Abstract
Background
Interactions between the heterotopic ossification (HO) and the neurological injuries have got lots of interest but still poorly understood. The present study aims to determine the effect of neuroinflammation related calcitonin gene-related protein (CGRP) on HO following spinal cord injury (SCI).
Method
C57Bl6 mice were anesthetized to make an SCI model, together with the ectopic injection of snake venom cardiotoxin (SVC) or CGRP protein into tibialis anterior (TA) muscles. The TA muscles were then harvested for radiologic, histologic, and immunohistology examinations to determine the formation of bone and the expression of CGRP. Fibro/adipogenic progenitors (FAPs) were isolated and cultured. CGRP protein was added into the medium and then detected for chondrogenic markers.
Result
The callus formed following SVC injection and SCI successfully, with much more formed in the group with SCI, as well as an increased level of CGRP. Similarly, the injection of CGRP directly was able to induce the formation of bone-like tissues in vivo. And, the additional CGRP protein was able to induce chondrogenic differentiation as shown in RT-PCT and immunofluorescence, in a dose-dependent manner.
Conclusion
CGRP was able to induce chondrogenic differentiation of FAPs, which may be an important part of Neurological heterotopic ossification developing.
Introduction
Heterotopic ossification (HO) is common in clinic, which is a poorly characterized degenerative disorder with ectopic bone formation in soft tissue that no effective treatment has been developed ever. Neurological heterotopic ossification (NHO) is common in result of traumatic brain injury (TBI) and spinal cord injury (SCI) [Citation1], with the percentage of 5–20% of severe TBI patients and 20–29% of SCI patients [Citation2]. NHO often forms around large joints like the hip, knee and shoulder joints, causing severe pain and deformation [Citation3]. Similarly, an interesting thing is the fractures combined with traumatic brain injury/spinal cord injury often recover much more rapidly than those without, as well as the more formation of ectopic bones in the soft tissues. Similar results can also be found even in cases peripheral nerve injury (such as brachial plexus injury and tibial fracture). Till now, the cellular and molecular mechanisms of the formation of NHO were poorly understood. Surgical excision is the only treatment for NHO; however, the recurrence of NHO cannot be avoided after surgery [Citation4].
The neurological factors may be the basis for the developing of NHO resulting from traumatic injury. The process of NHO formation is initiated by the peripheral injury with a concomitant central/peripheral neuronal injury which stimulates the bone formation at the site of the peripheral injury via endochondral ossification [Citation5]. The injury of the CNS initiates an inflammatory cascade, together with a range of growth factors and cytokines, which stimulates the recruit and transfer of stem-like cells and finally chondrogenic differentiation at the peripheral injury sites [Citation6]. The neuroinflammation after neurol injury introduces the release of the pain mediators like substance P (sP) and calcitonin gene-related protein (CGRP). Substance P is able to initiate expression of almost all known immunological chemical messengers or cytokines [Citation7], as well as specifically recruit of mast cells. Till now, a lot of researches have linked the CGRP with ectopic bone formation [Citation8–10]; however, the mechanism how CGRP involves in NHO remains unknown. CGRP, released by peripheral sensory nerves, was also reported to cause neurogenic vasodilatation, mast cell degranulation, cytokine release, and vascularization [Citation11].
Also, CGRP has been shown to interact with the BMPs, particularly BMP2 and BMP4, two of the main BMPs involved in HO [Citation9,Citation12]. CGRP mediates its effects through both the receptors named calcitonin receptor-like receptor (CALCRL) and activity-modifying protein (RAMP1) [Citation13], which are found throughout the body [Citation14]. The present study aims to investigate the role of CGRP in heterotopic ossification following spinal cord injury.
Method
Animal procedures
The C57Bl6 mice were purchased from Shanghai Slaccas Animal center. All the procedures were approved by the handled as ethics committee of the hospital. The animal procedures were performed according to a previous published literature [Citation15]. Generally, mice were anaesthetized with isoflurane gas and placed in prone position. Skin incision was made on top of thoracic kyphosis to expose the spinal cord. Complete transection of spinal cord was made followed by careful hemostasis. The control group performed only the exposure of the spinal cord but no transection. Tibialis anterior (TA) muscles of the left legs were injured (TAI) by injection 40 ml of snake venom cardiotoxin (10 mM) in both groups. Similarly, to investigate the effect of CGRP in ectopic bone formation, the CGRP (Bachem, Torrance, CA) (10 mM) was injected into the TA muscle of the left leg directly, with the right leg injected with equal volume of PBS for control.
Cell culture
Fibro/adipogenic progenitors (FAPs) were isolated by FACS as described [Citation16]; briefly, hind limb muscles were harvested, minced, and then incubated for 60 min at 37 °C in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco) supplemented with 400 U/ml Collagenase Type II (Sigma). The cell suspension was filtrated through 70-μm cell strainers (Falcon) and then centrifuged at 500 × g for 5 min. The cells were then resuspended in FACS buffer and immune-labelled with primary antibodies anti-CD31, anti-CD45, anti-Sca-1, and anti-α7 integrin for 30 min at 4 °C. Cells were then washed and filtered through a 40-μm strain filter. Flow cytometry analysis and cell sorting were performed on a FACSAria instrument, and the FAPs were gated based on size, shape, viability, and cell surface markers (CD45-/CD31-/integrin α7-/Sca1+). Then the cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 20% fetal bovine serum (FBS), 100 mg/ml streptomycin, and 100 U/ml penicillin in a 37 °C–5% CO2 incubator. When cultures become almost confluent, the cells were treated with 0.5 ml of 0.25% trypsin containing 0.02% EDTA for 2 min at room temperature. The third passage cells were used for further experiments.
For chondrogenic differentiation analysis, 1.0 × 105 cells/well was seeded into six-well plates in duplicate. Twenty-four hours after seeding, medium was replaced with chondrogenic medium (CYAGEN) according to the manufactory’s protocol. Cells were then incubated continuously in chondrogenic medium for 14 days. CGRP (Bachem, Torrance, CA) (10, 100, or 1000 ng/ml) was added to the chondrogenic medium.
Radiographic evaluation
The radiographic images were made with the use of CABINET X ray system to evaluate the formation of the callus at 2- and 4-weeks post-surgery. Generally, anaesthetized the mice and put them in prone position. The radiology condition was set at 25 KV and 10 s. Also, at four weeks after modelling, the samples of the legs were collected for evaluation of the cross-sectional area of the callus with the vivaCT 40.
Histology and immunofluorescence
The muscle tissue was isolated, fast frozen in liquid nitrogen, and then embedded in OCT compound. Frozen sections (6 um) were made, mount onto superfrost slides, and then stored at −80 °C refrigerator. Before histology/immunohistochemistry staining, the sections were got out of the refrigerator and fixed in 4% PFA immediately for 15 min. The H&E and Safranin O & staining were performed according to the instructions of the manufacturer.
For immunofluorescence staining, the sections were washed twice with PBS after fixation, treated with PBS containing 0.25% Triton-X for 5 min, blocked with 4% BSA for 30 min in 37 °C, and incubated with primary antibody diluted in 1% BSA for 1 h in 37 °C, followed by washed twice and incubated with proper secondary antibodies. Finally, the slides were incubated with DAPI, dehydrated, and mounted them for further evaluation in microscope.
Quantitative real-time PCR
Total RNA was extracted from tissue samples or cultured cells with Trizol (Qiagen). cDNA was got with PrimeScript™ RT Reagent Kit (Takara) following manufacturer’s protocol. Quantitative real-time PCR (qRT-PCR) was performed using TB Green™ Fast qPCR Mix (Takara) and Applied Biosystems 7500 Real-Time PCR System (Life Technologies) with the standard protocol. All values were obtained triple in a total of three independent evaluations. Calculation for the values was made using the ΔΔCt method.
Western blot
Total protein was extracted from cells using RIPA (Beyotime technology) according to the manufacturer’s protocol. Generally, protein samples were loaded onto and separated using SDS-PAGE, then transferred to PVDF membranes, blocked, and incubated with the primary antibodies at 4 °C overnight. After washing, the membranes were incubated with HRP-conjugated secondary antibodies (Beyotime) for 1 h under room temperature. Immunoreactivity was enhanced using a chemiluminescence kit (Beyotime, Inc, China) and exposed to film. The density of bands on Western blots was quantified using a Bio-Rad image system (Hercules, CA).
Statistical analysis
GraphPad Prism 7 (GraphPad Software Inc., La Jolla, CA) was used for analysis of the data. The one-way analysis of variance followed by the Student’s t-test was performed to determine the statistical differences between groups. Results were presented as the mean ± SD, and p values of less than 0.05 were considered statistically significant.
Result
Elevated level of CGRP in HO following spinal cord injury
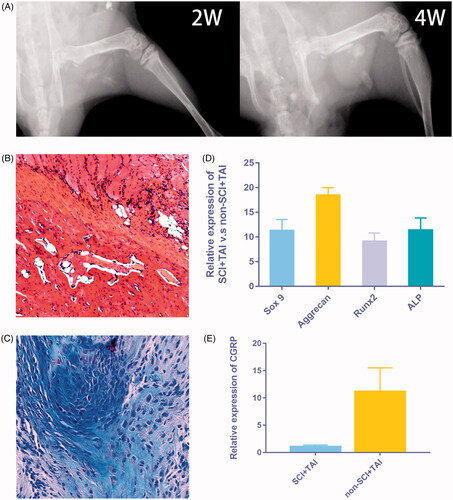
The X ray imaging was performed at 2 and 4 weeks after modelling to confirm the formation of callus following injury and SCI (), together with the H&E and Alcian Blue staining (). qRT-PCR also demonstrated the formation of chondrogenic and osteogenic callus as the chondrogenic and osteogenic genes were elevated significantly at day 14 (). Also, increased level of CGRP was observed in muscle samples at 14 days after modelling, as shown by qRT-PCR evaluation ().
Figure 1. Elevated level of CGRP was observed in HO following spinal cord injury. (A) The X ray imaging demonstrated the formation of callus 2 and 4 weeks after modelling. (B,C) The H&E and Alcian Blue staining showed the formation of cartilage and bone-like tissues. (D) The chondrogenic and osteogenic genes also elevated significantly at day 14. (E) An increased level of CGRP was observed in muscle samples at 14 days after modelling.
CGRP promotes callus formation in vivo
Four weeks after the modelling, qualitative radiology examination of the callus showed the trabeculae to be much larger in the SCI group than in the control (). The cross-sectional area of the callus was 25.3 ± 2.26 mm2 in the SCI group and 20.3 ± 2.05 mm2 in the control group (p = .003).
Figure 2. Directly injection of CGRP can promote callus formation in vivo. (A) The cross-sectional area of the callus is much larger in the SCI + TAI group than in the control (p = .003). (B) Directly injection of CGRP was able to induce the formation of cartilage/bone like tissues as shown by X ray of 2w post injection. (Red arrow: no cartilage/bone like tissue forms after PBS injection; Blue arrow: cartilage/bone like tissues forms after CGRP injection.) (C) The Alcian Blue staining of the samples 2w post injection. (Green arrow: limited areas of inflammatory infiltration; Yellow arrow: Chondrocytes formation after CGRP injection.)
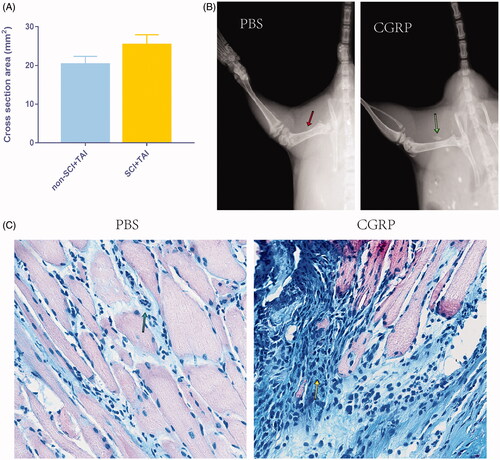
Similarly, the injection of CGRP directly was able to induce the formation of cartilage/bone like tissues as shown by X ray and histological evaluation (), with no such tissue forms in the control group.
CGRP stimulates chondrogenic differentiation of FAPs in vitro
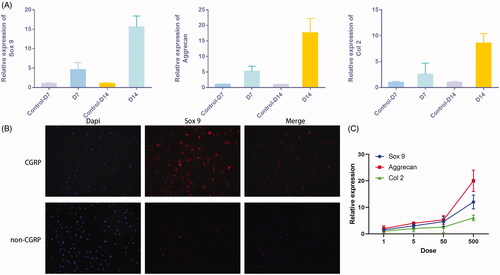
The additional CGRP was able to induce increased levels of chondrogenic markers like Sox9, Aggrecan, and Col II than the control groups, as shown by qRT-PCR of cells at day 7 and 14 days after induction (). Immunofluorescence of Sox 9 also confirmed the ability of CGRP in inducing chondrogenic differentiation (). Moreover, the expression of these genes was also in a dose-dependent manner with the volume of the additional CGRP ().
Figure 3. CGRP stimulates chondrogenic differentiation of FAPs in vitro. (A) Additional CGRP was able to induce chondrogenic differentiation of FAPs, as shown by increased expression of Sox9, Aggrecan, and Col II. (B) Immunofluorescence of Sox 9 was more significant with additional CGRP. (C) The expression of chondrogenic genes was in a dose-dependent manner with the dose of the additional CGRP.
Discussion
Heterotopic ossification is a disorder involving rapid ectopic bone formation within soft tissues like muscle, tendon, and ligaments, and it has also been linked to neuronal injuries [Citation17]. Here, we determined whether neuroinflammation related mediator calcitonin gene-related protein is involved in NHO. Our results suggest that the CGRP is high expressed in NHO samples and is also able to promote the chondrogenic differentiation of FAPs, and directly injection of CGRP into muscle is able to induce ectopic bone formation.
FAP is a multipotent progenitor population resident in skeletal muscle; it is useful during development and regeneration, which provides trophic support to myogenic progenitors for muscle fiber maturation and specification [Citation18,Citation19]. Fibro/adipogenic infiltration is a common outcome of chronic muscle disease as seen in elderly population, fibrosis diseases, and myophagism patients; it is also suggested that the FAPs should be responsible for developing it in pathological environments [Citation20,Citation21]. Despite the established importance of FAPs in both regeneration and degeneration, the signals that regulate the choice between these alternative fates are still poorly characterized, as well as the development of ossification. Previous studies have demonstrated that FAPs are a major source of cells for both injury-induced and spontaneous HO [Citation22,Citation23]. Here, we also demonstrated the FAPs can be induced by CGRP for chondrogenic differentiation during the pathologies of neurologic ossification.
CGRP is a neuropeptide released from both the peripheral and the spinal cord terminals of nociceptive neurons in response to various stimulations. CGRP-positive sensory fibers are present in bone which are considered to work as a feedback of fracture pain [Citation24,Citation25]. In the tissues other than bone, CGRP is also known to work as a balance between tissue and sympathetic nervous system. In the bone, CGRP is found able to directly influent the function of osteoclast. In vitro, CGRP could inhibit the formation of osteoclasts from bone-marrow precursors [Citation26]. CGRP has been directly linked to the HO pathophysiology with its function on immunoregulatory, such as immune cell recruitment and cytokine release [Citation27,Citation28]. Also, as known to us all the importance role of BMP signalling in hereditary and acquired HO, CGRP was found to function along with the BMPs [Citation29]. The neuropeptides up-regulated BMP expression and induced osteogenic differentiation in osteoprogenitor stem cells through up-regulation of cAMP levels [Citation30]. Here, we also demonstrated similar results that besides the role of inflammation regulation; CGRP was also able to induce chondrogenic differentiation of FAPs. However, the molecular basis of it remains to be determined.
Conclusion
NHO is common in clinic with a huge impact on patients’ quality of life. The reason of HO remains unknown, as well as the reliable treatment. To better understand the mechanism of NHO pathology, we developed a spinal cord injury model and found CGRP was able to induce chondrocyte differentiation of FAPs, which may be an important part of NHO developing.
References
- Genet F, Jourdan C, Schnitzler A, et al. Troublesome heterotopic ossification after central nervous system damage: a survey of 570 surgeries. PloS One. 2011;6:e16632.
- Genet F, Kulina I, Vaquette C, et al. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J Pathol. 2015;236:229–240.
- Ranganathan K, Loder S, Agarwal S, et al. Heterotopic ossification: basic-science principles and clinical correlates. J Bone Joint Surg Am. 2015;97:1101–1111.
- Sakellariou VI, Grigoriou E, Mavrogenis AF, et al. Heterotopic ossification following traumatic brain injury and spinal cord injury: insight into the etiology and pathophysiology. J Musculoskelet Neuronal Interact. 2012;12:230–240.
- Davies OG, Grover LM, Eisenstein N, et al. Identifying the cellular mechanisms leading to heterotopic ossification. Calcif Tissue Int. 2015;97:432–444.
- Tannous O, Stall AC, Griffith C, et al. Heterotopic bone formation about the hip undergoes endochondral ossification: a rabbit model. Clin Orthop Relat Res. 2013;471:1584–1592.
- Garza A, Weinstock J, Robinson P. Absence of the SP/SP receptor circuitry in the substance P-precursor knockout mice or SP receptor, neurokinin (NK)1 knockout mice leads to an inhibited cytokine response in granulomas associated with murine Taenia crassiceps infection. J Parasitol. 2008;94:1253–1258.
- Tuzmen C, Verdelis K, Weiss L, et al. Crosstalk between substance P and calcitonin gene-related peptide during heterotopic ossification in murine Achilles tendon. J Orthop Res. 2018;36:1444–1455.
- Salisbury E, Rodenberg E, Sonnet C, et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem. 2011;112:2748–2758.
- Wang XY, Guo X, Qu SX, et al. Temporal and spatial CGRP innervation in recombinant human bone morphogenetic protein induced spinal fusion in rabbits. Spine. 2009;34:2363–2368.
- Sara C, Consuelo A, Massimo N, et al. Capsaicin promotes a more aggressive gene expression phenotype and invasiveness in null-TRPV1 urothelial cancer cells. Carcinogenesis. 2011;32:686–694.
- Liu X, Kang H, Shahnazari M, et al. A novel mouse model of trauma induced heterotopic ossification. J Orthop Res. 2014;32:183–188.
- Poyner DR, Sexton PM, Marshall I, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246.
- Arulmani U, Maassenvandenbrink A, Villalon CM, et al. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharmacol. 2004;500:315–330.
- Debaud C, Salga M, Begot L, et al. Peripheral denervation participates in heterotopic ossification in a spinal cord injury model. PloS One. 2017;12:e0182454.
- Biswas AA, Goldhamer DJ. FACS fractionation and differentiation of skeletal-muscle resident multipotent Tie2+ progenitors. Methods Mol Biol. 2016;1460:255–267.
- Forsberg JA, Pepek JM, Wagner S, et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91:1084–1091.
- Joe AWB, Lin Y, Anuradha N, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163.
- Farup J, Madaro L, Puri PL, et al. Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell Death Dis. 2015;6:e1830.
- Gabi S, Monika WL, Zipora YR. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 2004;117:5393–5404.
- Yong L, Johnny H. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161:895–907.
- Lees-Shepard JB, Yamamoto M, Biswas AA, et al. Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nat Commun. 2018;9:471.
- Dey D, Bagarova J, Hatsell SJ, et al. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci Transl Med. 2016;8:366ra163.
- Martin CD, Juan Miguel JA, Ghilardi JR, et al. Organization of a unique net-like meshwork of CGRP + sensory fibers in the mouse periosteum: implications for the generation and maintenance of bone fracture pain. Neurosci Lett. 2007;427:148–152.
- Jian L, Andris K, Jonas BM, et al. Site-specific CGRP innervation coincides with bone formation during fracture healing and modeling: a study in rat angulated tibia. J Orthop Res. 2010;25:1204–1212.
- Kyoko I, Koji H, Hiroshi N, et al. Inhibitory effect of CGRP on osteoclast formation by mouse bone marrow cells treated with isoproterenol. Neurosci Lett. 2005;379:47–51.
- Zhou B, Zhou Y, Tang K. The effects of substance P on pluripotent tendon cells: an in vitro and in vivo study. J of Musculoskelet Neuronal Interact. 2014;14:349–358.
- Ackermann PW, Li J, Lundeberg T, et al. Neuronal plasticity in relation to nociception and healing of rat achilles tendon. J Orthop Res. 2003;21:432–441.
- Bucelli RC, Gonsiorek EA, Kim WY, et al. Statins decrease expression of the proinflammatory neuropeptides calcitonin gene-related peptide and substance P in sensory neurons. J Pharmacol Exp Ther. 2008;324:1172–1180.
- Tian G, Zhang G, Tan YH. Calcitonin gene-related peptide stimulates BMP-2 expression and the differentiation of human osteoblast-like cells in vitro. Acta Pharmacol Sin. 2013;34:1467–1474.
