guillot-Delost M, Cappuccio A, Bichet J-C, Chouchane-Mlik O, Donnadieu M-H, Barillot E, Hupé P, Chlichlia K, Efremidou EI et al. Multiple-checkpoint inhibition of thymic stromal lymphopoietin-induced TH2 response by TH17-related cytokines. J Allergy Clin Immunol 2012; 130:233-40.e5; PMID:22664159; http://dx.doi.org/10.1016/j.jaci.2012.04.038
