?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
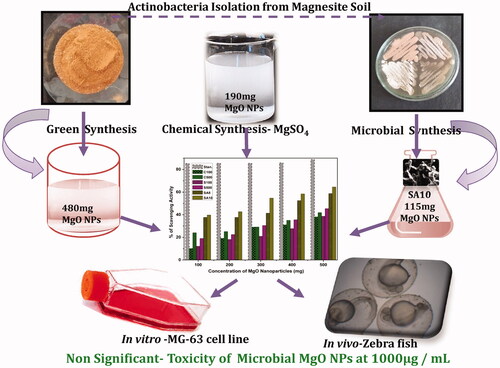
Owing to the hazards of chemical and physical syntheses of magnesium oxide nanoparticles (MgO NPs), an eco-friendly, high-yield, and promising biological method is highly desirable for biomedical applications. Hence, in this study, an extremophilic actinobacterial population (SA8 and SA10) from Salem magnesite mining soil was used as precursors for MgO NP synthesis. The prepared nanoparticles were subjected to X-ray diffraction study and showed face-centred cubic structure with an average particle size of 18–24 nm. Among all, high yield was obtained in SA10 actinobacteria-mediated synthesis of MgO NPs (480 mg/100 mL). In addition, the prepared MgO NPs (10 mg/well) showed 15–17 mm zone of inhibition against bacterial pathogens, especially bigger zones around SA10. The 64.5% antioxidant activity and nonsignificant toxicity of actinobacteria-synthesized MgO NPs in MG-63 cell lines at 100 µg/mL and nonsignificant in vivo toxicity in zebrafish at 0.1 mg/mL were remarkable. In addition, this is the first study to focus on MgO NP synthesis using extremophilic actinobacteria collected from Salem magnesite mining soil for high yield (115 mg/100 mL), reliable with potential antioxidant, and in vitro and in vivo compatibility. These results provided useful information for advanced research and mass production of NP for biomedical applications.
GRAPHICAL ABSTRACT
Introduction
Magnesium oxide nanoparticles (MgO NPs) are an interesting basic oxide that has potential surface reactivity, high concentration of edge/corner sites, less toxicity and biocompatibility, and can be prepared from economical precursors [Citation1]. Among various metal oxides, MgO is the most promising candidate because of its unique and excellent optical, electrical, thermal, mechanical and chemical properties and because of its high ionic character [Citation2]. MgO is used in many applications such as heat-resistant, high-temperature insulating materials in fuel–oil additives [Citation3], superconducting materials [Citation4], catalyst, synthesis of refractory ceramics [Citation5,Citation6], toxic waste remediation, and an adsorbent for removing dyes and removing heavy metals from wastewater [Citation6]. MgO NPs are effective antimicrobial agents showing bactericidal activity against both Gram-positive and -negative bacteria, spores and viruses [Citation7]. As an electrochemical biosensor [Citation8,Citation9], with the ability of membrane disruption and reactive oxygen species (ROS) generation, it also inhibits the essential enzymes of bacteria [Citation9–11]. Especially, MgO-based nanobiomaterials mimic the architecture and triggering of soluble ions as in natural bone tissue matrices. A number of studies have demonstrated that the fourth most abundant divalent cations of our body, i.e. Mg++ ion, has an important role in controlling bone apatite structure and density by cell–extracellular matrix interactions. In addition, Mg2+ ions are required to preserve Ca2+ ion and calcitonin hormone in the blood to support bone remodelling and skeletal development [Citation10]. Hence, the present study aimed to prepare high-yield, nontoxic, superior antioxidant, and eco-friendly MgO NPs with enhanced biocompatible potential (due to the existence of bio compound) from magnesite-associated extremophilic actinobacteria for contemporary bone regeneration and other biomedical applications.
Salem magnesite reserves are unique for their cryptocrystalline structure, which is best suited for manufacturing refractory bricks. Magnesite of the Salem region is relatively low in calcium oxide and high in silica content [Citation12]. The mineral composition of magnesite mine contains magnesite-rich ultra-basic and low concentration of silicon oxide (SiO2), 2.38%; aluminium oxide (Al2O3), 0.10%; ferric oxide (Fe2O3), 0.08%; ferrous oxide (FeO), 0.06%;calcium oxide (CaO), 0.42%; and MgO, 46.35%. Magnesite deposits cover an area of ∼5000 km; the pH of the soil is alkaline; and the temperature is high and dry in plains. In addition, the microorganism existing in the magnesite soil has special abilities to survive in the extremophilic environment. Among them, actinobacteria reside in a unique state due to their high G + C content and their ability to produce a variety of bioactive secondary metabolites [Citation12–14]. The extremophilic actinobacteria show several adaptive strategies such as antibiosis, switching between different metabolic modes (i.e. autotrophy, heterotrophy and saprobes), and production of specific enzymes to survive under unfavourable environmental conditions. Actinobacteria are an ecologically important group and widely found in terrestrial and aquatic ecosystems that play an important role in several biological processes such as biogeochemical cycles, bioremediation [Citation14,Citation15], bioweathering and plant growth promotion [Citation16]. Many researchers have synthesized silver and gold NPs using actinobacteria [Citation14–17].
Currently, nanoparticles are produced by chemical or physical approaches that produce toxic wastes, are capital intensive, and are inefficient with regard to bioactivity and energy use. Besides the applicative aspects of nanoparticles, another domain that is of great concern is the risk assessment of these nanoparticles to avoid unwanted hazards. Many of the researchers have shown the toxicity of metal oxide NPs in humans [Citation18,Citation19]; therefore, we have used the biological method to synthesize the NPs, which provides stability to nanoparticles and is cost-effective, eco-friendly, as well as non-toxic [Citation14,Citation18,Citation19]. Both unicellular and multicellular organisms such as bacteria, fungi, and yeast produce inorganic materials either intra-cellularly or extra-cellularly [Citation14,Citation20–25]. Biological synthesis is not only a good way to fabricate benign nanostructure materials, but also to reduce the use or generation of hazardous substances to human health and the environment. Hayat et al. [Citation26] conducted cytotoxicity experiments using the neutral red (NR) assay and revealed that MgO NPs were non-toxic to HeLa cells at 15–120 μg/mL concentration. In addition, the in vitro study confirmed that MgO NPs can be used effectively and safely as an anti-biofilm agent to inhibit multidrug-resistant bacterial biofilms. However, the cytotoxic effect, DNA damage, cell death, and oxidative damage effects of MgO NPs should raise concern about the safety associated with their applications in consumer products. Hence, this study aimed to prepare high-yield, nontoxic, superior antioxidant, elevated biocompatible potentials (due to the existence of bio-compound), and eco-friendly MgO NPs from magnesite-associated extremophilic actinobacteria for contemporary bone regeneration and other biomedical applications.
Materials and methods
Chemical synthesis of MgO NPs
MgO NPs were prepared from magnesium nitrate Mg (NO3)⋅6H2O and MgSO4 using sol–gel method [Citation27]. First, 500 ml NaOH (2.0g) was added to 500 ml magnesium source (12.8g) drop-by-drop under vigorous stirring. After 3 h of stirring, the solution was kept on a table at rest for 3 h to allow deposition of the precipitation. This precipitation was washed several times using distilled water until the pH reached 7. The final product was kept in an oven at 100 °C for 6 h (C100). The product made a very fine powder by using a mortar and pestle. The fine powder of MgO was sintered at 500 °C for 1 h for removing impurities present in it and the final product was termed as C500.
Synthesis of MgO NPs from magnesite soil
The magnesite soil was collected from the magnesite mining area located in Chalk hills, Salem, Tamil Nadu, India, at the depth of ∼20 cm. The samples were transported into a sterile polythene bag to the laboratory. Thereafter, the samples were crushed with the help the sterile mortar and pestle and then sieved through a 2-mm pore-size mesh to remove large debris. The sieved soils were stored in vacuum-free desiccators for further use.
For the synthesis of MgO NPs, 50 g magnesite soil sample was roasted for 1 h at 100 °C. After that, 50 ml hydrochloric acid was added drop-by-drop and stirred for 1 h at 1500 rpm at 50 °C. After cooling of the extract, 50 ml double-distilled water was added and again stirred for 2 h. The whole set-up was placed on a stand for a few hours until a clear solution was obtained, and then the supernatant was collected. Along with supernatant, 10 ml of 1 N NaOH was added and stirred for 0.5 h to obtain the precipitates. Then, it was washed with double-distilled water several times and the dried precipitates were grained with the help of the sterile mortar and pestle (S100). The fine powder was placed in a muffle furnace at 500 °C to remove the impurities as well as hydroxide groups present [Citation20,Citation28].
Isolation of actinobacteria from magnesite mining soil
For isolation of actinobacteria, 1 g magnesite soil was serially diluted from 10−2 to 10−6. Then 0.1 ml sample from the serial dilution was inoculated in starch casein medium using L-rod (spread plate technique) and incubated at room temperature (28 °C) for 7 days. After the incubation period, morphologically different colonies were isolated and sub-cultured into International Streptomyces Project-2 (ISP2) medium and the plates were stored at 4 °C in a refrigerator for further studies [Citation14].
Identifications of actinobacteria
For the primary morphological analysis, one isolated colony was taken from each agar plate, smeared and heat-fixed on a clean glass slide, and then examined under a microscope [Citation14,Citation20,Citation29]. Therefore, field-emission scanning electron microscopy with an accelerating voltage of 25 kV was used to study the surface morphology of the isolated actinobacteria at a magnification of 1000× and a resolution of 200 nm.
Microbial synthesis of MgO NPs
The ISP2 broth was prepared and sterilized at 121 °C for 15lbs. After sterilization, the broth was cooled and a loopful of isolated actinobacterial (SSA2, SSA8, SSA9, and SSA10) culture was inoculated. Then, the broth was placed on a shaking incubator at 28 °C for 7 days. After the incubation period, biomasses were observed and centrifuged at 5000 rpm at 4 °C for 20 min to separate the supernatant [Citation14,Citation19,Citation20,Citation23,Citation30]. The supernatant was filtered through Whatmann No. 1 filter paper and stored for further synthesis.
Thereafter, 100 ml sterile distilled water was added to 0.1 M of each chemical (magnesium nitrate, magnesium chloride, and magnesium sulphate) separately and 50 ml SSA2 actinobacterial supernatant was drop-wise added into each flask and kept under a cooling shaker for 2–3 days. After the incubation period was over, the flasks were kept at rest for a few hours to form a white precipitate at the bottom of the conical flasks. The supernatant was carefully removed from the container and the particles settling in the container were air-dried. After drying, the particles were grained using the sterile mortar and pestle. Finally, the products were kept under the muffle furnace at 500 °C to remove the hydroxide groups as well as impurities from those particles and the products were termed as SA2. Similarly, all the remaining isolated actinobacteria were subjected for MgO NP synthesis like the earlier method and termed as SA8, SA9, and SA10.
Characterization methods
The prepared MgO NPs were subjected to a double-beam UV–visible spectrophotometer (Shimadzu, Japan) at a wavelength range of 200–800 nm. The crystalline phase formation and size of MgO NPs were analyzed using an X-ray diffractometer (X’Pert Pro; PANalytical, Almelo, the Netherlands) with CuKα radiation (k = 0.154056 nm) at 40 kV and 30 mA. The data were recorded over a 2 h period from 200 to 800 nm in the step scan mode with a step size of 0.02 nm at room temperature. The X-ray diffraction (XRD) spectra of the synthesized nanoparticles were obtained using an XRD diffractometer (Bruker AXS D8 Advance, Bruker, MA, USA) for 2θ values ranging from 10° to 80° using CuKα radiation at λ = 1.5406 Å.
Antibacterial activity
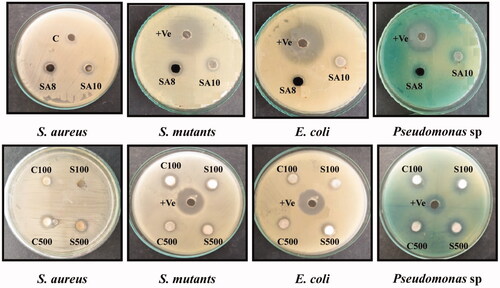
Antibacterial activity of the prepared MgO NPs was examined by agar well-diffusion method [Citation14,Citation20,Citation31] using Muller–Hinton agar (MHA). Both Gram-positive and -negative bacterial pathogens such as Staphylococcus aureus, Streptococcus mutants, Escherichia coli and Pseudomonas sp. were obtained from Actinobacterial Research Laboratory (Microbiology Department, Periyar University, Salem, Tamil Nadu, India) and used for the test. The cultures were sub-cultured into the nutrient broth and incubated at 37 °C for 6–10 and 24 h. The freshly grown bacterial culture was uniformly swabbed onto the MHA plate and used for studying antimicrobial activity. Thereafter, 50 mg/mL MgO NPs was loaded onto the 5 mm well size of MHA plates and incubated for 48 h and observed for the zone of inhibition. The antibacterial assessment was performed in triplicate, and the average zone of inhibition was measured and compared with that of control.
Antioxidant activity
Antioxidant activity of the sample was determined by the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical, which was reflected by the reduction of the absorbance of the DPPH methanol solution of MgO NPs. The sample containing hydrogen as a donor (A-H) when combined with DPPH gives strong absorbance at 517 nm where the colour changes from deep violet to pale yellow due to the acceptance of electron from MgO NPs and the transformation of free radical to non-radical form [Citation14,Citation32]. First, 0.2 mM DPPH reagent was prepared before 24 h to protect it from sunlight. Methanol acts as a blank and ascorbic acid acts as the standard value. Then, 3 ml methanol was added into each of the test tubes and different concentrations of the test samples (100, 200, 300, 400, and 500 µl) were added and mixed thoroughly. To this 0.5 ml DPPH was added under the dark condition and allowed to incubate for 30 min. The OD value was observed at 517 nm and a graph was plotted.(1)
(1)
Neutral red uptake assay in MG-63 cell line
The human bone-derived, adherent osteosarcoma (MG-63) cell lines were purchased from National Centre for Cell Science (Pune, Maharashtra, India) and used for NR uptake assay of the prepared nanoparticles [Citation32]. The cells were cultured in a minimum essential medium with essential supplementations. After the confluent growth, the cells were passaged and allowed to attach on 24-well plates for 48 h. Before the treatment, the medium was replaced with a fresh medium and subsequently filtered and ultraviolet (UV) light-sterilized nanoparticles were added to the 24-well plates and incubated at 37 ± 1 °C for 24 h. Then, the treatment medium was removed and NR medium was added in the entire well and incubated for 3 h. After incubation, a desorbing medium was added in the wells and read at 540 nm in a photometer [Citation14,Citation20,Citation33]. The test was repeated in triplicates and the cell viability was calculated by following formula:(2)
(2)
The results of all the analyses were represented as mean and SD using Graph Pad Prism statistical software, version 3.02. The level of significance was evaluated at p < .05 (*) and p < .01 (**).
In vivo toxicity in Danio rerio embryos:
The wild-type adult male and female zebrafish (Danio rerio) were allowed to breed during the full moon and new moon sessions in a net-separated laboratory tank with defined temperature (27 ± 2 °C) and aeration for obtaining maximum number of embryos. Sixty-three (7 × 3 = 21 × 3 = 63) healthy, eight-cell staged D. rerio embryos were collected and three were placed in each well of 24-well plates with Hanks’ solution. Then, the embryos were treated with two concentrations (1 and 0.1 mg mL−1) of prepared C500, S500 and SA10 MgO NPs, and then incubated for a specific incubation period (12, 36 and 60 h) to explore their effect on embryonic development and changes, whereas untreated zebrafish were maintained as control [Citation32–36]. After the defined incubation period, the embryos were screened through the fluorescent microscopy (Euromex-Holland, B-plus, Netherlands) for observing growth retardation and malformation [Citation36]. Similarly, the mortality and hatching rates were also monitored and recorded as a mean value from the experiments performed in triplicates.
Results
Magnesium oxide NPs from chemical and magnesite soil
In chemical synthesis, white colour precipitation was formed at the bottom of the beaker, which indicates the synthesis of MgOH NPs (C100) [Citation27]. Then, it was sintered at 500 °C to remove the hydroxide group and obtain MgO NPs. The obtained NPs were termed as C500. Similarly, MgO NPs were successfully obtained from the magnesite mining soil by hydrochloric acid leaching treatment. Whenever NaOH was added to this mixture, Mg(OH (S100) and sodium chloride were formed. Finally, the precipitated Mg(OH)was sintered at 500 °C to remove the hydroxide group and impurities present in that sample and to obtain MgO NPs (S500).
Isolation and screening of actinobacteria from magnesite mining soil
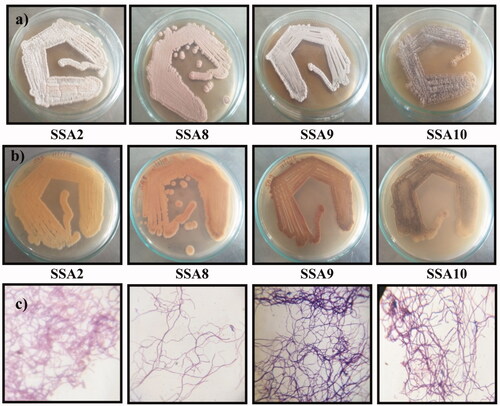
Magnesite-soil-associated actinobacteria were isolated by starch casein agar plates after 5–7 days of incubation (). The total number of the isolated actinobacterial colony was observed and noted as 10−3 (19) and 10−4 (3). The isolated actinobacterial colonies were named as SSA1–SSA22 () and streaked on ISP2 medium and incubated at 28 °C for 7 days. After the incubation period, the organisms were grown well and showed similar morphological characters on ISP2 medium, confirming the four actinobacteria colonies, namely SSA2, SSA8, SSA9 and SSA10 (;). The reverse side pigments are shown in . The microscopic and electron microscopic views were used to find the substrate mycelium and true branching of filamentous structures. The filamentous structure confirmed that the isolated microorganisms were actinobacteria, which is in accordance with many previous studies [Citation10,Citation19,Citation23,Citation29].
Table 1. Culture characteristics of isolates.
Microbial reduction of magnesium oxide
The isolated SSA2, SSA8, SSA9 and SSA10 were inoculated into ISP2 broth medium and biomass and extracellular contents were obtained. The MgO NPs were produced by using the above mentioned microbial supernatant under defined condition. The visual inspection of the flasks for change in white precipitation at the bottom confirmed the reduction of MgO (). There was no microbial synthesis on SSA2 and SSA9 cultures, whereas SSA8 strain showed the reduction in MgCl2 and MgSO4 chemicals, and there was no reduction in MgNO3. Similarly, SSA10 culture showed an excellent reduction in all the chemicals used for this test. After that, all the precipitates were filtered and washed with double-distilled water several times and then dried [Citation18,Citation19,Citation27]. The dried precipitates were sintered at 500 °C for 1 h and termed as SA8 and SA10. The obtained results showed that SSA8 and SSA10 actinobacteria have an excellent extracellular enzyme-reducing capacity.
Figure 3. Microbial reductions of MgO NPs from extracellular actinobacteria (C: Control, S: Supernatant, T: Test).

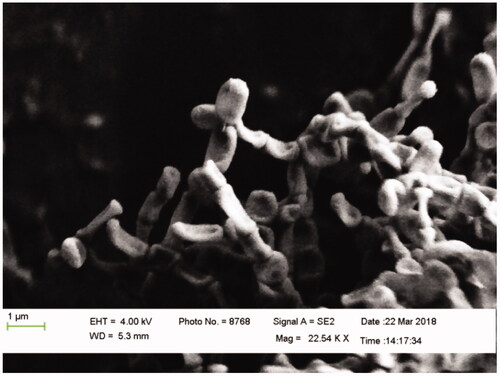
The comparison between the yields of chemical, soil and microbial synthesis of MgO NPs is shown in In microbial synthesis method, SSA2 and SSA9 showed no reduction of any chemical. However, the other two actinobacteria showed better yield than the previous methods [Citation11]. While using magnesite soil as a precursor, 480 mg/100 ml MgO NP yield was obtained, which is better than that obtained from chemical and microbial synthesis methods as well as that reported in previous studies. The quantity of chemically synthesized MgO NPs for MgSO4 and MgNO3 was 190 and 144 mg/100 ml respectively. Four actinobacterial colonies were isolated from magnesite soil and two (SSA8 and SSA10) were found highly potential in the synthesis of MgO NPs. Three chemical sources were used to optimize the yield and productivity. For SSA8, 66 mg/100 ml yield was obtained in MgSO4, 54 mg/100 ml in MgCl2, and no yield was obtained in MgNO3. Similarly, for SSA10, 115 mg/100 ml yield was obtained in MgSO4, 105 mg/100 ml in MgCl2 and 92 mg/100 ml in MgNO3. According to the obtained results, SSA10 in MgSO4 showed better yield than SSA8. Then the SSA10 was further analyzed by FE-SEM and the obtained image confirmed the morphology of Streptomyces Sp. ().
Table 2. MgO NPs: Yield comparison b/w chemical, green and microbial synthesis.
Characterization studies
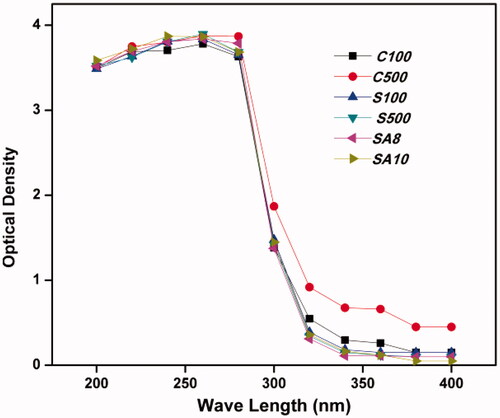
Results obtained from the UV–visible spectrophotometer suggested a sharp peak at 260 nm, which was obtained in all the prepared MgO NPs (), confirming the formation of MgO. These results are in accordance with previous reports [Citation11,Citation28]. Hence, all the prepared samples were selected for further studies.
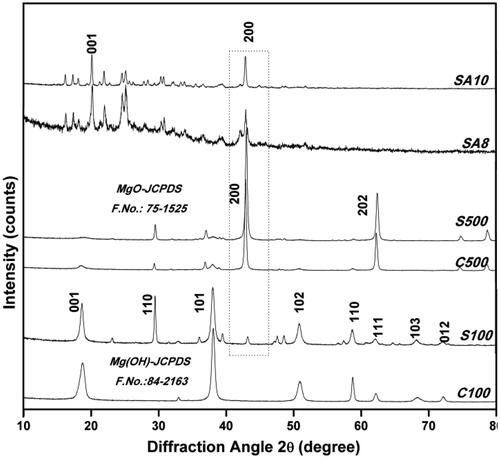
shows the XRD pattern of MgO NPs synthesized from different methods. The sharp peaks are clearly observed in the sintered MgO (C500 and S500), which confirms the existence of the crystalline phase of face-centred cubic (FCC) structure as well as high purity. The obtained diffraction peaks are assigned to 39.60(111), 42.90(200), 62.86(220), 74.62(311) and 78.62(222) (JCPDS file numbers 87–0653 and 75–1525) [Citation18,Citation19,Citation28]. Similarly, the XRD patterns of C100 and S100 clearly suggest the presence of a hexagonal structure of Mg (OH) NPs determined with diffraction peaks at 2θ values 18.54(001), 32.80(110), 37.95(101), 50.93(102), 58.61(110), 62.02(111), 68.03(103), 72.04(012) (JCPDS file numbers 84–2163 and 002–1207) [Citation11,Citation28,Citation29]. The XRD patterns of SA8 and SA10 show a high-intensity peak at 42.80(200) along with some other peaks. This result confirms that the actinobacteria-mediated MgO NPs show FCC MgO with impurities than those obtained from other methods. In accordance with previous reports and UV results obtained, the prepared samples were confirmed to be MgO NPs. The average crystallite size of the obtained MgO NPs was calculated from the broadening of the corresponding sharp peaks using Scherrer formula [Citation20] and was found to be 22 nm (C100), 35 nm (C500), 18 nm (S100), 28 nm (S500), 23 nm (SA8) and 24 nm (SA10) corresponding to the higher intense peak positions. It shows a smaller NP size in the presence of Mg (OH) rather than that of MgO. Compared to the chemical and soil syntheses, microbe-mediated MgO NP synthesis showed a very smaller crystallite size, which may be due to the synthesis mechanism and involvement of a potential bioactive compound [Citation19,Citation28].
Antimicrobial and antioxidant activities
Microbial and chemically synthesized MgO NPs showed better microbial activity against S. aureus, S. mutants, E. coli, and Pseudomonas sp. at 50 mg/mL (; ) [Citation34,Citation35]. The prepared MgO NPs confirmed that the 50 mg/mL (10 mg/well) concentration shows good antibacterial activity against clinical pathogens, which is lesser (i.e. 100 mg/mL) compared to that in the Anantharaman et al. [Citation22] study. Moreover, the chemical source, Salem magnesite mining soil source, and Sterptomyces sp.-mediated synthesis of MgO NPs show similar zones of inhibition against all the clinical pathogens. However, the slight increase in the zone size is observed in SA10, especially against S. aureus. This could be due to the influence of microbial compound present in magnesite-soil-isolated Sterptomyces sp. and nature of the cell wall [Citation14,Citation17–24].
Table 3. Agar well diffusion method of magnesium oxide.
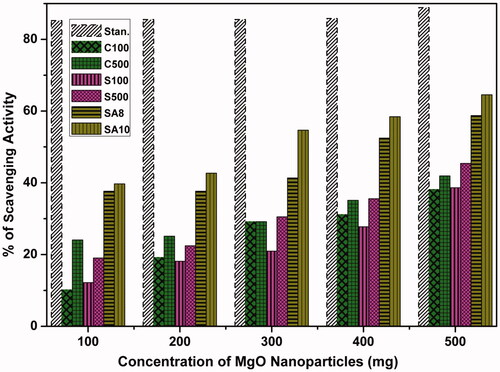
From , it can be observed that MgO NPs sintered at 500 °C show higher activity than the as-prepared samples. Similarly, soil-synthesized MgO NPs (S100 and S500) also show better scavenging activity than chemically synthesized MgO NPs. The antioxidant activity of all MgO NPs was determined by DPPH radical. Sintered, i.e. crystalline (C500) MgO NPs, showed better scavenging activity than amorphous Mg(OH) (C100). This is in accordance with MgO NPs from magnesite soil and shows better activity than chemically synthesized MgO NPs (). The microbially synthesized MgO NPs show the highest inhibition (SA8, 56%; SA10, 64.5%) of free radicals rather than soli-synthesized and chemically synthesized ones. Especially, SSA10 actinobacteria-mediated MgO NPs (SA10, 64.5%) showed maximum scavenging activity.
In vitro cell line study
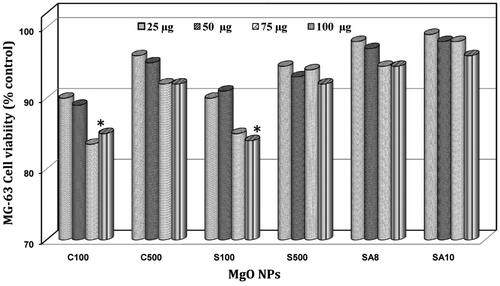
The toxicity level of the prepared and optimized MgO NPs was studied using NR uptake assay in MG-63 cell line and a nonsignificant toxicity level was observed in all the samples. However, shows the toxic nature of amorphous MgO NPs (C100 and S100) at higher concentration (100 µg/mL) and dose-dependent toxicity in all the MgO NPs. In particular, the actinobacteria-mediated MgO NPs (SA8 and SA10) showed no cellular-level damages at even the higher (100 μg/mL) concentration whereas chemically- and soil-synthesized MgO NPs showed significant toxicity based on dose and structure of the NPs, which is in agreement with previous reports [Citation10,Citation25,Citation36,Citation37].
In vivo toxicity study
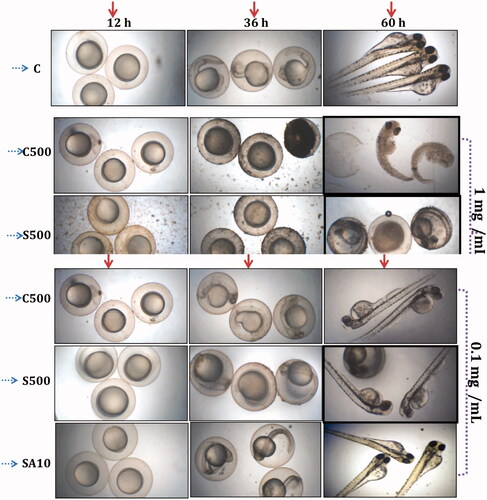
On the basis of in vitro studies, it was found that C500, S500 and SA10 produced embryonic-level toxicity in zebrafish according to dosage level. The percentage of mortality, hatching rate and deformation in organogenesis are shown in and . The figure shows that 1 mg/ml concentration leads to significant toxicity whereas no significant toxicity was observed at 0.1 mg/mL. In addition, the toxicity of the nanoparticles based on concentration and time is shown the linear graph (). Particularly, the chemically synthesized nanoparticles (C500, S500 and SA10) show a gradual increase in toxicity at both concentrations. C500 shows significant malformation in early developmental stages and leads to destruction and mortality at higher concentration. Microbially synthesized MgO NPs (SA10) shows non-significant toxicity in zebrafish at low and high concentration.
Table 4. Mortality and hatching rate of prepared MgO nanoparticles in zebrafish (D. rerio) embryos after 60 h of incubation.
Discussion
The requirement and usage of NPs, in day-to-day life, is dramatically increasing because of their small size and higher surface area. They can interact with biomolecules present on both the surface and inner side of the body cells [Citation25,Citation37]. Consequently, the production of nanoparticles is also increasing without concern about their toxicity and side effects. Nanoparticles produced by chemical or physical approaches lead to an increasing amount of toxic wastes; these methods are capital intensive and inefficient with respect to material and energy use [Citation25]. Ghobadian et al. [Citation36] and Mahmoud et al. [Citation25] reported significant toxic effects of chemically synthesized MgO NPs at in vitro and in vivo levels. Hence, we focused on the biosynthesis of MgO NPs without side effects and toxicity.
Many reports are available on the use of green (plants) [Citation18–22] and microbial (bacteria, mushroom and other fungi) [Citation23–25] syntheses of MgO NPs. However, high yield and potential biological activity of MgO NPs are not reported. The mineral composition of the magnesite mine in Salem contains 46.35% MgO and has extremophilic microbial population; actinobacteria should have special enzymes and bioactive compounds in them to survive [Citation14,Citation20,Citation30]. Hence, this magnesite soil was taken as a source for MgO NP synthesis.
Among the synthesized MgO NPs, SSA10 showed high yield in MgSO4 (115 mg), which is lesser than that obtained from chemical synthesis (190 mg) and magnesite soil-assisted synthesis (480 mg); however, the microbially obtained and green-synthesized MgO NP yield was higher than that reported previously [Citation11,Citation21–28]. Ibrahem et al. [Citation35] reported the highest MgO NP yield at 56 mg/100 ml while using Aspergillus niger. Similarly, Dobrucka [Citation21] and Anatharaman et al. [Citation22] synthesized 10 nm MgO NPs from Artemisia abrotanum and 8.6 nm from Aloe vera and obtained a maximum of 65 mg yield and used them for various environmental applications.
Compared to the sintered NPs (C500 and S500), C100 and S100 showed potential activity against clinical pathogens, which is in accordance with that reported by Kavitha et al. [Citation32,Citation33] and confirms that amorphous particles show higher activity than the crystalline because of the random packing of the lattice. In addition, the difference in microbial activity between the Gram-positive and -negative bacteria is due to the nature of the cell wall [Citation32,Citation33]. MgO NPs synthesized using A.vera extract completely inhibited the growth of S. aureus, Pseudomonas sp. B. subtilis and E. coli at a higher concentration of 100 mg/mL. However, at a lower concentration, they were not able to inhibit them [Citation22]. The result of our study confirmed that 50 mg/mL (10 mg/well) concentration of chemically and biologically synthesized MgO NPs shows a clear zone against bacterial pathogens.
XRD and UV results also confirmed the formation of FCC MgO NPs in all the prepared samples, which is in accordance with the results of Sushma et al. [Citation29]. The average crystallite size of MgO NPs suggests that the presence of Mg (OH) leads to smaller particle size. Compared to chemical and soil, microbial-mediated synthesis of sintered MgO NPs showed a smaller crystallite size (23–24 nm). This may be because of the extracellular synthesis mechanism [Citation19], which is similar to that reported by Jeevanantham et al. [Citation18], who synthesized 18–80 nm MgO NPs from Amaranthus sp. and Eucalyptus globules. Similar to plant sources, microorganisms also play an excellent role in nanoparticle synthesis. Mohanasrinivasan et al. [Citation19] synthesized 22 nm size of MgO NPs from Lactobacillus sp., Raliya and Tarafdar [Citation23] synthesized 6.4 nm size of MgO NPs from A.terreus, A.tubingensis, A. niger, R. bataticolaand A. oryzae. And finally, Jhansi et al. [Citation24] reported the synthesis of 15 nm size of MgO NPs from button mushroom. The obtained result confirms that with the increase in temperature, the average particle size increases. Microbial synthesis yields fine particles due to the presence of active metabolites in the organism to form fine powder. However, chemically synthesized MgO NPs are effectively free from impurities. But biologically synthesized MgO NPs have impurities. Depending upon the biological activity of obtained MgO NPs, further optimization and purification can be undertaken.
In this study, SSA10 actinobacteria-mediated MgO NPs (SA10) showed maximum scavenging activity (64.5%). This maximum inhibition is due to the presence of bioactive components in SSA10 actinobacteria. Sushma et al. [Citation29] and Dobrucka et al. [Citation21] reported similar antioxidant activity for C. ternatea leaf extract-mediated synthesis of MgO NPs. The microbially synthesized MgO NPs showed the highest inhibition of free radicals than those synthesized using soil and chemical methods.
The toxicity level of the prepared MgO NPs in MG-63 cell line at a maximum of 100 μg/mL administration is similar to that reported in many previous cell line studies [Citation10,Citation25,Citation36,Citation37]. NR uptake assay of MgO NPs in HT-29 cell line showed a time- and concentration-dependent response with loss of cell viability at 20–40 mg/ml gradually and demonstrated cytotoxic and inflammatory effects in HT-29 cells, which may be mediated through ROS generation and oxidative stress [Citation36]. Mahmoud et al. [Citation25] reported that at 323.39 mg/mL, MgO NPs caused 50% inhibition in cell viability in HepG2 and A549 cell lines and led to genotoxic damage ranked as HepG2 < A549 < Caco-2 < NRK-52E. Hayat et al. [Citation37] and Akhtara et al. [Citation10] reported 40–50 nm MgO NPs in human lung epithelial (A549) cell and HeLa cells induced concentration-dependent cytotoxicity when measured by MTT and NR uptake assay. The authors also suggested that this cytotoxicity is primarily dependent on GSH depletion and ROS induction [Citation10,Citation36]. The result of our studies showing higher antioxidant activity in actinobacteria-mediated MgO NPs (SA8, 56%; SA10, 64.5%) agrees well with nonsignificant toxicity in observed in MG-63 cell line and the above statement.
Ghobadian et al. [Citation36] reported that 0.1 mg/mL with 20 nm MgO NPs led to 10–20% mortality with malformation in organogenesis in zebrafish. In this study, chemically synthesized MgO NPs (C500) with 22 nm size showed relevant toxicity profile (10% mortality with malformation of organogenesis) which is in accordance with the Ghobadian et al. study [Citation36]. But in the case of S500 and SA10, the rates of mortality and malformation of organogenesis were reduced to 5–10% and 0%, respectively. However, S500 at 0.1 mg/mL showed delayed hatching. An interesting result of this study is the survival of embryo at 1 mg/mL in microbially synthesized MgO NPs with only 10% of mortality and no malformation ( and ), which is unique in MgO NP toxicity profile and which will be beneficial for biomedical and environmental applications. Moreover, further purification and identification of the compound responsible for the MgO synthesis could provide essential information for application-oriented studies.
Conclusion
MgO NPs were prepared by simple sol–gel (chemical), magnesite soil (SA, green), and extracellular components of extremophilic actinobacteria extracts of Salem magnesite soil (SSA8 and SSA10: microbial). The characterization studies such as UV and XRD showed the obtained particles had FCC structure with slight impurities with an average particle size of 22 nm (C100), 35 nm (C500), 18 nm (S100), 28 nm (S500), 23 nm (SA8) and 24 nm (SA10). The extracellular synthesis of MgO NPs from extremophilic actinobacteria extracts from Salem magnesite soil has been reported for the first time and provided high yield (480 mg/100 ml) compared to all other methods. The antibacterial activity showed that 50 mg/mL (10 mg/well) prepared MgO NPs demonstrate15–17 mm zone of inhibition against bacterial pathogens. The antioxidant activity confirms that SA10 has a maximum percentage of scavenging activity (64.5%) than SA8, S500, C500, S100 and C100 respectively. In addition, the non-toxic nature of the synthesized MgO NPs in MG-63 and survival of rate in zebrafish embryos shows tremendous potential of extremophilic actinobacteria in MgO NPs synthesis. Finally, this study provides a non-toxic, high yield and elevated antioxidant property containing potential MgO NPs for environmental, bone and dental regeneration applications.
Acknowledgements
Authors acknowledge DST-FIST [S.No. 69, 31/5/2016], New Delhi for the instrumentation facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Reference
- Sathyamoorthy R, Mageshwari K, Mali S. Effect of organic capping agent on the photocatalytic activity of MgO nanoflakes obtained by thermal decomposition route. Ceram Int. 2013;39:323–330.
- Silva RTD, Mantilaka MMMGPG, Ratnayake SP, et al. Nano-MgO reinforced chitosan nanocomposites for high performance packaging applications with improved mechanical, thermal and barrier properties. Carbohydr Polym. 2017;157:739–747.
- Ercan I, Kaygili O, Ates T, et al. The effects of urea content on the structural, thermal and morphological properties of MgO nanopowders. Ceram Int. 2018;44:14523–14527.
- Sabah C, Mulla B, Altan H, et al. Thermally and optically tunable sub-terahertz superconducting fishnet metamaterial. Physica C: Supercond Appl. 2018;544:46–53.
- Ma B, Yu N, Han Y, et al. Effect of magnesium oxide nanoparticles on microbial diversity and removal performance of sequencing batch reactor. J Environ Manage. 2018;222:475–482.
- Gajengi L, Sasaki T, Bhanage BM. Mechanistic aspects of formation of MgO nanoparticles under microwave irradiation and its catalytic application. Adv Powder Technol. 2017;28:1185–1192.
- Yadav LSR, Lingaraju K, Manjunath K, et al. Synergistic effect of MgO nanoparticles for electrochemical sensing, photocatalytic-dye degradation and antibacterial activity. Mat Res Exp. 2017;4:1–12.
- Rashid1 FL, Hadi A, Al-Garah NH, et al. Novel phase change materials, MgO nanoparticles, and water based nanofluids for thermal energy storage and biomedical applications. Int Pharmaceu Phytopharmacol Res. 2018;8:46–56.
- Das B, Moumita S, Ghosh S, et al. Biosynthesis of magnesium oxide (MgO) nanoflakes by using leaf extract of Bauhinia purpurea and evaluation of its antibacterial property against Staphylococcus aureus. Mater Sci Eng C Mater Biol Appl. 2018;91:436–444.
- Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int. 2014;25:423–439.
- Alqahtani S, Alomar SY. Induction of apoptosis and cytokine markers in colon cancer cells by magnesium oxide (MgO) nanoparticles. Toxicol Environ Chem. 2016;99(2):302–314.
- Satyanarayanan M, Eswaramoorthi S, Subramanian S, et al. Factor analysis of rock, soil and water geochemical data from Salem magnesite mines and surrounding area, Salem, southern India. Appl Water Sci. 2017;7:2607–2616.
- Bull AT, Stach JM, Ward AC, et al. Marine actinobacteria perspectives, challenges, future directions. Antonie Van Leeuwenhoek. 2005;87:65–79.
- Shanmugasundaram T, Balagurunathan R. Biomedically active zinc oxide nanoparticles synthesized by using extremophilic actinobacterium, Streptomyces sp. (MA30) and its characterization. Artif Cells, Nanomed Biotechnol. 2016;45(8):1–9.
- Chen M, Xu P, Zeng G, et al. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications. Biotechnol Adv. 2015;33:745–755.
- Palaniyandi SA, Yang SH, Zhang L, et al. Effects of actinobacteria on plant disease suppression and growth promotion. Appl Microbiol Biotechnol. 2013;97:9621–9636.
- Otari SV, Patil RM, Ghosh SJ, et al. Intracellular synthesis of silver nanoparticle by actinobacteria and its antimicrobial activity. Spectrochimica Acta A. 2015;136:1175–1180.
- Jeevanandam J, Chan YS, Danquah MK. Biosynthesis and characterization of MgO nanoparticles from plant extracts via induced molecular nucleation. New J Chem. 2017;41:2800–2814.
- Mohanasrinivasan V, Devi CS, Mehra A. Biosynthesis of MgO nanoparticles using Lactobacillus Sp. and its Activity Against Human Leukemia Cell Lines HL-60. Bionanosci. 2018;8:249–253.
- Kavitha K, Priya G, Banu Priya G, et al. bioactive compound production and microbial reduction of nanographene oxide using mushroom associated actinobacteria. Ind J Appl Microbiol. 2017;20:1–10.
- Dobrucka R. Synthesis of MgO nanoparticles using Artemisia abrotanum herba extract and their antioxidant and photocatalytic properties. Iran J Sci Technol Trans Sci. 2018;42:547–555.
- Anantharaman A, Sathyabhama S, George M. Green synthesis of magnesium oxide nanoparticles using Aloe Vera and its applications. Int J Scientific Res Dev. 2016;4:109–111.
- Raliya R, Tarafdar JC. Biosynthesis and characterization of zinc, magnesium and titanium nanoparticles: an eco-friendly approach. Int Nano Lett. 2014;4:93.
- Jhansi K, Jayarambabu N, Reddy KP. Biosynthesis of MgO nanoparticles using mushroom extract: effect on peanut (Arachis hypogaea L.) seed germination. 3 Biotech. 2017;7:263.
- Mahmoud A, Ezgi Ö, Merve A, et al. In vitro toxicological assessment of magnesium oxide nanoparticle exposure in several mammalian cell types. Int J Toxicol. 2016;35:429–437.
- Hayat S, Muzammil S, Rasool MH, et al. In vitro antibiofilm and antiadhesion effects of magnesium oxide nanoparticles against antibiotic resistant bacteria. Microbiol Immunol. 2018;62:211–220.
- Tamilselvi P, Yelilarasi A, Hema M, et al. Synthesis of hierarchical structured MgO by sol–gel method. Nano Bull. 2013;2(1):130106.
- Sandengen K, Josang LO, Kaasa B. Simple method for synthesis of magnesite (MgCO3). Ind Eng Chem Res. 2008;47:1002–1004.
- Sushma NJ, Prathyusha D, Swathi G, et al. Facile approach to synthesize magnesium oxide nanoparticles by using Clitoria ternatea—characterization and in vitro antioxidant studies. Appl Nanosci. 2016;6(3):437–444.
- Balagurunathan R, Radhakrishnan M, Somasundaram SM. L-glutaminase producing actinomycetes from marine sediments- selective isolation, semi quantitative assay and characterization of potential strain. Australian J Basic Appl Sci. 2010;698–705.
- Shanmugasundaram T, Radhakrishnan M, Gopikrishnan V, et al. Biocompatible silver, gold and silver/gold alloy nanoparticles 1 for enhanced 2 cancer therapy: an in vitro and in vivo perspectives. Nanoscale 2017;9:16773–16790.
- Kavitha K, Sutha S, Prabhu M, et al . In situ synthesized novel biocompatible titania-chitosan nanocomposites with high surface area and antibacterial activity. Carbohydr Polym. 2013;93:731–739.
- Kavitha K, Chunyan W, Rajendran V, et al. In vitro and preliminary in vivo toxicity screening of high-surface-area TiO2-chondroitin 4-sulfate nanocomposites for bone regeneration application. Colloids Surf B. 2015;128:347–356.
- Kavitha K, Prabhu M, Selvam, et al. TiO2–graphene nanocomposites for enhanced osteocalcin induction. Mater Sci Eng C. 2014;48:252–262.
- Ibrahem J, Karkaz M, Amin T, et al. Antibacterial potential of magnesium oxide nanoparticles synthesized by Aspergillus niger. BJI. 2017;18:1–7.
- Ghobadian M, Nabiuni M, Parivar K, et al. Toxic effects of magnesium oxide nanoparticles on early developmental and larval stages of zebrafish (Danio rerio). Ecotoxicol Environ Saf. 2015;122:260–267.
- Kavitha K, Navaneethan D, Vinod A, et al. Scavenging free radicals and soaring osteoinduction by extra cellular matrix protein–based nanocomposites on degenerative bone treatments. Mater Sci Eng C. 2017;77:1189–1195.