Abstract
Hearing loss is the most common neurosensory impairment worldwide. While conductive hearing loss can be managed by surgery, the management of sensorineural hearing loss (SNHL), related to the damage of sensory cells of the inner ear is more challenging to manage medically. Many causes of SNHL such as sudden idiopathic SNHL, Meniere’s disease, noise-induced hearing loss, autoimmune hearing loss or hearing loss from exposure to ototoxic substances can benefit from delivery of otoprotective drugs to the inner ear. However, systemic drug delivery through oral, intravenous and intramuscular methods leads to undesirable side effects due to the inner ear’s limited blood supply and the relatively poor penetration of the blood–inner ear barrier (BLB). Therefore, there has been an increased interest for the targeted drug delivery to the inner ear using nanoparticles. Drug delivery through nanoparticles offers several advantages including drug stabilization for controlled release and surface modification for specific targeting. Understanding the biocompatibility of nanoparticles with cochlea and developing novel non-invasive delivery methods will promote the translation of nanoparticle-mediated drug delivery for auditory disorders from bench to bedside.
Introduction
Hearing loss is the most common sensory impairment affecting humans. It is estimated that over 5% of the world’s population experiences disabling hearing loss, with over 20% of people experiencing some form of mild or unilateral hearing loss [Citation1]. Presbycusis, inner ear infection, Meniere’s disease, noise-induced hearing loss, autoimmune hearing loss, genetic diseases and hearing loss from ototoxic substances are examples of pathologies leading to sensorineural hearing loss [Citation2–6]. Most causes of sensorineural hearing loss are irreversible, and symptoms of deafness are managed by assistive devices such as hearing aids or cochlear implants (CIs) [Citation7,Citation8]. The pathophysiology of sensorineural hearing loss is not completely understood and therapeutic options are limited by a lack of both effective drugs and non-invasive targeted delivery systems to the structures of the inner ear.
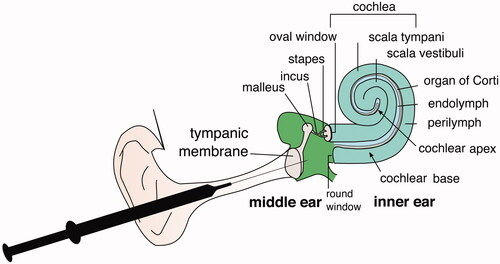
Treating inner ear disorders remain difficult due to the anatomical and physiological barriers. The inner ear has round and oval windows that prevent the permeability of larger molecules into the cochlea (). Currently, systemic drug delivery is considered as the first line approach as the treatment modality for inner ear disorders such as idiopathic sudden sensorineural hearing loss (ISSNHL) including noise-induced hearing loss, vertigo and Meniere’s disease [Citation9–14]. The main benefit associated with systemic drug delivery is the ease of administration especially when given orally in the form of pills. However, systemic drug delivery leads to undesirable side effects due to the inner ear’s limited blood supply and the relatively poor penetration of the blood–inner ear barrier (BLB) [Citation15]. This leads to sub-therapeutic local concentration of the drug and the need for higher systemic doses in order to reach therapeutic range. It increases the numerous adverse side effects of systemically delivered drugs such as corticosteroids that are commonly used to treat inner ear conditions [Citation2]. Modes of local drug administration to the inner ear include intratympanic (IT) injections, which deliver drugs to the inner ear directly through the round window membrane (RWM), bypassing the BLB and labyrinthine artery. IT injections were first used in 1956 for applying streptomycin to mitigate the symptoms of Meniere’s disease [Citation16]. The use of IT injections in Meniere’s disease came with significant drawbacks, such as clearance of drugs through the eustachian tube or the drugs not making contact with the round window. Although IT injections of steroids have been used to treat a variety of inner ear pathologies from tinnitus to idiopathic sudden sensorineural hearing loss though there is a lack of standardization of these procedures [Citation2]. IT injections deliver drugs into the middle ear space, allowing for drug diffusion across the RWM into the inner ear. Drug concentrations measured in inner ear fluids, perilymph and endolymph are significantly higher using IT injections compared to oral or parenteral administration of drugs [Citation17]. Although IT injections allow for a more direct method of drug delivery to the middle ear, drug concentrations with IT injections often still remain sub-therapeutic [Citation17]. Furthermore, IT injections do not offer increased drug stability to labile agents. To overcome these drawbacks, a chitosan glycerophosphate (CGP) hydrogel matrix was employed as a vehicle for local drug delivery. The use of hydrogel allowed for the sustained release of the administration of gentamicin in Meniere’s disease; however, this also leads to unselective gentamicin ototoxicity in the hair cells of both the cochlear and vestibular systems [Citation18].
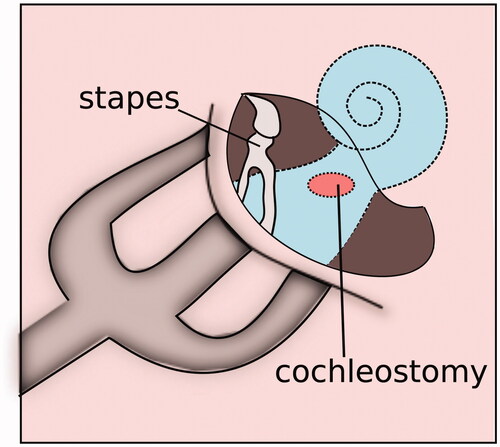
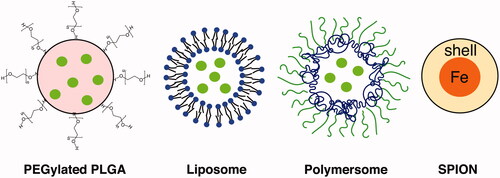
Due to the limitations of conventional drug delivery, there has been an increased interest in the auditory community to explore the potential of nanoparticles (NPs) for intracochlear drug delivery. NPs are carriers ranging from 1 to 1000 nm and can be used to both stabilize drugs in vivo and carry drugs to the cochlea [Citation19]. Nanocarriers were developed in the 1950s but have only been increasingly used for drug delivery within the last two decades [Citation19–21]. NPs can be engineered to allow for non-invasive application and specific site targeting. NPs can encapsulate therapeutic agents and are capable of transport across membranes such as RWM. However, in the auditory system, NPs must be biocompatible with cochlea and non-toxic for treating hearing disorders. The need for safety poses a challenge in NP research and development in otology. There are several methods of NP administration, such as locally through IT injection [Citation22], gelfoam applied to the RWM [Citation23,Citation24], or magnetic transfer through iron oxide NPs placed on the RWM [Citation25]. Surgical approaches for direct intracochlear drug delivery includes administration through RWM or through a cochleostomy, a hole drilled surgically through the cochlear bone (). Since NP-based drug delivery systems have already proven to be successful in many fields such as oncology, it holds a great potential for intracochlear drug delivery [Citation26–28]. Presently, there are several types of NPs that appears to be suitable for inner ear drug delivery, which have been classified according to their composition including poly (lactic-co-glycolic acid) (PLGA) NPs, magnetic NPs, lipid NPs, liposomes, polymersomes, hydroxyapatite NPs and silica NPs [Citation19] ().
Advantages of NPs for drug delivery to inner ear
The biggest advantage of NP-based drug delivery is that they can be targeted to a particular structure in the auditory system. Local injections into the middle ear have been the preferred method of delivery of drugs, as the drug would subsequently enter the oval window or round window membrane. The targeting system’s capability of NPs allows their widespread application in other delivery systems, including hydrogels and scaffolds [Citation29]. The ability to use NP with other delivery systems allows for higher a concentration of drugs to be delivered. Following administration, NPs can distribute in the perilymph as well as endolymph compartments () and can be targeted to a specific site of interest by altering surface chemistry with functional groups and ligands. NPs carrying nerve growth factor-derived peptide were found to target spiral ganglion cells through ligand-mediated binding of p75 neurotrophin receptors and tyrosine kinase receptors in an in vitro model of organotypic explant cultures prepared from mouse cochleae [Citation30]. Another study determined the distribution and safety of NPs within the inner ear by placing Cy3-labelled silica NPs onto the RWM of adult mice. There were no adverse effects of NPs on the auditory systems as hearing thresholds were comparable in NP-treated and control mice. The study found that NPs can also reach central auditory nuclei as silica NPs carrying Cy3 reached the dorsal cochlear nucleus and superior olive through retrograde axon transport [Citation22]. NP-based drug delivery offers numerous advantages over IT injections. With IT injections, the concentration of drug that comes into contact with the RWM determines the concentration delivered to the inner ear cavity [Citation31,Citation32]. The concentration of drug that does not reach the RWM is cleared through the Eustachian tube (ET). On the other hand, NPs can be conjugated to cell penetrating peptides or can be surface modified to enhance their contact with RWM leading to efficient inner ear drug delivery [Citation33–35].
The surface of NPs can be modified to target specific structures by conjugating ligands to the surface using bio-conjugation techniques. Some newer methods include expressed protein ligation (EPL) and click chemistry [Citation36,Citation37]. In EPL, a site-specific chemical ligation is made between a recombinant protein with a C-terminal thioester and a peptide or protein with an N-terminal cysteine. A C-terminal thioester can then be added onto a targeting ligand through the use of inteins (autoprocessing proteins) which are then placed between the ligand and an affinity tag. After bacterial expression and affinity purification steps, the ligand is released from the affinity tag to create a reactive thioester at the C-terminus. The thioester can then react with any peptide containing an N-terminal cysteine [Citation17]. On the other hand, click chemistry adds ligands to NPs through a Cu I- catalyzed terminal alkyne-azide cycloaddition [Citation17]. A drawback of click chemistry is the reaction process, which may degrade or modify the target of interest. Recently, EPL and click chemistry techniques have been combined to allow for the conjugation of targeting ligands to NPs that are site specific. The combined method allows for a stereospecific ligand attachment onto the NP surface. This method has been used to conjugate full antibodies, peptides and drugs onto the NP surface [Citation38].
Local delivery of NPs by IT injection through the round window has been shown to be effective in targeted drug delivery to specific structures in the inner ear. Prior studies have observed that IT-injected silica and PLGA NPs were able to reach the inner hair cells, spiral ganglion neurons, various structures in the organ of Corti, and even central auditory pathways in the brainstem [Citation22]. NPs can encapsulate an array of therapeutic agents. Delivery of NPs has been shown to allow for non-invasive application, drug stabilization, controlled release and surface modification for specific targeting.
Methods for NP delivery
A wide variety of methods have been employed for nanoparticle delivery into the inner ear. Hydrogels such as chitosan–glycerophosphate are an efficient localized drug delivery system with the advantage of slow controlled drug release as the material is gradually degraded by enzymes such as chitosanases present in the middle ear. Hydrogels embedded with NPs have been used to deliver corticosteroids and antibiotics into inner ear perilymph [Citation39,Citation40] and have been shown to have a lower risk of hearing loss compared to drugs delivered through IT injection [Citation40].
Drugs can also be delivered transtympanically through a cochleostomy (), although this is more invasive and traumatic than delivery through the round or oval window membrane or IT injection. Studies have shown that IT injection or RWM application of contrast agents yielded higher signal intensity in the scala vestibule compared to the scala tympani. Studies delivering different types of NPs through the round window membrane have shown localization in the spiral ganglion neuron cell bodies [Citation24,Citation41]. NP delivery was more variable in the organ of Corti, lateral wall and spiral ganglion neuron axons.
Cochlear implant-based delivery is another possibility for NP impregnated drugs in patients already undergoing surgery for device implantation into the scala tympani. NP-based drug delivery can be used to decrease post-implantation inflammation and fibrosis or to preserve residual hearing [Citation42,Citation43]. The penetration of cochlear implant electrodes deep into the cochlea would also allow for lower drug concentrations. However, further studies are warranted for the implementation of NP-based drug delivery during cochlear implantation.
Challenges for nanoparticle delivery into the inner ear
There are limitations to the implementation of NP delivery to the inner ear, mainly due to limited access to the inner ear and the poor uptake of therapies into the inner ear cells. Anatomically, the inner ear provides limited access for local and targeted drug delivery. In addition, RWM is difficult to access for intracochlear drug delivery. Physiologically, the middle-inner ear, blood-perilymph, blood–endolymph and perilymph–endolymph barriers limit the systemic delivery of drugs to the inner ear [Citation44,Citation45]. The blood–perilymph barrier is located at the modiolar vascular region. The perilymph–endolymph barrier is most porous at the modular wall of the first and the second turn of the scala vestibule and scala tympani [Citation46,Citation47]. The arterial supply to the labyrinth is sparse and this requires an increase in the dose of intravenous or oral drugs given to target the sensory cells in the inner ear. Thus, the need to achieve therapeutic drug levels in the inner ear while minimizing toxicity and systemic side effects is of utmost importance for intracochlear drug delivery.
Opportunities for inner ear drug delivery
Drug delivery through the cochlear round and oval window membranes is another possibility as these membranes are permeable to small molecules. The thickness of the RWM is about 70 microns in humans [Citation48]. The RWM consists of three layers having an outer epithelium, a fibrous connective tissue layer, and an inner epithelium surrounding the perilymph cavity [Citation15]. The middle collagenous layer may attenuate transport and permeability is determined by drug particle size, charge and solubility. However, there can be issues with RWM permeability and the trafficking of particles through it is still relatively poorly understood. The pharmacokinetics of RWM delivery is also unpredictable due to variable clearance of drug through the Eustachian tube and variable patency of the cochlear aqueduct in humans [Citation15]. However, prior studies have shown that molecules up to 1 µm in size can permeate the RWM and the small positively charged molecules are more easily transported [Citation49]. Permeability of the RWM can also be affected by inflammatory disease processes such as otitis media due to the scarring and fibrosis of granulation tissue hindering transport [Citation49]. Attempts have been made to increase membrane permeability and porosity using agents such as O-streptolysin [Citation50]. However, it is difficult to attain uniform drug distribution throughout the cochlea. The highest drug concentrations remain at the cochlear base adjacent to the RWM and the lowest concentrations have been observed at the apex [Citation51]. Low NP concentration in the apical section of the cochlea makes it more difficult to treat hearing loss of low frequencies. Current studies have shown that the administration of positively charges NPs, especially 1,2-dioleoyl-3-trimethylammonium-propane [DOTAP] modified NP have been observed to be distributed broadly in the inner ear after RWM application [Citation33]. Another study demonstrated that the surface-modified NPs reached the organ of Corti and were transported into the outer hair cells (OHCs) at a higher level compared to unmodified NPs [Citation34]. NPs that were surface modified with poloxamer 407 (P407) showed the greatest cellular uptake and prominent fluorescence in cochlear imaging compared to chitosan, or methoxy poly(ethylene glycol) and the unmodified NPs. Thus, surface modification provides an advantage for enhanced drug delivery into the inner ear. In addition, drug-loaded NPs have been combined with cell penetrating peptides to enhance their penetration across RWM. A study showed that the combination of P407-PLGA-NPs (mean diameter: 100–200 nm) and low molecular weight protamine provided a synergistic enhancement in NP entry to the organ of Corti and stria vascularis without inducing pathological alteration of cochlear tissues and RWM [Citation35]. Besides permeability, NP properties also affect the speed of entry. There is some evidence that NPs of certain sizes are able to enter the inner ear faster. A study observed that NPs between 150 and 300 nm entered the inner cochlea faster than NPs of 80 nm [Citation35]. NPs with positive surface charge were also found to enter the cochlea faster. Another study found that NPs <200 nm in size efficiently pass through the RWM [Citation52].
NP trafficking to the cytoplasm is another challenge that must be overcome with careful surface chemistry design. Internalization can be facilitated by attaching certain surface peptide motifs or functional ligands and in specific densities. Surface charge can also be altered, such as poly(ethylene glycol) (PEG), or PEGylation, to confer a neutral charge to the nanoparticle carrier [Citation53]. Viral protein attachment such as transactivator of transcription peptide (TAT) to nanoparticle surfaces [Citation54] is another modality that has been studied for increasing nanoparticle uptake into cells.
Targeted NP-based intracochlear drug delivery
NPs are now increasingly utilized for targeted drug delivery into the inner ear. A recent study looked at nanoparticle delivery of edaravone, an antioxidant used in the treatment of noise-induced hearing loss [Citation17]. Guinea pigs were treated with an NP-based delivery of edaravone in vivo. Results of the study suggested that IT delivery of solid lipid nanoparticle (SLN) encapsulated edaravone inhibited free radical generation in the cochlea and decreased hearing thresholds following noise-induced injury, although it did not show significant hearing recovery. These findings suggest that perhaps more targeted therapy is needed [Citation55].
Another study used lipid NPs to deliver rolipram to spiral ganglion cells in vitro [Citation17]. Results of this study showed increased neuronal survival and elongation of neurite outgrowth by inhibition of phosphodiesterase 4 in a dose-dependent manner [Citation17]. The study showed that lipid NP-encapsulated rolipram led to higher SGC survival rates and neurite outgrowth than rolipram delivery alone. However, the results of the study could not be replicated in vivo [Citation56].
Another study used a nanohydrogel delivery system, which combines nanotechnology with a hydrogel delivery system. Hydrogels are soft materials networked by physically or chemically crosslinked biopolymers in aqueous solutions and has the ability to transform from a liquid to a solid at a wide range of temperatures; once in the round window niche (RWN) the hydrogel gradually reaches body temperature and becomes a gel. The hydrogel once in RWN is able to withstand constant shear forces and allow for the slow, gradual and steady release of therapeutic agents into the round window [Citation57]. Another study showed that liposomal NPs delivered through nanohydrogel in vitro was able to persist in the middle ear for at least 2 weeks, and therefore sustained release of the drug was obtained [Citation58]. The same study found that liposomal NPs could be delivered in vivo across the RWM to reach structures in the scala media [Citation58]. Currently, nanohydrogel drug delivery system that successfully delivers nanoparticles into the inner ear have been introduced with a targeting peptide that recognizes prestin, a transmembrane electromotile protein uniquely expressed in outer hair cells (OHCs). This system allowed for the successful delivery of a JNK pathway inhibitor, D-JNKi-1, which attenuated hearing loss caused by acoustic trauma [Citation57].
Endosomal escape
Endosomes deliver intracellular contents to lysosomes which host an acidic environment for proteolysis and recycling of unneeded cellular building blocks [Citation59]. NPs delivered to the cell must be able to escape the endosome-lysosomal compartments to maintain drug integrity and efficiency. Different strategies have been developed to ensure endosomal release, one mechanism proposed was the “proton sponge,” which are typically polyamines that work by absorbing free protons in the endosomes. Protonable molecules induce an inflow of ions and water into the endosome, subsequently rupturing the endosome and releasing endosome contents into the cell [Citation60]. Another mechanism utilizes an approach modelled on how enveloped viruses induce endosomal escape by fusion of the viral envelope with the endosome membrane, allowing the viral capsid to enter the cytoplasm. Particles assembled from lipids can fuse with the endosome membrane, inverting the structure of the liposome and delivering instead the drug core to the cytoplasm [Citation61]. In addition, membrane disruption and pore formation can destabilize the endosome membrane and allow the NP containing the drug to diffuse out of the endosomal compartment through pores into the cytoplasm. Pore formation can occur through direct interaction of polymers or peptides that self-assemble across the endosomal membrane. An engineered peptide GALA is capable of releasing molecules up to ∼5000 Da [Citation61].
NPs for drug delivery into the inner ear
A vast variety of different NPs can be explored for drug delivery into the inner ear ( and ). NPs can be either organic or inorganic and may be fabricated to carry hydrophilic and/or hydrophobic drugs to the site of interest. For efficient drug delivery, particles should generally be no larger than 200 nm to avoid opsonization and elimination by the host immune system [Citation62].
Table 1. A summary of nanoparticles used for drug delivery into the inner ear.
There are many advantages to drug delivery through NPs. Polymer NPs are among the most well-studied and characterized NPs [Citation63]. Polymers are advantageous for their ability to increase drug half-life and their ability to facilitate controlled drug release [Citation63]. Most NPs that are FDA approved are PEGylated drugs (polymer–drug conjugates) and degradable multi-unit polymers. PEGylation increases the biological half-life in circulation, and drugs that are attached to such hydrophilic polymers. PEGylation also increases biocompatibility and solubility [Citation64]. Clinically, this prolongs the dosing interval between drug administrations. Besides being more convenient for the patient, this also decreases the exposure to drugs thereby limiting side effects, toxicity and immune reactions to drugs that are biological agents [Citation64].
Hydrophobic molecules such as micelles can enable delivery of hydrophobic drugs through circulation. The external surface of a micelle can enable solubilization in an aqueous environment while poorly water-soluble components can be encapsulated inside. In addition, unstable drugs such as biological molecules can be stabilized within micelles and delivered to the site of interest. Liposomal NPs are another platform for drug delivery [Citation65,Citation66]. A liposome NP utilizes a double layer of amphipathic molecules, creating a hydrophilic external and internal surface such as that seen in a phospholipid bilayer. Liposomes are easily synthesized due to their ability to self-assemble in aqueous solutions and were among the first NPs used in clinical trials. Liposomes increase the distribution of drugs with low bioavailability. Liposomes also decrease the negative side effects of highly toxic drugs en route to the drug’s target organ. Liposomes are rapidly cleared from the body and have short half-lives, which reduces the toxicity of liposomal drugs such as doxorubicin.
Another advantage of using NPs for intracochlear drug delivery is the ability to titrate and control the release of therapeutic dosing. Biomaterials can be selected to slowly degrade, releasing the drug in a controlled manner [Citation67]. For example, large multi-unit polymers decompose into single units over specific time periods [Citation68,Citation69]. Protein NPs are created when drugs are conjugates to endogenous protein carriers, thereby increasing therapeutic delivery to tissue [Citation70]. There are numerous advantages to using protein nanocarriers. Specific protein motifs can be used to target tissues [Citation71]. Conjugation to endogenous proteins can also reduce the toxicity of compounds previously needed to increase drug solubility such as Cremophor. Proteins also create favourable pharmacokinetics by increasing endothelial binding and transport of drug through epithelial surfaces [Citation70,Citation71].
Inorganic NPs of metals and metal oxides constitute another major class of nanocarrier-mediated drug delivery [Citation72]. Metallic NPs have the ability to be used in both therapeutics and imaging applications, known as theranostics. Iron oxide NPs for anemia treatment constitute the majority of FDA approved inorganic NPs.
Gold is another NP having tremendous potential for intracochlear drug delivery due to its optical and thermal properties as well as the ability to modify its size, shape and surface chemistry [Citation73]. Gold also exhibits surface plasmon resonance making it possible to measure interactions of these NPs in real-time with high sensitivity and without the need of labels [Citation74]. Although gold is widely used in research applications, very few gold NPs are being studied in clinical trials and no gold NPs are currently FDA approved therapies. One study determined the ability of targeted gold NPs as contrast agents for inner ear imaging using in vitro and in vivo mouse models employing micro-computed tomography (micro-CT) [Citation75]. Unfortunately, gold NPs were not able to provide enhanced micro-CT imaging of the inner ear compared to the control group. Further studies are warranted to decipher the potential of gold NPs for cochlear imaging and inner ear drug delivery.
Conclusions and future directions
Treatment of inner ear disorders poses a challenge both due to anatomical and physiological barriers of the ear. The anatomic inaccessibility of the inner ear coupled with poor penetration through the BLB and permeation of drug molecules through RWM results in sub-therapeutic concentrations of the drug at the site of action. One other key factor that limits the treatment options is the instability of the drug molecules in the inner ear for a prolonged period of time.
The use of nanotechnology is a rapidly evolving field in the treatment of inner ear disorders. NPs often possess physical, chemical and biological properties that can lead to efficient intracochlear drug delivery. These unique properties allow NPs to stabilize drug molecules and achieve not only site-specific targeting but also a controlled release of the drug molecules to the site of action. These properties can be further enhanced through alteration of surface chemistry or surface charge of NPs leading to efficient intracochlear drug delivery.
Despite the adverse effects, systemic drug delivery through oral, intravenous or intramuscular methods are still accepted as the first line approach when treating inner ear disorders. Although IT injections have been utilized to deliver NPs directly to the middle ear, they do come with their share of challenges. Enabling close contact with the RWM to allow efficient drug release in the inner ear, and also avoiding rapid clearance by the Eustachian tube are some of the issues that result in highly variable pharmacokinetic parameters following IT administration. The intracochlear route, which includes but not limited to, injections, implants, osmotic mini-pumps as well as reciprocation perfusion systems, has the highest efficiency among the inner ear delivery methods but poses a significant risk of deafness due to perforations and surgical manipulation.
The lessons learnt from the successful implementation of NP-mediated drug delivery for other organs can be translated for the inner ear. For example, to increase the penetration of NPs into the cochlea, NPs can be conjugated to cell penetrating peptides [Citation76]. The antitumor activity of drug epirubicin (EPR) has been shown to be enhanced following conjugation of drug loaded NPs with cell penetrating peptides [Citation77]. These findings hold a great potential to improve the penetration of drug loaded NPs into the cochlea.
Recently, there has been an increase in the number of FDA approvals utilizing NPs for treatment of various auditory disorders, with a substantial increase in the number of on-going clinical trials. While FDA approved NPs are primarily polymeric, liposomal and nanocrystal formulations, there is a trend towards the development of more complex NPs comprising micelles and protein-based NPs, as well as the emergence of a variety of inorganic NPs of metals and metal oxides in clinical trials. Currently, there is a need to determine the biocompatibility of new and emerging NPs employing in vitro and in vivo models of auditory system. Despite all the challenges that are presently being encountered by the nanotechnology platform, it is believed that nanodrugs will ultimately provide the necessary solutions that will significantly enhance the field of intracochlear drug delivery and will alter clinical practice in the future.
Acknowledgements
The authors are thankful to Dr. Valerie Gramling for critical reading of the manuscript.
Disclosure statement
Dr. Eshraghi received research funding from MED-EL corporation. All other authors have no conflict of interest.
Additional information
Funding
References
- Chadha S, Cieza A. Promoting global action on hearing loss: World Hearing Day. Int J Audiol. 2017;56:145–147.
- Kim DK. Nanomedicine for inner ear diseases: a review of recent in vivo studies. Biomed Res Int. 2017;2017:3098230.
- Mittal R, Patel AP, Nguyen D, et al. Genetic basis of hearing loss in Spanish, Hispanic and Latino populations. Gene. 2018;647:297–305.
- Cunningham LL, Tucci DL. Hearing loss in adults. N Engl J Med. 2017;377:2465–2473.
- Nieman CL, Reed NS, Lin FR. Otolaryngology for the internist: hearing loss. Med Clin North Am. 2018;102:977–992.
- Eshraghi AA, Jung HD, Mittal R. Recent advancements in gene and stem cell based treatment modalities: Potential implications in noise induced hearing loss. Anatomical Record 2018. In press.
- Eshraghi AA, Nazarian R, Telischi FF, et al. The cochlear implant: historical aspects and future prospects. Anat Rec (Hoboken). 2012;295:1967–1980.
- Roche JP, Hansen MR. On the horizon: Cochlear Implant Technology. Otolaryngol Clin North Am. 2015;48:1097–1116.
- Kil J, Lobarinas E, Spankovich C, et al. Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2017;390:969–979.
- Chen WT, Lee JW, Yuan CH, et al. Oral steroid treatment for idiopathic sudden sensorineural hearing loss. Saudi Med J. 2015;36:291–296.
- Gilles A, Ihtijarevic B, Wouters K, et al. Using prophylactic antioxidants to prevent noise-induced hearing damage in young adults: a protocol for a double-blind, randomized controlled trial. Trials. 2014;15:110.
- Coelho DH, Lalwani AK. Medical management of Ménière’s disease. Laryngoscope. 2008;118:1099–1108.
- Uri N, Doweck I, Cohen KR, et al. Acyclovir in the treatment of idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. 2003;128:544–549.
- Tucci DL, Farmer JC Jr, Kitch RD, et al. Treatment of sudden sensorineural hearing loss with systemic steroids and valacyclovir. Otol Neurotol. 2002;23:301–308.
- Bowe SN, Jacob A. Round window perfusion dynamics: implications for intracochlear therapy. Curr Opin Otolaryngol Head Neck Surg. 2010;18:377–385.
- Schuknecht HF. Ablation therapy for the relief of Ménière's disease. Laryngoscope. 1956;66:859–870.
- Li L, Chao T, Brant J, et al. Advances in nano-based inner ear delivery systems for the treatment of sensorineural hearing loss. Adv Drug Deliv Rev. 2017;108:2–12.
- Luo J, Xu L. Distribution of gentamicin in inner ear after local administration via a chitosan glycerophosphate hydrogel delivery system. Ann Otol Rhinol Laryngol. 2012;121:208–216.
- Pyykko I, Zou J, Schrott-Fischer A, et al. An overview of nanoparticle based delivery for treatment of inner ear disorders. Methods Mol Biol. 2016;1427:363–415.
- Mittal R, Patel AP, Jhaveri VM, et al. Recent advancements in nanoparticle based drug delivery for gastrointestinal disorders. Expert Opin Drug Deliv. 2018;15:301–318.
- Mittal R, Jhaveri VM, McMurry HS, et al. Recent treatment modalities for cardiovascular diseases with a focus on stem cells, aptamers, exosomes and nanomedicine. Artif Cells Nanomed Biotechnol. 2018;15:1–10.
- Praetorius M, Brunner C, Lehnert B, et al. Transsynaptic delivery of nanoparticles to the central auditory nervous system. Acta Otolaryngol. 2007;127:486–490.
- Tamura T, Kita T, Nakagawa T, et al. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope. 2005;115:2000–2005.
- Zou J, Saulnier P, Perrier T, et al. Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J Biomed Mater Res. 2008;87:10–18.
- Ge X, Jackson RL, Liu J, et al. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol Head Neck Surg. 2007;137:619–623.
- Nakano K, Matoba T, Koga JI, et al. Safety, tolerability, and pharmacokinetics of NK-104-NP. Int Heart J. 2018;59:1015–1025.
- Singh SK, Singh S, Lillard JW Jr, et al. Drug delivery approaches for breast cancer. Int J Nanomedicine. 2017;12:6205–6218.
- Singh P, Pandit S, Mokkapati VRSS, et al. Gold nanoparticles in diagnostics and therapeutics for human cancer. IJMS. 2018;19:1979.
- Lee JH, Lee MY, Lim Y, et al. Auditory disorders and future therapies with delivery systems. J Tissue Eng. 2018;9:2041731418808455.
- Roy S, Johnston AH, Newman TA, et al. Cell-specific targeting in the mouse inner ear using nanoparticles conjugated with a neurotrophin-derived peptide ligand: potential tool for drug delivery. Int J Pharm. 2010;390:214–224.
- Salt AN, Plontke SK. Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications. Hear Res. 2018;368:28–40.
- Salt AN, Hirose K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear Res. 2018;362:25–37.
- Liu H, Chen S, Zhou Y, et al. The effect of surface charge of glycerol monooleate-based nanoparticles on the round window membrane permeability and cochlear distribution. J Drug Target. 2013;21:846–854.
- Wen X, Ding S, Cai H, et al. Nanomedicine strategy for optimizing delivery to outer hair cells by surface-modified poly(lactic/glycolic acid) nanoparticles with hydrophilic molecules. Ijn. 2016; 11:5959–5969.
- Cai H, Liang Z, Huang W, et al. Engineering PLGA nano-based systems through understanding the influence of nanoparticle properties and cell-penetrating peptides for cochlear drug delivery. Int J Pharm. 2017;532:55–65.
- Frutos S, Hernández JL, Otero A, et al. Site-specific antibody drug conjugates using streamlined expressed protein ligation. Bioconjug Chem. 2018; 29:3503–3508.
- Valero T, Delgado-González A, Unciti-Broceta JD, et al. Drug “Clicking” on cell-penetrating fluorescent nanoparticles for in cellulo chemical proteomics. Bioconjug Chem. 2018;29:3154–3160.
- Yi G, Son J, Yoo J, et al. Application of click chemistry in nanoparticle modification and its targeted delivery. Biomater Res. 2018;22:13.
- Paulson DP, Abuzeid W, Jiang H, et al. A novel controlled local drug delivery system for inner ear disease. Laryngoscope. 2008;118:706–711.
- Xu L, Heldrich J, Wang H, et al. A controlled and sustained local gentamicin delivery system for inner ear applications. Otol Neurotol. 2010;31:1115–1121.
- Zhang W, Zhang Y, Löbler M, et al. Nuclear entry of hyperbranched polylysine nanoparticles into cochlear cells. Int J Nanomedicine. 2011;6:535–546.
- Eshraghi AA, Lang DM, Roell J, et al. Mechanisms of programmed cell death signaling in hair cells and support cells post-electrode insertion trauma. Acta Otolaryngol. 2015;135:328–334.
- Eshraghi AA, Ahmed J, Krysiak E, et al. Clinical, surgical, and electrical factors impacting residual hearing in cochlear implant surgery. Acta Otolaryngol. 2017;137:384–388.
- Sun W, Wang W. Advances in research on labyrinth membranous barriers. J Otol. 2015;10:99–104.
- Juhn SK. Barrier systems in the inner ear. Acta Otolaryngol Suppl. 1988;458:79–83.
- Eckhard A, Müller M, Salt A, et al. Water permeability of the mammalian cochlea: functional features of an aquaporin-facilitated water shunt at the perilymph–endolymph barrier. Pflugers Arch - Eur J Physiol. 2014;466:1963–1985.
- Eckhard A, Dos Santos A, Liu W, et al. Regulation of the perilymphatic–endolymphatic water shunt in the cochlea by membrane translocation of aquaporin-5. Pflugers Arch – Eur J Physiol. 2015;467:2571–2588.
- Mikulec AA, Hartsock JJ, Salt AN. Permeability of the round window membrane is influenced by the composition of applied drug solutions and by common surgical procedures. Otol Neurotol. 2008;29:1020–1026.
- Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001;121:437–447.
- Engel F, Blatz R, Kellner J, et al. Breakdown of the round window membrane permeability barrier evoked by streptolysin O: possible etiologic role in development of sensorineural hearing loss in acute otitis media. Infect Immun. 1995;63:1305–1310.
- Salt AN, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear Res. 2001;154:88–97.
- Martin-Saldana S, Palao-Suay R, Aguilar MR, et al. Polymeric nanoparticles loaded with dexamethasone or α-tocopheryl succinate to prevent cisplatin-induced ototoxicity. Acta Biomater. 2017;53:199–210.
- Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951.
- Dong X, Liu L, Zhu D, et al. Transactivator of transcription (TAT) peptide-chitosan functionalized multiwalled carbon nanotubes as a potential drug delivery vehicle for cancer therapy. Int J Nanomed. 2015;10:3829–3840.
- Gao G, Liu Y, Zhou CH, et al. Solid lipid nanoparticles loaded with edaravone for inner ear protection after noise exposure. Chin Med J. 2015;128:203–209.
- Meyer H, Stover T, Fouchet F, et al. Lipidic nanocapsule drug delivery: neuronal protection for cochlear implant optimization. Int J Nanomed. 2012;7:2449–2464.
- Kayyali MN, Wooltorton JRA, Ramsey AJ, et al. A novel nanoparticle delivery system for targeted therapy of noise-induced hearing loss. J Control Release. 2018;279:243–250.
- Lajud SA, Nagda DA, Qiao P, et al. A novel chitosan-hydrogel-based nanoparticle delivery system for local inner ear application. Otol Neurotol. 2015;36:341–347.
- Hu YB, Dammer EB, Ren RJ, et al. The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Transl Neurodegener. 2015;4:18.
- Xu C, Haque F, Jasinski DL, et al. Favorable biodistribution, specific targeting and conditional endosomal escape of RNA nanoparticles in cancer therapy. Cancer Lett. 2018;414:57–70.
- Selby LI, Cortez-Jugo CM, Such GK, et al. Nanoescapology: progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1452.
- Ulbrich K, Hola K, Subr V, et al. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem Rev. 2016;116:5338–5431.
- Lu XY, Wu DC, Li ZJ, et al. Polymer nanoparticles. Prog Mol Biol Transl Sci. 2011;104:299–323.
- Veronese FM, Mero A. The impact of PEGylation on biological therapies. Biodrugs. 2008;22:315–329.
- Zylberberg C, Gaskill K, Pasley S, et al. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017;24:441–452.
- Panahi Y, Farshbaf M, Mohammadhosseini M, et al. Recent advances on liposomal nanoparticles: synthesis, characterization and biomedical applications. Artif Cells Nanomed Biotechnol. 2017;45:788–799.
- Gao W, Zhang Y, Zhang Q, et al. Nanoparticle-hydrogel: a hybrid biomaterial system for localized drug delivery. Ann Biomed Eng. 2016;44:2049–2061.
- Clark A, Milbrandt TA, Hilt JZ, et al. Mechanical properties and dual drug delivery application of poly(lactic-co-glycolic acid) scaffolds fabricated with a poly(β-amino ester) porogen. Acta Biomater. 2014;10:2125–2132.
- Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011;3:1377–1397.
- Lohcharoenkal W, Wang L, Chen YC, et al. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int. 2014;2014:180549.
- Salatin S, Jelvehgari M, Maleki-Dizaj S, et al. A sight on protein-based nanoparticles as drug/gene delivery systems. Ther Deliv. 2015;6:1017–1029.
- Chen S, Hao X, Liang X, et al. Inorganic nanomaterials as carriers for drug delivery. J Biomed Nanotechnol. 2016;12:1–27.
- Pérez-Ortiz M, Zapata-Urzúa C, Acosta GA, et al. Gold nanoparticles as an efficient drug delivery system for GLP-1 peptides. Colloids Surf B Biointerfaces. 2017;158:25–32.
- Amendola V, Pilot R, Frasconi M, et al. Surface plasmon resonance in gold nanoparticles: a review. J Phys Condens Matter. 2017;29:203002.
- Kayyali MN, Brake L, Ramsey AJ, et al. A novel nano-approach for targeted inner ear imaging. J Nanomed Nanotechnol. 2017;8:456.
- Zakeri-Milani P, Mussa Farkhani S, Shirani A, et al. Cellular uptake and anti-tumor activity of gemcitabine conjugated with new amphiphilic cell penetrating peptides. Excli J. 2017;16:650–662.
- Mohammadi S, Zakeri-Milani P, Golkar N, et al. Synthesis and cellular characterization of various nano-assemblies of cell penetrating peptide–epirubicin–polyglutamate conjugates for the enhancement of antitumor activity. Artif Cells Nanomed Biotechnol. 2018;46:1572–1585.