Abstract
Methoxypoly(ethylene glycol) Poly(caprolactone) (mPEG–PCL) copolymers are important synthetic biomedical materials with amphiphilicity, controlled biodegradability, and great biocompatibility. They have great potential application in the fields of nanotechnology, tissue engineering, pharmaceutics, and medicinal chemistry. mPEG–PCL copolymers are biodegradable, biocompatible, and semi-crystalline copolymers having a very low glass transition temperature. Due to their slow degradation, mPEG–PCLs are ideally suitable for long-term delivery extending over a period of more than one year. This has led to its application in the preparation of different delivery systems in the form of microspheres, nanospheres, micelles, polymersomes, nanogels, and implants. Various categories of drugs have been encapsulated in mPEG–PCL for targeted drug delivery and for controlled drug release. As a research acmes incurrent years, mPEG–PCL copolymers and their end group-derived nanoparticles can progress the drug loading of hydrophobic drugs, enhancement bioavailability, escape being engulfed by phagocytes, reduce the burst release, and augment the circulation time of drugs in blood. Furthermore, these nanoparticles can assemble in inflammation or target locations to improve drug efficiency and diminish toxicity because of their smaller particle size and modified surface. Recent advances in mPEG–PCL block copolymer nanoparticles, counting the synthesis of mPEG–PCL and the preparation of mPEG–PCL nanoparticles, were discussed in this study, especially the drug release and adjustable characteristics of mPEG–PCL nanoparticles and their application in pharmaceutical preparations.
Public Interest Statement
Tremendous progress has been attained in evaluating and optimizing the application of mPEG–PCL copolymers in DDS. mPEG–PCL copolymers are biodegradable, biocompatible copolymers having a very low glass transition temperature. Due to their slow degradation, mPEG–PCLs are ideally suitable for long-term delivery extending over a period of more than one year. The reported studies on the mPEG–PCL copolymers involved syntheses of the copolymers, preparation, and application as micro/nanoparticles carrying various substances. Owing to the appearance of the PEG segment in mPEG–PCL copolymer, the degradation and biocompatibility of the PCL polyester have been improved. The synthetic procedures vary in reactants and catalysts to obtain different types of di-copolymers of mPEG–PCL. From the obtained copolymers, the fabrication of micro/nanoparticles can be adopted and modified from numerable techniques according to the attributes of materials and loaded substances. mPEG–PCL nanoparticles can be predictable to supply more apparatus and have a potential for the clinical management of diseases with extensive use prediction in pharmaceutical arrangements.
Competing interests
The author declares no competing interest.
1. Introduction
Drug delivery is a rapidly developing and evolving discipline underpinned by the principles and developments in related fields from classic chemistry to modern biotechnology. The development of drug delivery technology aims at enhancing the screening and evaluation of new compounds and “rescuing” failed compounds, optimizing them by improving their effectiveness or tolerability, and simplifying their administration (Rosen & Abribat, Citation2005). In past decades, the application of synthetic copolymers in drug delivery system (DDS) as drug carriers led to technological advances which bypassed the pharmacokinetic limitations of conventional, rapid release dosage forms. The improved DDS based on synthetic polymers generally appears in three types: micro/nanoparticles, implants (containing hydrogels), and fibers (Choi, Chae, & Nah, Citation2006). Drug is dissolved, entrapped, adsorbed, attached, or encapsulated in the polymeric material to make controlled release and localized drug delivery possible. Among the synthetic polymers adopted in DDS, biodegradable aliphatic polyesters such as poly(caprolactone) (PCL), poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and their copolymers received considerable attention (Chiellini & Solaro, Citation1996; Danafar et al., Citation2016; Masahiko, Citation2002; Robert & Vancanti, Citation1993). PCL is a semi-crystalline, hydrophobic polymer with a relatively polar ester group and five on-polar methylene groups in its repeating unit and has gained much attention as an ideal material for drug delivery and other applications through the decades (Mondrinos et al., Citation2006; Seregin & Coffer, Citation2006). And it is mostly synthesized by ring-opening polymerization method from caprolactone (CL) monomers. The high olefin content imparts polyolefin-like properties to PCL (Kim & Bae, Citation2004). However, owing to the high degree of crystallinity and hydrophobicity, PCL degrades rather slowly and is less biocompatible with soft tissues, which restrict its further clinical application. Therefore, the modification of PCL is proposed. Poly(ethylene glycol) (PEG), for its hydrophilicity, non-toxicity, and absence of antigenicity and immunogenicity, can be selected to be attached to PCL, forming mPEG–PCL copolymers. Thus, their hydrophilicity, biodegradability, and mechanical properties can be improved and may find much wider applications (Chen, Bei, & Wang, Citation2000; Huang et al., Citation2004; Moon, Lee, Han, & Byun, Citation2002). Due to the presence of hydrolytically labile ester groups in the macromolecular main chain, PCL is biodegradable under physiological conditions. And it was reported that mPEG–PCL copolymers with long PCL chains presented the same crystalline structure as PCL homopolymer, whereas PEG-bearing short PCL blocks retained the crystalline structure of PEG. However, both of them exhibited higher degradability compared with the PCL homopolymer (Wang & Qiu, Citation1993). In addition to the improved biodegradability, the copolymers showed higher hydrophilicity and better performance in the cell culture studies than the PCL homopolymer. Amphiphilic mPEG–PCL polymer can find wide applications as micro/nanoparticles or thermo-sensitive hydrogels. In this review, we deal with the recent developments in the application of mPEG–PCL block polymer in DDS. In Section 2, we focus on the synthesis mPEG–PCL copolymers. In the others Sections, we survey the preparation techniques and applications of nanoparticles from mPEG–PCL copolymers as drug carriers in DDS. Finally, conclusion and perspectives were given.
2. Synthesis of mPEG–PCL block copolymers
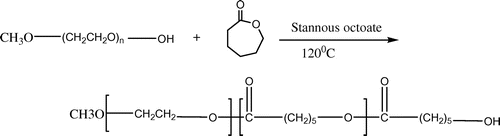
These copolymers could be synthesized by ring-opening polymerization among PEG or its end-group products (such as methoxy polyethylene glycol [mPEG]) and caprolactone. mPEG–PCL diblock copolymers have been synthesized using the ring-opening polymerization of 3-caprolactone (3-CL) using mPEG as the macro-initiator with stannous octoate[Sn(Oct)2] as the most conformist catalyst (). (Danafar, Rostamizadeh, Davaran, Valizadeh, & Hamidi, Citation2014; Yuan, Wang, Li, Xiong, & Deng, Citation2000). Tin salts are generally charity catalysts, particularly stannous compounds with a higher catalytic effectiveness. The sightseeing of new catalysts for mPEG–PCL synthesis never ends. A small number of new catalysts such as calcium (Cerrai et al., Citation1994; Rashkov, Manolova, Li, Espartero, & Vert, Citation1996; Youxin & Kissel, Citation1993), aluminum, bismuth, and yttrium tris(2,6-di-tert-butyl-4-methylphenolate)[Y(DBMP)3] complexes have been established. Rare earth complexes have been conveyed as effectual catalysts for the ring-opening polymerization of lactones, lactides, and cyclocarbonates (Deng et al., Citation2005; Nyce et al., Citation2003). Arrangement of rare earth trisphenolats for 3-CL homopolymerization with high movement and good controllability would formulate PCL with low toxicity (Connor, Nyce, Myers, Möck, & Hedrick, Citation2002; Myers et al., Citation2002). It was established that in the copolymerization system of l-lactide and PEG tetramer, the length of the polymer chain might be precise by altering the quantity of monomer and initiator, and copolymers with diverse molecular structures might be synthesized, such as A-B stellate copolymer, A-B-A triblock copolymer, multiblock copolymer, and reticular copolymer.
3. Preparation and processing of mPEG–PCL polymeric nanoparticles
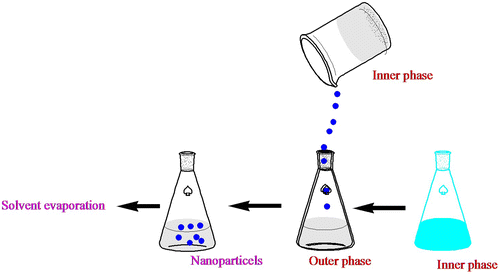
Biodegradable nanoparticles for pharmaceutical use are prepared from mPEG–PCL copolymers. Numerous systems are available for the synthesis of nanoparticles. After synthetic mPEG–PCL copolymers are applied, they are naturally dissolved in a suitable solvent monitored by precipitation in a liquid environment to nanoparticle creation. The drug envisioned to be encapsulated in the particles is usually merged in the development through the solvation and precipitation of mPEG–PCL copolymers. Emulsification/solvent diffusion, emulsification/solvent evaporation, nanoprecipitation, and salting-out systems exist as widely functional systems and have been conferred in numerous reviews (Hans & Lowman, Citation2002). In emulsification/solvent evaporation (), the liquefied mPEG–PCL copolymers are blended with the aqueous phase with the assistance of a high-energy cause, such as ultrasound or homogenization, monitored by solvent evaporation (vacuum and heat). In the salting-out system, the precipitation of mPEG–PCL copolymers is gained via phase farewell (organic solvent–aqueous phase) by the calculation of a salting-out agent, e.g. electrolytes in the event of acetone as a solvent for the mPEG–PCL copolymers. Likewise, in emulsification/solvent diffusion, nanoparticles are formed when the satiety limit of a partially water-miscible solvent (e.g. benzyl alcohol) is surpassed by adding water. In together systems, phase separation is fused by energetic stirring. The disjointed solvent is impassive, e.g. by cross-flow purification. In nanoprecipitation (), announced by Fessi and co-workers, the particle establishment is grounded on precipitation and successive solidification of the polymer is achieved at the boundary of a solvent and a non-solvent. So, the method is regularly called solvent supplanting or interfacial deposition. Systems such as supercritical fluid machineries (Jarmer, Lengsfeld, & Randolph, Citation2003) are unlike methods like nano-sized particle training. Two strategies happen. First, polymers with grafted molecules, such as poly(ethylene glycol) (PEG), can be used at the surface (covalent attachment) (Gref et al., Citation1994). Categorization of demonstrated mPEG–PCL nanoparticles based on the method of preparation was showed in .
Figure 2. Schematic illustration of preparation of mPEG–PCL nanoparticles; (a) o/w emulsion solvent evaporation.
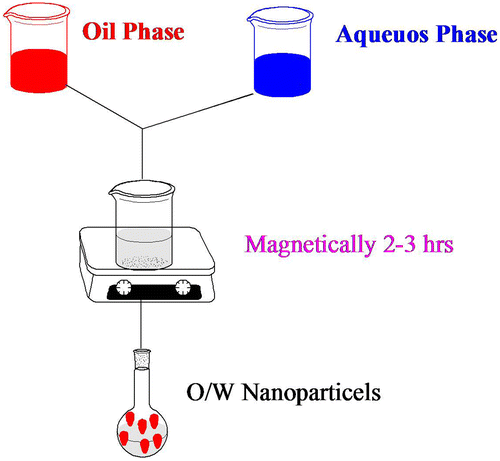
Table 1. Categorization of demonstrated mPEG–PCL nanoparticles based on the method of preparation
3.1. Nanoparticle characterization
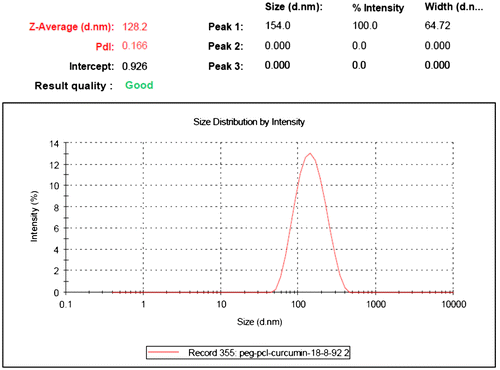
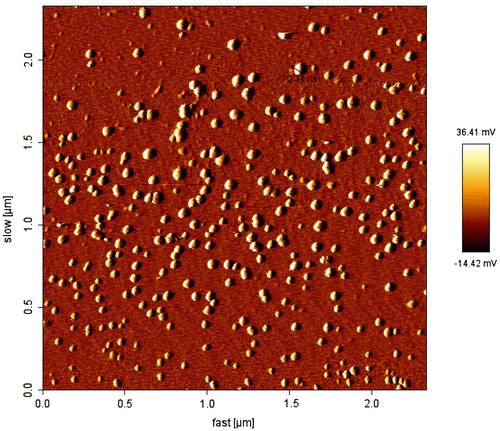
Methods for the classification of nanoparticle size and morphology are scanning electron microscopy (SEM) (Middleton & Tipton, Citation2000) and transmission electron microscopy (TEM) (Park, Citation1994). Currently, nanoparticles are typically calculated with 5,000–30,000-fold intensifications. In a SEM setup, the nanoparticulate sample, treated to be conductive (e.g. platinum), is scanned in a high vacuum chamber with an attentive electron beam (Ropert et al., Citation1993). Lesser electrons, emitted from the sample, are perceived and the image is formed. The environmental scanning microscope (ESEM) allows occupied uniform with moist samples, without conductive varnishes and high vacuum. In TEM, electrons sprinkled by the sample are detected; the sample is between the electron gun and the detectors (Hariharan et al., Citation2006). Another performance in pharmaceutical nanoparticle categorizations is atomic force microscopy (AFM).The formation of micellar nanostructures was confirmed by AFM. Apparently, mPEG–PCL micelles showed a homogeneous spherical morphology, as expected () (Kheiri, Sharafi, Danafar, & Ghasemi, Citation2016). Photon correlation spectroscopy (PCS), a system based on dynamic (laser) light scattering, is broadly used in size categorization purposes of nanoparticles (Maulding et al., Citation1986). PCS processes the strength dissimilarity of scattered light, and narrates it to the particle size with the benefit of an autocorrelation function (Pecora, Citation2000). Surface charge of nanoparticles, for its portion, regulates the presentation of the nanoparticle system in the body, e.g. interactions with cell membranes. Zeta (ζ) potential measurements provide information about the particle surface charge (Danafar, Rostamizadeh, Davaran, & Hamidi, Citation2014b). The size of nanoparticles was measured by dynamic light scattering technique. As shown in , the z-average curcumin-loaded mPEG–PCL micelles were found to be about 128 nm with their corresponding PDI being 0.166.
Figure 4. AFM image of micelles.
Source: Kheiri et al. (Citation2016).
3.2. Drug–polymer interactions
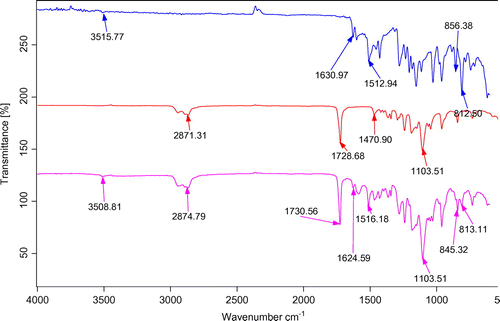
Using FTIR analysis, it is possible to obtain some information about the occurrence of possible interaction(s) between substances involved in a nanocarrier system. Generally, the interaction between drug and polymer is investigated through the band shifts exerted by the functional groups as well as through broadening in IR spectra compared to their individual spectra. To confirm the presence of any interactions between the drug and polymer, the FTIR spectra of solid micelles were compared with pure drug and individual polymers. Freeze-dried samples were pressed to form the standard disks and the FTIR spectra of the KBr disks were recorded using the aforementioned instrument from 600 to 4,000 cm−1. illustrates the FTIR spectra of mPEG–PCL copolymer, curcumin, and curcumin-loaded mPEG–PCL micelles. Comparing these data with the drug-loaded micelles’ spectrum exhibits the presence of curcumin characteristic peaks in the spectrum of micelles which could demonstrate the successful loading of curcumin in the nanoparticles. The most striking feature of the FTIR spectra of micelles was the blue shift of the C=O vibration, from 1,728.68 to 1,730.56 cm−1 for drug-loaded mPEG–PCL micelles compared to the copolymer spectrum. The shift in the micelles spectrum indicates that there exist some form(s) of association between curcumin and C=O functional group of the copolymer (Danafar, Rostamizadeh, Davaran, Valizadeh, et al., Citation2014). Any possible drug–polymer interaction(s) as well as the physical changes occurred on the drug or polymer can be studied using the thermal analysis. DSC analysis was carried out on pure drug and drug-loaded micelles. Samples were heated at a rate of 10°C min−1 and the data were recorded from 0 to 200°C (Danafar, Rostamizadeh, Davaran, Valizadeh, et al., Citation2014).
Figure 6. FTIR spectra of (a) curcumin, (b) mPEG–PCL and, (c) CUR-mPEG–PCL micelles.
Note: Spectrum blue is for curcumin, the red belongs to mPEG-PCL and pink belong to micelles. Source: Danafar, Rostamizadeh, Davaran, Valizadeh, et al. (Citation2014).
3.3. Drug loading and release
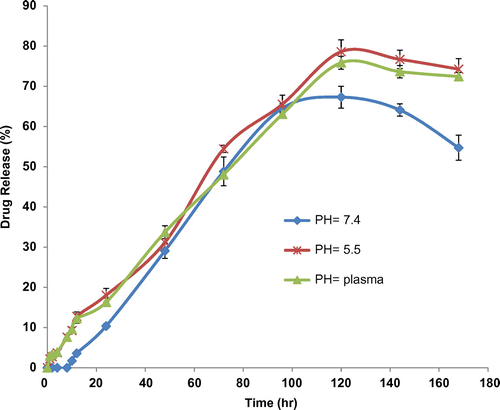
Drug entrapment or encapsulation efficacy is a proportion value that describes the amount of the substantial drug in the nanoparticles out of the total amount cast-off in the development. The drug contented (or drug loading) proportion is the drug amount associated to the nanoparticle mass. Due to the colloidal situation of nanoparticles, purpose of drug encapsulation, drug loading, and in vitro release could be challenging. Nevertheless, the encapsulation is dogged, e.g., by unraveling nanoparticles from the spreading medium by ultracentrifugation or ultrafiltration. Drug contented is then quantified from the supernatant or after solvation of the nanoparticle capsule (Danafar, Rostamizadeh, Davaran, & Hamidi, Citation2014b). In the container of drug quantization following freeze-drying, the polymer and drug are first dissolved (Painbeni, Venier-Julienne, & Benoit, Citation1998). For example, mPEG–PCL nanoparticles containing curcumin were dissolved in chloroform and the drug was quantified from the solution by UV spectroscopy since the polymer was established not to show absorbance intervention at the wavelengths applied (Kawashima, Yamamoto, Takeuchi, Hino, & Niwa, Citation1998). In a different case, D,L–PLA nanoparticles containing acyclovir were dissolved in dichloromethane followed by removal of the drug by an aqueous buffer (Barichello, Morishita, Takayama, & Nagai, Citation1999). The drug quantization was assigned by high performance liquid chromatography (HPLC). Drug release from nanoparticles could be achieved due to the dispersion of the particle, by desorption from the outside, or after poverty. Ultracentrifugation is frequently subjugated (Jarmer et al., Citation2003; Suryanarayanan, Citation1995). E.g. PLA–PEG–PLA nanoparticles containing atorvastatin and lisinopril were stimulated in a buffer solution (Ahlin, Kristl, Kristl, & Vrečer, Citation2002). As shown in , the percentage of curcumin released from the micelles slightly increased as the pH value decreased from 7.4 to 5.5. The sustained release of curcumin can be attributed to the entrapment of curcumin at the core of micelles. Therefore, mPEG–PCL copolymeric micelles can be regarded as highly attractive nanocarriers for both time-controlled drug delivery for hydrophobic drugs and for the achievement of different therapeutic objectives.
Figure 7. The release profiles of curcumin from CUR-mPEG–PCL micelles in different release media (a) pH 7.4, (b) plasma, and (c) pH 5.5.
Source: Danafar, Rostamizadeh, Davaran, Valizadeh, et al. (Citation2014).
3.4. Nanoparticles in drug delivery applications
The mPEG–PCL copolymer has the remuneration of both PEG and PCL. As a drug delivery system, mPEG–PCL copolymer nanoparticles have some compensation, e.g: (1) dipping the first-pass achieve and increasing bioavailability; (Jain et al., Citation2010) (2) growing drug loading and encapsulation competence; (Wei et al., Citation2008) (3) dipping particle size and burst release while flourishing targeting; (Lim Soo et al., Citation2008) (4) avoid discovery and elimination by the reticuloendothelial system, thus prolonging the circulation time of drugs in the blood and civilizing steadiness; (Maeda et al., Citation2001; Molina, Urbina, Gref, Brener, & Junior, Citation2001) and (5) good security. In a lot of studies, mPEG–PCL block copolymer nanoparticles were applied as carriers for vaccine, protein, and gene drugs, particularly in a sustained/controlled release drug delivery system and targeted-drug delivery method that might increase drug effectiveness and decrease drug struggle (Molina et al., Citation2001). mPEG–PCL copolymer nanoparticles are largely dispersal- and deprivation-controlled release systems. Hydrophobic drugs mostly are collected in the hydrophobic matrix. In dispersal-controlled systems, drugs are dissolved or isolated in PCL polymers, and the release rate is restricted by drug diffusion in a PCL matrix. The drugs neighboring the membrane surface can be released efficiently, while drugs seaside are needed to mellow to the membrane surface first and then are released effectively. In the deprivation-controlled system, drugs are dispersed in PCL, and the drug release time is assigned by degradation rate outstanding to effect from PCL chain length, drug loading of nanoparticles, release means, and additional factors. mPEG–PCL nanoparticles with a slight sharing of particle size have a minor particle size than PCL nanoparticles. Therefore, they can simply accumulate at inflammatory sites and then gradually release the drugs. Specially, targeting molecules can be synthesized in the PEG end to increase energetic targeting. Thus, the mixture of inactive and vigorous targeting can perform efficiently on abrasion locations.
4. Conclusions
Tremendous progress has been attained in evaluating and optimizing the application of mPEG–PCL copolymers in DDS. The reported studies on the mPEG–PCL copolymers involved syntheses of the copolymers, preparation, and application as micro/nanoparticles carrying various substances. Owing to the appearance of PEG segment in mPEG–PCL copolymer, the degradation and biocompatibility of the PCL polyester have been improved. The synthetic procedures vary in reactants and catalysts to obtain different types of di-copolymers of mPEG–PCL. From the obtained copolymers, the fabrication of micro/nanoparticles can be adopted and modified from numerable techniques according to the attributes of materials and loaded substances. The present review on mPEG–PCL nanoparticles are motionless performed in the laboratory, and the extended technique to go on how to construct technology steadier and fully developed and eventually encourage large-scale construction for clinical purpose. mPEG–PCL nanoparticles can be predictable to supply more apparatus and potential for the clinical management of diseases with extensive use prediction in pharmaceutical arrangements.
Additional information
Funding
Notes on contributors
Hossein Danafar
Hossein Danafar is an assistant professor of Medicinal Chemistry and dean of Department of Medicinal Chemistry at the School of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran. He received his PhD (2013) degree in Medicinal Chemistry from Tabriz University of Medical Sciences, Tabriz, Iran. His current research interests are analytical chemistry, pharmaceutical analysis, design, synthesis, and characterization of polymeric drug delivery systems, including micelles, nanogels, and molecularly imprinted carriers, bioconjugates, and magnetic-targeted drug delivery systems. The research study titled is (mPEG–PCL copolymeric nanoparticles in drug delivery systems). The future study is application of other block copolymers in drug delivery systems.
References
- Ahlin, P., Kristl, J., Kristl, A., & Vrečer, F. (2002). Investigation of polymeric nanoparticles as carriers of enalaprilat for oral administration. International Journal of Pharmaceutics, 239, 113–120.10.1016/S0378-5173(02)00076-5
- Ahmed, F., Discher, D. E. (2004). Self-porating polymersomes of PEG-PLA and PEG-PCL: Hydrolysis-triggered controlled release vesicles, Journal of Controlled Release, 96, 37–53.10.1016/j.jconrel.2003.12.021
- Barichello, J. M., Morishita, M., Takayama, K., & Nagai, T. (1999). Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Development and Industrial Pharmacy, 25, 471–476.10.1081/DDC-100102197
- Cerrai, P., Guerra, G. D., Lelli, L., Tricoli, M., Sbarbati Del Guerra, R., Cascone, M. G., & Giusti, P. (1994). Poly(ester-ether-ester) block copolymers as biomaterials. Journal of Materials Science: Materials in Medicine, 5, 33–39.
- Chawla, J. S., & Amiji, M. M. (2002). Biodegradable poly(ε-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. International Journal of Pharmaceutics, 249, 127–138.10.1016/S0378-5173(02)00483-0
- Chen, D. R., Bei, J. Z., & Wang, S. G. (2000). Polycaprolactone microparticles and their biodegradation. Polymer Degradation and Stability, 67, 455–459.10.1016/S0141-3910(99)00145-7
- Chiellini, E., & Solaro, R. (1996). Biodegradable polymeric materials. Advanced Materials, 8, 305–313.10.1002/(ISSN)1521-4095
- Choi, C. Y., Chae, S. Y., & Nah, J. W. (2006). Thermosensitive poly(N-isopropylacrylamide)-b-poly(ε-caprolactone) nanoparticles for efficient drug delivery systemfi. Polymer, 47, 4571–4580.10.1016/j.polymer.2006.05.011
- Choi, M. J., Ruktanonchai, U., Soottitantawat, A., & Min, S. G. (2009). Morphological characterization of encapsulated fish oil with beta-cyclodextrin and polycaprolactone. Food research international, 42, 989–997.
- Connor, E. F., Nyce, G. W., Myers, M., Möck, A., & Hedrick, J. L. (2002). First example of N-heterocyclic carbenes as catalysts for living polymerization: Organocatalyticring-opening polymerization of cyclic esters. Journal of the American Chemical Society, 124, 914–915.10.1021/ja0173324
- Danafar, H., Rostamizadeh, K., Davaran, S., & Hamidi, M. (2016). Drug-conjugated PLA–PEG–PLA copolymers: A novel approach for controlled delivery of hydrophilic drugs by micelle formation. Pharmaceutical Development and Technology, doi: 10.3109/10837450.2015.1125920.
- Danafar, H., Rostamizadeh, K., Davaran, S., Valizadeh, H., & Hamidi, M. (2014). Biodegradable m- PEG/PCL core-Shell Micelles: Preparation and characterization as a sustained release formulation for curcumin. Advanced Pharmaceutical Bulletin., 4, 501–510.
- Danafar, H., Rostamizadeh, K., Davaran, S., & Hamidi, M. (2014). PLA-PEG-PLA copolymer-based polymersomes as nanocarriers for delivery of hydrophilic and hydrophobic drugs: Preparation and evaluation with atorvastatin and lisinopril. Drug Development and Industrial Pharmacy, 40, 1411–1420.10.3109/03639045.2013.828223
- Deng, C., Rong, G., Tian, H., Tang, Z., Chen, X., & Jing, X. (2005). Synthesis and characterization of poly(ethylene glycol)-b-poly (l-lactide)-b-poly(l-glutamic acid) triblock copolymer. Polymer, 46, 653–659.10.1016/j.polymer.2004.11.100
- Gref, R., Minamitake, Y., Peracchia, M. T., Trubetskoy, V., Torchilin, V., & Langer, R. (1994). Biodegradable long-circulating polymeric nanospheres. Science, 263, 1600–1603.10.1126/science.8128245
- Hans, M. L., & Lowman, A. M. (2002). Biodegradable nanoparticles for drug delivery and targeting. Current Opinion in Solid State and Materials Science, 6, 319–327.10.1016/S1359-0286(02)00117-1
- Hariharan, S., Bhardwaj, V., Bala, I., Sitterberg, J., Bakowsky, U., & Ravi Kumar, M. N. V. (2006). Design of estradiol loaded PLGA nanoparticulate formulations: A potential oral delivery system for hormone therapy. Pharmaceutical Research, 23, 184–195.10.1007/s11095-005-8418-y
- Huang, M. H., Li, S., Hutmacher, D. W., Schantz, J. T., Vacanti, C. A., Braud, C., & Vert, M. (2004). Degradation and cell culture studies on block copolymers prepared by ring opening polymerization of ?-caprolactone in the presence of poly(ethylene glycol). Journal of Biomedical Materials Research, 69, 417–427.10.1002/(ISSN)1097-4636
- Jain, A. K., Goyal, A. K., Mishra, N., Vaidya, B., Mangal, S., & Vyas, S. P. (2010). PEG–PLA–PEG block copolymeric nanoparticles for oral immunization against hepatitis B. International Journal of Pharmaceutics, 387, 253–262.10.1016/j.ijpharm.2009.12.013
- Jarmer, D. J., Lengsfeld, C. S., & Randolph, T. W. (2003). Manipulation of particle size distribution of poly(l-lactic acid) nanoparticles with a jet-swirl nozzle during precipitation with a compressed antisolvent. The Journal of Supercritical Fluids, 27, 317–336.10.1016/S0896-8446(02)00245-0
- Kawashima, Y., Yamamoto, H., Takeuchi, H., Hino, T., & Niwa, T. (1998). Properties of a peptide containing dl-lactide/glycolide copolymer nanospheres prepared by novel emulsion solvent diffusion methods. European Journal of Pharmaceutics and Biopharmaceutics, 45, 41–48.10.1016/S0939-6411(97)00121-5
- Kheiri, H., Sharafi, A., Danafar, H., & Ghasemi, H. (2016). Poly (caprolactone)-poly (ethylene glycol) - poly (caprolactone) (PCL-PEG-PCL) nanoparticles: A costly and efficient system for in vitro and in-vivo delivery of curcumin. RSC Advances, 6, 14403–14415.
- Kim, J., & Bae, Y. (2004). Albumin loaded microsphere of amphiphilic poly(ethylene glycol)/ poly(α-ester) multiblock copolymer. European Journal of Pharmaceutical Sciences, 23, 245–251.10.1016/j.ejps.2004.07.011
- Lee, J., Oh, S., Joo, M. K., & Jeong, B. (2008). Solvent-free preparation of caprolactone oligomer microspheres. Journal of Physics and Chemistry of Solids, 69, 1596–1599.10.1016/j.jpcs.2007.09.016
- Li, G., Cai, Q., Bei, J., & Wang, S. (2003). Morphology and levonorgestrel release behavior ofpolycaprolactone/poly(ethylene oxide)/polylactide tri-component copolymericmicrospheres. Polymers for Advanced Technologies, 14, 239–244.10.1002/(ISSN)1099-1581
- Lim Soo, P., Cho, J., Grant, J., Ho, E., Piquette-Miller, M., Allen, C. (2008). Drug release mechanism of paclitaxel from a chitosan–lipid implant system: Effect of swelling, degradation and morphology. European Journal of Pharmaceutics and Biopharmaceutics. ,69, 149–157.10.1016/j.ejpb.2007.11.003
- Lowery, J. L., Datta, N., & Rutledge, G. C. (2009). Effect of fiber diameter, pore sizeand seeding method on growth of human dermal fibroblasts in electrospunpoly(epsilon-caprolactone) fibrous mats. Biomaterials, 31, 491–504.
- Maeda, H., Wu, J., Sawa, T., Matsumura, Y., & Hori, K. (2000). Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. Journal of Controlled Release, 65, 271–284.10.1016/S0168-3659(99)00248-5
- Masahiko, O. (2002). Chemical syntheses of biodegradable polymers. Progress in Polymer Science, 27, 87–133.
- Maulding, H. V., Tice, T. R., Cowsar, D. R., Fong, J. W., Pearson, J. E., & Nazareno, J. P. (1986). Biodegradable microcapsules: Acceleration of polymeric excipient hydrolytic rate by incorporation of a basic medicament. Journal of Controlled Release, 3, 103–117.10.1016/0168-3659(86)90071-4
- Molina, J., Urbina, J., Gref, R., Brener, Z., Junior, J. M. R., (2001). Cure of experimental Chagas’ disease by the bis-triazole DO870 incorporated into ‘stealth’ polyethyleneglycol-polylactide nanospheres. Journal of Antimicrobial Chemotherapy, 47, 101–104.
- Middleton, J. C., & Tipton, A. J. (2000). Synthetic biodegradable polymers as orthopedic devices. Biomaterials, 21, 2335–2346.10.1016/S0142-9612(00)00101-0
- Mondrinos, M. J., Dembzynski, R., Lu, L., Byrapogu, V. K., Wootton, D. M., Lelkes, P. I., & Zhou, J. (2006). Porogen-based solid freeform fabrication of polycaprolactone–calcium phosphate scaffolds for tissue engineering. Biomaterials, 27, 4399–4408.10.1016/j.biomaterials.2006.03.049
- Moon, H. T., Lee, Y. K., Han, J. K., & Byun, Y. J. (2002). Improved blood compatibility by sustained release of heparin–deoxycholic acid conjugates in a PCL–PEG multiblock copolymer matrix. Journal of Biomaterials Science, Polymer Edition, 13, 817–828.10.1163/156856202760197438
- Myers, M., Connor, E. F., Glauser, T., Möck, A., Nyce, G. W., & Hedrick, J. L. (2002). Phosphines: Nucleophilic organic catalysts for the controlled ring-opening polymerization of lactides. Journal of Polymer Science Part A: Polymer Chemistry, 40, 844–851.10.1002/pola.v40:7
- Nyce, G. W., Glauser, T., Connor, E. F., Möck, A., Waymouth, R. M., & Hedrick, J. L. (2003). Insitu generation of carbenes: A general and versatile platform for organo catalytic living polymerization. Journal of the American Chemical Society, 125, 3046–3056.10.1021/ja021084+
- Park, T. G. (1994). Degradation of poly(d,l-lactic acid) microspheres: Effect of molecular weight. Journal of Controlled Release, 30, 161–173.10.1016/0168-3659(94)90263-1
- Park, E. K., Kim, S. Y., Lee, S. B., & Lee, Y. M. (2005). Folate-conjugated methoxy poly(ethylene glycol)/poly(ɛ-caprolactone) amphiphilic block copolymeric micelles for tumor-targeted drug delivery. Journal of Controlled Release, 109, 158–168.10.1016/j.jconrel.2005.09.039
- Painbeni, T., Venier-Julienne, M. C., & Benoit, J. P. (1998). Internal morphology of poly(d,l-lactide-co-glycolide) BCNU-loaded microspheres. Influence on drug stability. European Journal of Pharmaceutics and Biopharmaceutics, 45, 31–39.10.1016/S0939-6411(97)00120-3
- Pecora, R. (2000). Dynamic light scattering measurement of nanometer particles in liquids. Journal of Nanoparticle Research, 2, 123–131.10.1023/A:1010067107182
- Rashkov, I., Manolova, N., Li, S. M., Espartero, J. L., & Vert, M. (1996). Synthesis charac-terization, and hydrolytic degradation of PLA/PEO/PLA triblock copolymerswithshort poly(l-lactic acid) chains. Macromolecules, 29, 50–56.10.1021/ma950530t
- Ropert, C., Bazile, D., Bredenbach, J., Marlard, M., Veillard, M., & Spenlehauer, G. (1993). Fate of 14C radiolabeled poly(dl-lactic acid) nanoparticles following oral administration to rats. Colloids and Surfaces B: Biointerfaces, 1, 233–239.10.1016/0927-7765(93)80023-R
- Robert, L., & Vancanti, J. P. (1993). Tissue engineering. Science, 260, 920–926.
- Rosen, H., & Abribat, T. (2005). Timeline: The rise and rise of drug delivery. Nature Reviews Drug Discovery, 4, 381–385.10.1038/nrd1721
- Seregin, V. V., & Coffer, J. L. (2006). Biomineralization of calcium disilicide in porous polycaprolactone scaffolds. Biomaterials, 27, 4745–4754.10.1016/j.biomaterials.2006.04.031
- Suryanarayanan, R. (1995). X-ray powder diffractometry. In H. G. Brittain (Ed.), Physical characterization of pharmaceutical solids, vol. 70 (pp. 187–221). New York, NY: Marcel Dekker.10.1201/IHCDRUPHASCI
- Youxin, L., & Kissel, T. (1993). Synthesis and properties of biodegradable ABA triblock copolymers consisting of poly(l-lactic acid) or poly (l-lactic-co-glycolic acid) A-blocks attached to central poly ( oxyethylene ) B-blocks. Journal of Controlled Release, 27, 247–257.10.1016/0168-3659(93)90155-X
- Yuan, M. L., Wang, Y. H., Li, X. H., Xiong, C. D., & Deng, X. M. (2000). Polymerizatioof lactides and lactones. 10. Synthesis, characterization, and application oamino-terminated poly(ethylene glycol)-co-poly(-caprolactone) block copolymer. Macromolecules, 33, 1613–1617.10.1021/ma991388p
- Wang, S. G., & Qiu, B. (1993). Polycaprolactone–poly(ethylene glycol) block copoly-mer. I. Synthesis and degradability in vitro. Polymers for Advanced Technologies, 4, 363–366.
- Wei, Q., Wei, W., Tian, R., Wang, L. Y., Su, Z. G., & Ma, G. H. (2008). Preparation of uniform-sized PELA microspheres with high encapsulation efficiency of antigen by premix membrane emulsification. Journal of Colloid and Interface Science, 323, 267–273.10.1016/j.jcis.2008.04.058