 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Difference in thyroid function depending on the etiology of end-stage renal disease (ESRD) was evaluated in 124 Japanese patients on haemodialysis (HD) due to either chronic glomerulonephritis (CGN, n = 82) or lifestyle related systemic disease (non-CGN, n = 42), such as diabetes mellitus (n = 30) or hypertension (n = 12). There was no significant difference in serum free thyroxine, free triiodothyronine and thyroid-stimulating hormone (TSH) level, but serum thyroglobulin (Tg) level was significantly higher in CGN (p = 0.0151). Prevalence of the patients with hypothyroidism (TSH > 4.83 mU/l) was 11 or 13.4% in CGN and 4 or 9.5% in non-CGN (p = 0.017). The most striking finding was the elevated Tg in 38 or 46.3% in CGN and in 11 or 26.2% in non-CGN (p = 0.034). Logistic regression analysis revealed elevated serum TSH level and higher thyroid volume were the significant factors associated with elevated Tg level. Extreme Tg elevation over 100 ng/ml was found only in CGN (12 or 14.6%), and 2 of the patients were overtly hypothyroid but became euthyroid after iodide restriction. Elevated Tg responding to elevated TSH mainly found in CGN suggested the relatively preserved thyroid tissue and reversible recovery of the thyroid function.
Public interest statement
After the introduction of haemodialysis, the prognosis of the patients with renal failure improved dramatically. However, several problems became apparent during haemodialysis. One of them is the high prevalence of thyroid dysfunction. In this paper, we evaluated the clinical differences depending on the etiology of renal failure such as (1) nephritis or (2) nephropathy due to systemic disease such as diabetes mellitus or hypertension. The complication of hypothyroidism was higher in nephritis (13.4%) than systemic nephropathy (9.5%). However, two patients with overt hypothyroidism, found in nephritis group, became euthyroid spontaneously after iodide restriction. The interesting finding was the elevated serum thyroglobulin level, a marker for the reversible recovery of thyroid function, especially in nephritis group. These findings suggested that elevated serum thyroglobulin level in renal failure due to nephritis is a sensitive marker of reversible thyroid dysfunction induced by some exacerbating factors such as excess iodide ingestion.
Competing interests
The authors declare no competing interests.
1. Introduction
The association between renal dysfunction and thyroid function has been extensively studied (Ang, Kaptein, & Massry, Citation1987; Asvold, Bjoro, & Vatten, Citation2011; Chonchol et al., Citation2008; Forest, Dube, & Talbot, Citation1982; Gardner, Mans, & Thomas, Citation1986; Hardy, Ragbeer, & Nascimento, Citation1988; Hershman et al., Citation1978; Kang et al., Citation2008; Kaptein, Citation1996; Kaptein, Quion-Verde, & Chooljian, Citation1988; Kutlay et al., Citation2005; Lim, Fang, Katz, & Refetoff, Citation1977; Lo, Chertow, Go, & Hsu, Citation2005; Shantha et al., Citation2011; Targher et al., Citation2009). Recent studies using chemiluminescent immunoassay suggested extraordinary high prevalence of hypothyroidism in the patients with renal dysfunction (18–28%) (Asvold et al., Citation2011; Chonchol et al., Citation2008; Kang et al., Citation2008; Kaptein, Citation1996; Lo et al., Citation2005; Shantha et al., Citation2011; Targher et al., Citation2009).
In the previous study, we evaluated the thyroid function in the 145 patients with end-stage renal disease (ESRD) on haemodialysis (HD), compared with sex and age matched healthy controls (Sanai et al., Citation2015). In that study, we pointed out the importance of differentiating two kinds of thyroid dysfunction found in ESRD, such as (1) complication of primary thyroid dysfunction and (2) apparent thyroid dysfunction due to ESRD itself as non-thyroidal illness (NTI) including significantly lower serum levels of both free thyroxine (fT4) and free triiodothyronine (fT3) and slightly but significantly elevated serum thyroid stimulating hormone (TSH) in ESRD. We further emphasized the importance of the specific reference values for each NTI such as ESRD in order to evaluate the prevalence of the patients with primary thyroid dysfunction, especially in the patients with borderline thyroid dysfunction (Sanai et al., Citation2015).
Even compared with the specific reference values for ESRD, the prevalence of the patients with primary hypothyroidism was found to be very high, including subclinical hypothyroidism in 10.3% and overt hypothyroidism in 1.4% (Sanai et al., Citation2015). Interestingly, most of the patients with overt hypothyroidism became euthyroid after iodide restriction suggesting reversible hypothyroidism induced by excess iodide ingestion in Japan (Sanai et al., Citation2008).
However, the difference in clinical features depending on the etiology of ESRD has been unclear. Therefore, we evaluated the difference in the clinical features including thyroid dysfunction depending on the etiology of ESRD, such as (1) chronic glomerulonephritis (CGN) as an example of primary kidney disease, and (2) non-CGN group or nephropathy due to lifestyle related systemic disease, including diabetic nephropathy (DM) and hypertensive nephrosclerosis (HTN).
2. Subject and methods
2.1. Patients selection
In this study, the thyroid function of 124 Japanese ESRD patients on maintenance HD at the Abe Clinic were examined by blood sampling and ultrasonography, and evaluated the clinical difference depending on the pathogenesis of ESRD, such as the CGN group (n = 82) and the non-CGN group (n = 42) including DM (n = 30) and HTN (n = 12).
Clinical data were compared with 166 healthy individuals without renal disease.
We conducted a retrospective analysis at a single center.
2.2. Blood sampling methods, dialysis and cardiothoracic ratio
The serum levels of creatinine, blood urea nitrogen, calcium, phosphate, intact parathyroid hormone, Kt/V (urea kinetics; K, the whole-body clearance rate of urea; t, effective treatment time; V, total body water), fT3, fT4, TSH, thyroglobulin (Tg), total cholesterol, creatine phosphokinase (CPK) levels were assayed from blood sample drawn immediately before HD. The haematocrit and the cardiothoracic ratio in chest X-ray were measured immediately before HD. The serum fT4, fT3, and TSH levels were measured by electrochemiluminescence (Blackburn et al., Citation1991). The reference values for healthy control patients and ESRD patients were, 0.8–1.7 and 0.6–1.3 ng/dl for fT4, 2.2–3.8 and 1.4–3.2 pg/ml for fT3, 0.42–3.81 and 0.65–4.83 mU/l for TSH and 1.4–3.6 and 1.4–3.6 for fT3/fT4 ratio, respectively (Sanai et al., Citation2015). Serum levels of Tg, well known thyroid specific protein as a marker for reversible recovery of thyroid function, were measured with immunoradiometric assay (IRMA) kit (Bayer & Kriss, Citation1979) (reference value; 30 ng/ml).
2.3. Ultrasonographic study
Thyroid gland morphology was examined by ultrasonography (ProSound SSD-4000; Aloka Co., Ltd., Tokyo, Japan) using a frequency probe (8.5 MHz Electronic Linear Probe, Aloka Co., Ltd., Tokyo, Japan). The estimated TV was measured as follow:
TV (ml) = TV right (ml) + TV left (ml),
TV right or left = long axis × short axis × thickness × 0.7 ml (Yokozawa & Morita, Citation1999).
As the normal range of TV is 16–20 ml in Japanese people, a “goiter” was defined as a TV > 20 ml.
2.4. Statistical analysis
Continuous data are presented as the mean ± SD or median (range), as appropriate for each variable. Statistical differences were calculated by the one-way analysis variance, the unpaired τ-test with the Bonferroni’s method or Kruskal–Wallis test and a multivariate logistic regression analysis. Serum TSH level was evaluated after logarithmic conversion. Correlation between serum Tg and TSH levels was evaluated by Spearman’s rank correlation coefficient. χ2 test was used to perform group comparisons of the categorical variables. The analyses were performed using the JMP 10 software program (SAS Institute, Inc., Cary, North Carolina). A p value below 0.05 was considered to be statistically significant.
3. Results
3.1. Comparison of clinical data between the CGN and the non-CGN groups as the causes of ESRD
The clinical data were shown in Table . Compared with the healthy controls, haematocrit and total cholesterol levels were significantly lower and creatinine, blood urea nitrogen and inorganic phosphate levels were higher both in the CGN and the non-CGN group (p < 0.05). There was no significant difference in CPK and calcium level. Between the CGN and the non-CGN group, there were no significant differences in clinical data including sex, haematocrit, the cardiothoracic ratio in chest X-ray or intact parathyroid hormone levels, although the age was significantly older (p < 0.05) and the duration of HD was significantly shorter (p < 0.05) in the non-CGN group. As the metabolic marker of hypothyroidism, there was no significant difference in serum total cholesterol levels. Serum CPK and calcium levels was slightly significantly higher in the CGN group (p < 0.05).
Table 1. Clinical data and thyroid function in end-stage renal disease on haemodialysis: Comparison between CGN and non-CGN
3.2. Comparison of thyroid function between CGN and non-CGN groups as the causes of ESRD
Thyroid function was shown in Table . Compared with healthy control, there was no significant difference in serum fT4 levels but serum fT3 levels was significantly lower both in the CGN and the non-CGN group. Serum TSH level was higher in the CGN group and fT3/fT4 levels was lower in the non-CGN group (p < 0.05). Between the CGN and the non-CGN group, there were no significant differences in serum fT4 or fT3 levels and estimated thyroid volume. Serum TSH levels seemed slightly higher in the CGN and fT3/fT4 level lower in the non-CGN group, but the difference was not significant. Serum Tg level was significant higher in CGN group (p < 0.05).
3.3. Prevalence of hypothyroidism and patients with elevated serum Tg level in ESRD on HD: Comparison between the CGN and the non-CGN groups
As shown in Table , the prevalence of hypothyroidism was 13.4% in the CGN group and 9.5% in the non-CGN group (p = 0.0170). The difference was significant and overt hypothyroidism was found in 2 patients only in the CGN group. Goiter was found in about 15% of the patients in each group but the size was small and there was no difference between the groups.
Table 2. Prevalence of hypothyroidism in end-stage renal failure on haemodialysis: Comparison between the chronic glomerulonephritis (CGN) and the non-CGN groups
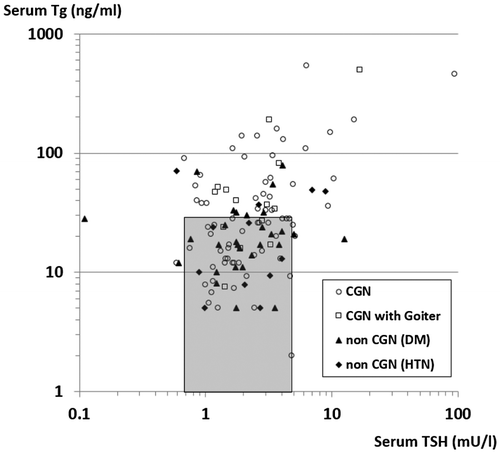
The most striking finding was the prevalence of the patients with elevated serum Tg levels. Serum Tg levels was high in 38 or 46.3% of the patients in the CGN group and only in 11 or 26.2% in the non-CGN group (p = 0.0335). Extremely high serum Tg levels (>100 ng/ml) was observed in 12 or 14.6% of the patients in the CGN group and two of them were suffering from overt hypothyroidism (Table ). As shown in Figure , there was a very good correlation between serum Tg and TSH levels after logarithmic conversion (r = 0.4918, p < 0.001). However, the correlation was significant only in the CGN group (r = 0.5921, p < 0.001), and there was no significant correlation in the non-CGN group (r = 0.0838).
Figure 1. Correlation between elevated serum thyroglobulin (Tg) and TSH levels in the patients with ESRD on maintenance haemodialysis in the CGN group or the non-CGN group (nephropathy due to lifestyle related systemic disease, such as DM or HTN).
In the patients with hypothyroidism, serum TSH levels was followed in 9 of 11 patients in the CGN group and 3 or 4 patients in the non-CGN group with iodide restriction. Serum TSH levels decreased in all the patients examined and became within the reference values for ESRD after 3 months in 7 of 9 patients in the CGN group and in 2 of 3 patients in the non-CGN group.
3.4. Comparison of clinical data between the patients with normal or elevated serum Tg level
As shown in Table , a multivariate logistic regression analysis revealed that significant factors associated with elevated serum Tg level were the serum TSH level (p = 0.0014) and TV (p = 0.0121). There was a tendency that elevated serum Tg level seemed to be more frequently found in CGN group, however the difference was not significant (p = 0.0529).
Table 3. Clinical data and thyroid function in end-stage renal disease on haemodialysis: Comparison between the patients with normal or elevated serum thyroglobulin
4. Discussion
In this study, we confirmed the high prevalence of the patients with primary hypothyroidism in the ESRD patients on HD, as reported by previous papers (Kaptein, Citation1996; Kaptein et al., Citation1988; Kutlay et al., Citation2005). Although the very high prevalence of hypothyroidism such as 18–28% has been reported recently (Asvold et al., Citation2011; Chonchol et al., Citation2008; Kang et al., Citation2008; Kaptein, Citation1996; Lo et al., Citation2005; Shantha et al., Citation2011; Targher et al., Citation2009), the prevalence was about 13.4% in the CGN group and 9.5% in the non-CGN group (Table ), not so high when compared with reference values of ESRD.
Whether or not these patients require thyroid hormone replacement therapy is a clinically important problem (Kaptein, Citation1996). During our study on the prevalence of thyroid dysfunction in the ESRD patients on HD, we came across an interesting finding of TSH-dependent elevation of the serum Tg levels in patients suffering from CGN. It was then suggested the difference in the pattern of hypothyroidism depending on the pathogenesis of ESRD. Overt but reversible hypothyroidism was more frequent with TSH dependently elevated serum Tg levels in the CGN group compared with the non-CGN group.
Acquired hypothyroidism in the adult is not the end result of chronic thyroiditis and reversible recovery of the thyroid function has been reported (Okamura et al., Citation1988; Okamura, Inoue, & Omae, Citation1978; Sato et al., Citation1996; Yoshinari et al., Citation1983). The characteristics of reversible hypothyroidism are (1) the presence of goiter (Yoshinari et al., Citation1983) and (2) TSH-dependent high thyroidal uptake of radioactive iodine (Okamura et al., Citation1988), suggesting the presence of intact thyroid tissues which is transiently damaged by some factors such as excess iodide and stimulated by elevated serum TSH levels (Okamura et al., Citation1978). Although thyroidal uptake test is rather inconvenient in general practice, Sato et al. (Citation1988) reported that the measurement of serum level of Tg, thyroid specific protein important for thyroid hormone synthesis, is also a good marker for reversible recovery of thyroid function. In iodine-deficient area, measurement of the serum Tg level was also reported to be a sensitive marker for hypothyroidism (Jukic et al., Citation2015). On the other hand, history of excess iodide ingestion and/or renal dysfunction suggests the reversible recovery of thyroid function, especially when serum Tg level is also elevated (Sato et al., Citation1992). Not only excess iodide ingestion but also impaired excretion of iodide into urine might be responsible for well-known Wolff–Chaikoff effect (excess iodide induced iodide organification block) in the thyroid, as originally reported by Wolff and Chaikoff in heminephrectomized rats (Wolff & Chaikoff, Citation1948).
As to the difference in clinical features between the CGN and the non-CGN (Table ), the age was higher and the duration of HD was shorter in non-CGN probably reflecting the later onset and the poor prognosis in this group. In Japan, five year survival rate among HD patients is around 70% in the CGN, 48% in the DM and 45% in the HT groups. Lower fT3/fT4 ratio in the non-CGN group might suggest the presence of micro-inflammation and poor prognosis as reported by Zoccali, Tripepi, Cutrupi, Pizzini, and Mallamaci (Citation2005).
The most interesting finding in this study was the elevated serum Tg levels in 38 (46.3%) of 82 patients in the CGN group (Table ). We observed a very good correlation between the serum Tg and TSH levels after logarithmic conversion in the CGN group (r = 0.5921, p < 0.001), suggesting TSH-dependent elevation of the serum Tg levels (Figure ). However, there was no significant correlation in the non-CGN group. Thus, elevated Tg responding to elevated TSH found in the CGN group suggested the relatively preserved thyroid tissue and the recovery of thyroid function.
Impaired renal handling of iodide in the kidney, not only in the glomerulus but also in the renal tubules, may be responsible for the retention of iodide resulting in reversible hypothyroidism (Sanai et al., Citation2008; Sato et al., Citation1992). The pattern of hypothyroidism found in the CGN group with elevated serum Tg levels suggested this might be the mechanism for thyroid dysfunction in this group.
Comparison of the clinical data between the patients with normal or elevated Tg level (Table ) suggested that significant factors for elevated serum Tg level were the elevated serum TSH level and bigger TV, suggesting the enlarged thyroid gland responding to endogenous TSH in patients with elevated serum Tg level. Although the difference was not significant, high serum Tg level was more frequently found in the CGN group.
Another possible mechanism for thyroid dysfunction is the senile change in the thyroid gland with or without immunological perturbation, because the ESRD patients were usually old and frequently associated with hypertension or atherosclerosis. The elevation of serum TSH levels was suggested in the elderly patients (Hollowell, Staehling, & Flanders, Citation2002; Okamura et al., Citation1989). It was then suggested that the patients with lifestyle related systemic disease, such as DM or HTN, might be more susceptible to senile visceral damage compared with the CGN group, damaging not only kidney, heart, brain but also thyroid and other organs. Although the number was small, TV of the 12 patients in the HTN group was small (11.0 ± 2.9 ml). Considering the high prevalence of hypothyroidism in the elderly people in general (Hollowell et al., Citation2002; Okamura et al., Citation1989), effect of longstanding HTN or DM not only on kidney but also on vascular-rich thyroid gland may require further investigation. Complication of HTN even in the CGN group may precipitate the visceral damages even in the CGN group.
As to the therapeutic point of view, low T4 and low T3 syndrome usually found in ESRD as NTI may not require replacement therapy. Whether the patients with primary subclinical hypothyroidism should be treated or not is a controversial matter, but replacement therapy should be considered in younger patients (<65–70 years) (Pearce, Brabant, & Duntas, Citation2013). There may be no doubt that overt primary hypothyroidism complicated with ESRD should be treated. Our study suggested that, it may be better to try iodide restriction before starting l-thyroxine replacement therapy when serum levels of both TSH and Tg become elevated in the ESRD patients due to CGN, because the thyroid tissue may be well preserved to synthesize Tg.
In conclusion, we found the high prevalence of the patients with not only elevated serum TSH levels but also proportionately elevated serum Tg levels in ESRD due to CGN. Reversible inhibition of thyroid hormone synthesis was suggested and it is better to seek and avoid inhibitory factors such as excess iodide or amiodarone. Although (1) the presence of goiter, (2) high thyroidal uptake of radioactive iodine and (3) elevated serum Tg levels were the good markers for reversible recovery of thyroid function, our study suggested that elevated serum Tg levels is the most convenient marker.
Funding
The authors received no direct funding for this research.
Author contributions
Toru Sanai: conceived and designed study, analyzed the data, contributed reagent, materials, analysis tools, wrote the paper.
Ken Okamura: conceived and designed study, analyzed the data, contributed reagent, materials, analysis tools, wrote the paper.
Shuichi Rikitake, Tsuyoshi Takashima, Motoaki Miyazono: contributed reagent, materials, analysis tools.
Yuji Ikeda: contributed reagent, materials, analysis tools.
Takanari Kitazono: contributed reagent, materials, analysis tools.
Acknowledgements
We are grateful to Dr T. Abe for his valuable advice.
Additional information
Notes on contributors
Toru Sanai
Toru Sanai has a track record of obtaining Clinical Professor of Nephrology of Saga University, Chief of Kidney Care Unit of Kyushu University, Fellowship of Sheffield Kidney Institute (UK), and Chief of Nephrology of National Kyushu Medical Center.
Ken Okamura
We will take a great interest in thyroid and kidney in the future.
References
- Ang, W. W., Kaptein, E. M., & Massry, S. G. (1987). Diagnosis of hypothyroidism in patients with end-stage renal disease. American Journal of Nephrology, 7, 192–197.
- Asvold, B. O., Bjoro, T., & Vatten, L. J. (2011). Association of thyroid function with estimated glomerular filtration rate in a population-based study: The HUNT study. European Journal of Endocrinology, 164, 101–105.10.1530/EJE-10-0705
- Bayer, M. F., & Kriss, J. P. (1979). Immunoradiometric assay for serum thyroglobulin: Semiquantitative measurement of thyroglobulin in antithyroglobulin-positive sera. Journal of Clinical Endocrinology and Metabolism, 49, 557–564.10.1210/jcem-49-4-557
- Blackburn, G. F., Sheh, H. P., Kenten, J. H., Leland, J., Kammin, R. A., Link, J., … Talley, D. B. (1991). Electrochemiluminescence detection for development of immunoassays and DNA probe assay for clinical diagnostics. Clinical Chemistry, 37, 1534–1539.
- Chonchol, M., Lippi, G., Salvagno, G., Zoppini, G., Muggeo, M., & Targher, G. (2008). Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clinical Journal of American Society of Nephrology, 3, 1296–1300.10.2215/CJN.00800208
- Forest, J., Dube, J., & Talbot, J. (1982). Thyroid hormone in patients with chronic renal failure undergoing maintenance hemodialysis. American Journal of Clinical Pathology, 77, 580–586.10.1093/ajcp/77.5.580
- Gardner, D. F., Mars, D. R., & Thomas, R. G. (1986). Iodine retention and thyroid dysfunction in patients on hemodialysis and continuous ambulatory peritoneal dialysis. American Journal of Kidney Diseases, 7, 471–476.10.1016/S0272-6386(86)80187-1
- Hardy, M. J., Ragbeer, S. S., & Nascimento, L. (1988). Pituitary-thyroid function in chronic renal failure assessed by a highly sensitive thyrotropin assay. Journal of Clinical Endocrinology and Metabolism, 66, 233–236.10.1210/jcem-66-1-233
- Hershman, J. M., Krugman, L. G., Kopple, J. D., Reed, A. W., Azukizawa, M., & Shinaberger, J. H. (1978). Thyroid function in patients undergoing maintenance hemodialysis: Unexplained low serum thyroxine concentration. Metabolism, 27, 755–759.10.1016/0026-0495(78)90209-3
- Hollowell, J. G., Staehling, N. W., & Flanders, W. D. (2002). Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition examination survey (NHANES III). Journal of Clinical Endocrinology and Metabolism, 87, 489–499.10.1210/jcem.87.2.8182
- Jukic, T., Zimmerman, M. B., Granic, R., Prpic, M., Krilic, D., Juresa, V., … Kusic, Z. (2015). Sufficient iodine intake in schoolchildren from the Zagreb area: Assessment with dried blood spot thyroglobulin as a new functional biomarker for iodine deficiency. Acta Clinica Croatica, 54, 424–431.
- Kang, E. W., Nam, J. Y., Yoo, T. H., Shin, S. K., Kang, S.-W., Han, D.-S., & Han, S. H. (2008). Clinical implications of subclinical hypothyroidism in continuous ambulatory peritoneal dialysis patients. American Journal of Nephrology, 28, 908–913.10.1159/000141933
- Kaptein, E. M. (1996). Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocrine Reviews, 17, 45–63.10.1210/edrv-17-1-45
- Kaptein, E. M., Quion-Verde, H., & Chooljian, C. J. (1988). The thyroid in end-stage renal disease. Medicine, 67, 187–197.10.1097/00005792-198805000-00005
- Kutlay, S., Atli, T., Koseogullari, O., Nergizoglu, G., Duman, N., & Gullu, S. (2005). Thyroid disorders in hemodialysis patients in an iodine-deficient community. Artificial Organs, 29, 329–332.10.1111/aor.2005.29.issue-4
- Lim, V. S., Fang, V. S., Katz, A. I., & Refetoff, S. (1977). Thyroid dysfunction in chronic renal failure. A study of the pituitary-thyroid axis and peripheral turnover kinetics of thyroxine and triiodothyronine. Journal of Clinical Investigation, 60, 522–534.10.1172/JCI108804
- Lo, J. C., Chertow, G. M., Go, A. S., & Hsu, C.-Y. (2005). Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney International, 67, 1047–1052.10.1111/j.1523-1755.2005.00169.x
- Okamura, K., Inoue, K., & Omae, T. (1978). A case of Hashimoto’s thyroiditis with thyroid immunological abnormality manifested after habitual ingestion of seaweed. Acta Endocrinology (Copenh), 88, 703–712.
- Okamura, K., Sato, K., Ikenoue, H., Yoshinari, M., Nakagawa, M., Kuroda, T., & Fujishima, M. (1988). Reevaluation of the thyroidal radioactive iodine primary uptake test, with special reference to reversible primary hypothyroidism with elevated thyroidal radioiodine uptake. Journal of Clinical Endocrinology and Metabolism, 67, 720–726.10.1210/jcem-67-4-720
- Okamura, K., Ueda, K., Sone, H., Ikenoue, H., Hasuo, Y., Sato, K., … Fujishima, M. (1989). A sensitive thyroid stimulating hormone assay for screening of thyroid functional disorders in elderly Japanese. Journal of American Geriatrics Society, 37, 317–322.10.1111/jgs.1989.37.issue-4
- Pearce, S. H. S., Brabant, G., & Duntas, L. H. (2013). ETA guideline: Management of subclinical hypothyroidism. European Thyroid Journal, 2, 215–228.10.1159/000356507
- Sanai, T., Inoue, T., Okamura, K., Sato, K., Yamamoto, K., Abe, T., … Iida, M. (2008). Reversible primary hypothyroidism in Japanese patients undergoing maintenance hemodialysis. Clinical Nephrology, 69, 107–113.10.5414/CNP69107
- Sanai, T., Okamura, K., Kishi, T., Miyazono, M., Ikeda, Y., & Kitazono, T. (2015). Importance of specific reference values for evaluation of the deteriorating thyroid function in patients with end-stage renal disease on hemodialysis. Journal of Endocrinological Investigation, 38, 47–56.10.1007/s40618-014-0121-6
- Sato, K., Okamura, K., Hirata, T., Yamasaki, K., Ikenoue, H., Kuroda, T., … Fujishima, M. (1996). Immunological and chemical type of reversible hypothyroidism; clinical characteristic and long-tern prognosis. Clinical Endocrinology (Oxl), 45, 519–528.10.1046/j.1365-2265.1996.00858.x
- Sato, K., Okamura, K., Ikenoue, H., Shiroozu, A., Yoshinari, M., & Fujishima, M. (1988). TSH dependent elevation of serum thyroglobulin in reversible primary hypothyroidism. Clinical Endocrinology (Oxl), 29, 231–237.10.1111/j.1365-2265.1988.tb01220.x
- Sato, K., Okamura, K., Yoshinari, M., Kuroda, T., Ikenoue, H., Okazawa, K., … Fujishima, M. (1992). Reversible primary hypothyroidism and elevated serum iodine level in patients with renal dysfunction. Acta Endocrinologica (Copenh), 126, 253–259.
- Shantha, G. P., Kumar, A. A., Bhise, V., Khanna, R., Sivagnanam, K., & Subramanian, K. K. (2011). Prevalence of subclinical hypothyroidism in patients with end-stage renal disease and the role of serum albumin: A cross-sectional study from South India. Cardiorenal Medicine, 1, 255–260.10.1159/000332757
- Targher, G., Chonchol, M., Zoppini, G., Salvagno, G., Pichiri, I., Franchini, M., & Lippi, G. (2009). Prevalence of thyroid autoimmunity and subclinical hypothyroidism in persons with chronic kidney disease not requiring chronic dialysis. Clinical Chemistry of Laboratory Medicine, 47, 1367–1371.
- Wolff, J., & Chaikoff, I. L. (1948). Plasma inorganic iodide, a chemical regulator of normal thyroid function. Endocrinology, 42, 468–471.10.1210/endo-42-6-468
- Yokozawa, T., & Morita, S. (1999) Ultrasonographic thyroid examination. In K. Kuma (Eds.), Atlas of thyroid and parathyroid ultrasound (2nd ed., pp. 18–38). Tokyo: Japan Vector Core (in Japanese).
- Yoshinari, M., Okamura, K., Tokuyama, T., Shiroozu, A., Nakashima, T., Inoue, K., & Omae, T. (1983). Clinical importance of reversibility in primary goitrous hypothyroidism. British Medical Journal, 287, 720–722.10.1136/bmj.287.6394.720
- Zoccali, C., Tripepi, G., Cutrupi, S., Pizzini, P., & Mallamaci, F. (2005). Low triiodothyronine: A new facet of inflammation in end-stage renal disease. Journal of American Society of Nephrology, 16, 2789–2795.10.1681/ASN.2005040356
