?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, effects of sucrose concentration (40–60% w/v), immersion time (1–5 h), and temperature (25–65°C) on water loss (WL) and solute gain (SG) during osmotic dehydration of broccoli stalk slices were quantitatively investigated using response surface methodology. It was found that concentration of sucrose solution, immersion time, and temperature affected the WL during osmotic dehydration process. Significant factors affecting the SG are the temperature and immersion time. The operating conditions to obtain water removal of 62% with solute intake of 7% were found to be at a sucrose concentration of 56% w/v, temperature of 42°C, and immersion time of 4 h.
Public Interest Statement
The broccoli stalk has the potential to be used as a food rather than farmers plowing back the crop into the soil. Drying could offer remarkable value-added product of this leftover plant part. However, drying is an energy-intensive process. Therefore, osmotic dehydration pre-treatment prior to drying process has gained interest in recent year for its quality advantage and energy saving. This work investigated the effects of operating factors on osmotic dehydration to achieve higher water removal with minimal solute gain as a pre-drying step. The information provided in this study is useful to achieve better energy conservation for further drying in the development of dried broccoli stalk slices.
Competing interests
The authors declare no competing interests.
1. Introduction
Drying is an important process to increase the shelf life of food products by removing the moisture to a point where the product is shelf stable. During drying, exposure of biological products to high temperatures for long periods of time often results in quality degradation. Therefore, there are a various improved drying technologies spurred in food application nowadays to produce better quality product and to improve energy efficiency during the drying process.
In the last few years, there has been a plethora study on osmotic dehydration of various agricultural products. This phenomenon indicates that osmotic dehydration has been considered as an important process in food production (An et al., Citation2013; Changrue & Orsat, Citation2009; Corrêa, Dev, Gariepy, & Raghavan, Citation2011; Kowalski & Mierzwa, Citation2013; Therdthai & Visalrakkij, Citation2012; Udomkun et al., Citation2015; Verma, Kaushik, & Rao, Citation2014; Wang, Zhang, & Mujumdar, Citation2010; Zhao et al., Citation2013). Osmotic dehydration utilizes osmotic principles when the food products are immersed in a hypertonic solution. During the immersion process, osmotic pressure acts as a driving force across the cell wall of the food product. This pressure results in cell membrane disintegration that causes the occurrences of mass transfer processes (Rastogi, Raghavarao, Niranjan, & Knorr, Citation2002). The two major mass transfers occurring are the water outflow from the product and the solute inflow from the solution to product concurrently. There are also natural solutes (such as minerals, organic acids, pigments, and flavor) leaching from the product to the solution during the immersion process (Taiwo, Eshtiaghi, Ade-Omowaye, & Knorr, Citation2003; Tortoe, Citation2010). All these mass transfer exchange might affect the final quality of food product. Thus, it is important to identify the optimum operating conditions to increase the mass transfer and produce a desirable quality product.
Osmotic dehydration is a process where the water is removed without a phase change. Thus, it can facilitate less energy requirement which is a major concern in the drying process (Raghavan & Valerie, Citation2008). Although this process will not result in sufficiently low moisture content to be considered as a shelf-stable product, still it can reduce the mass of fresh sample by up to 50%. Hence, it has often been applied as a pre-treatment step prior to finish drying. Previous study has shown that osmotic dehydration was found advantageous in improving the final quality of food product (An et al., Citation2013; Changrue, Citation2006; Therdthai, Zhou, & Pattanapa, Citation2011). In general, food products that undergo the osmotic step before drying results in modified functional properties of food; which are favorable for drying and offers a various taste of end products that can provides more options to consumers.
Broccoli is considered as the best known cruciferous vegetable that is rich in health-promoting components (Latté, Appel, & Lampen, Citation2011; Moreno, Carvajal, López-Berenguer, & García-Viguera, Citation2006). Each part of the broccoli plants has significant amounts of bioactive compounds (Campas-Baypoli et al., Citation2009; Singh, Chaturvedi, Walia, Kaushik, & Thakur, Citation2011; Vallejo, Tomás-Barberán, & García-Viguera, Citation2002). In broccoli market, the florets are the main raw commodities, meanwhile, the stalk and leaves are left as a crop remains. Therefore, there is a crucial need in adding value to these byproducts and thus bring profit to the broccoli industry.
In this study, we are interested to add value to the broccoli stalk by applying osmotic dehydration as a pre-treatment step before drying process. Previous study done by Ohnishi and Miyawaki (Citation2005) reported that broccoli tissue pretreated osmotically in sucrose solutions has higher retention of rheological properties compared to untreated samples. However, little information is available on the statistical modeling of osmotic dehydration of broccoli stalk slices.
Furthermore, the broccoli stalk consists of a woody outer layer that can limit the mass transfer during osmotic dehydration process due to its rigid cell wall. For this particular circumstance, skin peeling becomes an important step to enhance the mass transfer rate (Lewicki & Lenart, Citation1995). In addition to that, the rate of mass transfer is also dependent upon various operating variables such as the temperature, type of osmotic agent, osmotic solution concentration, immersion time, the ratio of solution to product, size, and geometry of the food sample.
Response surface methodology (RSM) is an effective statistical tool to evaluate the effects of operating factors even in the presence of complex interactions and to seek the optimum condition for multivariable response according to the target specification. RSM also is a useful technique in building empirical model for performance prediction that relates the response to the process parameter (Song & Hwang, Citation2003). Other advantage of employing RSM is associated with the design of experimentation which helps in reducing the number of experimental runs needed to evaluate the various operating factors and their interactions; leading to substantial reduction in the cost and experiment time (Giovanni, Citation1983; Mohsen, Afshin, Abdul Karim Sabo, & Nazamid, Citation2013).
Therefore, the objectives of this study are to investigate the effects of sucrose concentration, temperature, and immersion time on the mass transfer exchange during osmotic dehydration process of broccoli stalk and to find the optimum operating condition that maximizes the water loss (WL) while minimizing the solute gain (SG) using response surface methodology.
2. Materials and Methods
2.1. Materials
The broccoli stalks (Brassica oleracea L. var. italica) were washed, peeled, and sliced to a uniform shape with thickness of 6 mm and diameter of 23 mm. Moisture content of samples were determined by drying in a hot air oven at 105°C (Thermo Scientific Model 6528, Rockford, IL, USA) for until constant mass is reached (Association of Official Analytical Chemists, Citation2000). Sucrose (Redpath Sugars, Montreal, QC, Canada), used as an osmotic agent, was purchased locally. The osmotic solutions were prepared by dissolving the sucrose with the proper amount of distilled water.
2.2. Osmotic dehydration
Containers filled with sucrose solution are placed in a water bath controlled by heating/cooling unit (Isotemp 1013S, Fisher Scientific Inc., Pittsburgh, PA, USA). All containers were covered with a lid during the experiment in order to prevent evaporation from the osmotic solution. Once the osmotic solution reached the required temperature, the weighed broccoli stalk slices were immersed into the container and kept for the required time period. Samples were then removed from the osmotic solution and rinsed immediately in flowing water, drained on a tissue paper to remove surface moisture. The mass and moisture content of sample before and after osmotic process were determined. For each experiment, fresh sucrose solution was used. A ratio of sample to solution was kept constant at 1:20.
2.3. Determination of WL and SG
Gravimetric approach has been used extensively to analyze osmotically dehydrated products (Tortoe, Citation2010). The terminology used to quantify the mass exchange of sample and solution between broccoli stalk slices and sucrose solution during osmotic dehydration are the WL and SG. The WL and SG were calculated using the following equations (Hawkes & Flink, Citation1978):(1)
(1)
(2)
(2)
where Wwo is the mass of water in sample before dehydration (g), Wt is the mass of the sample after dehydration (g), Wso is the mass of the solids in sample before dehydration (g), and Wst is the mass of the solids in sample after dehydration (g).
2.4. Experimental design and statistical analysis
The response surface methodology was used to estimate the main effects of osmotic dehydration process. The experiments were designed according to central composite rotatable design using the statistical software JMP version 11 (SAS Institute, Cary, NC, USA). The rotatable design provides a constant variance under any rotation of the coordinate axes (Jones, Citation1996). The design required 20 runs derived from 23 full factorial design combined with 6 central points and 6 axial points. The six central point runs were added in this study with a purpose of: (1) Providing a measure of process stability (i.e. reduces model prediction error); (2) Capturing any inherent variability; and (3) Providing a check for curvature (Reddy, Citation2011).
The three continuous independent variables were sucrose concentration (X1), temperature (X2), and immersion time (X3) which were examined at five different levels as shown in Table . The column called Pattern identifies the coding of the factors; “ + ” for high factor, “–” for low factor, “a” and “A” for low and high axial values, and “0” for midrange. The levels of the independent variables were selected based on the preliminary study. The WL (Y1) and SG (Y2) obtained from experimental data are the dependent variables, also referred as responses that were subjected to an analysis of variance (ANOVA) in order to determine the significant effects of independent variables on each response. The following second-order polynomial model was used for the statistical analysis:(3)
(3)
Table 1. Process variables and the levels for the osmotic dehydration of broccoli stalk slices
where Yi is the response (i.e. is WL and
is SG); bo, bi, bii, bij are the regression coefficients for intercept, linear, quadratic and interaction terms; X1,
and X3 are the coded independent variables.
The identification of the operating conditions for osmotic dehydration of broccoli stalk slices as a pre-drying stage, which aimed to achieve higher water removal with minimal solute intake were evaluated using desirability function performed by the same software as mentioned earlier. Desirability function is a simple and useful technique in optimizing multiple response to provide the desirable product characteristics (Costa, Lourenço, & Pereira, Citation2011; Eren & Kaymak-Ertekin, Citation2007; Vieira, Pereira, & Hubinger, Citation2012). Different desirability functions can be used depending on particular response whether to be maximized or minimized (Derringer & Suich, Citation1980). For each response, the desirability functions assign numbers between 0 and 1, and it corresponds to the desirability interpretation such as: very good (1.0–0.8); good (0.8–0.63); fair (0.63–0.37); poor (0.37–0.2); and very poor (0.2–0) (Harrington, Citation1965). In practice, fitted response value at operating conditions x are used in place of Yi. Thus, for WL to be maximized, the desirability function, d1 is written as:
(4)
(4)
where L1 is a lower value for , and T1 is the target value. For SG to be minimized, the desirability function, d2 with the target value T2 and the upper value U2 has the form:
(5)
(5)
The individual desirabilities are then combined using the geometric mean, which gives the overall desirability function, D:(6)
(6)
3. Results and discussion
3.1. Effect of process variables on WL and SG
Experimental data obtained for WL and SG of broccoli stalk slices subjected to different run of osmotic dehydration conditions are presented in Table . The response for WL and SG are in the range of 37.0–65.0% and 3.7–15.6%, respectively. The results of multiple linear regressions conducted using second order response surface model for describing the effect of independent variables on the WL and SG are presented in Table (A) and (B), respectively.
Table 2. Experimental conditions with values of response variables
Table 3. Regression coefficients and ANOVA of the second-order polynomial model for the overall effects of the three process variables (sucrose concentration (X1), temperature (X2), and immersion time (X3)) on (A) water loss (WL) and (B) solute gain (SG)
According to the results given in Table , it can be seen that WL was affected linearly and quadratically by all the independent variables. The sucrose concentration had the most important effect on WL, followed by immersion time and temperature. However, the sucrose concentration did not significantly influence SG. Only the effect of temperature and immersion time positively affected SG. To visualize the effects of the independent variables on WL and SG, response surface curves were generated (Figures ). These surface plot reflects the influence of two independent variables on WL and SG, while the third variable was kept constant at their central point.
Figure 1. 3D surface plot as a function of concentration and immersion time during osmotic dehydration of broccoli stalk slices on WL.
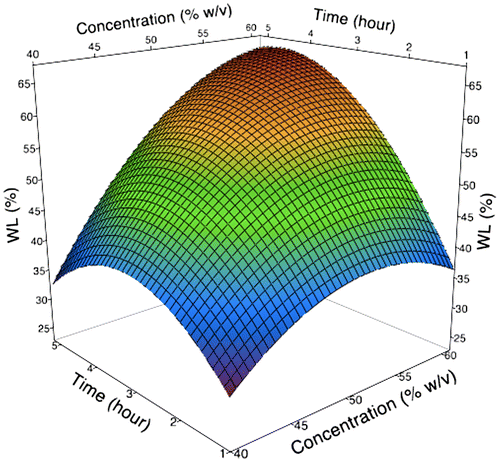
Figure 2. 3D surface plot as a function of temperature and concentration during osmotic dehydration of broccoli stalk slices on WL.
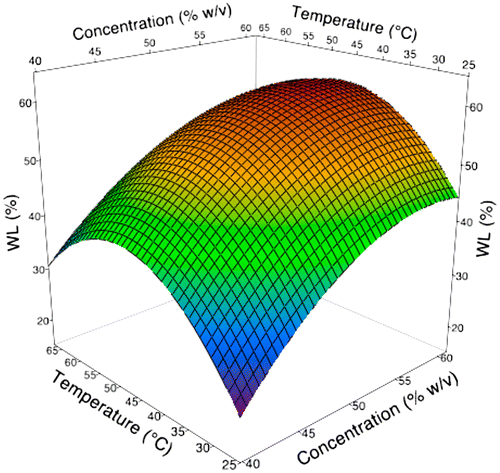
Figure 3. 3D surface plot as a function of immersion time and temperature during osmotic dehydration of broccoli stalk slices on SG.
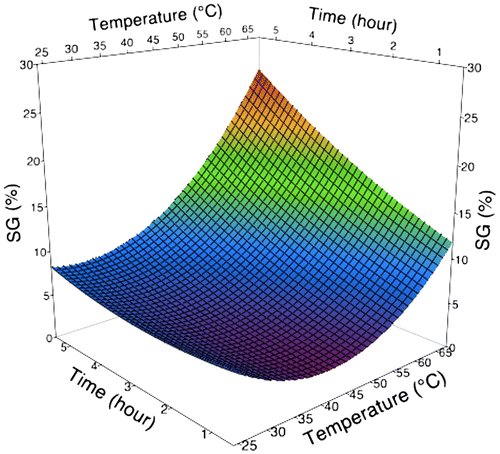
Figure shows the effect of sucrose concentration and immersion time on WL. It shows that WL is increasing gradually with solution concentration over the entire process due to high osmotic driving force between the sample and solution. In addition, the WL increases rapidly at the beginning stage of the process, after which the rate of water transfer is slowed down. These findings match those observed in earlier studies on potato (Eren & Kaymak-Ertekin, Citation2007). The higher water removal from the sample during the early stage of the process reduces concentration gradient around the sample and subsequently decreases the driving force, thus lowering the mass transfer rate.
On the other hand, WL increased at the lower temperatures, while the rate gradually decreased at higher temperature as shows in Figure . Higher temperatures seem to increase the solubility of solids in liquids. Therefore, the osmotic solution becomes more dilute (less concentrated) and it reduces the driving force during the osmotic dehydration process. Although the rate of water removal has decreased at the higher temperature, the SG kept increasing (Figure ). This is alluded to the membrane swelling effect which might increase the cell membrane permeability to sucrose molecules (Lazarides, Gekas, & Mavroudis, Citation1997). The increases in SG as temperature increased are in line with those of previous studies reported by Azoubel and Da Silva (Citation2008) for mangoes; Lee and Lim (Citation2011) for pumpkin; and Badwaik, Choudhury, Borah, and Deka (Citation2013) for bamboo shoots. It is to be noted in Figure that higher values of temperature in combination with longer immersion time produced sharper increases in SG. Through these findings, it demonstrates that higher temperature applied on osmotic dehydration of broccoli stalk contributes to the decrease in WL performance with increases in SG.
3.2. Predictive model for WL and SG
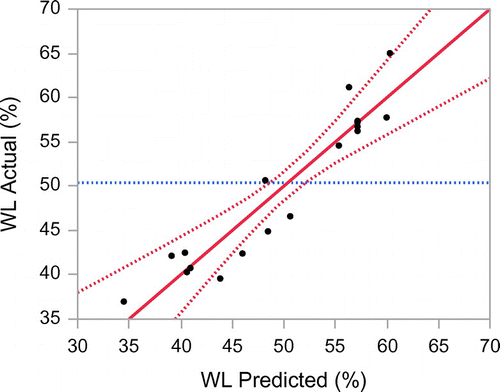
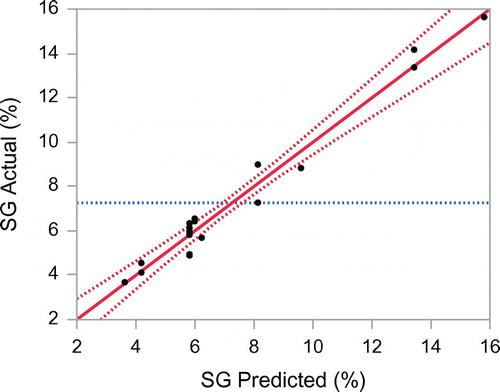
The predictive model was obtained for each response with stepwise regression where factors were rejected when their significance level was less than 95% (Draper, Smith, & Pownell, Citation1966). Table shows the analysis of variance for fitting the second-order polynomial models to the experimental data for both responses. The correlation value of these models for WL (R2 = 0.90) and SG (R2 = 0.96) indicate a good fit of experimental data. The plot of predicted versus of experimental data for WL is shown in Figure and SG in Figure . The mathematical expression to describe the relationship to the response with variables is shown in Equations (7) and (8) for WL and SG, respectively. Both models are valid within the applied range of the experimental processing variables used in this study.(7)
(7)
(8)
(8)
Table 4. Summary of fit and ANOVA of the stepwise regression for water loss (WL) and solute gain (SG)
where;
3.3. Operating conditions for osmotic dehydration as pre-drying step
The studied dehydration concept has showed partial water removal with less energy use for finish drying. However, the solute uptake would cause a change in sensory perception and acceptability. Moreover, excessive solute uptake during the osmotic process can block the surface layer of the product and this additional resistance can lower the water diffusion rate during the finish drying process (Menting, Hoogstad, & Thijssen, Citation1970).
For this reason, it is important to determine optimal operating conditions of the three independent variables: (1) sucrose concentration at a range of 40–60 % w/v; (2) temperature at a range of 25–65°C; and (3) immersion time at a range of 1–5 h to achieve higher WL at minimal SG which can be used at the pre-drying stage. By applying desirability function generated by the JMP statistical software, the results indicate that to achieve 61.5 (g/100 g fresh sample) of WL with corresponding SG of 6.7 (g/100 g fresh sample) where the conditions required are: a concentration of 56 % w/v operating at 42°C for 4 h of immersion time. The individual desirability at these conditions was found to be 0.74 for WL and 0.65 for SG; which provides an overall desirability of 0.70. These conditions show that the water content in broccoli stalk can be reduced by more than 50% through the osmotic dehydration. Meanwhile, less than 7% SG are achieved in four hours of immersion time.
4. Conclusion
In this study, the effects of sucrose concentration, temperature, and immersion time on mass transfer exchange during osmotic dehydration of broccoli stalk slices were investigated using response surface methodology. Analysis of variance shows that sucrose concentration, temperature and immersion time significantly affected WL, whereas, temperature and immersion time significantly affected SG. The predictive model for both responses was well fitted to the experimental data with high value of R2 (≥0.9). From the analysis, water removal of 61.5% with only 6.7% gain of solute can be accomplished through sucrose concentration of 56 % w/v at 42°C for 4 h immersion time. Also, energy conservation for further drying is achievable through the establishment of these pre-drying conditions.
Additional information
Funding
Notes on contributors
Nora Salina Md Salim
Our research group is working on the innovations of technologies in food, energy, and environmental nexus. Product developments using appropriate drying technologies are required in the transformation of the edible waste that still has the potential to be used as food. Thus, this value-added product will lead to minimization of crop losses and wastage problems, which in turn helps in the better economic return for the producers. Information provided from the manuscript is a key to provide energy saving for further drying process and also to enhance the drying performance and the quality of the dried product.
References
- An, K., Li, H., Zhao, D., Ding, S., Tao, H., & Wang, Z. (2013). Effect of osmotic dehydration with pulsed vacuum on hot-air drying kinetics and quality attributes of cherry tomatoes. Drying Technology, 31, 698–706.10.1080/07373937.2012.755192
- Association of Official Analytical Chemists. (2000). Official methods of analysis (17th ed.). Gaithersburg, MD: Author.
- Azoubel, P. M., & Da Silva, F. O. (2008). Optimisation of osmotic dehydration of ‘Tommy Atkins’ mango fruit. International Journal of Food Science & Technology, 43, 1276–1280.
- Badwaik, L. S., Choudhury, S., Borah, P. K., & Deka, S. C. (2013). Optimization of osmotic dehydration process of bamboo shoots in mixtures of sucrose and sodium chloride solutions. Journal of Food Processing and Preservation, 37, 1068–1077.10.1111/jfpp.2013.37.issue-6
- Campas-Baypoli, O. N., Sánchez-Machado, D. I., Bueno-Solano, C., Núñez-Gastélum, J. A., Reyes-Moreno, C., & López-Cervantes, J. (2009). Biochemical composition and physicochemical properties of broccoli flours. International Journal of Food Sciences and Nutrition, 60, 163–173.10.1080/09637480802702015
- Changrue, V. (2006). Hybrid (osmotic, microwave-vacuum) drying of strawberries and carrots. Montreal: McGill University.
- Changrue, V., & Orsat, V. (2009). Osmotically dehydrated microwave vacuum drying of carrots. Canadian Biosystems Engineering, 51, 311–319.
- Corrêa, J., Dev, S., Gariepy, Y., & Raghavan, G. (2011). Drying of pineapple by microwave-vacuum with osmotic pretreatment. Drying Technology, 29, 1556–1561.10.1080/07373937.2011.582558
- Costa, N. R., Lourenço, J., & Pereira, Z. L. (2011). Desirability function approach: A review and performance evaluation in adverse conditions. Chemometrics and Intelligent Laboratory Systems, 107, 234–244.10.1016/j.chemolab.2011.04.004
- Derringer, G., & Suich, R. (1980). Simultaneous optimization of several response variables. Journal of Quality Technology, 12, 214–219.
- Draper, N. R., Smith, H., Pownell, E. (1966). Applied regression analysis. New York, NY: Wiley.
- Eren, İ., & Kaymak-Ertekin, F. (2007). Optimization of osmotic dehydration of potato using response surface methodology. Journal of Food Engineering, 79, 344–352.10.1016/j.jfoodeng.2006.01.069
- Giovanni, M. (1983). Response surface methodology and product optimization. Food Technology, 37, 41–45.
- Harrington, E. (1965). The desirability function. Industrial Quality Control, 21, 494–498.
- Hawkes, J., & Flink, J. M. (1978). Osmotic concentration of fruit slices prior to freeze dehydration. Journal of Food Processing and Preservation, 2, 265–284.10.1111/jfpp.1978.2.issue-4
- Jones, S. P. (1996). Stability and response surface methodology. In J. H. d. B. Margriet, M. W. B. Hendriks, K. S. Age (Eds.), Data handling in science and technology (Vol. 19, pp. 11–77). Seattle, WA: Elsevier.
- Kowalski, S. J., & Mierzwa, D. (2013). Influence of osmotic pretreatment on kinetics of convective drying and quality of apples. Drying Technology, 31, 1849–1855.10.1080/07373937.2013.833518
- Latté, K. P., Appel, K.-E., & Lampen, A. (2011). Health benefits and possible risks of broccoli–An overview. Food and Chemical Toxicology, 49, 3287–3309.10.1016/j.fct.2011.08.019
- Lazarides, H. N., Gekas, V., & Mavroudis, N. (1997). Apparent mass diffusivities in fruit and vegetable tissues undergoing osmotic processing. Journal of Food Engineering, 31, 315–324.10.1016/S0260-8774(96)00084-2
- Lee, J. S., & Lim, L. (2011). Osmo-dehydration pretreatment for drying of pumpkin slice. International Food Research Journal, 18, 1223–1230.
- Lewicki, P., & Lenart, A. (1995). Osmotic dehydration of fruits and vegetables. Handbook of Industrial Drying, 1, 691–714.
- Menting, L., Hoogstad, B., & Thijssen, H. (1970). Diffusion coefficients of water and organic volatiles in carbohydrate-water systems. International Journal of Food Science & Technology, 5, 111–126.
- Mohsen, Z., Afshin, E., Abdul Karim Sabo, M., & Nazamid, S. (2013). Modeling of glutamic acid production by Lactobacillus plantarum MNZ. Electronic Journal of Biotechnology, 16, 2013.
- Moreno, D. A., Carvajal, M., López-Berenguer, C., & García-Viguera, C. (2006). Chemical and biological characterisation of nutraceutical compounds of broccoli. Journal of Pharmaceutical and Biomedical Analysis, 41, 1508–1522.10.1016/j.jpba.2006.04.003
- Ohnishi, S., & Miyawaki, O. (2005). Osmotic dehydrofreezing for protection of rheological properties of agricultural products from freezing-injury. Food Science and Technology Research, 11, 52–58.10.3136/fstr.11.52
- Raghavan, G. V., & Valerie, O. (2008). Nonconventional heating sources during drying. In C. Ratti (Ed.), Advances in food dehydration (pp. 401–422). Boca Raton, FL: CRC Press.
- Rastogi, N. K., Raghavarao, K. S. M. S., Niranjan, K., & Knorr, D. (2002). Recent developments in osmotic dehydration: methods to enhance mass transfer. Trends in Food Science & Technology, 13, 48–59.
- Reddy, T. A. (2011). Design of experiments. In Applied data analysis and modeling for energy engineers and scientists (pp. 183–208). New York, NY: Springer.10.1007/978-1-4419-9613-8
- Singh, B., Chaturvedi, S., Walia, S., Kaushik, G., & Thakur, S. (2011). Antioxidant potential of broccoli stalk: A preliminary investigation. Mediterranean Journal of Nutrition and Metabolism, 4, 227–230.10.1007/s12349-011-0058-7
- Song, M., & Hwang, S. (2003). 12 - Recycling food processing wastes. In B. Mattsson & U. Sonesson (Eds.), Environmentally-friendly food processing (pp. 205–217). Cambridge: Woodhead Publishing.10.1533/9781855737174.2.205
- Taiwo, K. A., Eshtiaghi, M. N., Ade-Omowaye, B. I. O., & Knorr, D. (2003). Osmotic dehydration of strawberry halves: influence of osmotic agents and pretreatment methods on mass transfer and product characteristics. International Journal of Food Science & Technology, 38, 693–707.
- Therdthai, N., & Visalrakkij, T. (2012). Effect of osmotic dehydration on dielectric properties, microwave vacuum drying kinetics and quality of mangosteen. International Journal of Food Science & Technology, 47, 2606–2612.
- Therdthai, N., Zhou, W., & Pattanapa, K. (2011). Microwave vacuum drying of osmotically dehydrated mandarin cv. (Sai-Namphaung). International Journal of Food Science & Technology, 46, 2401–2407.
- Tortoe, C. (2010). A review of osmodehydration for food industry. African Journal of Food Science, 4, 303–324.
- Udomkun, P., Nagle, M., Mahayothee, B., Nohr, D., Koza, A., & Müller, J. (2015). Influence of air drying properties on non-enzymatic browning, major bio-active compounds and antioxidant capacity of osmotically pretreated papaya. LWT - Food Science and Technology, 60, 914–922.10.1016/j.lwt.2014.10.036
- Vallejo, F., Tomás-Barberán, F., & García-Viguera, C. (2002). Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. European Food Research and Technology, 215, 310–316.
- Verma, D., Kaushik, N., & Rao, P. S. (2014). Application of high hydrostatic pressure as a pretreatment for osmotic dehydration of banana slices (Musa cavendishii) finish-dried by dehumidified air drying. Food and Bioprocess Technology, 7, 1281–1297.10.1007/s11947-013-1124-6
- Vieira, G. S., Pereira, L. M., & Hubinger, M. D. (2012). Optimisation of osmotic dehydration process of guavas by response surface methodology and desirability function. International Journal of Food Science & Technology, 47, 132–140.
- Wang, R., Zhang, M., & Mujumdar, A. S. (2010). Effect of osmotic dehydration on microwave freeze-drying characteristics and quality of potato chips. Drying Technology, 28, 798–806.10.1080/07373937.2010.482700
- Zhao, D., Zhao, C., Tao, H., An, K., Ding, S., & Wang, Z. (2013). The effect of osmosis pretreatment on hot-air drying and microwave drying characteristics of chili (Capsicum annuum L.) flesh. International Journal of Food Science & Technology, 48, 1589–1595.