Abstract
The nervous system is known as a crucial part of the body and derangement in this system can cause potentially lethal consequences or serious side effects. Unfortunately, the nervous system is unable to rehabilitate damaged regions following seriously debilitating disorders such as stroke, spinal cord injury and brain trauma which, in turn, lead to the reduction of quality of life for the patient. Major challenges in restoring the damaged nervous system are low regenerative capacity and the complexity of physiology system. Synthetic polymeric biomaterials with outstanding properties such as excellent biocompatibility and non-immunogenicity find a wide range of applications in biomedical fields especially neural implants and nerve tissue engineering scaffolds. Despite these advancements, tailoring polymeric biomaterials for design of a desired scaffold is fundamental issue that needs tremendous attention to promote the therapeutic benefits and minimize adverse effects. This review aims to (i) describe the nervous system and related injuries. Then, (ii) nerve tissue engineering strategies are discussed and (iii) physiochemical properties of synthetic polymeric biomaterials systematically highlighted. Moreover, tailoring synthetic polymeric biomaterials for nerve tissue engineering is reviewed.
Introduction
Nerve system disorders annually affect millions of people around the world and might create dire consequences owing to the limited ability of the nerve system especially the central nervous system (CNS) for the regeneration of damaged regions in mammals [Citation1]. On the other hand, nerve system disorders are associated with a reduction in quality of life and debilitating economic and social burdens [Citation2]. There are several glycoproteins in the myelin of the CNS that act as inhibitors for the regeneration of injured nerves [Citation3]. The pathophysiological response in the CNS differs from the peripheral nervous system (PNS). Macrophage infiltration in the CNS is very slow relative to PNS that in turn result in delaying the elimination of inhibitory myelin [Citation4].
An intrinsic regenerative ability of the PNS might provide the possibility for repairing of damaged nerves after injury while the adult CNS fails to spontaneously rehabilitate after injury [Citation5]. In the case of small injuries, peripheral nerves have the ability to rehabilitate on their own over partly short distances in a suitable condition. When peripheral nerves transected, a sequential chain of molecular and cellular events has occurred that is known as Wallerian degeneration [Citation6]. This process includes reduction of the myelin sheaths to granular and amorphous debris in the distal stump of transected nerve by the Schwann cells within 24 h, transformation of the myelin sheath toward the short segment within 48 h, phagocytosis of the myelin debris and axon fibers by activated macrophages into the degenerating nerve stumps, proliferation and migration of the Schwann cells toward the damaged site and formation of longitudinal cell columns (known as Bands of Bungner) that in turn result in production of neurotrophic factors and regeneration of damaged nerve () [Citation7].
Figure 1. (A) Schematic representation of the Wallerian degeneration [Citation9]. (B) Schematic representation of end-to-end surgical reconnection of the injured nerve ends [Citation10]. (C) Schematic representation of the autologous nerve grafting [Citation11].
![Figure 1. (A) Schematic representation of the Wallerian degeneration [Citation9]. (B) Schematic representation of end-to-end surgical reconnection of the injured nerve ends [Citation10]. (C) Schematic representation of the autologous nerve grafting [Citation11].](/cms/asset/c1255b28-44d0-42e7-979d-7b0e64926551/ianb_a_1639723_f0001_c.jpg)
The autologous nerve grafting and end-to-end surgical reconnection of the injured nerve ends are current gold standard techniques to repair damaged peripheral nerves [Citation8] ().
Although reconnecting the two nerve ends can be an efficient approach for repair of small defects or gaps, it not suitable for large gaps since the presence of any tension in the site of injury inhibits regeneration. In the case of large gaps, an autologous nerve graft can contribute to the span of the damaged area [Citation12]. Likewise, this process possesses several major disadvantages including the possibility of a mismatch between donor and recipient nerves, limited availability of donor tissue, secondary deformities, donor site morbidity, and requirement of a second surgery to provide the donor nerve [Citation13,Citation14]. In the case of CNS injuries, particularly SCI, clinical treatment options are anti-inflammatory drugs that can provide protection against secondary injury. Indeed, there is no current treatment to improve function or hinder primary injury after CNS injuries. Currently, many novel therapeutic agents and methods have been introduced to treat neurological disorders and other diseases [Citation15–19]. Tissue engineering is defined as fabrication of tissue substitutes that imitate the structural and physiological nature of native tissue by merging principles and methods of cell biology, engineering, and material science [Citation20,Citation21]. In recent years, researchers have developed neural tissue engineering strategies as potential treatments for CNS and PNS injuries to overcome drawbacks of current therapeutic approaches or techniques [Citation22,Citation23]. Owing to unique properties, Polymer biomaterials have attracted much attention from the scientific community for neural tissue engineering purposes and controlling neuronal cell behaviors such as proliferation, differentiation, neurite outgrowth as well as nerve gap bridging [Citation24]. Generally, a comprehensive understanding of the advantages and disadvantages of synthetic polymeric biomaterials and possible solution for tailoring them neural tissue engineering is needed in their future translation for routine clinical use. A pointed understanding of the synthetic polymeric biomaterials and their physiochemical properties contribute to the promotion of the therapeutic benefits and reduction of adverse effects.
Here, nerve tissue overview and nerve tissue engineering strategies are highlighted. Then, the physiochemical properties of synthetic polymeric biomaterials are discussed and possible solution for tailoring these polymers for neural tissue engineering is reviewed.
Nerve tissue overview
The nervous system is composed of two main parts including the CNS and the peripheral nervous system (PNS). The CNS includes brain, spinal cord, olfactory, optic and auditory systems that are composed of neuronal cells, glial cells, supporting connective tissue and blood vessels. The main function of CNS is the reception, interpretation of signals and sending excitatory stimuli to the PNS [Citation25].
Owing to intricate tasks and even supporting the most daily living functions, the CNS possesses a high degree of anatomical complexity [Citation26]. The PNS is formed from peripheral, cranial (arising from the brain) and spinal nerves as well as their motor and sensory endings. A multilayered building containing bone, meninges, connective tissue and skin support the nervous system. In fact, the spinal cord acts as a bridge for connecting the brain to the rest of the body and facilitates receiving and sending sensory and motor information via electrochemical impulses to and from the PNS [Citation27]. There are two types of cells within the nerve system including neurons and neuroglia. Neurons are known as the basic structural and functional components of the nervous system and are comprised of soma, axons, and dendrites. Dendrites provide the possibility for transmission of electrical signals to soma and axons steer impulses away. Glial cells, or neuroglia act as supporting cells for neuronal function and are classified into three type cells including astrocytes, oligodendrocytes (present in the CNS) and Schwann cells (present in the PNS). The amount of glial cells is more than neurons in the nervous system. Although neurons are not capable to perform mitosis, glial cells have the ability in cell division. It is also worth to note that neurons can rehabilitate or trigger sprouting in certain conditions. The spinal cord is comprised of dendrites, axons, and cell bodies.
The spinal cord structure is simpler relative to the brain but it is more vulnerable in trauma [Citation28]. Although the spinal cord is protected by several structures including cerebrospinal fluid, three meningeal layers, and vertebrae relative to extending peripheral nerves, a growing list and high incidence of SCI cases have been reported around the world in recent years (between 12,000–15,000 people in North America each year) [Citation29]. Generally, the spinal cord is formed from grey matter (butterfly-shaped area) that is surrounded by white matter. The grey matter includes lower motor neurons (a receipt of signals from upper motor neurons in the brain), sensory neurons (a receipt of signals from the periphery), glial cells and blood vessels. The axons and glial cells such as astrocytes, oligodendrocytes, and microglia are the main components of the white matter in the spinal cord.
In the white matter, the ascending tracts of myelinated axons carry sensory signals to the brain and descending tracts of myelinated axons relay motor information or modulatory signals from the brain to the muscles [Citation30]. Therefore, damage to these tracts can result in serious functional deficits in the human body. On the other hand, the rehabilitation of large crushed or sectioned segments of peripheral nerves has been often challenged owing to the high complexity in the biological environment and the lack of proper treatment options towards foster reconstruction. Peripheral neuropathy is known as damage to a peripheral nerve that is caused by interference in nerve conduction and can result in derangement of motor and sensory functions within the PNS. Generally, the leading causes of peripheral neuropathies are compression, elongation or laceration. The most type of traumatic neuropathy is elongation that is created when tension is more than its capacity for resistance. The nerve laceration includes about 30% of severe peripheral nerve injuries and several fragments of the nerve maintain nerve continuity even in the presence of a complete tear. In the case of nerve compression, continuity is maintained but the loss of motor and sensory function may occur due to several mechanisms such as ischemia, mechanical compression, and deformation. A common consequence of prolonged mechanical compression is damage to endoneurial channels that in turn can create a sequential chain of endoneurial oedema, alteration of the ionic balance and fascicular microcirculation, and subsequently, ischemia owing to the increase in the endoneurial fluid pressure [Citation31]. In the case of CNS, injuries are classified into two major types including acute and chronic. Acute injuries often create irreversible structural and functional changes and are including stroke, traumatic brain injury (TBI), and acute SCI. The designing therapeutic and regenerative systems for treatment are important fundamental issues that need great attention owing high incidence of these injuries (800,000 strokes and 1.7 million TBIs in the United States (US) each year). Current treatment option for ischemic stroke is mechanical thrombectomy or using an intravenous thrombolytic agent. One of the side effects of tissue plasminogen activator (tPA) as the gold standard treatment for ischemic stroke is the enhancement of inflammatory response in brain capillaries and consequently cell death [Citation32]. There are no current options to improve clinical outcomes after a hemorrhagic stroke. In the case of TBIs, although surgical treatment can be used to prevent or reduce secondary injuries, it is inadequate to minimize primary damages and improve regeneration of damaged tissues. The chronic degenerative disorders in CNS are including Alzheimer’s disease (AD), Parkinson’s disease (PD), and brain neoplasm. AD is the most common type of neurodegenerative diseases around the world, leading cause of dementia and the sixth leading cause of death in the US. There are no treatment options for AD and cholinesterase inhibitor and memantine only relieve AD symptoms. PD has been known as the second most common type of neurodegenerative disorders and its medical costs in 2010 were $14 billion in the US [Citation33]. It has been reported that brain tumours have reached a total cost of about 5.2 billion euros in 2010 in Europe [Citation34].
Nerve tissue engineering strategies
In the case of peripheral nerve injuries, big gaps need clinical techniques to autologous approaches to provide the desired recovery. Discovery of an alternative to the autologous nerve graft and requirements for elimination of two surgeries and the removal of tissue from the patient has been posed as challenges of PNS injuries [Citation35].
A current possible solution is a design and development of engineered ‘‘nerve guidance channels’’ (NGCs) that contribute to physically guide the regeneration of damaged nerve along with lesions () [Citation36]. Generally, an NGC is comprised from a tubular device with a single lumen that acts as a bridging device to repair damaged peripheral nerves by isolating the regenerating axons from scar tissue and its preservation against compression by the surrounding tissue. NGCs are advantageous in that they reduce the ingrowth of scar tissue in the site of injury; contribute to the sprouting of axons from the proximal nerve; centralize growth factors secreted by the damaged nerve ends [Citation37]. Currently, researchers have widely used natural or synthetic biomaterials to fabricate NGCs with various structural and physicochemical features () [Citation38–40]. Tailoring NGC chemical structure and composition provide a possibility to obtain a conduit with desired properties such as excellent mechanical strength, good biocompatibility, biodegradability, and permeability. In fact, NGCs should be capable of improving cellular behaviours and provide mechanical and molecular signalling towards nerve generation without creating adverse effects on cells and nerve tissues [Citation41]. On the other hand, degradation pattern of NGCs fabricated by biomaterials is a fundamental issue that needs significant attention since a suitable degradation rate provides the possibility for such device to withstand the mechanical compression stresses of surrounding tissues until perfect nerve rehabilitation and avoid guide collapse [Citation42]. Moreover, degradation of NGCs should happen with minimal foreign body reaction and swelling [Citation43]. In the case of porous NGCs, the surface erosion rate is greater than non-porous NGCs because degradation pattern relies on the free surface in contact with the medium. Mechanical strength of NGC should be close to natural nerves to tolerate physiological loads [Citation44]. It is also worth noting that, mechanical properties of NGCs can be affected by porosity, the composition of biomaterials, presence of lumen fillers, and wall thickness [Citation45]. Another crucial factor for design of NGC is the permeability that gives rise to the supply of nutrients and oxygen to cells as well as prevention the missing of the neurotrophic factors secreted by Schwann cells at the site of injury. Some properties of polymeric biomaterials such as hydrophilicity/hydrophobicity, degree of crystallinity and porosity, the chemical composition, and functionalization groups can affect permeability of NGC. Several complex guide designs have introduced by different researchers including i) Single hollow lumen porous or non-porous NGCs that consist of longitudinally oriented grooves in their lumen surface that can be modified with bioactive molecules. Since such conduits may confer not complete reinnervation owing to axon dispersion or polyinnervation of various targets by the axons of the same motoneuron, they are commonly used for small lesions (<30 mm) in the sensory nerves, ii) Porous or non-porous single lumen NGCs are comprised from fillers (porous sponges, gels, and aligned fibers) as topographical cues that promote rehabilitation by mimicking the endoneurial-like structure of autologous nerve grafts. In fact, fillers should imitate the morphology and composition of ECM protein based intraluminal matrix to support repairment of the damaged nerves, iii) Multichannel NGCs imitate the natural compartment structure of nerves [Citation46]. These conduits possess several advantages compared to single lumen NGCs including reduction of axon dispersion, the higher surface area for modification, migration, and attachment of cells. On the other hand, their major drawbacks such as reduced permeability and mechanical flexibility limit using them for nerve tissue engineering. In the case of the CNS, challenges are greater compared to the PNS since there is no efficient method to prevent primary and secondary injuries.
Figure 2. Nerve guidance channels improve axonal growth without the formation of mismatches. (A) Schematic representation of inhibitory elements for axonal regeneration after peripheral nerve injury and the possible formation of mismatches. (B) Presence of a rigid tubular structure provides mechanical support and promotes axonal regeneration [Citation47]. (C,D) SEM images of a nerve guidance channel (NGC) fabricated by RGD-conjugated polyurea and polycaprolactone. (E) Sciatic nerve isolation. (F) Sutured autograft and (G) sutured conduit fabricated by RGD-conjugated polyurea and polycaprolactone [Citation38]. (H) Representative digital image of an NGC fabricated by chitosan-gelatin-polypyrrole polymers. (I) Sciatic nerve isolation and (J) sutured conduit fabricated by chitosan-gelatin-polypyrrole polymers [Citation48]. In the next sections, the physiochemical properties of synthetic polymeric biomaterials are discussed and possible solution for tailoring these polymers for neural tissue engineering is reviewed. In the next section, we reviewed the physiochemical properties of synthetic polymeric biomaterials that make them suitable candidates for nerve tissue engineering purposes.
![Figure 2. Nerve guidance channels improve axonal growth without the formation of mismatches. (A) Schematic representation of inhibitory elements for axonal regeneration after peripheral nerve injury and the possible formation of mismatches. (B) Presence of a rigid tubular structure provides mechanical support and promotes axonal regeneration [Citation47]. (C,D) SEM images of a nerve guidance channel (NGC) fabricated by RGD-conjugated polyurea and polycaprolactone. (E) Sciatic nerve isolation. (F) Sutured autograft and (G) sutured conduit fabricated by RGD-conjugated polyurea and polycaprolactone [Citation38]. (H) Representative digital image of an NGC fabricated by chitosan-gelatin-polypyrrole polymers. (I) Sciatic nerve isolation and (J) sutured conduit fabricated by chitosan-gelatin-polypyrrole polymers [Citation48]. In the next sections, the physiochemical properties of synthetic polymeric biomaterials are discussed and possible solution for tailoring these polymers for neural tissue engineering is reviewed. In the next section, we reviewed the physiochemical properties of synthetic polymeric biomaterials that make them suitable candidates for nerve tissue engineering purposes.](/cms/asset/926f4f14-87d9-4b8d-bb63-bcbd43dbcbc2/ianb_a_1639723_f0002_c.jpg)
Synthetic polymers
Owing to the possibility of tailoring the physiochemical and mechanical properties for mimicking of biological tissues, synthetic polymers have attracted tremendous attention of the scientific community for nerve tissue engineering and regenerative medicines purpose. These polymers are advantageous for tissue engineering purpose because their degradation pattern is based on simple hydrolysis and remains constant for every host [Citation49].
Poly(α-hydroxy esters)
Poly(α-hydroxy esters) polymers are formed from poly(lactic acid) [PLA] and poly(glycolic acid) [PGA] that the successful use of them was reported in the late 1960s.
The unique properties of Poly (α-hydroxy esters) such as easy processing ability, good mechanical strength, high biocompatibility, and non-immunogenicity, low toxicity, and biodegradability make them good candidates for drug delivery and tissue engineering [Citation50]. Currently, they are widely used for design and fabrication controlled drug delivery nanosystems in medicine because the United States Food and Drug Administration (USFDA) approved their parenteral administration. Their degradation is based on hydrolytic cleavage of the ester bonds that in turn result in the production of lactic acid [LA] and glycolic acid[GA] groups [Citation49].
Poly (glycolic acid) [PGA]
The polyglycolic acid is a biodegradable, thermoplastic polymer and simplest linear aliphatic polyester with high molecular weight about 20,000e1,45,000. PGA is derived from glycolide synthesized by fermenting sugarcane or pineapples. The ester groups are responsible for stabilizing the polymeric structure. Likewise, desired molecular packing is responsible for the excellent mechanical properties, crystallinity (45–55%) and high melting point (224–228 °C) of this polymer. The final product of its degradation is the glycolic acid group that is excreted in the urine. This polymer can be fabricated by ring-opening polymerization of GA [Citation51].
Polylactic acid or polylactide (PLA)
PLA is a thermoplastic polymer and a product of starch from renewable resources like potatoes, beets, corn, and sugarcane. It can be synthesized by several methods including ring-opening polymerization of lactide dimer in the presence of a suitable catalyst, polycondensation of lactic acid or enzymatic synthetic processes by lipases without the presence of metallic catalysts [Citation52–54]. PLA can be fabricated as PDLLA, PDLA, and PLLA because it has two stereoisomers of lactic acid including L- and D-lactic acid [Citation55]. The outstanding properties of PLA such as good mechanical strength (Young's modulus = 1.2–3 GPa), processability, biocompatibility, and biodegradability make it a suitable material for biomedical application such as drug delivery [Citation56] and tissue engineering [Citation57]. Slow degradation rate, hydrophobicity, and low impact toughness of PLA may limit its biomedical applications. A possible solution is its blending with other polymers [Citation58]. The Methyl side chain of PLA makes it more hydrophobic compared to PGA.
The degradation pattern of PLA is based on non-enzymatic hydrolysis and its byproduct is lactic acid that is easily eliminated in normal cell metabolism [Citation59].
Poly (lactic-co-glycolic acid) [PLGA]
Poly (lactic-co-glycolic acid) or PLGA is a synthetic and block copolymer that is comprised from lactic acid and glycolic acid monomers. Glass transition temperature of PLGA is about 45–55 °C that confers rigid chain structure in nature. Some properties of PLGA such as mechanical strength, glass transition temperature, crystallinity, swelling, and capability of biodegradation can be adjusted by the molar ratio of the individual monomer components.
Among the copolymers of PLA and PGA, PLGA hold great promise in various medical fields such as drug delivery [Citation60], nanobiosensors [Citation61], tissue engineering [Citation62,Citation63] as well as fabrication of medical and surgical devices [Citation64]. Owing to sustained-release properties, film-forming ability, favourable thermal stability, biocompatibility, and biodegradability, PLGA has been approved by the US FDA for biomedical applications [Citation65,Citation66]. PLGA-based materials are advantageous for tissue engineering purposes in that they have micropores on the surface that lead to high nutrient permeability and subsequently improvement of cell attachment and proliferation [Citation67].
The degradation pattern of PLGA highly depends on the sequence of monomers into its structure. Random PLGA exhibit rapid degradation compared to sequenced PLGA [Citation68]. When PLGA come into contact with biological media, its backbone is randomly hydrolyzed owing to the presence of ester linkages that in turn result in the production of one OH group and one COOH group. Scission of long polymer chains and subsequently the decrease in the molecular weight result in the production of water-soluble polymer fragments. Finally, further degradation of these fragments produce glycolic and lactic acids which are biologically inert to the cells and are expurgated in the body via metabolic pathways [Citation69].
Polyanhydride polymers
For the first time, Polyanhydride, one type of degradable synthetic biopolymers, were fabricated through activation of the aromatic dicarboxylic acids using acetic anhydride. Good mechanical properties, prolonged shelf-life and degradation time of different types of polyanhydride polymers make them good candidates for the controlled delivery of drugs [Citation70]. Most Polyanhydride polymers can be synthesized via simple reactions of activated dicarboxylic acids under vacuum and heat. Limited shelf-life, high reactivity, and instability are the major shortcomings of such polymers [Citation49].
Polycaprolactone
Polycaprolactone (PCL) is an FDA approved aliphatic polyester that is made of hexanoate repeat units. It is known as a semicrystalline polymer and its crystallinity degree can reach to 69%. PCL can be synthesized by two main methods including the ring-opening polymerization of a lactone (e-caprolactone) and the polycondensation of a hydroxycarboxylic acid (6-hydroxyhexanoic acid) [Citation71]. Superior viscoelastic properties and its low melting temperature provide the possibility to prepare it in a large range of shapes and sizes [Citation72]. Despite the PLGA polymer, the production cost of PCL is inexpensive. A two-phase of degradation pattern has been described for PCL polymer under in-vivo conditions. Frist, PCL polymer is hydrolyzed into low-molecular-weight oligocaprolactone (OCL) with higher crystallinity. In the next step, macrophage and giant cells eat the OCL with a molecular weight of fewer than 3,000 Daltons through phagocytosis. Finally, the intracellular degradation of these fragments occurs by esterases [Citation73]. The degradation profile of PCL is very slow and takes up to 24 months [Citation74]. Owing to Outstanding properties such as good mechanical strength, high crystallinity, bioadsorbable and biocompatible nature, PCL has widely been used for drug delivery and tissue engineering purposes [Citation75–77].
Poly (propylene fumarate) (PPF)
Poly (propylene fumarate) (PPF), a linear polyester, is fabricated from fumaric acid. Fumaric acid is a component of the Krebs cycle and its unsaturated carbon-carbon bonds provide the possibility for cross-linking of this polymer into a covalent polymer network. One advantage of PPF is the creation of excretable and f biocompatible degradation products including fumaric acid and propylene glycol [Citation78]. Indeed. Degradation pattern of PPF is based on simple hydrolysis of the ester bonds and required time for degradation relies on molecular weight, and cross-linker type and density. It has recognized that the degree of cross-linking of PPF can affect cellular behaviour [Citation79].
The major limitation of PPF is its viscous liquid at room temperature that, in turn, is resulted in cumbersome handling of this polymer. The unique properties of PPF-based Polymers such as biocompatibility, absorbability, injectability, and biodegradability provide the possibility for their extensive usages in biomedicine fields such as scaffolds for tissue regeneration and controlled nanodrug delivery system, preparation of orthopaedic implants and nano-vehicles for cell transplantation [Citation80].
Polyethylene glycol (PEG)
Polyethylene glycol (PEG) is a hydrophilic FDA approved polymer with low toxicity in humans that have been widely used cosmetic products and drug industry [Citation81]. PEG can prevent attachment of other proteins to the scaffold surface which in turn is resulted in minimizing adverse immune responses [Citation82]. It is known as the most widely used “stealth” polymer in the field of drug delivery that can promote systemic circulation time [Citation83]. The molecular weight of PEG can affect swelling and mechanical properties of hydrogels for tissue engineering purposes [Citation84].
Polyvinyl alcohol
Polyvinyl alcohol (PVA), a biocompatible and solubilized crystalline structure polymer, is fabricated from polyvinyl acetate by hydrolysis process. PVA is widely used for medical and industrial and commercial applications [Citation85]. Owing to the presence of hydroxyl groups on the carbon atoms, degradability of PVA occurs via hydrolysis. Hydrophilic nature, the ability of swelling, lack of toxicity and biodegradability of the polymer make it benign to living tissue and suitable for preparation of scaffolds. Transformation of PVA into hydrogels is very easy since the reactive alcohol group can be decorated by crosslinkers. Incomplete degradation property of PVA polymer is a major drawback that might limit its applications in biomedicine field.
Polyurethane
For the first time, Polyurethane was fabricated from a disocyanate and polyols by Otto Bayer in 1937 [Citation86]. Polyurethanes are a class of polymers containing a chain of organic units connected by urethane links [Citation87]. Crystallinity, presence of chemical groups in the molecular chains, molecular orientation and cross-linking are determinant factors in biodegradation of polyurethane [Citation88]. A wide range of degradation patterns has been reported for polyurethane including hydrolysis, oxidation, thermal and enzymatic processes [Citation89,Citation90].
Polyurethanes are advantageous for tissue engineering purposes in that they have many unique properties such as fatigue resistance, flexibility, strong adhesion to many substances, durability, and elastomer-like characteristics [Citation91,Citation92].
Tailoring synthetic polymeric biomaterials for nerve regeneration
PGA
A randomized prospective study by Weber et al showed that PGA conduits exerted higher improved sensation for nerve gaps of 4 mm or less relative to the end-to-end repair of digital nerves. Moreover, the authors mentioned that PGA conduits provided better results compared with nerve grafts and omitted the donor-site morbidity caused by nerve graft harvesting [Citation93].
At variance with silicone tube conduits, PGA based scaffolds are semipermeable and slowly hydrolyzed in vivo [Citation94]. The slow degradation pattern and good mechanical properties of PGA make it a suitable material for nerve regeneration. For instance, Matsumoto et al. fabricated PGA–collagen tube filled with laminin-coated collagen fibres to determine peripheral nerve regeneration. They used PGA mesh to reinforce the nerve conduit for preventing from fast degradation of the conduit and maintain the required space for axonal elongation [Citation95].
Although PGA fibres are suitable materials and substrate for neuronal stem cells, their hydrophobicity may limit their applications in nerve tissue engineering. A possible solution is surface modification with adhesive materials [Citation96]. In an interesting example, Kojima et al. coated PGA fibres with linear and dendrigraft polylysines to increase cell attachment to them. They found an increased cell adhesion to the PGA fibres coated with dendrigraft polylysine even at a low concentration (0.05 μg/ml) [Citation97].
PLA
The unique features of PLA make it an excellent material to prepare scaffolds for nerve tissue engineering. A major limitation of polyester surfaces is suboptimal hydrophilicity that decreases cell adhesion. Currently, researchers have focused on variations in physiochemical properties of PLA scaffolds to improve their efficiency for nerve tissue engineering. To this end, Zhang et al. decorated PLLA nanofibrous scaffolds with the heparin/collagen encapsulating nerve growth factor (NGF) multilayers through layer-by-layer (LbL) self-assembly method to improve their hydrophilicity for peripheral nerve tissue engineering purposes. They found that sustained release of bioactive NGF from the designed scaffold contributed to the proliferation of Schwann cells and differentiation of PC12 cells [Citation98].
Likewise, Haddad et al. firstly decorated PLA scaffolds with polyallylamine to introduce amine groups and then functionalized them with epidermal growth factor to create a medium for supporting the proliferation of Neural Stem-Like Cells even in the absence of soluble growth factors for 14 days [Citation99]. In another interesting example, Hoveizi et al. modified PLA scaffold with chitosan to increase its biocompatibility and affinity to PC12 cells for nerve tissue engineering purposes [Citation100]. It has been reported that the concentration of PLA and its blending with other polymers or biomaterials can affect physiochemical properties of scaffolds for nerve tissue engineering. For instance, Yang et al. reported that the concentration of PLLA highly affected the porosity and the fibre diameters as well as the behaviour of nerve stem cells (NSCs) [Citation101]. The authors mentioned that increasing the concentration of PLLA solution significantly increased the fibre diameters and decreased surface area to volume ratio and porosity of nano-fibrous scaffolds ().
Figure 3. The effects of PLLA on porosity of scaffold. (A) 2% wt/v, (B) 5% wt/v and (C) 9% wt/v [Citation101].
![Figure 3. The effects of PLLA on porosity of scaffold. (A) 2% wt/v, (B) 5% wt/v and (C) 9% wt/v [Citation101].](/cms/asset/62b53a69-cb07-4e79-8fd6-0f9bc46a4ab8/ianb_a_1639723_f0003_b.jpg)
They found that not only 2% wt/v PLLA/THF solution had better porosity and surface area to volume ratio but also its mechanical strength was weak. Since in vitro cell culture of NSCs needs both high porosity (towards cell migration and nutrient supply) and good mechanical strength of scaffolds, they chose 5% wt/v PLLA/THF solution [Citation101]. Likewise, Yang et al. reported that NSCs exhibited higher differentiation on the surface of PLLA nanofibers compared to microfibers [Citation102]. In another work by Zhang et al. aligned PLLA nanofibrous scaffolds were coated with GO nanosheets through aminolysis process. The authors reported that aminolysis and GO nanosheets coating led to the introduction of OH, COOH, and NH2 groups onto the surface and subsequently improved proliferation, differentiation and neurite growth of PC12 cells [Citation103].
The bioelectricity present in the human body contributes to maintaining normal biological functions like wound healing, muscle contraction and signalling of the nerve system [Citation104]. High-rate transportation of electrical charges leads to neural differentiation through the improvement of cell-to-cell or cell-to-substrate interactions. In order to provide optimal conductivity for nerve tissue engineering, coated 3 D PLLA fibrous scaffold with polypyrrole (a conductive polymer) nano-layer through situ surface polymerization [Citation105].
PLGA
The weak electrical conductivity of PLGA can limit its application for nerve tissue engineering. To this end, Lee et al. coated electrospun PLGA nanofibers with polypyrrole to investigate their effect on the growth and differentiation of hippocampal neurons and PC12 under electrical stimulation (). Generally, they concluded that PPy–PLGA scaffolds, stimulated with a potential of 10 mV/cm, exhibited 40–90% more neurite formation and 40–50% longer neuritis relative to unstimulated cells on PPy–PLGA scaffolds [Citation106].
Figure 5. Schematic representation of Poly-L-lysine coated PLGA/MWCNTs scaffold design and differentiation of PC12 cells into it with electrical stimulation [Citation108].
![Figure 5. Schematic representation of Poly-L-lysine coated PLGA/MWCNTs scaffold design and differentiation of PC12 cells into it with electrical stimulation [Citation108].](/cms/asset/ade416e3-ee67-467f-8a6c-bc2dff9e15fc/ianb_a_1639723_f0005_c.jpg)
High hydrophobicity of PLGA results in lack of surface cell discrimination points and subsequently poor attachment of cells on the surface of scaffolds. In this scenario, a possible solution is to combine biocompatible materials or bioactive molecule into the PLGA backbone. For example, Mehrasa et al. fabricated a scaffold containing PLGA/gelatin nanofibers embedded with silica nanoparticles for nerve tissue engineering. They found that incorporation with gelatin as well as embedding with silica nanoparticles led to the increase of hydrophilicity and tensile mechanical properties. Finally, they observed that PLGA/gelatin nanofibers embedded with silica nanoparticles exhibited higher attachment and proliferation of PCl2 cells compared to pure PLGA scaffolds [Citation107]. Another research group has created a hydrophilic surface onto the surface of aligned nanofibrous scaffold composed of PLGA/multi-walled carbon nanotubes (MWCNTs) by poly-L-lysine coating to prepare it for nerve tissue engineering [Citation108]. Moreover, MWCNTs were used to improve the electrical conductivity of scaffold. Poly-L-lysine coated PLGA/MWCNTs scaffolds provided a better environment for adhesion of PC12 cells and exogenous electrical stimulation resulted in better neuronal differentiation of PC12 cells compared to unstimulated cells [Citation108] ().
Drugs and growth factors can be chemically or physically entrapped into PLGA scaffolds for protection of degradation and sustained release in the site of interest of repair of damaged nerves. For instance, Reis et al. synthesized core-shell PLGA microfibers and encapsulated FGF-2 into these structure by coaxial electrospinning. They found that PLGA/FGF-2 coaxial microfibers supported attachment and proliferation of PC12 cells as well as improved locomotor recovery after 28 days in a rat model of SCI [Citation109].
Polyanhydride polymers
In the case of polyanhydride polymers, Griffin prepared aligned PLGA bioactive polyanhydride electrospun fibres that were capable to support neurite outgrowth and the formation of linear Schwann cell processes as the building blocks of the bands of Bungner [Citation110]. In addition to supporting the adhesion and proliferation of neuronal cells, sustained release of anti-inflammatory drugs in the site of injury can be achieved by entrapping drug into the backbone of polyanhydride scaffolds.
In another study, the same group synthesized salicylic acid (SA)‐based poly(anhydride‐ester) NGCs as scaffold and a drug delivery system with the controllable release of SA, a nonsteroidal anti‐inflammatory drug, and implanted it in femoral nerves of mice for 16 weeks to investigate functional recovery. Generally, they concluded SA‐NGCs significantly reduced inflammation and scar tissue formation as well as improved functional recovery [Citation111].
PCL polymer
As mentioned above for other polymers, PCL polymer can be tailored for nerve tissue engineering purposes by manipulating its properties. For instance, Panahi-Joo et al evaluated the behaviour of PC12 cells on the surface of highly aligned fibres (2 P samples), Semi-aligned fibres (M1), and random fibres (M2) of PCL. They reported that tensile strength and the crystallinity degree of 2 P samples was more than M1 and M2 fibres that in turn resulted in better migration, distribution, growth, and elongation of cells on uniform aligned fibres [Citation112]. The need for tailoring of PCL arises from its major drawbacks such as hydrophobicity, lack of functional groups, and slow degradation rate that can limit its applications for nerve tissue engineering. One strategy to achieve the desired hydrophilicity is to blend it with other polymers. For example, Lorenzo et al. designed electrospun PCL/chitosan scaffolds for nerve regeneration. They reported that increasing chitosan content decreased the water contact angle from 113° (for PCL) to 52° and subsequently improved attachment and proliferation of fibroblasts and Schwann cells on the surface of scaffolds [Citation113]. In another work, Saderi et al. doped gold nanoparticles into PCL/chitosan nanofibrous scaffolds to improve their conductivity for nerve tissue engineering.
They observed that the conductivity of scaffolds was significantly enhanced following the inclusion of gold nanoparticles that in turn promoted attachment and proliferation of Schwann cells compared to PCL/chitosan nanofibrous scaffolds () [Citation114].
Figure 6. (A,B) FE-SEM micrographs of the AuNPs-doped PCL/chitosan scaffolds exhibited the presence of AuNPs on the substrate without any destruction of microstructure. (C) The evaluation of Schwann cell proliferation on the substrate by MTT assay after 1 and 5 days. (D,E) FE-SEM micrograph of Schwann cell morphology on PCL/chitosan and the AuNPs-doped PCL/chitosan [Citation114].
![Figure 6. (A,B) FE-SEM micrographs of the AuNPs-doped PCL/chitosan scaffolds exhibited the presence of AuNPs on the substrate without any destruction of microstructure. (C) The evaluation of Schwann cell proliferation on the substrate by MTT assay after 1 and 5 days. (D,E) FE-SEM micrograph of Schwann cell morphology on PCL/chitosan and the AuNPs-doped PCL/chitosan [Citation114].](/cms/asset/54f928c0-0dc5-4b8e-a4a0-a0f9a38ffe2e/ianb_a_1639723_f0006_b.jpg)
Likewise, Entekhabi et al. combined Hyaluronic acid (one of the major components of the ECM) with PCL to improve hydrophilicity and biological properties of scaffold for nerve tissue engineering [Citation115]. In other work, PAN et al. developed NGCs with PPy-PCL- nanoyarns as fillers to evaluate their effects on nerve regeneration. They reported that using PPy as coating improved conductivity and Young’s modulus (from 10.55 ± 0.23 MPa to 11.73 ± 0.05) towards the better proliferation of Schwann cell than PPy-PCL-nanoyarns [Citation116]. It is well documented that PPF can be blended with other polymers to improve their physical properties for nerve tissue engineering. For example, wang et al. reported that weak mechanical properties of polycaprolactone fumarate (PCLF) limit its application for nerve regeneration since the nerve tubes formed of amorphous PCLF network cannot act as a bridge between the distal and proximal ends of an injured nerve tube and subsequently promote axon growth. To this end, they prepared a series of blends comprised of two photo-cross-linkable polymers including PPF and PCLF. Generally, they concluded that photo-cross-linked hybrid polymeric disks highly supported viability, attachment, and proliferation of SPL201 cells compared to only PCLF networks () [Citation117].
Figure 7. (A) Schematic representation of SPL201 cell on the cross-linked polymer disk. (B) Morphology (a-c × 25;d-f, ×200) of SPL201 cells on the cross-linked disks 7 days after seeding. (a) PCLF, (b) PPF/PCLF (25%), (c) PPF/PCLF (50%), and (d) tissue culture polystyrene (TCPS). (C) MTS absorption of SPL201 cells on the cross-linked disks of PCLF and PPF/PCLF (25%) and (50%) relative to cell-seeded TCPS as positive (+) control and empty TCPS as negative (−) control. (p < 0.05 between PPF/PCLF (50%) and PCLF, PPF/PCLF (25%)).
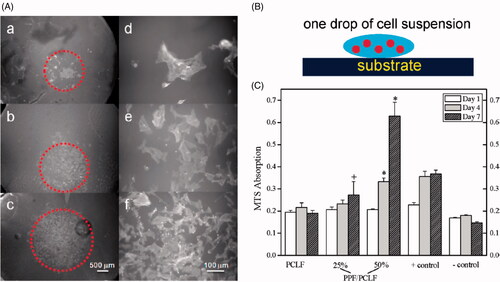
The design of a scaffold for nerve tissue engineering requires biomaterials with high mechanical strength. Another interesting approach is the use of PPF to enforce mechanical strength of scaffolds. To illustrate this concept, Chen et al. combined PPF with collagen biomaterial to provide biocompatible scaffold with high mechanical strength and minimum deformation in a short period compared to collagen alone. They observed that multichannel PPF-Collagen scaffold with collagen-binding neurotrophic factor 3 provides more efficient regrowth-supportive microenvironment for guiding neural tissue regeneration and inhibition of fibrotic scar formation following SCI compared to control, PPF and PPF-Collagen groups () [Citation80].
Figure 8. (A) Schematic representation of conduit containing PPF, collagen, and CBD-NT3 for treatment of peripheral nerve injury. (B) The greatest connectivity and integration with a normal spinal cord was found for the PPF + C+NT3 group. (C,D) The highest reduction of the chondroitin sulfate proteoglycans (CSPGs) as an inhibitor of regeneration was observed in the PPF + C+NT3 group. (E,F) The highest reduction of fibrotic scarring (Laminin) was observed in the PPF + C+NT3 group [Citation80].
![Figure 8. (A) Schematic representation of conduit containing PPF, collagen, and CBD-NT3 for treatment of peripheral nerve injury. (B) The greatest connectivity and integration with a normal spinal cord was found for the PPF + C+NT3 group. (C,D) The highest reduction of the chondroitin sulfate proteoglycans (CSPGs) as an inhibitor of regeneration was observed in the PPF + C+NT3 group. (E,F) The highest reduction of fibrotic scarring (Laminin) was observed in the PPF + C+NT3 group [Citation80].](/cms/asset/9d3fc1f8-0417-4781-94de-de5a5a7f938f/ianb_a_1639723_f0008_c.jpg)
In addition to mechanical properties, the porosity of scaffolds is an utmost important issue since it allows effective and uniform transport of nutrition for metabolic requirements of cells. Guo et al. reported that the concentration of PPF and solvents ratio strongly affected the formation of pores into the structure of scaffold. They mentioned that among different concentrations of (4, 8, and 12 wt%) polymer, 8 wt% demonstrated the best pore interconnectivity for nerve tissue engineering applications [Citation118].
PEG has been found to be an excellent candidate for the regeneration of damaged nerves due to its unique properties [Citation119]. For example, Ghergherehchi et al. reported that PEG-fusion contains neurorrhaphy blended with four pharmaceutical agents could contribute to the restoration of axonal continuity across the site of injury within minutes and fast recovery of voluntary behaviours relative to neurorrhaphy alone [Citation120]. At variance with the above-mentioned studies, Robinson et al. reported that PEG fusion disrupted the beneficial trophic influence of muscle on motor neuron reinnervation accuracy in rats [Citation121]. Moreover, Brown et al. reported that PEG did not exert beneficial effects in the regeneration of facial nerve injury in rats [Citation122].
For these controversial findings, Bittner et al. mentioned that neither they nor Robinson and coworkers have yet published any information on the accuracy of reinnervation of motoneurons following successful PEG fusion. They explained that detailed understanding of the success of PEG fusion technologies needs a collection of appreciating information on cellular/molecular mechanisms of axonal plasticity and specificity [Citation123].
Stocco et al. selected and prepared oxidized polyvinyl alcohol (OxPVA), PVA and SF as conduits to investigate their effects on peripheral nerve regeneration [Citation124]. It was found that OxPVA scaffolds exhibited better outcomes in terms of axon density relative to neat PVA conduits. These better outcomes for OxPVA scaffolds were attributed to the chemical structure of the polymer since it improved possible Schiff-base interactions between carbonyl groups of oxidized PVA and amino-groups of neurotrophic factors secreted by stumps which in turn resulted in the promotion of protein-loading [Citation124]. Weak cell affinity and the lack of antigenicity might disrupt the high efficiency of synthetic polymers such as PVA for nerve tissue engineering purposes. A possible solution is a combination of PVA with other polymers [Citation125].
To this end, Golafshan et al. carefully studied the effects of blending graphene nanosheets with sodium alginate (SA)/PVA on the improvement of scaffold properties and behaviour of PC12 cells [Citation126]. The graphene nanosheets into the structure of PVA were found to act as electrical nanobridges and improve electrical conductivity, toughness, and mechanical properties of scaffolds compared with pure SA/PVA. Likewise, the PC12 cells demonstrated stronger interactions and proliferation on the surface of the hybrid scaffold compared to neat SA/PVA [Citation126]. Recently, another report by Guo et al. demonstrated that modification of PVA nanofibers with keratose nanoparticles at different concentrations resulted in higher wettability, stronger mechanical properties, as well as higher adhesion and proliferation of relative to oxidative keratin (KOS)/PVA blend nanofibers () [Citation127].
Figure 9. (A,B) The fluorescence images of PC12 cells a and C6 cells b on PVA nanofibers, KNPs/PVA nanofibers with different mass ratios of KOS to PVA, and KOS/PVA blend nanofibers after 24 h. (C,D) Quantitative analyses of Cell numbers and length ratio on different nanofibers [Citation127].
![Figure 9. (A,B) The fluorescence images of PC12 cells a and C6 cells b on PVA nanofibers, KNPs/PVA nanofibers with different mass ratios of KOS to PVA, and KOS/PVA blend nanofibers after 24 h. (C,D) Quantitative analyses of Cell numbers and length ratio on different nanofibers [Citation127].](/cms/asset/88b4e3d9-8051-4e53-ad3e-8a11b46b28b3/ianb_a_1639723_f0009_c.jpg)
Many previous studies have shown the successful application of scaffolds based on polyurethane in nerve regeneration [Citation128,Citation129].
It was found that fabrication of polyurethane conduit based on PCL, PEG, and 1,6-hexamethyl diisocyanate is more efficient for peripheral nerve regeneration owing to better degradation rate compared to PCL one. They found that although PCL segments into the structure of polyurethane might decrease biodegradation owing to its hydrophobic property, the PEG segments provide the possibility for easier hydrolysis and enzymolysis due to its hydrophilic characteristics. The authors mentioned elastomeric polyurethane conduit lost 66.7% of its weight in 14 weeks owing to the presence of 33.3% PEG into the structure of conduit while degradation of PCL is more than one year [Citation130]. Likewise, Niu et al. reported that scaffolds from block polyurethanes based on PCL/PEG demonstrated much better regeneration behaviour than PCL, silicone tube due to the presence of biochemical and topographic cues and highly surface-area porous [Citation131]. It has been reported that coating of polyurethane with conductive polymers or blending with other polymers has resulted in improvement of the physicochemical properties such as mechanical strength, cytocompatibility, and electrical conductivity for nerve tissue engineering [Citation132].
Conclusions
Numerous attempts by many researchers worldwide have been made to promote the design of medical devices and biomaterials with high efficiency for the regeneration of damaged nervous system since seriously debilitating disorder such as SCI, brain trauma, and ischemic stroke result in a reduction in quality of life and economic and social burdens for patients. Low degradation rate, high hydrophobic nature and low conductivity of some synthetic polymeric biomaterials remain the main challenges in their usage with the highest efficiency and minimum side effects for the regeneration of damaged nerves in the routine clinic. On the other hand, utilizing the polymeric biomaterials has been often challenged by concerns regarding lack of bioactivity of these materials and the foreign body response. Understanding the pathophysiological changes in the damaged nervous system and smart design of synthetic polymeric biomaterials can overcome the barriers for future efficient and safe regeneration of damaged nerves in the routine clinic.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mahar M, Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci. 2018;19:323.
- Chamorro Á, Dirnagl U, Urra X, et al. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881.
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617.
- Zigmond RE, Echevarria FD. Macrophage biology in the peripheral nervous system after injury. Prog Neurobiol. 2018;173:102–121.
- Chen P, Piao X, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015;130:605–618.
- Zuo M, Guo H, Wan T, et al. Wallerian degeneration in experimental focal cortical ischemia. Brain Res Bull. 2019;149:194–202.
- Belanger K, Dinis TM, Taourirt S, et al. Recent strategies in tissue engineering for guided peripheral nerve regeneration. Macromol Biosci. 2016;16:472–481.
- Houschyar KS, Momeni A, Pyles MN, et al. The role of current techniques and concepts in peripheral nerve repair. Plastic Surg Int. 2016;2016:1.
- Alvites RD, Santos ARC, Varejão ASP, et al., Olfactory mucosa mesenchymal stem cells and biomaterials: A new combination to regenerative therapies after peripheral nerve injury, in mesenchymal stem cells–isolation, characterization and applications. Rijeka: InTech; 2017.
- Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347.
- Luca L. Chapter 10 – Nerve repair. In: Saunders RJ, et al., editors. Hand and upper extremity rehabilitation. 4th ed. St. Louis: Churchill Livingstone; 2016. p. 95–102.
- Meyer C, Stenberg L, Gonzalez-Perez F, et al. Chitosan-film enhanced chitosan nerve guides for long-distance regeneration of peripheral nerves. Biomaterials. 2016;76:33–51.
- Gu X, Ding F, Yang Y, et al. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93:204–230.
- Santos D, Wieringa P, Moroni L, et al. PEOT/PBT guides enhance nerve regeneration in long gap defects. Adv Healthcare Mater. 2017;6:1600298.
- Zhu W, Tringale KR, Woller SA, et al. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater Today. 2018;21:951–959.
- Amani H, Ajami M, Nasseri Maleki S, et al. Targeting signal transducers and activators of transcription (STAT) in human cancer by dietary polyphenolic antioxidants. Biochimie. 2017;142:63–79.
- Zarch AV, Toroudi HP, Soleimani M, et al. Neuroprotective effects of diazoxide and its antagonism by glibenclamide in pyramidal neurons of rat hippocampus subjected to ischemia-reperfusion-induced injury. Int J Neurosci. 2009;119:1346–1361.
- Javedan G, Shidfar F, Davoodi SH, et al. Conjugated linoleic acid rat pretreatment reduces renal damage in ischemia/reperfusion injury: unraveling antiapoptotic mechanisms and regulation of phosphorylated mammalian target of rapamycin. Mol Nutr Food Res. 2016;60:2665–2677.
- Habibey R, Pazoki‐Toroudi H. Morphine dependence protects rat kidney against ischaemia–reperfusion injury. Clin Exp Pharmacol Physiol. 2008;35:1209–1214.
- Langer R, Vacanti J. Advances in tissue engineering. J Pediatr Surg. 2016;51:8–12.
- Amani H, Mostafavi E, Arzaghi H, et al. Three-dimensional graphene foams: synthesis, properties, biocompatibility, biodegradability, and applications in tissue engineering. ACS Biomater Sci Eng. 2018;5:193–214.
- Spearman BS, Desai VH, Mobini S, et al. Tissue‐engineered peripheral nerve interfaces. Adv Funct Mater. 2018;28:1701713.
- Harris JP, Struzyna LA, Murphy PL, et al. Advanced biomaterial strategies to transplant preformed micro-tissue engineered neural networks into the brain. J Neural Eng. 2016;13:016019.
- Mobasseri A, Faroni A, Minogue BM, et al. Polymer scaffolds with preferential parallel grooves enhance nerve regeneration. Tissue Eng A. 2015;21:1152–1162.
- Wong FS, Tsang KK, Lo AC. Nanoengineered biomaterial for brain tissue reconstruction and functional repairment. In: Mozafari M, Rajadas J, Kaplan D, editors. Nanoengineered biomaterials for regenerative medicine. Amsterdam; Oxford; Cambridge (MA): Elsevier; 2019. p. 145–166.
- Gaudin A, Yemisci M, Eroglu H, et al. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nature Nanotech. 2014;9:1054.
- Assunção-Silva R, et al., Nanoengineered biomaterials for spinal cord regeneration. In: Mozafari M, Rajadas J, Kaplan D, editors. Nanoengineered biomaterials for regenerative medicine. Amsterdam; Oxford; Cambridge (MA): Elsevier; 2019. p. 167–185.
- Kayalioglu G. The vertebral column and spinal meninges. In: Watson C, Paxinos G, Kayalioglu G, editors. The spinal cord. Cambridge (MA): Elsevier; 2009. p. 17–36.
- Falavigna A, Righesso O, Guarise da Silva P, et al. Epidemiology and management of spinal trauma in children and adolescents <18 years Old. World Neurosurg. 2018;110:e479–e483.
- Guertin PA. Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front Neur. 2013;3:183.
- Badea S, Wu W. Nanoengineered biomaterials for bridging gaps in damaged nerve tissue. In: Mozafari M, Rajadas J, Kaplan D, editors. Nanoengineered biomaterials for regenerative medicine. Cambridge (MA): Elsevier; 2019. p. 187–214.
- Amani H, Habibey R, Shokri F, et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep. 2019;9:6044.
- Kowal SL, Dall TM, Chakrabarti R, et al. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord. 2013;28:311–318.
- Olesen J, Gustavsson A, Svensson M, et al. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19:155–162.
- Dalamagkas K, Tsintou M, Seifalian A. Advances in peripheral nervous system regenerative therapeutic strategies: a biomaterials approach. Mater Sci Eng C. 2016;65:425–432.
- Chang W, Shah MB, Lee P, et al. Tissue-engineered spiral nerve guidance conduit for peripheral nerve regeneration. Acta Biomater. 2018;73:302–311.
- Lackington WA, Ryan AJ, O’Brien FJ. Advances in nerve guidance conduit-based therapeutics for peripheral nerve repair. ACS Biomater Sci Eng. 2017;3:1221–1235.
- Lee DJ, et al. Biomimetic nerve guidance conduit containing intraluminal microchannels with aligned nanofibers markedly facilitates in nerve regeneration. ACS Biomater Sci Eng. 2016;2:1403–1410.
- Zhou Z-F, et al. Electrospinning of PELA/PPY fibrous conduits: promoting peripheral nerve regeneration in rats by self-originated electrical stimulation. ACS Biomater Sci Eng. 2016;2:1572–1581.
- Ebrahimi M, Ai J, Biazar E, et al. In vivo assessment of a nanofibrous silk tube as nerve guide for sciatic nerve regeneration. Artif Cells Nanomed Biotechnol. 2018;46:394–401.
- Mohamadi F, Ebrahimi-Barough S, Nourani MR, et al. Use new poly (ε-caprolactone/collagen/NBG) nerve conduits along with NGF for promoting peripheral (sciatic) nerve regeneration in a rat. Artif Cells Nanomed Biotechnol. 2018;46:34–45.
- Wang G-W, Yang H, Wu W-F, et al. Design and optimization of a biodegradable porous zein conduit using microtubes as a guide for rat sciatic nerve defect repair. Biomaterials. 2017;131:145–159.
- Tuan RS, Alexander P. Adult stem cell-based enhancement of nerve conduit for peripheral nerve repair. Pittsburgh: University of Pittsburgh; 2018.
- Chiono V, Tonda-Turo C. Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog Neurobiol. 2015;131:87–104.
- Salmoria GV, Paggi RA, Kanis LA. Manufacturing of PCL/SAg tubes by melt-extrusion for nerve regeneration: structure and mechanical properties. Polymer Testing. 2016;55:160–165.
- Pawelec KM, Koffler J, Shahriari D, et al. Microstructure and in vivo characterization of multi-channel nerve guidance scaffolds. Biomed Mater. 2018;13:044104.
- Carballo-Molina OA, Velasco I. Hydrogels as scaffolds and delivery systems to enhance axonal regeneration after injuries. Front Cell Neurosci. 2015;9:13.
- Vishnoi T, Singh A, Teotia AK, et al. Chitosan-gelatin-polypyrrole cryogel matrix for stem cell differentiation into neural lineage and sciatic nerve regeneration in peripheral nerve injury model. ACS Biomater Sci Eng. 2019;5:3007
- Ratheesh G, Venugopal JR, Chinappan A, et al. 3D fabrication of polymeric scaffolds for regenerative therapy. ACS Biomater Sci Eng. 2017;3:1175–1194.
- Yao L, O'Brien N, Windebank A, et al. Orienting neurite growth in electrospun fibrous neural conduits. J Biomed Mater Res. 2009;90:483–491.
- Lee S, Hongo C, Nishino T. Crystal modulus of poly (glycolic acid) and its temperature dependence. Macromolecules 2017;50:5074–5079.
- Södergård A, Stolt M. Properties of lactic acid based polymers and their correlation with composition. Prog Polym Sci. 2002;27:1123–1163.
- Chanfreau S, Mena M, Porras-Domínguez JR, et al. Enzymatic synthesis of poly-L-lactide and poly-L-lactide-co-glycolide in an ionic liquid. Bioprocess Biosyst Eng. 2010;33:629–638.
- Omay D, Guvenilir Y. Synthesis and characterization of poly (d, l-lactic acid) via enzymatic ring opening polymerization by using free and immobilized lipase. Biocatal Biotransf. 2013;31:132–140.
- Zeng J-B, Li K-A, Du A-K. Compatibilization strategies in poly (lactic acid)-based blends. Rsc Adv. 2015;5:32546–32565.
- Tyler B, Gullotti D, Mangraviti A, et al. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv Drug Deliv Rev. 2016;107:163–175.
- Santoro M, Shah SR, Walker JL, et al. Poly (lactic acid) nanofibrous scaffolds for tissue engineering. Adv Drug Deliv Rev. 2016;107:206–212.
- Saini P, Arora M, Kumar MR. Poly (lactic acid) blends in biomedical applications. Adv Drug Deliv Rev. 2016;107:47–59.
- Lasprilla AJR, Martinez GAR, Lunelli BH, et al. Poly-lactic acid synthesis for application in biomedical devices - a review. Biotechnol Adv. 2012;30:321–328.
- Mir M, Ahmed N, ur Rehman A. Recent applications of PLGA based nanostructures in drug delivery. Colloids and Surfaces B: Biointerfaces. 2017;159:217–231.
- Baican MC. Polymeric nanobiosensors. In: Vasile C, editors. Polymeric nanomaterials in nanotherapeutics. Cambridge (MA): Elsevier; 2019. p. 151–181.
- Liang X, et al. Bilayered PLGA/PLGA-HAp composite scaffold for osteochondral tissue engineering and tissue regeneration. ACS Biomater Sci Eng. 2018;4:3506–3521.
- Qian C, et al. Targeting early apoptosis in acute ischemic stroke with a small-molecule probe. ACS Biomater Sci Eng. 2018;4:1862–1870.
- Jain RA. The manufacturing techniques of various drug loaded biodegradable poly (lactide-co-glycolide)(PLGA) devices. Biomaterials. 2000;21:2475–2490.
- Kapoor DN, Bhatia A, Kaur R, et al. PLGA: a unique polymer for drug delivery. Ther Deliv. 2015;6:41–58.
- dos Santos FP, Peruch T, Katami SJV, et al. Poly (lactide-co-glycolide)(PLGA) scaffold induces short-term nerve regeneration and functional recovery following sciatic nerve transection in rats. Neuroscience. 2019;396:94–107.
- Ferreira CL, Valente CA, Zanini ML, et al. Biocompatible PCL/PLGA/polypyrrole composites for regenerating nerves. In Macromolecular symposia. Hoboken (NJ): Wiley Online Library; 2019.
- Lanao RPF, Jonker AM, Wolke JGC, et al. Physicochemical properties and applications of poly (lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng B Rev. 2013;19:380–390.
- Lü J-M, Wang X, Marin-Muller C, et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Exp Rev Mol Diagn. 2009;9:325–341.
- Basu A, Domb AJ. Recent advances in polyanhydride based biomaterials. Adv Mater. 2018;30:1706815.
- Labet M, Thielemans W. Synthesis of polycaprolactone: a review. Chem Soc Rev. 2009;38:3484–3504.
- Mondal D, Griffith M, Venkatraman SS. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: current scenario and challenges. Int J Polym Mater Polym Biomater. 2016;65:255–265.
- Chang SH, Lee HJ, Park S, et al. Fast degradable polycaprolactone for drug delivery. Biomacromolecules. 2018;19:2302–2307.
- Sun H, Mei L, Song C, et al. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27:1735–1740.
- Surnar B, Jayakannan M. Structural engineering of biodegradable PCL block copolymer nanoassemblies for enzyme-controlled drug delivery in cancer cells. ACS Biomater Sci Eng. 2016;2:1926–1941.
- Palamà IE, Arcadio V, D’Amone S, et al. Therapeutic PCL scaffold for reparation of resected osteosarcoma defect. Sci Rep. 2017;7:12672.
- Zhou XHu, Shi G, Fan B, et al. Polycaprolactone electrospun fiber scaffold loaded with iPSCs-NSCs and ASCs as a novel tissue engineering scaffold for the treatment of spinal cord injury. IJN. 2018;13:6265.
- Kasper FK, Tanahashi K, Fisher JP, et al. Synthesis of poly(propylene fumarate)). Nat Protoc. 2009;4:518.
- Wang K, Cai L, Hao F, et al. Distinct cell responses to substrates consisting of poly (ε-caprolactone) and poly (propylene fumarate) in the presence or absence of cross-links. Biomacromolecules. 2010;11:2748–2759.
- Chen X, Zhao Y, Li X, et al. Functional multichannel poly (propylene fumarate)‐collagen scaffold with collagen‐binding neurotrophic factor 3 promotes neural regeneration after transected spinal cord injury. Adv Healthcare Mater. 2018;7:1800315.
- Cui J, Björnmalm M, Ju Y, et al. Nanoengineering of poly(ethylene glycol) particles for stealth and targeting. Langmuir. 2018;34:10817–10827.
- Pape A, Ippel BD, Dankers PY. Cell and protein fouling properties of polymeric mixtures containing supramolecular poly (ethylene glycol) additives. Langmuir. 2017;33:4076–4082.
- Suk JS, Xu Q, Kim N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51.
- Temenoff JS, Athanasiou KA, Lebaron RG, et al. Effect of poly (ethylene glycol) molecular weight on tensile and swelling properties of oligo (poly (ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J Biomed Mater Res. 2002;59:429–437.
- Gaaz T, Sulong A, Akhtar M, et al. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules. 2015;20:22833–22847.
- Bayer O. Das di‐isocyanat‐polyadditionsverfahren (polyurethane). Angew Chem. 1947;59:257–272.
- Zhang Q, Zhang G, Xu J, et al. Recent advances on ligin-derived polyurethane polymers. Rev Adv Mater Sci. 2015;40:146–154.
- Howard GT. Biodegradation of polyurethane: a review. Int Biodeter Biodegr. 2002;49:245–252.
- Duquesne S, Le Bras M, Bourbigot S, et al. Thermal degradation of polyurethane and polyurethane/expandable graphite coatings. Polym Degrad Stab. 2001;74:493–499.
- Gautam R, Bassi AS, Yanful EK. Candida rugosa lipase-catalyzed polyurethane degradation in aqueous medium. Biotechnol Lett. 2007;29:1081–1086.
- Wu Y, Wang L, Guo B, et al. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials. 2016;87:18–31.
- Hsieh F-Y, Lin H-H, Hsu S-h. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials. 2015;71:48–57.
- Weber RA, Breidenbach WC, Brown RE, et al. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000;106:1036–1045.
- Shimizu M, Matsumine H, Osaki H, et al. Adipose‐derived stem cells and the stromal vascular fraction in polyglycolic acid‐collagen nerve conduits promote rat facial nerve regeneration. Wound Rep and Reg. 2018;26:446–455.
- Matsumoto K, Ohnishi K, Kiyotani T, et al. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)–collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000;868:315–328.
- Amani H, Arzaghi H, Bayandori M, et al. Controlling cell behavior through the design of biomaterial surfaces: a focus on surface modification techniques. Adv Mater Interfaces. 2019;1900572.
- Kojima C, Fusaoka-Nishioka E, Imai T, et al. Dendrigraft polylysine coated‐poly (glycolic acid) fibrous scaffolds for hippocampal neurons. J Biomed Mater Res. 2016;104:2744–2750.
- Zhang K, Huang D, Yan Z, et al. Heparin/collagen encapsulating nerve growth factor multilayers coated aligned PLLA nanofibrous scaffolds for nerve tissue engineering. J Biomed Mater Res. 2017;105:1900–1910.
- Haddad T, Noel S, Liberelle B, et al. Fabrication and surface modification of poly lactic acid (PLA) scaffolds with epidermal growth factor for neural tissue engineering. Biomatter. 2016;6:e1231276.
- Hoveizi E, Tavakol S, Ebrahimi-Barough S. Neuroprotective effect of transplanted neural precursors embedded on PLA/CS scaffold in an animal model of multiple sclerosis. Mol Neurobiol. 2015;51:1334–1342.
- Yang F, Murugan R, Ramakrishna S, et al. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900.
- Yang F, Murugan R, Wang S, et al. Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610.
- Zhang K, Zheng H, Liang S, et al. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater. 2016;37:131–142.
- Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, et al. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng A. 2009;15:3605–3619.
- Jin L, Feng Z-Q, Zhu M-L, et al. A novel fluffy conductive polypyrrole nano-layer coated PLLA fibrous scaffold for nerve tissue engineering. J Biomed Nanotechnol. 2012;8:779–785.
- Lee JY, Bashur CA, Goldstein AS, et al. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30:4325–4335.
- Mehrasa M, Asadollahi MA, Ghaedi K, et al. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int J Biol Macromol. 2015;79:687–695.
- Wang J, Tian L, Chen N, et al. The cellular response of nerve cells on poly-l-lysine coated PLGA-MWCNTs aligned nanofibers under electrical stimulation. Mater Sci Eng C. 2018;91:715–726.
- Reis KP, Sperling LE, Teixeira C, et al. Application of PLGA/FGF-2 coaxial microfibers in spinal cord tissue engineering: an in vitro and in vivo investigation. Regen Med. 2018;13:785–801.
- Griffin J, Delgado-Rivera R, Meiners S, et al. Salicylic acid‐derived poly (anhydride‐ester) electrospun fibers designed for regenerating the peripheral nervous system. J Biomed Mater Res. 2011;97:230–242.
- Lee YS, Griffin J, Masand SN, et al. Salicylic acid‐based poly (anhydride‐ester) nerve guidance conduits: impact of localized drug release on nerve regeneration. J Biomed Mater Res. 2016;104:975–982.
- Panahi-Joo Y, Karkhaneh A, Nourinia A, et al. Design and fabrication of a nanofibrous polycaprolactone tubular nerve guide for peripheral nerve tissue engineering using a two-pole electrospinning system. Biomed Mater. 2016;11:025017.
- Bolaina-Lorenzo E, Martínez-Ramos C, Monleón-Pradas M, et al. Electrospun polycaprolactone/chitosan scaffolds for nerve tissue engineering: physicochemical characterization and Schwann cell biocompatibility. Biomed Mater. 2016;12:015008.
- Saderi N, et al. Fabrication and characterization of gold nanoparticle-doped electrospun PCL/chitosan nanofibrous scaffolds for nerve tissue engineering. J Mater Sci: Mater Med. 2018;29:134.
- Entekhabi E, Haghbin Nazarpak M, Moztarzadeh F, et al. Design and manufacture of neural tissue engineering scaffolds using hyaluronic acid and polycaprolactone nanofibers with controlled porosity. Mater Sci Eng C. 2016;69:380–387.
- Pan X, Sun B, Mo X. Electrospun polypyrrole-coated polycaprolactone nanoyarn nerve guidance conduits for nerve tissue engineering. Front Mater Sci. 2018;12:438–446.
- Wang S, Kempen DH, Simha NK, et al. Photo-cross-linked hybrid polymer networks consisting of poly (propylene fumarate) and poly (caprolactone fumarate): controlled physical properties and regulated bone and nerve cell responses. Biomacromolecules. 2008;9:1229–1241.
- Guo J, Liu X, Lee Miller A, et al. Novel porous poly (propylene fumarate‐co‐caprolactone) scaffolds fabricated by thermally induced phase separation. J Biomed Mater Res. 2017;105:226–235.
- Riley DC, Bittner GD, Mikesh M, et al. Polyethylene glycol‐fused allografts produce rapid behavioral recovery after ablation of sciatic nerve segments. J Neurosci Res. 2015;93:572–583.
- Ghergherehchi CL, Mikesh M, Sengelaub DR, et al. Polyethylene glycol (PEG) and other bioactive solutions with neurorrhaphy for rapid and dramatic repair of peripheral nerve lesions by PEG-fusion. J Neurosci Methods. 2019;314:1–12.
- Robinson GA, Madison RD. Polyethylene glycol fusion repair prevents reinnervation accuracy in rat peripheral nerve. J Neurosci Res. 2016;94:636–644.
- Brown BL, Asante T, Welch HR, et al. Functional and anatomical outcomes of facial nerve injury with application of polyethylene glycol in a rat model. JAMA Facial Plast Surg. 2019;21:61–68.
- Bittner GD, Sengelaub DR, Trevino RC, et al. Robinson and madison have published no data on whether polyethylene glycol fusion repair prevents reinnervation accuracy in rat peripheral nerve. J Neurosci Res. 2017;95:863–866.
- Stocco E, Barbon S, Lora L, et al. Partially oxidized polyvinyl alcohol conduitfor peripheral nerve regeneration. Sci Rep. 2018;8:604.
- Alhosseini SN, et al. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int J Nanomed. 2012;7:25.
- Golafshan N, Kharaziha M, Fathi M. Tough and conductive hybrid graphene-PVA: alginate fibrous scaffolds for engineering neural construct. Carbon. 2017;111:752–763.
- Guo T, Yang X, Deng J, et al. Keratin nanoparticles-coating electrospun PVA nanofibers for potential neural tissue applications. J Mater Sci: Mater Med. 2019;30:9.
- Singh A, Shiekh PA, Das M, et al. Aligned chitosan-gelatin cryogel-filled polyurethane nerve guidance channel for neural tissue engineering: fabrication, characterization, and in vitro evaluation. Biomacromolecules. 2019;20:662
- Wu Y, Wang L, Hu T, et al. Conductive micropatterned polyurethane films as tissue engineering scaffolds for Schwann cells and PC12 cells. J Colloid Interface Sci. 2018;518:252–262.
- Yin D, Wang XH, Yan Y, et al. Preliminary studies on peripheral nerve regeneration using a new polyurethane conduit. J Bioact Compat Polym. 2007;22:143–159.
- Niu Y, Chen KC, He T, et al. Scaffolds from block polyurethanes based on poly (ɛ-caprolactone)(PCL) and poly (ethylene glycol)(PEG) for peripheral nerve regeneration. Biomaterials. 2014;35:4266–4277.
- Shrestha S, Shrestha BK, Kim JI, et al. Electrodeless coating polypyrrole on chitosan grafted polyurethane with functionalized multiwall carbon nanotubes electrospun scaffold for nerve tissue engineering. Carbon. 2018;136:430–443.

