Abstract
This study aimed to assess the impact of a polycystic ovary syndrome (PCOS) diagnosis and other factors on health-related quality of life (HRQoL) in women of reproductive age. Online questionnaires were completed and study groups compared. Potential causal relationships were evaluated using path analysis. Analyses revealed that a PCOS diagnosis alongside BMI had the largest effect on HRQoL. Higher levels of physical activity (PA) were not associated with greater HRQoL, and PA was not directly affected by any other outcome. However, reduced self-esteem was identified as a key factor in the promotion of physical and mental health.
Introduction
Polycystic ovary syndrome (PCOS) is the most frequent endocrine disorder in women of reproductive age (ESHRE & ASRM Group, Citation2004), affecting up to 21% of this population depending on the applied diagnostic criteria and the studied cohort (Boyle et al., Citation2012). Women with PCOS typically have hyperandrogenaemia, menstrual irregularity, and/or polycystic ovaries (PCO) (Kyritsi et al., Citation2017; Lizneva et al., Citation2016). Furthermore, most women with PCOS exhibit metabolic (e.g., overweight/obesity, or insulin resistance) (Hutchison et al., Citation2011; Li et al., Citation2019; Lim et al., Citation2012; Shirazi et al., Citation2021) and/or psychological (e.g., anxiety and depression) (Karjula et al., Citation2017; Tay et al., Citation2019) comorbidities. Overall, the health burden of PCOS impacts adversely upon health-related quality of life (HRQoL) (Moghadam et al., Citation2018), an important outcome in the context of chronic disease treatment and management (Dokras et al., Citation2018), relating to patient-reported physical, social, and emotional well-being (Colwell et al., Citation2010). Indeed, consistently lower HRQoL has been noted in women with PCOS when compared with data from healthy populations (Asdaq et al., Citation2020; Panico et al., Citation2017; Sánchez-Ferrer et al., Citation2020), or those with other chronic diseases (Coffey et al., Citation2006; Naumova et al., Citation2021).
The physical benefits of increasing physical activity (PA) levels have been widely reported across a range of populations (Warburton & Bredin, Citation2017), including PCOS (Kite et al., Citation2019, Citation2022). Moreover, increased PA or engagement with exercise regimes may also improve HRQoL, particularly in those with chronic diseases, such as cancer (Fuller et al., Citation2018), chronic respiratory conditions (Eichenberger et al., Citation2013), and rheumatoid arthritis or osteoarthritis (Kelley et al., Citation2015). Interestingly, most of the studies that have compared the PA levels of women with PCOS against healthy controls reported that despite poorer physical and mental health in women with PCOS, there were no statistical differences in energy expenditure between these groups (Mario et al., Citation2012; Rodino et al., Citation2016; Wang et al., Citation2021). However, despite the high prevalence of PCOS, there is still limited evidence about the potential mediating role of PA in the physical and psychological manifestations of PCOS.
Accordingly, the objectives of the current study were to identify whether there are differences in HRQoL between women with PCOS and a healthy control group, and to explore whether higher levels of PA facilitate improved HRQoL. Furthermore, this study aimed to estimate any potential simultaneous impact of not only a PCOS diagnosis but also of other predictive factors (i.e., PA and its determinants, body mass index [BMI], self-esteem) upon the mental and physical domains of HRQoL.
Material and Methods
Ethical approval was granted by the Aston University Ethics Committee (project number: 1442). Recruitment of reproductive-aged (18–45 years) women with PCOS (self-reported diagnosis) and controls (self-reported being free from any chronic condition) took place between January 9 and May 9, 2019, via advertisements on social media, through PCOS support groups, and in online forums hosted by Verity, the UK-based PCOS charity. Using snowball sampling, potential respondents were encouraged to share the study advert within their networks to anyone they thought may be eligible/interested to participate.
Study Questionnaires
A range of questionnaires were used to collect the study data. All study questionnaires were completed online using the survey software, Qualtrics© XM (Qualtrics XM, Provo, Utah, USA) which was accessed through a study URL link.
Each participant completed a study-specific questionnaire to ascertain sociodemographic and anthropometric data, including self-reported age, height (m) and weight (kg), BMI (kg/m2), and waist circumference (cm). Furthermore, participants were asked if they had ever been diagnosed with PCOS; if they responded affirmatively, they were asked to specify the time since diagnosis and to identify the specific PCOS phenotype associated with their diagnosis (PCO, menstrual disruption, and excess androgens; PCO and menstrual disruption; PCO and excess androgens; or menstrual disruption and excess androgens; or alternatively answer as “do not know”). Questions about participant ethnicity, marital status, occupational status, education level, whether they have children, and their approximate household income were also included.
HRQoL was assessed by the validated 12-item Short Form (SF-12v2) Health Survey (Ware et al., Citation1996) which provides eight health scales (physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health), as well as physical and mental component summary scores. Values for each scale are calculated by transforming raw scores into norm-based scores (Gandek et al., Citation1998). For the path analysis performed for this study, the raw individual mental and physical health scores were combined to give an overall composite score for quality of life (Ware et al., Citation1994). Whilst not commonplace, this approach was taken due to a high degree of correlation between domain and composite scores () and for simplification of the path analysis model.
Participants in the PCOS study group were also given a PCOS-specific questionnaire, i.e., the PCOS-Q. The PCOS-Q is a 26-item questionnaire that was developed to assess the impact of PCOS symptoms, and their associated treatments, across five domains, each related to a common symptom of PCOS (Cronin et al., Citation1998), i.e., emotions, body hair, body weight, infertility, and menstrual problems. Participants respond to each of the 26 items on the PCOS-Q by selecting an answer on a 1–7 scale; seven is representative of optimal function and one the poorest function. Each item is weighted equally when scored, meaning that each domain is presented as a score out of seven regardless of the number of items.
Self-reported PA was assessed via the International Physical Activity Questionnaire Long Form (IPAQ-LF) (Hagströmer et al., Citation2006), which asks participants to recall their last seven days of PA and is widely used in clinical settings and PA research. Summation of self-reported PA duration, multiplied by weekly frequency and normative Metabolic Equivalent of Task (MET) data (Ainsworth et al., Citation2011) provides continuous data reported as MET-min/wk and categorical data based upon low, moderate, or high levels of PA.
The Self-Efficacy for Exercise Scale, a 9-item questionnaire, was used to measure self-efficacy barriers to exercise (Resnick & Jenkins, Citation2000) and to assess perceived motivational barriers to completion of PA. For this scale, participants are tasked with scoring from 0 to 10 (not confident: 0; very confident: 10) how confident they are that they could exercise for 20 minutes, three times per week, given a variety of situations. The total score of this scale is calculated by summing the responses to each question (possible scoring range: 0–90), with a higher score indicating higher self-efficacy for exercise.
In addition, the Exercise Benefits/Barriers Scale was used to broadly measure participants’ perceived benefits and barriers to participation in exercise (Sechrist et al., Citation1987). This scale requires respondents to rate their agreement with 43 statements (benefit items: 29; barrier items: 14) using a 4-point Likert scale. Answers are scored from 1 to 4 (strongly disagree: 1; strongly agree: 4), with the 14 barrier items being reverse scored. Total scores range from 43 to 172, with a lower score indicative of fewer perceived benefits and greater perceived barriers.
Finally, self-esteem was measured using the Rosenberg Self-Esteem Scale (Rosenberg, Citation1965), which utilizes a 4-point Likert scale allowing participants to respond to 10 statements about themselves (a higher score indicates a greater level of self-esteem).
Statistical Analysis
All study questionnaires were scored according to their individual criteria, and data were collated in Excel (Microsoft Excel v16.04849.1000; Microsoft Corporation, Washington, USA). Statistical analysis was completed in jamovi (the jamovi project, v.1.6) and in IBM SPSS Amos (IBM SPSS Amos, v.25.0.0, Amos Development Corporation, PA, USA).
Due to the sample size (≥ 20), the Shapiro-Wilk test of normality was completed on each variable, separated by group, and Q-Q plots were visually inspected; where data were non-normally distributed, median and interquartile range (IQR) were reported and Mann-Whitney U tests were completed to highlight between-group differences. Between group median difference, effect size (Cohen’s d), 95% confidence intervals (CIs) and statistical significance values (p) were reported for all non-parametric outcomes. Where data were normally distributed, mean ± standard deviation (SD) were reported, and Welch’s t-test was used (Delacre et al., Citation2017). In these analyses, pairwise exclusion was used to deal with missing values.
Due to the prevalence of nonparametric variables, Kendall’s rank correlation (τb) was chosen to measure the strength of association between two variables. Where variables were highly correlated and deemed to be reporting similar effects (e.g., body mass, BMI, and waist circumference), the variable with the largest sample size was retained. Where these variables were domains from a questionnaire (e.g., mental and physical domains of the SF-12v2), the aggregated score was used as the variable in the regression.
A separate analysis was completed on the domain scores from the PCOS-Q. Because these data were nonparametric, median difference (MD) and IQR were calculated between domains and a Durbin-Conover pairwise comparison was used to identify statistical differences.
In order to generate a complete data set for the path analysis of this study, full information maximum likelihood (FIML) regression imputation was used to account for missing data. Although pairwise or listwise deletion are commonly used, FIML was regarded as more favorable to preserve the sample size, whilst FIML also provides data estimates that are unbiased and more efficient than other methods (Enders & Bandalos, Citation2001). Moreover, the composite HRQoL score was used as the endogenous variable and the remaining variables were arranged into a path model to indicate causal relationships between the exogenous (diagnosis of PCOS, and BMI), mediating (self-efficacy for exercise, self-esteem, perceived benefits/barriers of exercise, and MET-min/wk) and endogenous variables. The decision to use the composite HRQoL score was taken since the effect of the exogenous and mediating variables upon HRQoL varied little when individual domain scores, or indeed the composite score, were inputted into the path model. Collapsing the two domain scores into a single measure for HRQoL simplified the path model whilst retaining meaning.
Results
Participant Characteristics
In total, 194 participants accessed the online surveys and consented to participate (). Based upon the exclusion criteria, 40 participants were deemed ineligible due to reporting one or more chronic conditions other than PCOS. Of the remaining 154 participants, 24 were deemed to have provided insufficient data to warrant inclusion in the analysis. As such, 130 eligible participants were included in the two study groups (PCOS: 64; controls: 66). Regarding the self-reported phenotype of the women with PCOS, the majority (52%) self-reported as having excess androgens, menstrual dysfunction, and PCO, whilst 17% were unsure of the phenotype for their PCOS diagnosis.
Figure 1. Flow diagram for the recruitment of women of reproductive age into the study group with polycystic ovary syndrome (PCOS) and the one without (control).
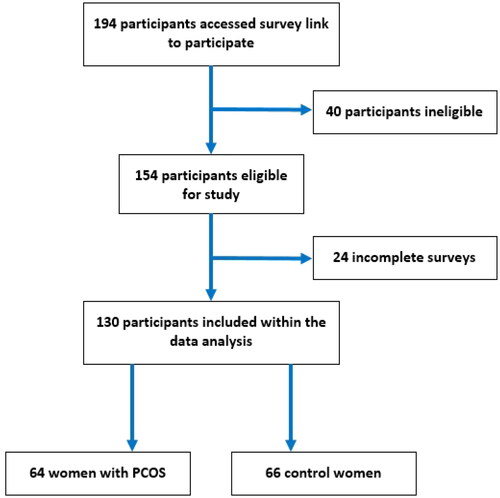
Certain demographic differences exist between the groups, with ∼63% of women with children being in the control group (). Women in the control group also tended to be educated to a higher level and have a greater household income than their counterparts. Furthermore, a larger number of women in the control group self-reported that they were currently a student. Whilst there were no statistically significant differences between the age and height of the two groups, women with PCOS had higher body weight, BMI, and waist circumference than the control group (). Moreover, women with PCOS reported lower scores in all domains of the SF-12v2, which is indicative of overall poorer HRQoL.
Table 1. Key sociodemographic characteristic of the study participants with polycystic ovary syndrome (PCOS) and without (control).
Table 2. Comparison of self-reported variables between women with polycystic ovary syndrome (PCOS) and the control group.
Results from the PCOS-Q are presented in . When domain scores were compared, there were statistical differences between five domains; the weight domain was statistically lower than emotions (MD = −1.15, p < .001), menstrual problems (MD = −1.10, p < .001), body hair (MD = −0.70, p = .004), and infertility (MD = −0.60, p = .025), whilst infertility was also lower than emotions (MD = −0.55, p = .043). It is evident that concerns about body weight and infertility are the most prevalent in this sample.
Table 3. Domain-specific scores of the health-related quality-of-life questionnaire for women with polycystic ovary syndrome (PCOS-Q) reported from the study participants with PCOS.
With regard to other measures, women with PCOS had statistically lower self-esteem than the controls, whilst also perceiving fewer benefits and greater barriers to exercise (). When the highest and lowest perceived benefits/barriers to exercise were split by study group and scored, there were similarities between the top scoring results, and some disparity with the lowest scoring (). The highest scoring benefits in both groups were similar and generally related to physical health and well-being; by contrast, the lowest scoring benefits tended to be linked to social well-being. There were also similar findings for the highest scoring barriers; both groups scored “exercise tires me,” “exercise is hard work for me,” and “I am fatigued by exercise” in their top three, whereas there was a marked difference in the lowest perceived barriers.
In contrast to the Exercise Benefits/Barriers Scale, there were no statistically significant between-group differences in self-efficacy for exercise. In addition, when data from the IPAQ was analyzed, there were no between-group statistical differences in either MET-min/wk or sitting time.
Path Analysis
Within our path model (), both a diagnosis of PCOS and BMI had direct effects on HRQoL (standardized β = 0.230, and β = 0.234, respectively) and self-esteem (β = 0.364 and β = −0.213, respectively). Furthermore, PCOS also had the largest indirect (β = 0.177) and total effect (β = 0.407) upon HRQoL. PCOS diagnosis was closely followed by BMI (indirect β = −0.116; total β = −0.351, respectively), which in addition had a direct effect on the Exercise Benefits/Barriers Scale score (β = −0.180). The Self-Esteem (β = 0.326) and Exercise Benefits/Barriers Scale (β = 0.254) scores also demonstrated a direct effect upon HRQoL and, although they had no statistically significant indirect effects (via PA as a mediator), both demonstrated a total effect upon HRQoL in this model (β = 0.324 and β = 0.265, respectively). The Exercise Benefits/Barriers Scale score also had a statistically significant total effect on weekly PA (β = 0.376). Self-efficacy for exercise and weekly PA had no effect (either direct or indirect) on any other variable in the model ().
Figure 2. Schematic presentation of the path analysis model based on the findings of the present study. Bidirectional arrows are indicative of correlation between exogenous variables. Single directional arrows indicate significant standardized path coefficients and bias-corrected 95% confidence intervals for direct effects. A dashed arrow indicates nonsignificant direct effects. All values rounded to two decimal places (PCOS: diagnosed with polycystic ovary syndrome; BMI: body mass index (self-reported); HRQoL: health-related quality of life as measured by aggregate SF-12 score; MET-min/wk: metabolic equivalent of task minutes per week as measured by the IPAQ-LF).
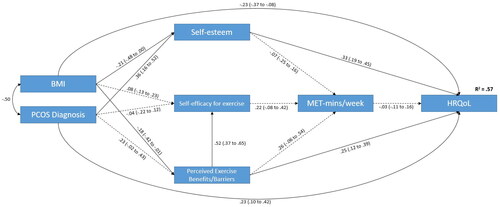
Table 4. Direct, indirect, and total effects of variables in the Health-Related Quality of Life (HRQoL) causal model.
Discussion
Between-Group Differences
The existing evidence consistently highlights impaired HRQoL in women with PCOS (Amiri et al., Citation2019; Naz et al., Citation2020; Yoldemir et al., Citation2017), that is also markedly lower than in women without PCOS (Asdaq et al., Citation2020; Benetti-Pinto et al., Citation2015; Drosdzol et al., Citation2007; Sánchez-Ferrer et al., Citation2020; Shishehgar et al., Citation2016). The present findings agree with this, showing statistically worse values across all eight summary health scores and both the physical and mental component scores of the SF-12v2. Studies utilizing the SF-12v2 to assess HRQoL in women with PCOS are scarce, meaning there are limited data for comparison. However, a recent case-control study found women with PCOS had poorer physical component scores compared to controls, and our findings agree with this (Sánchez-Ferrer et al., Citation2020). When the mental component score was considered, we reported a statistical difference favoring the control group, but Sánchez-Ferrer et al. (Citation2020) reported no such difference. Notably, the norm-based score for women with PCOS in our study was markedly lower than that reported by Sánchez-Ferrer et al. (mean 44.2, 95% CI: 42.4 to 46.1). Interestingly, another previous study that used only the raw scores (0–100) from the Bodily Pain subscale to compare women with PCOS to controls reported statistically higher bodily pain severity in women with PCOS. However, the magnitude of difference is greater, and mean scores lower, in the current study compared to those previously reported (Morán-Sánchez et al., Citation2021).
The paucity of previous studies using the SF-12v2 makes it difficult to infer clinical relevance from our findings. However, a previous study reported change scores for each domain of the SF-36 before and after an aerobic exercise intervention (Costa et al., Citation2018), identifying thresholds for clinical significance. Although these scores cannot be compared directly, the effect size (d) of key domains can be compared. In the present study, the between-group effect sizes for the role emotional and mental health summary scores were greater than the change from baseline effect sizes (d = 0.8, 95% CI: 0.0 to 1.6; and d = 1.0, 95% CI: 0.0 to 2.0, respectively) reported in Costa et al. (Citation2018), suggesting clinical importance in the current study. We also reported a statistically significant difference between mental and physical summary scores (mean difference = 19.2, 95% CI: 15.7 to 22.6) in women with PCOS, suggesting that PCOS has a greater psychological impact than it does physical, which agrees with findings reported by a previous study (Bazarganipour et al., Citation2013).
Since the PCOS-Q is specific to people living with PCOS, this validated PCOS-specific questionnaire was not administered to women in the control group. For those living with PCOS, statistically lower scores were reported for weight compared to other domains. This is in accord with other studies using the PCOS-Q (Barnard et al., Citation2007; Coffey et al., Citation2006, Thomson et al., Citation2010; Jones et al., Citation2004; McCook et al., Citation2005), or other HRQoL measurement tools (Kerchner et al., Citation2009), which have also reported that excess body weight has the greatest detrimental effect upon HRQoL in those with PCOS. For infertility, we found that this was the second lowest domain affecting HRQoL, and this is also in agreement with findings from Jones et al. (Citation2004) and Coffey et al. (Citation2006).
These findings should be further explored by future research focusing on identifying HRQoL thresholds for clinically important changes/differences in women with PCOS, and by incorporating assessments of psychological well-being and appropriate treatment/management strategies during the management of PCOS. Moreover, there is a need to standardize the version of the tool used, whilst ensuring it is robust and valid for use in a range of different cultural/social settings (Taghavi et al., Citation2015).
As commonly reported in the relevant literature, women with PCOS in our study also reported higher body weight, BMI, and waist circumference than the control group. The association between obesity and PCOS has been widely reported, with available older data indicating that the obesity prevalence in UK women with PCOS was 35–38% (Balen, Citation1995; Kiddy et al., Citation1990). Given the increasing obesity prevalence rates in the general population over the past few decades (Moody & Neave, Citation2016), it is a reasonable assumption that a similar growth may have been observed in women with PCOS. A previous study in the United States (Yildiz et al., Citation2008) reported temporal trends of obesity prevalence in local women with PCOS reflecting the increases in obesity prevalence in the general population, which is in concordance with the findings of this study.
Furthermore, we found no statistically significant differences in the amount of self-reported PA or sitting time between women with PCOS and the control group. Previous research also supports this, with data from a large cohort study showing no differences in total PA levels between women with PCOS and controls (Moran et al., Citation2013), which is also supported elsewhere (Álvarez-Blasco et al., Citation2011; Cutler et al., Citation2019; Douglas et al., Citation2006; Lin et al., Citation2019; Wright et al., Citation2004). The lack of significant differences in sitting time between women with PCOS and controls in the present study is also in accord with most of the evidence from the existing relevant literature (Ahmadi et al., Citation2013; Álvarez-Blasco et al., Citation2011; Lin et al., Citation2019; Wright et al., Citation2004). Indeed, to our knowledge, only one study has reported increased sitting time in women with PCOS compared to a control group (6.3 ± 2.8 vs 5.8 ± 2.9 h/day, respectively; p = 0.008) (Moran et al., Citation2013).
The current study also revealed lower self-esteem in women with PCOS than in the control group, which contrasts with a previous study which reported no statistical differences (Annagür et al., Citation2014); this may be attributed to the BMI of study groups being lower than in the current study. Indeed, previous studies have reported significantly lower self-esteem across subsets of women with PCOS (i.e., subgroups with hirsutism, infertility, and obesity) compared to healthy controls, but it was those with obesity and PCOS who were far more likely to have the lowest self-esteem (Açmaz et al., Citation2013; Tay et al., Citation2019). The relationship between a higher BMI and lower self-esteem has previously been reported in the general population (Biro et al., Citation2006; Hesketh et al., Citation2004; Strauss, Citation2000), and the direct effect of BMI upon self-esteem in the current study tends to support this, although there is a paucity of literature on this topic in women with PCOS.
Interestingly, despite the absence of significant differences in self-reported PA between our two study groups, women with PCOS perceived both fewer benefits and a greater number of barriers to participation in exercise. Whilst greater perceived benefits have been associated with increased exercise participation (Bonheur & Young, Citation1991; Grubbs & Carter, Citation2002; Jones & Nies, Citation1996), it is the perceived barriers that are the most powerful predictor of a health behavior (Janz & Becker, Citation1984). Although there is mixed evidence as to whether women with PCOS are less active than their non-PCOS counterparts, few studies have investigated the relationship between barrier/benefit perception and PCOS. One such study compared women with PCOS to controls and, as in the current study, found many similarities in barrier/benefit perception (Banting et al., Citation2014). A more recent study of women with PCOS reported that the most common perceived barriers to PA were those related to physical exertion (i.e., exercise is tiring, hard work, and fatiguing) (Thomson et al., Citation2016), and these findings exactly match the top-cited barriers identified in this study. Notably, Thomson et al. (Citation2016) further stated that exposure to a lifestyle intervention may improve these perceptions, particularly those relating to barriers, and this should be a focus for future research.
Path Analysis
The path analysis model showed little evidence that PA had influenced HRQoL; no single outcome had a direct effect on the amount of self-reported PA, and similarly PA did not have a direct effect on HRQoL scores. A statistical total effect of perceived exercise benefits/barriers on MET-min/wk is reported, but this is likely due to the strength of the direct effect of Exercise Benefits/Barriers Scale scores on self-efficacy for exercise (as a mediating variable). This relationship was the only instance where an outcome was deemed to have any effect upon PA levels.
Although there may be no true relationship between PA and HRQoL in this group, it should be highlighted that there are notable limitations with self-reported data, particularly for PA (Prince et al., Citation2008). In this context, it is of note that the self-reported PA data in this study is higher than the most active female group from normative UK population data (Love-Koh & Taylor, Citation2018). It is possible that the participants within the current study are indeed highly physically active (e.g., due to motivation or medical advice relating to controlling PCOS as a chronic medical condition), but it is also likely that social desirability bias and/or the healthy volunteer effect (Froom et al., Citation1999; Grimm, Citation2010; Prince et al., Citation2020) may have influenced these results.
In the present study, when direct and indirect effects were summed, our model identified a diagnosis of PCOS as having the largest total effect on participant’s HRQoL. When only direct effects were considered, the effect of PCOS upon HRQoL was comparable to the effect of self-reported BMI. A diagnosis of PCOS had a greater direct effect on self-esteem than did BMI, but both were statistically significant. Self-esteem had the largest direct effect upon HRQoL. In fact, self-esteem emerged as a key mediator between the exogenous variables and HRQoL. It is therefore likely that women with PCOS have a two-fold effect upon their self-esteem, namely managing a chronic disease and its associated symptoms promotes lower self-esteem but so too does increased BMI (Chu et al., Citation2019). Another key consideration is the bidirectional effect of self-esteem, where chronic disease reduces an individual’s self-esteem, and vice versa, since previous studies have identified that low self-esteem is associated with physical dysregulation (in the context of stress) (Liu et al., Citation2014), and physical health complications (Cott et al., Citation1999). Of note, the Rosenberg Self-Esteem Scale has been used in studies in women with PCOS. Similarly, self-esteem was shown to play an important role, as a mediating factor, in the HRQoL of these women (Bazarganipour et al., Citation2013, Citation2014).
Study Limitations
Certain potential limitations should be acknowledged in the context of the present study. Since the study was promoted mainly via internal university systems, calling for volunteers with or without PCOS, it is possible that a higher proportion of the control group may have originated from the local university population, as is implied by the higher number of participants in the control group who were students, and those with a doctorate-level qualification at the time of survey. The degree to which this may have influenced the results is difficult to assess.
Based on the eligibility criteria, participants had to be free from any other chronic condition which may impact on their ability to perform PA. The objective behind this decision was to isolate the impact of a PCOS diagnosis rather than any other comorbidity. However, it is widely reported that women with PCOS are more susceptible to a range of physical and psychological conditions; excluding these women means that the extent to which the present findings can be generalized for women with PCOS, and other chronic conditions may be limited.
Moreover, self-reported data always present a methodological concern, particularly regarding reporting PA (Prince et al., Citation2008) and sedentary behaviors (Prince et al., Citation2020). Such response bias for these outcomes may be a contributing factor to the absence of impact on the variance in HRQoL. It should also be noted that whilst the IPAQ has been previously validated, Lee et al. (Citation2011) report that correlations between the IPAQ and objective measures are lower than the acceptable standard, and that the IPAQ overreports PA behaviors by as much as 84%. This phenomenon is perhaps further compounded by the fact that the IPAQ has not been validated for use specifically in women with PCOS, which reduces confidence in the true effect of PA in this study.
Similar issues around reporting also apply to the self-reporting of anthropometric measures (e.g., overreporting height or underreporting body weight) (Gorber et al., Citation2007), with the greatest degree of inaccuracy tending to present in women and/or in those with a higher BMI (Bigaard et al., Citation2005). However, a previous study of mid-aged women reported substantial agreement between self-reported and measured height/weight data (Burton et al., Citation2010), and whilst this bias may typically be expected in this type of research, the online nature of the study, which offered complete anonymity, may have reduced the degree of such validity issues (Larson, Citation2019). Finally, the presence or absence of a PCOS diagnosis was also self-reported by the study participants; this may be regarded as a study limitation regarding the true categorization of study groups with and without PCOS. However, only 17% could not (or opted not to) report the exact phenotype for their PCOS diagnosis, meaning risk of misclassification may have been low.
Implications for Practice and/or Policy
Given that the United Nations’ Sustainable Development Goals (UN General Assembly, Citation2015) highlight the need to reduce health inequalities and to specifically prioritize women’s health, it is perhaps no surprise that the Department of Health and Social Care’s Women’s Health Strategy for England (Citation2022) was produced. This policy, which aims to reform healthcare to reduce health inequalities for women, identifies PCOS as a priority condition; the links between PCOS and impaired cardiovascular health are stated and so, too, is the need to increase awareness of PCOS amongst women and girls so that they know when, and indeed where, to seek health-care support. Furthermore, women should have access to high-quality, personalized care, which includes access to contraception for the management of menstrual problems and gynecological conditions.
In this context, the present findings that identify reduced self-esteem as a key factor in the promotion of physical and mental health in women with PCOS highlight self-esteem as an additional aspect which should not be overlooked in interventions/policies on women’s health. Moreover, given that women with PCOS report impaired HRQoL compared to women without, HRQoL assessment should also not be omitted by health-care practitioners in order to facilitate a more personalized/holistic management plan according to the needs of the patient. Indeed, it is likely that greater support for mental well-being is required for women with PCOS, with a potential emphasis on improving self-esteem which is currently lacking from PCOS management pathways/approaches. Accordingly, future research should also focus on identifying clinical thresholds so that HRQoL can be monitored more effectively in these women. In addition, behavior change techniques are likely to further support women with PCOS both to improve health literacy on the benefits of PA and with barrier identification and removal.
The evidence around the effectiveness of PA in mitigating the manifestations of PCOS is mixed. Given that PA (as part of broader lifestyle changes) is now included in the international evidence-based guideline for the assessment and management of PCOS (Teede et al., Citation2023), it is imperative that its true effectiveness is better studied so that it can be used effectively in this population. Accordingly, PA/lifestyle measures which are specifically designed (and validated) for those living with PCOS are needed. Whilst there is a need for rigorously designed and well-reported trials in women with PCOS (Kite et al., Citation2019), studies should also utilize device-measurement of PA and sedentary behavior to strengthen the certainty of the evidence.
Conclusions
Overall, the present study shows that poorer HRQoL is reported by women with PCOS and highlights self-esteem as a key factor in the promotion of health in this patient population. A PCOS diagnosis was also noted to have a greater impact upon mental health than on physical health, with both domains being impaired in women with PCOS. Whilst previous studies suggest that increasing PA has a key role at improving health in a range of populations, the link here was not apparent. This further highlights a need for future studies, preferably using device-measured PA methods, to better understand the health-related impact of PA as well as the potential role of PA in the promotion of self-esteem in women with PCOS.
Acknowledgments
All authors acknowledge and thank all participants of this study. Also, we thank Verity (the UK PCOS charity) and the PCOS support groups on Facebook for their valuable support to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Declaration of Interest Statement
The authors have no competing interests to declare that are relevant to the content of this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author [CK] upon reasonable request.
Additional information
Funding
References
- Açmaz, G., Albayrak, E., Acmaz, B., Başer, M., Soyak, M., Zararsız, G., & İpekMüderris, İ. (2013). Level of anxiety, depression, self-esteem, social anxiety, and quality of life among the women with polycystic ovary syndrome. The Scientific World Journal, 2013, 1–7. https://doi.org/10.1155/2013/851815
- Ahmadi, A., Akbarzadeh, M., Mohammadi, F., Akbari, M., Jafari, B., & Tolide-Ie, H. R. (2013). Anthropometric characteristics and dietary pattern of women with polycystic ovary syndrome. Indian Journal of Endocrinology and Metabolism, 17(4), 672–676. https://doi.org/10.4103/2230-8210.113759
- Ainsworth, B. E., Haskell, W. L., Herrmann, S. D., Meckes, N., Bassett, D. R., Tudor-Locke, C., Greer, J. L., Vezina, J., Whitt-Glover, M. C., & Leon, A. S. (2011). 2011 Compendium of Physical Activities: A second update of codes and MET values. Medicine and Science in Sports and Exercise, 43(8), 1575–1581. https://doi.org/10.1249/MSS.0b013e31821ece12
- Álvarez-Blasco, F., Luque-Ramírez, M., & Escobar-Morreale, H. F. (2011). Diet composition and physical activity in overweight and obese premenopausal women with or without polycystic ovary syndrome. Gynecological Endocrinology, 27(12), 978–981. https://doi.org/10.3109/09513590.2011.579658
- Amiri, M., Bidhendi Yarandi, R., Nahidi, F., Tohidi, M., & Ramezani Tehrani, F. (2019). The relationship between clinical and biochemical characteristics and quality of life in patients with polycystic ovary syndrome. Clinical Endocrinology, 90(1), 129–137. https://doi.org/10.1111/cen.13858
- Annagür, B. B., Tazegül, A., & Akbaba, N. (2014). Body image, self-esteem and depressive symptomatology in women with polycystic ovary syndrome. Noro psikiyatri arsivi, 51(2), 129–132. https://doi.org/10.4274/npa.y6778
- Asdaq, S. M. B., Jomah, S., Hasan, R., Al-Baroudi, D., Alharbi, M., Alsubaie, S., Buhamad, M. H., Alyahya, B., & Al-Yamani, M. J. (2020). Impact of polycystic ovary syndrome on eating behavior, depression and health related quality of life: A cross-sectional study in Riyadh. Saudi Journal of Biological Sciences, 27(12), 3342–3347. https://doi.org/10.1016/j.sjbs.2020.08.039
- Balen, A. (1995). Polycystic ovarian syndrome: The spectrum of the disorder in 1741 patients. Human Reproduction, 10, 2705–2712.
- Banting, L. K., Gibson-Helm, M., Polman, R., Teede, H. J., & Stepto, N. K. (2014). Physical activity and mental health in women with polycystic ovary syndrome. BMC Women’s Health, 14(1), 1–9.
- Barnard, L., Ferriday, D., Guenther, N., Strauss, B., Balen, A. H., & Dye, L. (2007). Quality of life and psychological well being in polycystic ovary syndrome. Human Reproduction, 22(8), 2279–2286. https://doi.org/10.1093/humrep/dem108
- Bazarganipour, F., Ziaei, S., Montazeri, A., Foroozanfard, F., Kazemnejad, A., & Faghihzadeh, S. (2013). Body image satisfaction and self-esteem status among the patients with polycystic ovary syndrome. Iranian Journal of Reproductive Medicine, 11(10), 829.
- Bazarganipour, F., Ziaei, S., Montazeri, A., Foroozanfard, F., Kazemnejad, A., & Faghihzadeh, S. (2014). Health‐related quality of life in patients with polycystic ovary syndrome (PCOS): A model‐based study of predictive factors. The Journal of Sexual Medicine, 11(4), 1023–1032. https://doi.org/10.1111/jsm.12405
- Benetti-Pinto, C. L., Ferreira, S. R., Antunes, A., & Yela, D. A. (2015). The influence of body weight on sexual function and quality of life in women with polycystic ovary syndrome. Archives of Gynecology and Obstetrics, 291(2), 451–455. https://doi.org/10.1007/s00404-014-3423-1
- Bigaard, J., Spanggaard, I., Thomsen, B. L., Overvad, K., & Tjønneland, A. (2005). Self-reported and technician-measured waist circumferences differ in middle-aged men and women. The Journal of Nutrition, 135(9), 2263–2270. https://doi.org/10.1093/jn/135.9.2263
- Biro, F. M., Striegel-Moore, R. H., Franko, D. L., Padgett, J., & Bean, J. A. (2006). Self-esteem in adolescent females. The Journal of Adolescent Health, 39(4), 501–507. https://doi.org/10.1016/j.jadohealth.2006.03.010
- Bonheur, B., & Young, S. W. (1991). Exercise as a health-promoting lifestyle choice. Applied Nursing Research, 4(1), 2–6. https://doi.org/10.1016/s0897-1897(05)80045-x
- Boyle, J. A., Cunningham, J., O’Dea, K., Dunbar, T., & Norman, R. J. (2012). Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin, Australia. The Medical Journal of Australia, 196(1), 62–66. https://doi.org/10.5694/mja11.10553
- Burton, N. W., Brown, W., & Dobson, A. (2010). Accuracy of body mass index estimated from self‐reported height and weight in mid‐aged Australian women. Australian and New Zealand Journal of Public Health, 34(6), 620–623. https://doi.org/10.1111/j.1753-6405.2010.00618.x
- Chu, D.-T., Minh Nguyet, N. T., Nga, V. T., Thai Lien, N. V., Vo, D. D., Lien, N., Nhu Ngoc, V. T., Son, L. H., Le, D.-H., Nga, V. B., Van Tu, P., Van To, T., Ha, L. S., Tao, Y., & Pham, V.-H. (2019). An update on obesity: Mental consequences and psychological interventions. Diabetes & Metabolic Syndrome, 13(1), 155–160. https://doi.org/10.1016/j.dsx.2018.07.015
- Coffey, S., Bano, G., & Mason, H. D. (2006). Health-related quality of life in women with polycystic ovary syndrome: A comparison with the general population using the Polycystic Ovary Syndrome Questionnaire (PCOSQ) and the Short Form-36 (SF-36). Gynecological Endocrinology, 22(2), 80–86. https://doi.org/10.1080/09513590600604541
- Colwell, K., Lujan, M. E., Lawson, K. L., Pierson, R. A., & Chizen, D. R. (2010). Women’s perceptions of polycystic ovary syndrome following participation in a clinical research study: Implications for knowledge, feelings, and daily health practices. Journal of Obstetrics and Gynaecology Canada, 32(5), 453–459. https://doi.org/10.1016/S1701-2163(16)34499-1
- Costa, E. C., De Sá, J. C. F., Stepto, N. K., Costa, I. B. B., Farias Junior, L. F., Moreira, S. D. N. T., Soares, E. M. M., Lemos, T. M. A. M., Browne, R. A. V., & Azevedo, G. D. (2018). Aerobic training improves quality of life in women with polycystic ovary syndrome. Medicine & Science in Sports & Exercise, 50(7), 1357–1366.
- Cott, C. A., Gignac, M. A., & Badley, E. M. (1999). Determinants of self rated health for Canadians with chronic disease and disability. Journal of Epidemiology and Community Health, 53(11), 731–736. https://doi.org/10.1136/jech.53.11.731
- Cronin, L., Guyatt, G., Griffith, L., Wong, E., Azziz, R., Futterweit, W., Cook, D., & Dunaif, A. (1998). Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS). The Journal of Clinical Endocrinology and Metabolism, 83(6), 1976–1987. https://doi.org/10.1210/jcem.83.6.4990
- Cutler, D. A., Pride, S. M., & Cheung, A. P. (2019). Low intakes of dietary fiber and magnesium are associated with insulin resistance and hyperandrogenism in polycystic ovary syndrome: A cohort study. Food Science & Nutrition, 7(4), 1426–1437. https://doi.org/10.1002/fsn3.977
- Delacre, M., Lakens, D., & Leys, C. (2017). Why psychologists should by default use Welch’s t-test instead of Student’s t-test. International Review of Social Psychology, 30(1), 92–101. https://doi.org/10.5334/irsp.82
- Dokras, A., Stener-Victorin, E., Yildiz, B. O., Li, R., Ottey, S., Shah, D., Epperson, N., & Teede, H. (2018). Androgen Excess-Polycystic Ovary Syndrome Society: Position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertility and Sterility, 109(5), 888–899. https://doi.org/10.1016/j.fertnstert.2018.01.038
- Douglas, C. C., Norris, L. E., Oster, R. A., Darnell, B. E., Azziz, R., & Gower, B. A. (2006). Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertility and Sterility, 86(2), 411–417. https://doi.org/10.1016/j.fertnstert.2005.12.054
- Drosdzol, A., Skrzypulec, V., Mazur, B., & Pawlińska-Chmara, R. (2007). Quality of life and marital sexual satisfaction in women with polycystic ovary syndrome. Folia Histochemica et Cytobiologica, 45(I), S93–S97.
- Eichenberger, P. A., Diener, S. N., Kofmehl, R., & Spengler, C. M. (2013). Effects of exercise training on airway hyperreactivity in asthma: A systematic review and meta-analysis. Sports Medicine, 43(11), 1157–1170. https://doi.org/10.1007/s40279-013-0077-2
- Enders, C. K., & Bandalos, D. L. (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling: A Multidisciplinary Journal, 8(3), 430–457. https://doi.org/10.1207/S15328007SEM0803_5
- ESHRE, & ASRM Group. (2004). Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility, 81(1), 19–25.
- Froom, P., Melamed, S., Kristal-Boneh, E., Benbassat, J., & Ribak, J. (1999). Healthy volunteer effect in industrial workers. Journal of Clinical Epidemiology, 52(8), 731–735. https://doi.org/10.1016/s0895-4356(99)00070-0
- Fuller, J. T., Hartland, M. C., Maloney, L. T., & Davison, K. (2018). Therapeutic effects of aerobic and resistance exercises for cancer survivors: A systematic review of meta-analyses of clinical trials. British Journal of Sports Medicine, 52(20), 1311–1311. https://doi.org/10.1136/bjsports-2017-098285
- Gandek, B., Ware, J. E., Aaronson, N. K., Apolone, G., Bjorner, J. B., Brazier, J. E., Bullinger, M., Kaasa, S., Leplege, A., Prieto, L., & Sullivan, M. (1998). Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA project. Journal of Clinical Epidemiology, 51(11), 1171–1178. https://doi.org/10.1016/s0895-4356(98)00109-7
- Gorber, S. C., Tremblay, M., Moher, D., & Gorber, B. (2007). A comparison of direct vs. self‐report measures for assessing height, weight and body mass index: A systematic review. Obesity Reviews, 8(4), 307–326. https://doi.org/10.1111/j.1467-789X.2007.00347.x
- Gov. UK. (2022). Women’s Health Strategy for England (cited November 2023). Available from: https://www.gov.uk/government/publications/womens-health-strategy-for-england/womens-health-strategy-for-england
- Grimm, P. (2010). Social desirability bias. In J. N. Sheth & N. K. Malhorta (Eds.), Wiley international encyclopedia of marketing. John Wiley and Sons.
- Grubbs, L., & Carter, J. (2002). The relationship of perceived benefits and barriers to reported exercise behaviors in college undergraduates. Family & Community Health, 25(2), 76–84. https://doi.org/10.1097/00003727-200207000-00009
- Hagströmer, M., Oja, P., & Sjöström, M. (2006). The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutrition, 9(6), 755–762. https://doi.org/10.1079/phn2005898
- Hesketh, K., Wake, M., & Waters, E. (2004). Body mass index and parent-reported self-esteem in elementary school children: Evidence for a causal relationship. International Journal of Obesity and Related Metabolic Disorders, 28(10), 1233–1237. https://doi.org/10.1038/sj.ijo.0802624
- Hutchison, S. K., Stepto, N. K., Harrison, C. L., Moran, L. J., Strauss, B. J., & Teede, H. J. (2011). Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism, 96(1), E48–E56. https://doi.org/10.1210/jc.2010-0828
- Janz, N. K., & Becker, M. H. (1984). The health belief model: A decade later. Health Education Quarterly, 11(1), 1–47. https://doi.org/10.1177/109019818401100101
- Jones, G. L., Benes, K., Clark, T. L., Denham, R., Holder, M. G., Haynes, T. J., Mulgrew, N. C., Shepherd, K. E., Wilkinson, V. H., Singh, M., Balen, A., Lashen, H., & Ledger, W. L. (2004). The polycystic ovary syndrome health‐related quality of life questionnaire (PCOSQ): A validation. Human Reproduction, 19(2), 371–377. https://doi.org/10.1093/humrep/deh048
- Jones, M., & Nies, M. A. (1996). The relationship of perceived benefits of and barriers to reported exercise in older African American women. Public Health Nursing, 13(2), 151–158. https://doi.org/10.1111/j.1525-1446.1996.tb00233.x
- Karjula, S., Morin-Papunen, L., Auvinen, J., Ruokonen, A., Puukka, K., Franks, S., Järvelin, M.-R., Tapanainen, J. S., Jokelainen, J., Miettunen, J., & Piltonen, T. T. (2017). Psychological distress is more prevalent in fertile age and premenopausal women with PCOS symptoms: 15-year follow-up. The Journal of Clinical Endocrinology and Metabolism, 102(6), 1861–1869. https://doi.org/10.1210/jc.2016-3863
- Kelley, G. A., Kelley, K. S., & Hootman, J. M. (2015). Effects of exercise on depression in adults with arthritis: A systematic review with meta-analysis of randomized controlled trials. Arthritis Research & Therapy, 17(1), 21. https://doi.org/10.1186/s13075-015-0533-5
- Kerchner, A., Lester, W., Stuart, S. P., & Dokras, A. (2009). Risk of depression and other mental health disorders in women with polycystic ovary syndrome: A longitudinal study. Fertility and Sterility, 91(1), 207–212. https://doi.org/10.1016/j.fertnstert.2007.11.022
- Kiddy, D. S., Sharp, P. S., White, D. M., Scanlon, M. F., Mason, H. D., Bray, C. S., Polson, D. W., Reed, M. J., & Franks, S. (1990). Differences in clinical and endocrine features between obese and non‐obese subjects with polycystic ovary syndrome: An analysis of 263 consecutive cases. Clinical Endocrinology, 32(2), 213–220. https://doi.org/10.1111/j.1365-2265.1990.tb00857.x
- Kite, C., Lahart, I. M., Afzal, I., Broom, D. R., Randeva, H., Kyrou, I., & Brown, J. E. (2019). Exercise, or exercise and diet for the management of polycystic ovary syndrome: A systematic review and meta-analysis. Systematic Reviews, 8(1), 51. https://doi.org/10.1186/s13643-019-0962-3
- Kite, C., Parkes, E., Taylor, S. R., Davies, R. W., Lagojda, L., Brown, J. E., Broom, D. R., Kyrou, I., & Randeva, H. S. (2022). Time to load up–resistance training can improve the health of women with polycystic ovary syndrome (PCOS): A scoping review. Medical Sciences, 10(4), 53. https://doi.org/10.3390/medsci10040053
- Kyritsi, E. M., Dimitriadis, G. K., Kyrou, I., Kaltsas, G., & Randeva, H. S. (2017). PCOS remains a diagnosis of exclusion: A concise review of key endocrinopathies to exclude. Clinical Endocrinology, 86(1), 1–6. https://doi.org/10.1111/cen.13245
- Larson, R. B. (2019). Controlling social desirability bias. International Journal of Market Research, 61(5), 534–547. https://doi.org/10.1177/1470785318805305
- Lee, P. H., Macfarlane, D. J., Lam, T. H., & Stewart, S. M. (2011). Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 8(1), 1–11. https://doi.org/10.1186/1479-5868-8-115
- Li, W., Chen, Q., Xie, Y., Hu, J., Yang, S., & Lin, M. (2019). Prevalence and degree of insulin resistance in Chinese Han women with PCOS: Results from euglycemic‐hyperinsulinemic clamps. Clinical Endocrinology, 90(1), 138–144. https://doi.org/10.1111/cen.13860
- Lim, S. S., Davies, M., Norman, R. J., & Moran, L. (2012). Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Human Reproduction Update, 18(6), 618–637. https://doi.org/10.1093/humupd/dms030
- Lin, A. W., Kazemi, M., Jarrett, B. Y., Vanden Brink, H., Hoeger, K. M., Spandorfer, S. D., & Lujan, M. E. (2019). Dietary and physical activity behaviors in women with polycystic ovary syndrome per the new international evidence-based guideline. Nutrients, 11(11), 2711. https://doi.org/10.3390/nu11112711
- Liu, S. Y., Wrosch, C., Miller, G. E., & Pruessner, J. C. (2014). Self-esteem change and diurnal cortisol secretion in older adulthood. Psychoneuroendocrinology, 41, 111–120. https://doi.org/10.1016/j.psyneuen.2013.12.010
- Lizneva, D., Suturina, L., Walker, W., Brakta, S., Gavrilova-Jordan, L., & Azziz, R. (2016). Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertility and Sterility, 106(1), 6–15. https://doi.org/10.1016/j.fertnstert.2016.05.003
- Love-Koh, J., & Taylor, M. (2018). Physical activity and the environment: Final report. National Institute for Health and Care Excellence.
- Mario, F., do Amarante, F., Toscani, M., & Spritzer, P. (2012). Lean muscle mass in classic or ovulatory PCOS: Association with central obesity and insulin resistance. Experimental and Clinical Endocrinology & Diabetes, 120(9), 511–516. https://doi.org/10.1055/s-0032-1309006
- McCook, J. G., Reame, N. E., & Thatcher, S. S. (2005). Health‐related quality of life issues in women with polycystic ovary syndrome. Journal of Obstetric, Gynecologic, and Neonatal Nursing, 34(1), 12–20. https://doi.org/10.1177/0884217504272945
- Moghadam, Z. B., Fereidooni, B., Saffari, M., & Montazeri, A. (2018). Measures of health-related quality of life in PCOS women: A systematic review. International Journal of Women’s Health, 10, 397–408. https://doi.org/10.2147/IJWH.S165794
- Moody, A., & Neave, A. (2016). Health Survey for England 2015: Adult overweight and obesity. Health and Social Care Information Centre.
- Moran, L. J., Ranasinha, S., Zoungas, S., McNaughton, S. A., Brown, W. J., & Teede, H. J. (2013). The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Human Reproduction, 28(8), 2276–2283. https://doi.org/10.1093/humrep/det256
- Morán-Sánchez, I., Adoamnei, E., Sánchez-Ferrer, M. L., Prieto-Sánchez, M. T., Arense-Gonzalo, J. J., Carmona-Barnosi, A., Hernandez-Peñalver, A. I., Mendiola, J., & Torres-Cantero, A. M. (2021). Assessment of optimism in women with polycystic ovary syndrome: A case control-study. International Journal of Environmental Research and Public Health, 18(5), 2352. https://doi.org/10.3390/ijerph18052352
- Naumova, I., Castelo-Branco, C., Kasterina, I., & Casals, G. (2021). Quality of life in infertile women with polycystic ovary syndrome: A comparative study. Reproductive Sciences, 28(7), 1901–1909. https://doi.org/10.1007/s43032-020-00394-1
- Naz, S., Anjum, N., & Gul, I. (2020). A community based cross sectional study on prevalence of polycystic ovarian syndrome (PCOS) and health related quality of life in Pakistani females. EEO, 20(6), 141–147.
- Panico, A., Messina, G., Lupoli, G. A., Lupoli, R., Cacciapuoti, M., Moscatelli, F., Esposito, T., Villano, I., Valenzano, A., Monda, V., Messina, A., Precenzano, F., Cibelli, G., Monda, M., & Lupoli, G. (2017). Quality of life in overweight (obese) and normal-weight women with polycystic ovary syndrome. Patient Preference and Adherence, 11, 423–429. https://doi.org/10.2147/PPA.S119180
- Prince, S. A., Adamo, K. B., Hamel, M. E., Hardt, J., Gorber, S. C., & Tremblay, M. (2008). A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 5(1), 56. https://doi.org/10.1186/1479-5868-5-56
- Prince, S. A., Cardilli, L., Reed, J. L., Saunders, T. J., Kite, C., Douillette, K., Fournier, K., & Buckley, J. P. (2020). A comparison of self-reported and device measured sedentary behaviour in adults: A systematic review and meta-analysis. International Journal of Behavioral Nutrition and Physical Activity, 17(1), 1–17. https://doi.org/10.1186/s12966-020-00938-3
- Resnick, B., & Jenkins, L. S. (2000). Testing the reliability and validity of the self-efficacy for exercise scale. Nursing Research, 49(3), 154–159. https://doi.org/10.1097/00006199-200005000-00007
- Rodino, I. S., Byrne, S., & Sanders, K. A. (2016). Obesity and psychological wellbeing in patients undergoing fertility treatment. Reproductive Biomedicine Online, 32(1), 104–112. https://doi.org/10.1016/j.rbmo.2015.10.002
- Rosenberg, M. (1965). Rosenberg self-esteem scale (RSE). Acceptance and Commitment Therapy. Measures Package, 61(52), 18.
- Sánchez-Ferrer, M. L., Adoamnei, E., Prieto-Sánchez, M. T., Mendiola, J., Corbalán-Biyang, S., Moñino-García, M., Palomar-Rodríguez, J. A., & Torres-Cantero, A. M. (2020). Health-related quality of life in women with polycystic ovary syndrome attending to a tertiary hospital in Southeastern Spain: A case-control study. Health and Quality of Life Outcomes, 18(1), 232. https://doi.org/10.1186/s12955-020-01484-z
- Sechrist, K. R., Walker, S. N., & Pender, N. J. (1987). Development and psychometric evaluation of the exercise benefits/barriers scale. Research in Nursing & Health, 10(6), 357–365. https://doi.org/10.1002/nur.4770100603
- Shirazi, F. K. H., Khodamoradi, Z., & Jeddi, M. (2021). Insulin resistance and high molecular weight adiponectin in obese and non-obese patients with Polycystic Ovarian Syndrome (PCOS). BMC Endocrine Disorders, 21(1), 45. https://doi.org/10.1186/s12902-021-00710-z
- Shishehgar, F., Ramezani Tehrani, F., Mirmiran, P., Hajian, S., & Baghestani, A. R. (2016). Comparison of the association of excess weight on health related quality of life of women with polycystic ovary syndrome: An age-and BMI-matched case control study. PLoS One, 11(10), e0162911. https://doi.org/10.1371/journal.pone.0162911
- Strauss, R. S. (2000). Childhood obesity and self-esteem. Pediatrics, 105(1), e15. https://doi.org/10.1542/peds.105.1.e15
- Taghavi, S. A., Bazarganipour, F., Montazeri, A., Kazemnejad, A., Chaman, R., & Khosravi, A. (2015). Health-related quality of life in polycystic ovary syndrome patients: A systematic review. Iranian Journal of Reproductive Medicine, 13(8), 473–482.
- Tay, C. T., Teede, H. J., Hill, B., Loxton, D., & Joham, A. E. (2019). Increased prevalence of eating disorders, low self-esteem, and psychological distress in women with polycystic ovary syndrome: A community-based cohort study. Fertility and Sterility, 112(2), 353–361. https://doi.org/10.1016/j.fertnstert.2019.03.027
- Teede, H. J., Tay, C. T., Laven, J. J. E., Dokras, A., Moran, L. J., Piltonen, T. T., Costello, M. F., Boivin, J., Redman, L. M., Boyle, J. A., Norman, R. J., Mousa, A., Joham, A. E., & International PCOS Network. (2023). Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. European Journal of Endocrinology, 189(2), G43–G64. https://doi.org/10.1093/ejendo/lvad096
- Thomson, R. L., Buckley, J. D., & Brinkworth, G. D. (2016). Perceived exercise barriers are reduced and benefits are improved with lifestyle modification in overweight and obese women with polycystic ovary syndrome: A randomised controlled trial. BMC Women’s Health, 16(1), 14. https://doi.org/10.1186/s12905-016-0292-8
- Thomson, R. L., Buckley, J. D., Lim, S. S., Noakes, M., Clifton, P. M., Norman, R. J., & Brinkworth, G. D. (2010). Lifestyle management improves quality of life and depression in overweight and obese women with polycystic ovary syndrome. Fertility and Sterility, 94(5), 1812–1816. https://doi.org/10.1016/j.fertnstert.2009.11.001
- UN General Assembly. (2015). Transforming our world: The 2030 Agenda for Sustainable Development, A/RES/70/1. Retrieved November 13, 2023, from https://www.refworld.org/docid/57b6e3e44.html
- Wang, Z., Groen, H., Cantineau, A. E. P., van Elten, T. M., Karsten, M. D. A., van Oers, A. M., Mol, B. W. J., Roseboom, T. J., & Hoek, A. (2021). Dietary intake, eating behavior, physical activity, and quality of life in infertile women with PCOS and obesity compared with non-PCOS obese controls. Nutrients, 13(10), 3526. https://doi.org/10.3390/nu13103526
- Warburton, D. E., & Bredin, S. S. (2017). Health benefits of physical activity: A systematic review of current systematic reviews. Current Opinion in Cardiology, 32(5), 541–556. https://doi.org/10.1097/HCO.0000000000000437
- Ware, J. E., Kosinski, M., & Keller, S. D. (1994). SF-36 physical and mental health summary scales: A user’s manual. Health Assessment Lab.
- Ware, J. E., Jr., Kosinski, M., & Keller, S. D. (1996). A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Medical Care, 34(3), 220–233. https://doi.org/10.1097/00005650-199603000-00003
- Wright, C., Zborowski, J., Talbott, E., McHugh-Pemu, K., & Youk, A. (2004). Dietary intake, physical activity, and obesity in women with polycystic ovary syndrome. International Journal of Obesity and Related Metabolic Disorders, 28(8), 1026–1032. https://doi.org/10.1038/sj.ijo.0802661
- Yildiz, B. O., Knochenhauer, E. S., & Azziz, R. (2008). Impact of obesity on the risk for polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism, 93(1), 162–168. https://doi.org/10.1210/jc.2007-1834
- Yoldemir, T., Angin, P., Ramoglu, S., & Atasayan, K. (2017). Health-related quality of life (HRQL) in women with polycystic ovary syndrome (PCOS). Maturitas, 100, 175. https://doi.org/10.1016/j.maturitas.2017.03.192
Appendix
Table A1. Correlation matrix (Kendall’s τb) for self-reported variables for all study participants.
Table A2. Highest and lowest scoring items from the Exercise Benefits/Barriers Scale separated by study group (women with PCOS vs. women without PCOS [control] group).
