Abstract
Background
Despite the medical uses of lidocaine has been well-characterized, the study of lidocaine’s pharmacological function other the anaesthetic effect was never stopped. This study designed to reveal the effect of lidocaine on the growth and metastasis of lung cancer in vitro.
Methods
A549 and NCI-H1299 cells were treated by lidocaine for 24 h. miR-539 expression in cell was silenced by transfection with the specific inhibitor. The changes in cell growth and metastasis were determined using CCK-8 assay and western blot. Luciferase activity assay was performed to assay if EGFR was a target of miR-539. Western blot was used to test the activation of EGFR downstream signalling.
Results
Lidocaine suppressed the viability, migration, and invasion of A549 and NCI-H1299 cells while induced apoptotic death. Lidocaine elevated the expression of miR-539. The anti-tumour properties of lidocaine towards A549 and NCI-H1299 cells were partially attenuated when miR-539 was silenced. EGFR was a target of miR-539. Lidocaine repressed the activation of ERK and PI3K/AKT pathways also via regulating miR-539.
Conclusion
The anti-growth and anti-metastatic effects of lidocaine towards lung cancer cells. The anti-tumour properties of lidocaine may be partial via up-regulation of miR-539, which blocked EGFR signalling by directly binding with EGFR.
Introduction
Lung cancer is one kind of malignant tumour that has been characterized by uncontrolled tumour cell growth. According to statistics, 1.8 million new cases of lung cancer occurred in 2012 and lead to 1.6 million death worldwide [Citation1]. The survival rate of lung cancer depends on the stage at diagnosis. Patients with distant stage disease possess lower 5-year survival rate than those with localized stage (4% vs. 54%) [Citation1]. Thus, suppressing lung cancer growth and metastasis has been considered as a promising therapeutic strategy for treating this cancer. Currently, the most main treatment for lung cancer is surgery, radiotherapy, chemotherapy and molecular targeted therapy of comprehensive treatment [Citation2,Citation3]. Although these therapies have been utilized in clinic, more effective strategies are still required to be developed which will be helpful for further increase the survival of patients.
Lidocaine is a commonly used local anaesthetic with fast onset, good ability to permeate through mucosa and broad range of safety [Citation4]. It is widely used in regional anaesthesia, peripheral nerve blocks, and epidural anaesthesia with excellent effects on the management of pain. Beyond that, lidocaine also has many other pharmacological effects, like anti-pyroptosis [Citation5], neuroprotection [Citation6], cerebral protection [Citation7], anti-inflammation [Citation8], preventing mitochondrial dysfunction [Citation9], and so forth. Moreover, recently paper reported the anti-tumour properties of lidocaine against diverse cancers, including colon adenocarcinoma [Citation10], gastric cancer [Citation11], breast cancer [Citation12], and lung cancer [Citation13]. Lidocaine significantly inhibited lung cancer cells proliferation [Citation14] and made cancer cells more sensitive to cisplatin [Citation13]. Further investigations revealed that lidocaine exerted its anti-tumor properties in lung cancer possibly via regulating GOLT1A [Citation14] and miR-21 [Citation13]. However, large amount of researches are still required to fully appreciate its complexity.
miR-539 is a 78 bp miRNA located at human chromosome 14q32.31 locus, which is the largest miRNA cluster identified so far in human genome [Citation15]. The role of miR-539 in several human cancers has been revealed and recent studies displayed a contradictory role of it in cancer. For example, miR-539 expression was elevated in epithelial ovarian cancer [Citation16] while was declined in Wilms' tumour [Citation17], colon adenocarcinoma [Citation18], and non-small cell lung cancer [Citation19]. Functional experiments demonstrated that miR-539 exhibited as an oncogene in epithelial ovarian cancer [Citation16] while as a tumour suppressor in other three types of cancers [Citation17–19]. Besides that, EGFR, an oncogenic driver of lung cancer [Citation20], was found as a target gene of miR-539 [Citation21]. Based on these reason, we focused on investigating whether lidocaine functions to lung cancer cells through regulating miR-539.
To this end, two lung cancer cells were treated by lidocaine and the effects of lidocaine and miR-539 dysregulation were studied. This paper may provide novel evidence to explain how lidocaine inhibited the growth and metastasis of lung cancer.
Materials and methods
Cell treatment
A549 (CCL-185™) and NCI-H1299 cells (CRL-5803™) both from ATCC (Manassas, VA) were, respectively, cultured in F-12K medium (ATCC) and RPMI-1640 medium (ATCC). Fetal bovine serum (Gibco, Grand Island, NY) was added with concentration of 10% to make complete growth medium. The cells were cultured in an atmosphere with 5% CO2 at 37 °C.
Analytical standard lidocaine was from Sigma-Aldrich (St. Louis, MO). A549 and NCI-H1299 cells were treated by 8 mM lidocaine for 24 h as previously described [Citation14].
Transfection
miR-539 inhibitor, mimic and the negative control (NC) were obtained from GenePharma (Shanghai, China). Cell transfection was performed by Lipofectamine 3000 (Invitrogen, Carlsbad, CA) for 48 h.
CCK-8 assay
A549 and NCI-H1299 cells were seeded in 96-well plates at 1000 cells/well. 48 h later of treatment by lidocaine, the viability of cells was tested by adding with 10 μL CCK-8 solution (Dojindo Molecular Technologies, Kyushu, Japan). The absorbance of each well following 4 h of incubation was tested at 450 nm using a Microplate Reader (Bio-Rad, Hercules, CA).
Apoptosis
A549 and NCI-H1299 cells in 6-well plates (1 × 105) were treated by lidocaine, after which the cells were harvested for use in the detection of apoptotic cell rate. Annexin V-FITC/PI Apoptosis Detection Kit (Sangon Biotech, Shanghai, China) was utilized in this process. The data were analyzed using the FlowJo software (Treestar, San Carlos, CA).
Transwell assay
A549 and NCI-H1299 cells pre-treated by lidocaine were resuspended in serum medium and placed into the upper side of the Transwell chamber (Costar-Corning, New York). The lower side was filled with the complete growth medium. After 24 h of incubation, the cells migrated to the lower side were stained by crystal violet and counted. The invasion of cells was tested the same as migration assay, except the insert was pre-coated with Matrigel (Millipore, Bedford, MA).
Luciferase activity assay
A pmirGlO vector (Promega, Madison, WI) was inserted with wild-type of EGFR sequences to construct a reporter plasmid (EGFR-wt). The vector inserted with mutated EGFR was constructed for use as a negative control (EGFR-mut). The reporter vectors were transiently transfected into HEK 293 T cells along with miR-539 mimic/NC. 48 h later, the luciferase activity was tested using Dual-Luciferase Reporter Assay System (Promega).
qRT-PCR
miRNAs in cell was extracted by miRNeasy Mini Kit (Qiagen, Shenzhen, China). All-in-One miRNA qRT-PCR Detection Kit (GeneCopoeia, Rockville, MD) was used to test the expression of miR-539 in accordance with the manufacturer’s instructions. U6 served as an internal control. Data were analyzed with the 2−ΔΔCt method.
Western blot
The proteins used in Western blot analysis were isolated by RIPA buffer (Beyotime, Shanghai, China). The proteins were immunoblotted with primary antibodies against anti-Bax (sc-20067, Santa Cruz Biotechnology, Santa Cruz, CA), anti-cleaved-PARP (sc-56196), anti-MMP-9 (sc-21733), anti-Vimentin (sc-80975), anti-EGFR (sc-71034), anti-cleaved-caspase-3 (ab2302, Abcam, Cambridge, MA), anti-t-ERK (ab32537), anti-p-ERK (ab194776), anti-t-PI3K (ab86714), anti-p-PI3K (ab182651), anti-t-AKT (ab8805), anti-p-AKT (ab38449), and anti-β-actin (ab8229). Goat anti-rabbit (ab6940), goat anti-mouse (ab97035), and donkey anti-goat (ab6566) IgGs were used as the secondary antibodies. The positive bands were developed by BeyoECL Moon (Beyotime) and analyzed using the Image Lab™ Software (Bio-Rad).
Statistics
Data presented as mean ± SD and statistical results presented as asterisks. Statistical difference was set at p < .05. SPSS 19.0 software (Chicago, IL) was used for calculating statistical difference. Student t-test and ANOVA followed by Duncan procedure were conducted to compare the difference between two or more groups.
Results
Lidocaine inhibited the growth and metastasis of lung cancer cells
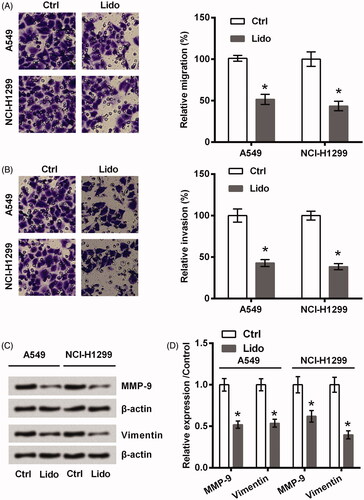
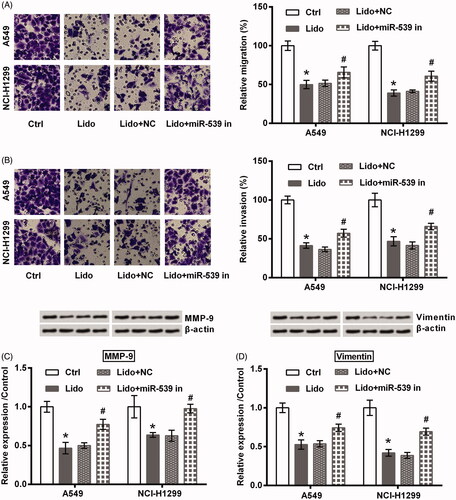
Two lung cancer cell lines (A549 and NCI-H1299) were treated by 8 mM lidocaine for 24 h. As seen in , the viability of these two cell lines was significantly declined by treating with lidocaine (p < .05). Of contrast, the apoptosis rate was raised by lidocaine (p < .05, ), which was coupled with the increased expression of Bax as well as the cleavage of PARP and caspase-3 (all p < .05, ). Meanwhile, displayed low capacities of migration and invasion after A549 and NCI-H1299 cells were treated by lidocaine (p < .05). Consistently, the protein expression of MMP-9 and Vimentin was decreased by lidocaine (p < .05, ). All these data suggested the anti-tumour properties of lidocaine in lung cancer cells towards the aspects of cell viability, apoptosis, migration, and invasion.
Figure 1. Lidocaine inhibited the growth of lung cancer cells. A549 and NCI-H1299 cells were treated by 8 mM lidocaine for 24 h. (A) Cell viability, (B) apoptosis rate, and (C–E) expression of apoptosis-related proteins were measured using CCK-8 assay, flow cytometry and western blot. * indicates p < .05 vs. control (Ctrl) group.
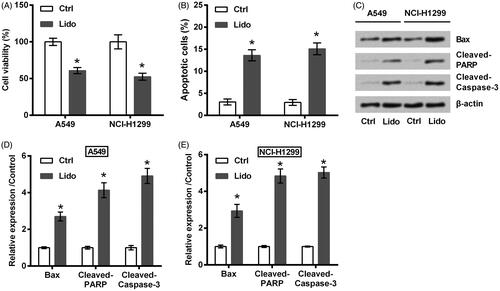
Figure 2. Lidocaine inhibited the metastasis of lung cancer cells. A549 and NCI-H1299 cells were treated by 8 mM lidocaine for 24 h. (A) Migration, (B) invasion, and (C,D) expression of metastasis-related proteins were measured using Transwell assay and western blot. * indicates p < .05 vs. control (Ctrl) group.
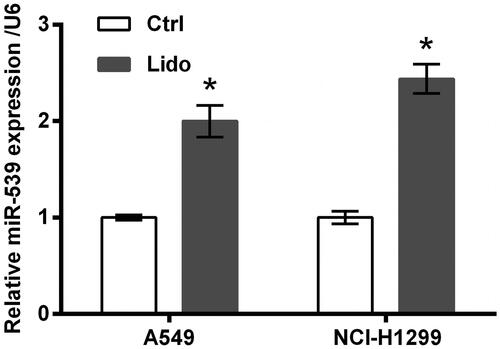
Lidocaine up-regulated the expression of miR-539
miR-539 expression in A549 and NCI-H1299 cells following lidocaine treatment was tested using qRT-PCR. Data in showed that miR-539 expression was significantly increased by lidocaine (p < .05), indicating the involvement of miR-539 in lidocaine’s anti-tumour properties.
Lidocaine suppressed the growth and metastasis of lung cancer cells via up-regulating miR-539
In order to test if miR-539 participates in the tumour suppressive effects of lidocaine on lung cancer cells, its expression in A549 and NCI-H1299 cells was suppressed by transfection with the specific inhibitor. Transfection efficiency tested by qRT-PCR displayed that miR-539 expression was successfully decreased (p < .05, ). Of note, the inhibitory effects of lidocaine on A549 and NCI-H1299 cells growth and metastasis were attenuated by miR-539 silence. Specifically, cell viability loss (p < .05, ), the induced apoptosis (p < .05, ), the altered expression of apoptosis-related proteins (p < .05, ), the decreased migration and invasion (p < .05, ), as well as the altered expression of metastasis-related proteins (p < .05, ) by lidocaine were all attenuated by transfection with miR-539 inhibitor.
Figure 4. Lidocaine inhibited the growth of lung cancer cells via up-regulating miR-539. (A) A549 and NCI-H1299 cells were transfected with miR-539 inhibitor or NC. The expression of miR-539 was tested using qRT-PCR. The transfected cells were treated by 8 mM lidocaine for 24 h. (B) Cell viability, (C) apoptosis rate, and (D–F) expression of apoptosis-related proteins were measured using CCK-8 assay, flow cytometry and western blot. * indicates p < .05 vs. control (Ctrl) or NC group. # indicates p < .05 vs. Lido + NC group.
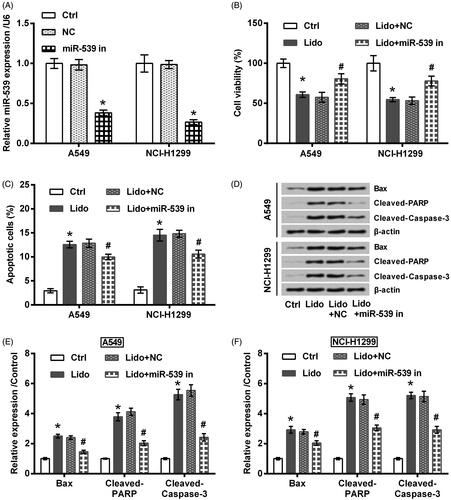
Figure 5. Lidocaine inhibited the metastasis of lung cancer cells via up-regulating miR-539. The transfected A549 and NCI-H1299 cells were treated by 8 mM lidocaine for 24 h. (A) Migration, (B) invasion, and (C,D) expression of metastasis-related proteins were measured using Transwell assay and western blot. * indicates p < .05 vs. control (Ctrl) group. # indicates p < .05 vs. Lido + NC group.
EGFR was a target of miR-539
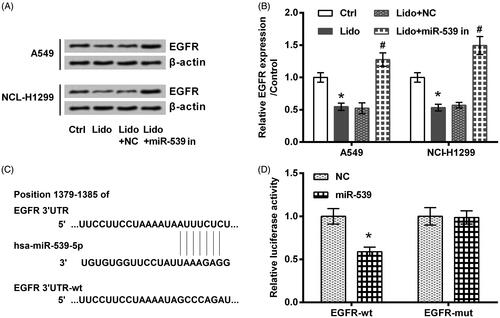
A previous study has reported that lidocaine has potent anti-tumour property against colorectal cancer through suppressing the expression of EGFR [Citation22]. Additionally, EGFR can act as a target gene for miR-539 [Citation21]. These previous findings linked the anti-tumour property of lidocaine with the regulation of miR-539/EGFR axis. The same phenomenon was observed in the present work. As seen in , lidocaine decreased the expression of EGFR while transfection with miR-539 inhibitor increased its expression (p < .05). displayed that relative luciferase activity was significantly decreased by transfection with miR-539 mimic and EGFR-wt (p < .05).
Figure 6. EGFR was a target of miR-539. (A,B) The transfected A549 and NCI-H1299 cells were treated by 8 mM lidocaine for 24 h. The expression of EGFR protein was tested using western blot. (C) The predicted binding sites between miR-539 and the 3’UTR of EGFR. (D) Luciferase activity assay was utilized to confirm the binding effects between miR-539 and EGFR. * indicates p < .05 vs. control (Ctrl) or NC group. # indicates p < .05 vs. Lido + NC group.
Lidocaine suppressed the activation of ERK and PI3K/AKT pathways via up-regulating miR-539
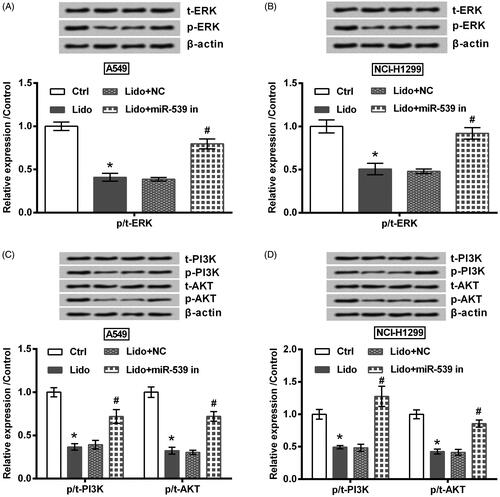
Finally, the underlying signalling pathways involved in lidocaine’s anti-tumour properties were studied. Results in showed that the phosphorylation levels of ERK, PI3K, and AKT were repressed by lidocaine (p < .05). However, the repressive effect of lidocaine on these proteins was attenuated by transfection with miR-539 inhibitor (p < .05).
Figure 7. Lidocaine suppressed the activation of ERK and PI3K/AKT pathways via up-regulating miR-539. The transfected A549 and NCI-H1299 cells were treated by 8 mM lidocaine for 24 h. The phosphorylation levels of (A,B) ERK, (C,D) PI3K and AKT were determined using western blot. * indicates p < .05 vs. control (Ctrl) group. # indicates p < .05 vs. Lido + NC group.
Discussion
As well-established previously, uncontrolled growth and distant metastasis make lung cancer become the most incurable cancer, with poor survival rate [Citation1]. In the current study, the capacities of viability, migration and invasion of two lung cancer cells (A549 and NCI-H1299) were repressed by treating with lidocaine, while the apoptotic death was induced by lidocaine. Meantime, lidocaine treatment elevated the expression of miR-539. The anti-tumour properties of lidocaine towards A549 and NCI-H1299 cells were partially attenuated when miR-539 was silenced by transfection with the specific inhibitor. Besides that, EGFR was found to be a target gene of miR-539. Lidocaine repressed the activation of ERK and PI3K/AKT pathways also via regulating miR-539.
Despite the medical uses of lidocaine has been widely characterized, the study of lidocaine’s pharmacological function other the anaesthetic effect was never stopped. The anti-tumour properties of lidocaine have been reported in human cancers towards various aspects. On the one hand, lidocaine was capable of repressing tumour cells growth, like colon adenocarcinoma cells [Citation10] and gastric cancer cells [Citation11]. On the other hand, lidocaine could inhibit tumour metastasis by controlling the capacities of migration and invasion [Citation11,Citation23]. Herein, the anti-growth and anti-metastatic effects of lidocaine towards A549 and NCI-H1299 cells were illustrated, indicating the anti-tumour properties of lidocaine on lung cancer. This phenomenon was coupled with the up-regulation of anti-apoptotic protein (Bax), the cleavage of pro-apoptotic proteins (PARP and caspase-3), as well as the alterations of Epithelial-mesenchymal transition (EMT)-related proteins (MMP-9 and Vimentin). The result indicated that lidocaine inhibited lung cancer cells growth and metastasis possibly through regulating mitochondria-dependent and EMT-related signalling.
In response to lidocaine treatment, the cells expressed different miRNAs patterns, possibly via which lidocaine exerted its specific function [Citation24,Citation25]. To date, several miRNAs have been mentioned as the effector genes of lidocaine, such as miR-520-3p [Citation22], miR-493 [Citation26], and miR-let-7b [Citation27]. Herein, miR-539 was first found to be an effector miRNA of lidocaine. miR-539 attracted our attention due to its anti-tumour effects towards various cancers, like Wilms' tumour [Citation17], colon adenocarcinoma [Citation18] and lung cancer [Citation19]. The data from this study found that miR-539 expression was elevated by lidocaine treatment. Moreover, the anti-tumour properties of lidocaine towards A549 and NCI-H1299 cells were attenuated by miR-539 silence. Above findings suggested that lidocaine inhibited lung cancer partially via up-regulation of miR-539. However, other miRNAs which in changes of lidocaine’s anti-tumour properties still need to be revealed in the further.
EGFR belongs to the family of type I EGF, which mainly contains four members HER1 (ErbB1, EGFR), HER2 (ErbB2, NEU), HER3 (Erb3), and HER4 (ErbB4) [Citation28]. EGFR is located in cell membrane and acts as an important target for facilitating intercellular communication [Citation29]. In cancer cells, EGFR can deregulate the downstream cascade and drive the initiation of tumour [Citation29]. EGFR has been widely recognized as an oncogenic driver of lung cancer [Citation20]. In the present work, EGFR was found to be a target of miR-539, which was consistency with the finding reported elsewhere [Citation21]. Besides that, lidocaine could inhibit the expression of EGFR, which collectively suggested that lidocaine conferred its anti-tumour effects possibly through up-regulating miR-539, which directly bind with EGFR and thereby blocked EGFR-signaling.
ERK and PI3K/AKT are known as two main downstream signalling of EGFR [Citation30,Citation31]. These two signalling pathways are also in charge of the growth and metastasis of human cancers, including lung cancer [Citation32]. The inhibitory effects of lidocaine on the activation of ERK and PI3K/AKT pathways have been found in melanoma cells [Citation26,Citation33]. However, only one literature indicated the effects of lidocaine on PI3K/AKT signalling in lung cancer cells [Citation13]. The present work was consistent with the previous finding suggested that lidocaine could inactive PI3K/AKT signalling and linked lidocaine’s anti-tumour effects with ERK and PI3K/AKT pathways. Besides that, the data from this study found that lidocaine inhibited these two signalling also via a miR-539-dependent fashion.
To conclude, this study demonstrated the anti-growth and anti-metastatic effects of lidocaine towards lung cancer cells. The anti-tumour properties of lidocaine may be partial via up-regulation of miR-539, which blocked EGFR signalling by directly binding with EGFR. However, further studies are required to confirm the properties of lidocaine in an animal model of lung cancer while may largely improve the findings of this paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Reference
- Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19.
- Bryan DS, Donington JS. The role of surgery in management of locally advanced non-small cell lung cancer. Curr Treat Options in Oncol. 2019;20:27.
- Azar FE, Azami-Aghdash S, Pournaghi-Azar F, et al. Cost-effectiveness of lung cancer screening and treatment methods: a systematic review of systematic reviews. BMC Health Serv Res. 2017;17:413.
- Beecham GB, Stephenson E. Lidocaine. Treasure Island (FL): StatPearls Publishing LLC; 2019.
- Yang X, Yang LX, Wu J, et al. Treatment of lidocaine on subacute thyroiditis via restraining inflammatory factor expression and inhibiting pyroptosis pathway. J Cell Biochem. 2019, [Epub ahead of print]. doi:10.1002/jcb.27675
- Sisti MS, Nishida F, Zanuzzi CN, et al. Lidocaine protects neurons of the spinal cord in an excitotoxicity model. Neurosci Lett. 2019;698:105–112.
- Mitchell SJ, Merry AF, Frampton C, et al. Cerebral protection by lidocaine during cardiac operations: a follow-up study. Ann Thoracic Surg. 2009;87:820–825.
- Wang L, Wang M, Li S, et al. Nebulized lidocaine ameliorates allergic airway inflammation via downregulation of TLR2. Mol Immunol. 2018;97:94–100.
- Li J, Zhu X, Yang S, et al. Lidocaine attenuates cognitive impairment after isoflurane anesthesia by reducing mitochondrial damage. Neurochem Res. 2019;44:1703. Apr 15.
- Tat T, Jurj A, Selicean C, et al. Antiproliferative effects of propofol and lidocaine on the colon adenocarcinoma microenvironment. J BUON. 2019;24:106–115.
- Sui H, Lou A, Li Z, et al. Lidocaine inhibits growth, migration and invasion of gastric carcinoma cells by up-regulation of miR-145. BMC Cancer. 2019;19:233.
- D'Agostino G, Saporito A, Cecchinato V, et al. Lidocaine inhibits cytoskeletal remodelling and human breast cancer cell migration. Br J Anaesth. 2018;121:962–968.
- Yang Q, Zhang Z, Xu H, et al. Lidocaine alleviates cytotoxicity-resistance in lung cancer A549/DDP cells via down-regulation of miR-21. Mol Cell Biochem. 2019;456:63–72. Jan 14.
- Zhang L, Hu R, Cheng Y, et al. Lidocaine inhibits the proliferation of lung cancer by regulating the expression of GOLT1A. Cell Prolif. 2017;50. doi:10.1111/cpr.12364
- Seitz H, Royo H, Bortolin ML, et al. A large imprinted microRNA gene cluster at the mouse Dlk1-Gtl2 domain. Genome Res. 2004;14:1741–1748.
- Gong YB, Fan XH. MiR-539-3p promotes the progression of epithelial ovarian cancer by targeting SPARCL1. Eur Rev Med Pharmacol Sci. 2019;23:2366–2373.
- Su H, Wang X, Song J, et al. MicroRNA-539 inhibits the progression of Wilms' tumor through downregulation of JAG1 and Notch1/3. CBM. 2019;24:125–133.
- Zhao J, Xu J, Zhang R. MicroRNA-539 inhibits colorectal cancer progression by directly targeting SOX4. Oncol Lett. 2018;16:2693–2700.
- Deng H, Qianqian G, Ting J, et al. miR-539 enhances chemosensitivity to cisplatin in non-small cell lung cancer by targeting DCLK1. Biomed Pharmacother. 2018;106:1072–1081.
- Liu X, Wang P, Zhang C, et al. Epidermal growth factor receptor (EGFR): a rising star in the era of precision medicine of lung cancer. Oncotarget. 2017;8:50209–50220.
- Guo J, Gong G, Zhang B. miR-539 acts as a tumor suppressor by targeting epidermal growth factor receptor in breast cancer. Sci Rep. 2018;8:2073.
- Qu X, Yang L, Shi Q, et al. Lidocaine inhibits proliferation and induces apoptosis in colorectal cancer cells by upregulating mir-520a-3p and targeting EGFR. Pathol Res Practice. 2018;214:1974–1979.
- Jiang Y, Gou H, Zhu J, et al. Lidocaine inhibits the invasion and migration of TRPV6-expressing cancer cells by TRPV6 downregulation. Oncol Lett. 2016;12:1164–1170.
- Rancan L, Simon C, Marchal-Duval E, et al. Lidocaine administration controls microRNAs alterations observed after lung ischemia-reperfusion injury. Anesth Analg. 2016;123:1437–1447.
- Sung SH, Lee JG, Yu SB, et al. The effects of lidocaine and procaine on microRNA expression of adipocyte-derived adult stem cells. Korean J Anesthesiol. 2012;62:552–557.
- Wang Y, Xie J, Liu W, et al. Lidocaine sensitizes the cytotoxicity of 5-fluorouacil in melanoma cells via upregulation of microRNA-493. Pharmazie. 2017;72:663–669.
- Wang Q, She Y, Bi X, et al. Dexmedetomidine protects PC12 cells from lidocaine-induced cytotoxicity through downregulation of COL3A1 mediated by miR-let-7b. DNA Cell Biol. 2017;36:518–528.
- Holowka D, Baird B. Mechanisms of epidermal growth factor receptor signaling as characterized by patterned ligand activation and mutational analysis. Biochim Biophys Acta Biomembr. 2017;1859:1430–1435.
- Iyer S, Prajapati R, Ramesh A, et al. The future of lung cancer therapy: striding beyond conventional EGFR and ALK treatments. Mol Clin Onc. 2019;10:469–475.
- Belo A, Cheng K, Chahdi A, et al. Muscarinic receptor agonists stimulate human colon cancer cell migration and invasion. Am J Physiol Gastrointest Liver Physiol. 2011;300:G749–60.
- Xie G, Cheng K, Shant J, et al. Acetylcholine-induced activation of M3 muscarinic receptors stimulates robust matrix metalloproteinase gene expression in human colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2009;296:G755–63.
- Wang H, Yu Z, Huo S, et al. Overexpression of ELF3 facilitates cell growth and metastasis through PI3K/Akt and ERK signaling pathways in non-small cell lung cancer. Int J Biochem Cell Biol. 2018;94:98–106.
- Chen J, Jiao Z. Lidocaine inhibits melanoma cell proliferation by regulating ERK phosphorylation. J Cell Biochem. 2019;120:6402–6408.