Abstract
Background
Understanding the mechanism of chondrocytes degeneration could provide a new potential therapeutic idea for rheumatoid arthritis (RA) treatment. MicroRNA-27b-3p (miR-27b-3p) has been shown to regulate a variety of cell behaviors in various cell types. However, the role of miR-27b-3p in RA remains unknown.
Materials and methods
Expression of miR-27b-3p and HIPK2 in cartilage tissues and chondrocytes was characterized using qRT-PCR and Western blot. MiR-27b-3p was overexpressed or suppressed in chondrocytes to observe the potential role of miR-27b-3p.
Results
We found declined miR-27b-3p and elevated HIPK2 in RA tissues and cells using qRT-PCR. Dual-luciferase reporter assay validated HIPK2 is a direct target of miR-27b-3p, confirmed by Western blot results. Pearson correlation presented that there was a significantly negative correlation between miR-27b-3p and HIPK2 mRNA. Overexpression of miR-27b-3p significantly reduced the expression of pro-apoptotic protein c-caspase3 and increased the expression of anti-apoptotic Bcl-2; however, downregulation of miR-27b-3p has a significant effect of inducing apoptosis. Furthermore, overexpression of miR-27b-3p combined with recombinant HIPK2 protein showed the inhibitory effect of miR-27b-3p was abolished by HIPK2.
Conclusion
We found declined miR-27b-3p and elevated HIPK2 in RA tissues and cells. Further in vitro studies demonstrated that miR-27b might inhibit chondrocyte apoptosis and thus attenuate RA development by directly inhibiting HIPK2 expression.
Introduction
Rheumatoid arthritis (RA) is a systematic autoimmune disease affecting multiple joints, for which no effective treatment has been established [Citation1]. RA is common in the clinic, with an incidence of about 0.3–1.1% of the population all over the world [Citation2,Citation3]. RA patients have always suffered from severe chronic pain and physical disability of the joint, and finally reduction of the life quality [Citation4]. RA has been one of the major causes of disability and huge economic pressures. Therefore, it is necessary to quickly identify the mechanism of RA development and new potential targets for RA treatment.
Typical characteristic pathological changes of RA includes cartilage destruction, chronic hyperplasia synovitis and inflammatory immune cell infiltration [Citation5]. Recent studies have found that insufficient apoptosis of chondrocytes plays an important role in cartilage destruction and finally developing RA [Citation6,Citation7]. Chondrocytes could produce and maintain the cartilaginous matrix, thus the degeneration of chondrocytes plays an important role in RA progression and finally affects the cartilage regeneration [Citation8]. Therefore, understanding of the mechanism of chondrocytes degeneration may provide a new potential therapeutic idea for RA treatment.
MicroRNAs (miRs) are a class of endogenous, small noncoding RNAs that could regulate gene expression by binding in the 3’-UTR of target mRNAs, resulting in its degradation or translational repression. MiRs are known to play a role in the development of all facets of the immune system, from hematopoiesis to effector functions [Citation9], and are currently believed to be therapeutically desirable for RA [Citation10,Citation11]. Recently, researchers have found several miRs playing a potential role in regulating the pathogenesis of RA, including miR-16, miR-155, miR-132, miR-146a, miR-223, miR-124a and miR-203 [Citation12,Citation13]. However, the relationship between miRs and the mechanism of RA progression remains unclear. More potential targets need to be found. miR-27b-3p was reported to be involved in many diseases by affecting cell growth, invasion and metastasis [Citation14,Citation15]. We, therefore, speculated that miR-27b-3p might play a significant role in RA development. The purpose of this study was to confirm the effect of miR-27b-3p in RA and its underlying mechanisms.
Materials and methods
Written informed consent was obtained from all participants. This study was approved by the ethics committee of the Institutional Animal Care and Use Committee of Hunan Provincial People’s Hospital.
Sample collection
Fresh cartilage tissue specimens were obtained from non-RA controls (n = 10) with no history of autoimmune diseases who underwent total hip arthroplasty due to femoral neck fracture, or patients with RA (n = 10) who received total knee arthroplasty in our hospital. In this study, all RA patients were diagnosed, according to the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 classification criteria [Citation16]. After the collection of cartilage tissue, samples were immediately transported to the laboratory for the following experiments.
Animals
Female C57 BL/6 mice of 4 weeks old of age were obtained from the Shanghai Laboratory Animal Centre, CAS (SLACCAS, Shanghai, China). All mice were fed with a normal diet for another 6 weeks before the establishment of RA mice models. Briefly, the mice were anaesthetized with 4% chloral hydrate (about 2.5 ml) and the right knees were subcutaneously injected with 1 ml type II collagenase. The same procedure was repeated 1 week later. At the same time, the left knees were injected with the same volume of normal saline. The study was performed with the approval Institutional Animal Care and Use Committee of the Hunan Provincial People’s Hospital.
Chondrocyte culture and cell transfection
Cartilage tissues of the knee were carefully collected from mice and washed with PBS twice. The tissues were cut into pieces of ∼1 mm3 and then digested with 0.2% type II collagenase overnight at 37 °C. Then centrifuged with 1200 rpm for 3 min and discarded the supernatant. The cells were resuspended with DMEM/F12 (Dulbecco’s Modified Eagle’s Medium/Ham’s Nutrient Mixture F-12) medium supplied with 10% FBS (fetal bovine serum) and 0.5% penicillin-streptomycin. The cells were incubated in a humidified 37 °C incubator saturated with 5% CO2 in 95% ambient air. Small interfering RNA (siRNA) and HIPK2 expression vector were used to knock down and increase HIPK2 expression as previously described, which were obtained from Santa Cruz Biotechnology.
Hematoxylin-eosin (HE) staining
The knees were isolated from the mice, fixed with 10% formalin for 24 h, and then decalcified with 10% EDTA demineralized solution for at least 1 month. Then the tissues were embedded in paraffin and sliced into 5-μm sections. The sections were stained with hematoxylin and eosin and observed under a light microscope.
Cell viability assay
Cell viability was determined by CCK-8. Briefly, the cells were seeded onto 96-well plates and then treated with different methods for 24 h. Then, the cells were incubated with CCK-8 solution at 37 °C for 3 h and finally measured with a microplate reader at 450 nm.
Apoptosis assays
The chondrocytes were managed with the different treatments and then were collected using trypsin (Gibco) and washed with PBS. The apoptosis of chondrocytes was detected using an Annexin V-FITC Apoptosis Kit (Invitrogen, Thornton, NSW, Australia). According to the manufacturer’s instructions, the collected cells were stained by Annexin V-FITC and PI for 15 min. The BD FACS flow cytometer (BD bioscience, Franklin Lakes, NJ, USA) was used to determine the cell apoptosis. This experiment was performed in triplicate.
Quantitative PCR
Total RNA was isolated from the cells using the Trizol with the RNA isolation kit (TIANGEN Biotech Co., Ltd., Beijing, China). Primers were synthesized by Sangon Biotech (Shanghai, China). The cDNA was synthesized using the PrimeScript RT Reagent Kit according to the manufacturer’s protocol and then served as templates for qPCR amplification using the SYBR Premix Ex TaqTM II Kit. RT-PCR was performed on an Applied Biosystems 7300 system (Foster City, CA, USA) using the following conditions: pre-denaturation at 95 °C for 30 s, fllowed by 40 cycles of denaturation at 95 °C for 5 s and annealing and extension at 60 °C for 30 s. β-actin was used as an internal reference and the relative mRNA expression of target genes was calculated using the 2−ΔΔCt method. The experiment was independently conducted 3 times.
Dual-luciferase reporter assay
The target gene Homeodomain-interacting protein kinase (HIPK2) of miR-27b-3p was screened by the TargetScan and Starbase online tools. The wild-type or mutant-type 3’-untranslated sequences (wt-3’-UTR or mut-3’-UTR) were synthesized by TransGen Biotech (Beijing, China). All the constructs were verified by DNA sequencing. Cells were co-transfected with the luciferase report constructs and pSilencer 4.1-miR-27b-3p or pSilencer 4.1-CMV puro plasmid (no-insert control) (0.2 μg/well each). After 24 h, the luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) on a Glomax Luminometer (Promega, Madison, WI).
Western blots
The proteins were isolated from the cells and tissues with the RIPA. The mixture of the same concentration of proteins (supplied with loading buffer) was boiled for 10 min at 95 °C. Then, 20 ml mixture (containing 30–50 μg samples) was added to the plate of the 10% polyacrylamide gel and underwent electrophoresis to isolate the proteins. The proteins were transferred from the gels to PVDF membrane, blocked out, and then incubated overnight at 4 °C with primary antibodies. The samples were washed with TBST and then incubated with secondary antibodies at room temperature for 1 h. Experiments were conducted for three times with the mean value obtained. Densitometry analysis was accomplished using Image J.
Statistical analysis
All statistical analyses were processed with GraphPad Prism 7.0 software (GraphPad Software, CA, USA). Experimental differences were determined with Student’s t-test, One-Way ANOVA, and Two-Way ANOVA. p-values of <.05 were considered as statistically significant.
Results
MicroRNA-27b-3p is involved in RA
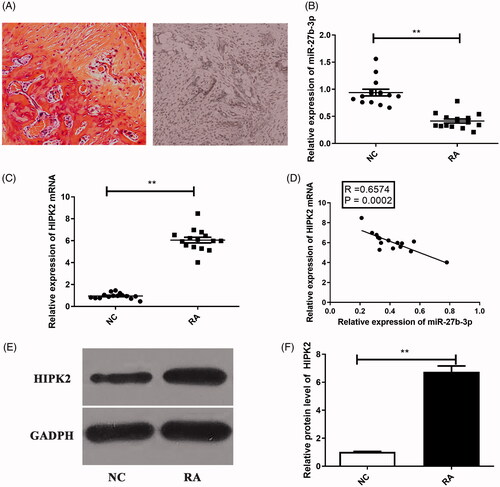
As shown in , HE staining presented the pathological features of RA. The levels of miR-27b-3p and HIPK2 in sample tissues were detected by qRT-PCR. We found that the expression of miR-27b-3p was decreased in sample tissues from RA patients, compared that of controls ().
Figure 1. Expression levels of miR-27b-3p and HIPK2 in cartilage tissues from RA and NC patients. (A) The H&E staining demonstrated the pathological features of RA.The expression of (B) miR-27b-3p and (C) HIPK2 mRNA in cartilage tissues. (D) The correlation between miR-27b-3p and HIPK2 mRNA expression in RA cartilage tissues. (E,F) The expression of HIPK2 protein in RA cartilage tissues. The normal articular cartilage tissues were served as control. **p < .01.
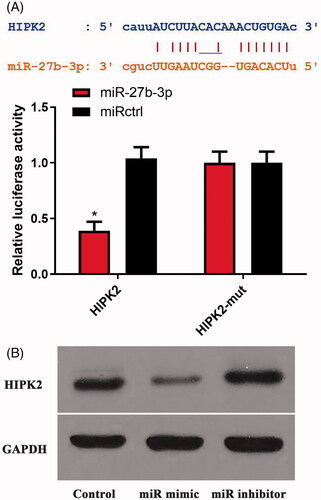
HIPK2 is a direct target of miR-27b-3p and is correlated with RA development
By searching in the TargetScan and Starbase databases, HIPK2 was found as a candidate target of miR-27b-3p (). Homeodomain-interacting protein kinase (HIPK2) is a member of the homeodomain-interacting protein kinase family. Then, Dual-luciferase reporter assay demonstrated that the inhibitory effect of miR-27b-3p on the luciferase reporter activity was abolished when the predicted miR-27b-3p site was mutated (). As shown in , Western blot results confirmed the luciferase reporter assay. Moreover, consistent with the luciferase reporter assay, HIPK2 mRNA and protein levels were higher in patients with RA than those in NC patients (). Pearson correlation presented that there was a significantly negative correlation between miR-27b-3p and HIPK2 mRNA ().
miR-27b-3p suppresses the apoptosis of chondrocytes by targeting HIPK2
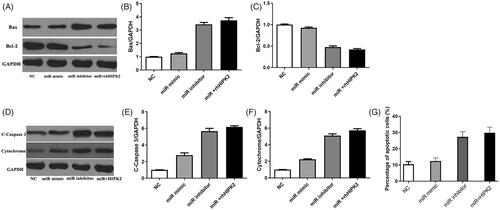
We then evaluated the effect of miR-27b-3p on the apoptosis of chondrocytes (). Overexpression of miR-27b-3p significantly reduced the expression of pro-apoptotic protein c-caspase3 and increased the expression of anti-apoptotic Bcl-2; however, downregulation of miR-27b-3p has a significant effect of inducing apoptosis. Furthermore, overexpression of miR-27b-3p combined with recombinant HIPK2 protein showed the inhibitory effect of miR-27b-3p was abolished by HIPK2.
Figure 3. Recombinant HIPK2 antagonized miR-27b-3p mimic-inhibited cell apoptosis. (A–F) The protein level of Bax, Bcl2, cleaved caspase-3 and cytochrome determined by Western blotting. (G) Percentage of apoptotic cells measured after treatment with miR-27b-3p mimic or combination of HIPK2 and miR-27b-3p inhibitor.
Discussions
RA is a chronic autoimmune disease without any effective therapy, which is a major health concern and economy burden all over the world [Citation17]. The characteristics of RA include abnormal hyperplasia and massive inflammatory cell infiltration [Citation18]. The etiology and pathogenesis of RA remain unclear; therefore, the pathogenesis of RA and disease prediction need to be under intensive investigation. In this study, we found that miR-27b-3p play an inhibitory role on the apoptosis of chondrocyte in RA by targeting HIPK2.
Many miRs have been reported to play a critical role in the regulation of many diseased cells, so they have become potential diagnostic or therapeutic targets for a variety of diseases [Citation19,Citation20]. Accumulated studies have reported during the development of RA the abnormal expression of miRs, including miR-155, miR-146a and miR-125 [Citation21–24]. In the current study, we found that the expression level of miR-27b-3p was decreased in cartilage tissue of patients with RA, compared with those in non-RA controls. Therefore, miR-27b-3p may become a therapeutic direction for RA treatment. A variety of studies have demonstrated that miR expression could have a key role in cell proliferation, apoptosis, migration and inflammation. Nakamachi et al. [Citation25] reported that miR-124a is a critical regulator of proliferation in fibroblast-like synoviocytes from patients with RA. Semman et al. [Citation26] suggested that miR-346 could control the stability of TNF-α mRNA and the release of TNF-α protein in RA. In our study, we investigated the effects of miR-27b-3p on chondrocyte apoptosis, and we found that delivery of miR-27b-3p mimics into chondrocyte could induce cell apoptosis by targeting HIPK2 protein.
Homeodomain-interacting protein kinase (HIPK2) is a member of the homeodomain-interacting protein kinase family, which is able to interact with homeodomain transcription factors and many other transcription factors like p53. It is also able to function as either corepressor or coactivator. HIPK2 is also able to regulate a wide range of biological processes, including cell proliferation, tumorigenesis, vasculogenesis, DNA damage response, apoptosis, tissue fibrosis, epithelial-mesenchymal transition and neural development [Citation27,Citation28]. Additionally, HIPK2 is also reported to participate in various diseases like tumor progression and fibrosis of pulmonary [Citation29]. HIPK2 is also reported to induce cell cycle arrest and apoptosis in response to genotoxic damage [Citation30,Citation31]. Moreover, the down-expression of HIPK2 also impairs the pro-apoptosis ability and induces drug resistance [Citation32]. The role of HIPK2 in RA has not been researched in detail ever, a previous study using whole-exome sequencing technique demonstrated variants of gene HIPK2 may induce functional impact on RA pathogenesis [Citation33]. Therefore, it is the first time to investigate the functional effects of HIPK2 protein, as well as the upstream regulator (miR-27b-3p), in RA development. This study might provide a potential therapeutic target for RA treatment in the clinic.
Conclusions
In summary, declined miR-27b-3p and elevated HIPK2 in RA tissues and cells was revealed. Further in vitro studies demonstrated that miR-27b might inhibit chondrocyte apoptosis and thus attenuate RA development by directly inhibiting HIPK2 expression. Therefore, miR-27b-3p might become a potential target for prevention and treatment of RA in the clinic.
Ethics approval and consent to participate
The present study was approved by the Institutional Animal Care and Use Committee of Materials and Methods
Written informed consent was obtained from all participants. This study was approved by the ethics committee of the Institutional Animal Care and Use Committee of Hunan Provincial People’s Hospital.
Disclosure statement
No potential conflict of interest was reported by the authors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Prabahar A, Natarajan J. MicroRNA mediated network motifs in autoimmune diseases and its crosstalk between genes, functions and pathways. J Immunol Meth. 2017;440:19–26.
- Tobon GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J Autoimmun. 2010;35:10–14.
- Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012;51:v3–11.
- Smolen JS, Breedveld FC, Burmester GR. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75:3–15.
- Garnero P, Rousseau JC, Delmas PD. Molecular basis and clinical use of biochemical markers of bone, cartilage, and synovium in joint diseases. Arthritis Rheum. 2000;43:953–968.
- Henc I, Kokotkiewicz A, Łuczkiewicz P, et al. Naturally occurring xanthone and benzophenone derivatives exert significant anti-proliferative and proapoptotic effects in vitro on synovial fibroblasts and macrophages from rheumatoid arthritis patients. Int Immunopharmacol. 2017;49:148–154.
- Bustamante MF, Garcia-Carbonell R, Whisenant KD, et al. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2017;19:110.
- Rohner E, Matziolis G, Perka C, et al. Inflammatory synovial fluid microenvironment drives primary human chondrocytes to actively take part in inflammatory joint diseases. Immunol Res. 2012;52:169–175.
- Davidson-Moncada J, Papavasiliou FN, Tam W. MicroRNAs of the immune system: roles in inflammation and cancer. Ann N Y Acad Sci. 2010;1183:183–194.
- Cuppen BV, Rossato M, Fritsch-Stork RD, et al. Can baseline serum microRNAs predict response to TNF-alpha inhibitors in rheumatoid arthritis? Arthritis Res Ther. 2016;18:189.
- Wang L, Wang C, Jia X, et al. Circulating exosomal miR-17 inhibits the induction of regulatory T cells via suppressing TGFBR II expression in rheumatoid arthritis. Cell Physiol Biochem. 2018;50:1754–1763.
- Li Z, Li Y, Li Q, et al. Role of miR-9-5p in preventing peripheral neuropathy in patients with rheumatoid arthritis by targeting REST/miR-132 pathway. In Vitro Cell Dev Biol Anim. 2019;55:52–61.
- Fu D, Xiao C, Xie Y, et al. MiR-3926 inhibits synovial fibroblasts proliferation and inflammatory cytokines secretion through targeting toll like receptor 5. Gene 2019;687:200–206.
- Ye J, Wu X, Wu D, et al. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis in colorectal cancer. PloS One. 2013;8:e60687.
- Wang Y, Rathinam R, Walch A, et al. ST14 (suppression of tumorigenicity 14) gene is a target for miR-27b, and the inhibitory effect of ST14 on cell growth is independent of miR-27b regulation. J Biol Chem. 2009;284:23094–23106.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581.
- El Miedany Y. Co-morbidity index in rheumatoid arthritis: time to think. Clin Rheumatol. 2015;34:1995–2000.
- Kim BH, Yoon BR, Kim EK, et al. Alleviation of collagen-induced arthritis by the benzoxathiole derivative BOT-4-one in mice: Implication of the Th1- and Th17-cell-mediated immune responses. Biochem Pharmacol. 2016;110–111:47–57.
- Tavasolian F, Abdollahi E, Rezaei R, et al. Altered expression of microRNAs in rheumatoid arthritis. J Cell Biochem. 2018;119:478–487.
- Churov AV, Oleinik EK, Knip M. MicroRNAs in rheumatoid arthritis: altered expression and diagnostic potential. Autoimmun Rev. 2015;14:1029–1037.
- Tang Y, Luo X, Cui H, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 2009;60:1065–1075.
- Pincus T, Sokka T, Wolfe F. Premature mortality in patients with rheumatoid arthritis: evolving concepts. Arthritis Rheum. 2001;44:1234–1236.
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450.
- Zhou Q, Haupt S, Kreuzer JT, et al. Decreased expression of miR-146a and miR-155 contributes to an abnormal Treg phenotype in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74:1265–1274.
- Nakamachi Y, Kawano S, Takenokuchi M, et al. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2009;60:1294–1304.
- Semaan N, Frenzel L, Alsaleh G, et al. miR-346 controls release of TNF-α protein and stability of its mRNA in rheumatoid arthritis via tristetraprolin stabilization. PLoS One. 2011;6:e19827.
- Saul VV, Schmitz ML. Posttranslational modifications regulate HIPK2, a driver of proliferative diseases. J Mol Med. 2013;91:1051–1058.
- Blaquiere JA, Verheyen EM. Homeodomain-interacting protein kinases: diverse and complex roles in development and disease. Curr Top Dev Biol. 2017;123:73–103.
- Huang Y, Tong J, He F, et al. MiR-141 regulates TGF-β1-induced epithelial-mesenchymal transition through repression of HIPK2 expression in renal tubular epithelial cells. Int J Mol Med. 2015;35:311–318.
- D’Orazi G, Cecchinelli B, Bruno T, et al. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat Cell Biol. 2002;4:11–19.
- Hofmann TG, Moller A, Sirma H, et al. Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat Cell Biol. 2002;4:1–10.
- Puca R, Nardinocchi L, Givol D, et al. Regulation of p53 activity by HIPK2: molecular mechanisms and therapeutical implications in human cancer cells. Oncogene 2010;29:4378–4387.
- Li Y, Lai-Han Leung E, Pan H, et al. Identification of potential genetic causal variants for rheumatoid arthritis by whole-exome sequencing. Oncotarget 2017;8:111119–111129.