ABSTRACT
Auditory frequency change (FC) might be crucial to respond to. We hypothesized that FC affects human motor behavior within short latency, and that upwards vs downwards changes exert distinct effects. Behavioral correlates of asymmetries in FC processing are scarcely researched. Previous work of ours showed direction-biased FC effect in a tapping task. Here we probed the effect’s specificity to the frequency domain, and its latency. Twenty-three musicians synchronized finger-tapping to isochronous beeps, set in sequences presenting increments/decrements, in frequency/intensity (Loud, Soft, Up, Down). We recorded tap-timing, finger acceleration, and muscle activity. Diverging behavior patterns implied domain-specificity: Intensity conditions yielded responses correlated with change-direction – increments elicited earlier taps of aroused action profile and vice versa, whereas FC to both directions elicited early action triggering. Within Frequency, Up’s effect arose 160 ms post-change, Down’s effect significantly later. These results cannot be accounted for by mere surprise effect. Swift modulation of motor behavior elicited by task-irrelevant FC was shown, biased by FC direction. Latencies imply early cortical components involved in the bias. Early latency covert human motor responses evoked by FC may form a facet of everyday auditory perception, and of specialized domains as music.
Introduction
Perception of exogenous cues enhances survival by guiding behavioral responses. Hence, specific motor activations may be yielded to specific cues, couplings ingrained over eons. Our research probes whether auditory frequency change (FC), and its direction, yield covert motor responses in adult humans. As this question is scarcely researched, we first summarize shortly some related evidence of FC yielding specific motor behaviors in perceivers. We find that this evidence, from ethological studies, from studies in communication with babies, and from some perceptual phenomena, supports our hypothesis that such effects do exist in adult humans too.
Behavioral Response to FC and Its Direction – Overview of Evidence
Frequency, FC, and the direction of FC, are major elements in acoustic cues evoking phonotactic approach/withdrawal behaviors of mate-choice or evasion (e.g.,: anurans: Ryan, Citation1980; small primates: Miller, Miller, Gil-da-Costa, & Hauser, Citation2001). Their role is vital also in referential alarm calls, yielding threat-specific evasion responses (ptarmigans: Ausmus & Clarke, Citation2014; suricates Manser, Seyfarth, & Cheney, Citation2002; BBC Earth, (Citationn.d.)). Signaler and responder may even be of different species (Haff & Magrath, Citation2013). The latency of responses to FC varies. In the signals yielding such behaviors, FC direction (rise vs fall) matters: a common linkage of rise to appeasement, and of fall to threat, elicits, respectively, approach or withdrawal behaviors, in short latencies; a “pitch code” has been postulated to rule, in animals and humans (Morton, Citation1977, in particular p. 861; Ohala, Citation1984; Pisanski, Cartei, McGettigan, Raine, & Reby, Citation2016).
Evidence in other realms, too, suggests that human motor acts are affected by FC and its direction. Infant-directed speech shapes babies’ behaviors, using typecast, enhanced FC patterns. These universal parental codes for warning, cheer, etc., elicit instantaneous, distinct modulations of babies’ attention and motion; abrupt frequency rise leads to a standstill, bell-shaped contour to eye-contact, etc. “Understood” by infants worldwide, cross-culturally (Fernald, Citation1992a, Citation1992b; Grieser & Kuhl, Citation1988), such codes may be “tutorials” of general human FC→behavior couplings.
FC and its direction affect motor acts not only in vocalizations contexts. Perception of auditory stimuli as “looming” (drawing nearer, possibly threatening, vs “receding”) is biased: they are perceived by animals (Maier & Ghazanfar, Citation2004) and humans (Väljamäe, Citation2009) as louder, longer (DiGiovanni & Schlauch, Citation2007), and faster-approaching (Neuhoff, Citation2016) than they are, evoking avoidance behavior even in babies (Freiberg, Tually, & Crassini, Citation2001). Though loom bias is identified with intensity increments, Baumgartner et al. (Citation2017) report spectral cues (in the frequency domain) elicit it, evoking a motion percept. Indeed, the bias is yielded also by upward frequency shifts: monkeys orient more toward tones of fast-rising frequency and associate them with looming visual cues (Ghazanfar & Maier, Citation2009).
Another short-latency auditory-motor response affected by FC and its direction is the Lombard effect – “online” modulation of vocalization to overcome masking. Evoked by intensity rise or frequency shifts in the auditory environment, it is mediated rapidly (100–150 ms), mainly at midbrain level (monkey: Hage, Jiang, Berquist, Feng, & Metzner, Citation2013; humans: Larson, Sun, & Hain, Citation2007). The exact modulation is determined by the frequency relation between the vocalization and the masking noise (review: Ruch, Zürcher, & Burkart, Citation2018).
Bearing an immediate “meaning” in manyfold behavioral contexts, FC can swiftly alter motor acts. Its early deciphering in subcortical stages (cf Krishnan, Xu, Gandour, & Cariani, Citation2004 [humans]; Gordon & O’neill, Citation2000 [bats]) earns perceivers critical time in optimizing response.
Probing Motor Response to FC: Our Previous Study
An effect of FC on the motor system, then, is acknowledged (more directly) to exist in animals, and (mostly indirectly acknowledged) in humans. Such an activation or modulation of motor activity may constitute an integral outcome of sound perception. Testing direct impact of FC on the motor system in humans, we used, in a previous study (Boasson & Granot, Citation2012), a finger tapping task – a well-studied paradigm, with low variance of both timing and action (see review in Repp, Citation2005). Participants, musicians and non-musicians, were asked to synchronize taps to isochronous beeps (InterOnset Interval [IOI] = 500 ms). The sequences in which the beeps were set formed various patterns of (discrete) FC. We studied the effects of FC on subjects’ Negative Mean Asynchrony (NMA) – a well-known effect of beat anticipation (Boasson & Granot, Citation2012).
We found that a frequency rise (of 288 cents) yielded larger NMA already on Tap_1 after change; the effect peaked at Tap_2. Thus, a planned, repetitive motor act, which humans perform with low variance, was modulated in response to a task-irrelevant FC, within less than 500 ms. Importantly, of the two “melodic” directions, the “tap earlier” bias was significant in rise alone. Frequency-related perceptual-behavioral coupling, and the rise-fall asymmetry, were evinced.
We are aware of one other study-line using tapping to research effects of FC (Ammirante & Thompson, Citation2012; Ammirante, Thompson, & Russo, Citation2011). They probed the “ideomotor” hypothesis, by which traits of physical locomotion, such as speed loss in zigzag motion, affect FC perception. A continuation-tapping paradigm was used: subjects synchronized finger taps to an auditory pacing signal; upon its cessation they tapped on, maintaining tempo independently. The now unpaced taps set off sound feedback: each tap a new pitch. Longer Inter-Tap Intervals (ITIs) were indeed elicited by frequency contour changes. Unpredicted, an effect of melodic direction emerged: descending contour-preserving tones elicited shorter ITIs. The first five unpaced taps were not analyzed. Thus, our (Boasson & Granot, Citation2012) report of melodic direction’s behavioral effect at first change, as well as evidence of FC effect on ITI under external pacing, were novel.
Changes in the Present Study
Verifying Specificity of Response to Frequency by Comparison to Intensity
To test whether early tap timing elicited by increments is specific to frequency or is part of a wider behavioral asymmetry yielded by auditory perception, we added intensity-change stimuli, paralleling those of FC. Several study-lines in the research literature point at basic differences in processing change between the domains of intensity (INT) and frequency (FRQ), which may support a hypothesis of domain specificity in responses to increments vs. decrements.
Differences in Coding
INT is coded principally by neural firing rate (of given neuron populations) (Plack & Carlyon, Citation1995), whereas FRQ is coded tonotopically (by dedicated neural bands) (Saenz & Langers, Citation2014). INT was suggested to be a “prothetic” percept, FRQ a “metathetic” one: INT discrimination is quantitative, based on additive mechanisms; that of FRQ is qualitative, based on substitutive mechanisms (Stevens, Citation1957).
Behavioral Differences in Detecting Changes
Different decay patterns of INT/FRQ memory traces imply distinct “storage” categories (Clément, Demany, & Semal, Citation1999). Superior memorization for pitch- vs loudness-change sequences is found: FC detectors “extract” shift size and direction (Cousineau, Demany, & Pressnitzer, Citation2009, review: Demany & Semal, Citation2018); shifts are detected even when masked (Demany, Carcagno, & Semal, Citation2013; Demany & Ramos, Citation2005).
Differences in Processing Change
INT/FRQ changes undergo different processing. At cortical level, at ~150–200 ms latency, the MisMatch Negativity (MMN) to deviants of INT/FRQ is seen at different loci (Giard et al., Citation1995). Larger right hemisphere dominance, and stronger and earlier N100m are elicited to FRQ than to INT modulation (Pardo, Mäkelä, & Sams, Citation1999).
At midbrain level, Stimulus-Specific Adaptation (SSA) attenuates neural activity to repeated sounds; activity re-awakens to deviants. Whereas FRQ deviance to both directions is detected by SSA, in INT detection is limited to increments (no true INT SSA) (Duque, Wang, Nieto-Diego, Krumbholz, & Malmierca, Citation2016; Grimm & Escera, Citation2012). Brief INT-rise glides elicit larger ABR inter-peak amplitude, in narrower latency, than FRQ-rise glides (Arlinger & Jerlvall, Citation1981).
Kohn, Lifshitz, and Litchfield (Citation1978, Citation1980) used a simple-response-time task, to test simultaneously cortical and behavioral response to INT/FRQ increments/decrements, by ramp (gradual) or step (discrete), within a continuous tone. In ramps, for both FRQ and INT, a larger signal was elicited at ~200 ms latency to onset of increments (vs decrements). In steps, response to both frequency directions was similar, whereas that to intensity increments was far larger than to decrements. Lower AEP amplitude entailed wider response-time dispersion.
Investigating Response Latencies and the Relay Levels They May Hint At
Auditory-motor Mediation: Searching for Evidence
Latency of the FC direction effect as noted in our previous study, ~500 ms, does not disclose the levels of relay from perception to action, as most FC processing is completed by then. However, the effect could have begun earlier, before Tap 1; if found early enough in the tap-action, it could suggest direct subcortical auditory-motor mediation of FC information. In the present study, we shortened IOI to 250 ms and recorded (aside tap-time) continuous indices of the tap-action (muscle activity and finger kinematics), aiming to gain insight on the neural wiring of the effect by its latency.
Has evidence been found of early enough biases in FC direction processing, and of “low-level” auditory-motor relays, to back a “direct relay” hypothesis?
Subcortical Evidence: Early Responses
Single neurons reacting to one preferred FC direction exist in the rat’s inferior colliculus (IC) (Kuo & Wu, Citation2012). In humans, rising ultra-fast (1 ms) frequency glides elicit more robust ABR than falling ones (Arlinger & Jerlvall, Citation1981).
SSA encodes deviant frequency already in the IC (in animals, review: Grimm & Escera, Citation2012; in humans: Cacciaglia et al., Citation2015). Importantly, it is in midbrain SSA only, not in A1-cortex SSA, that deviant frequency yields earlier response-peak latency (Escera & Malmierca, Citation2014). IC SSA detecting deviance of FC direction has not been reported yet, though detection of other higher-order frequency irregularities has just been shown (Malmierca et al., Citation2019).
Larger Frequency Following Response (FFR) is elicited by rising sweeps than by other frequency contours (tones: Krishnan & Parkinson, Citation2000; syllables: Krishnan et al., Citation2004). Larger FFR predicts shorter N100 latency (Slugocki, Bosnyak, & Trainor, Citation2017), larger N200 magnitude (Galbraith et al., Citation2004), and faster response-time (Galbraith et al., Citation2000).
Hage, Jürgens, and Ehret (Citation2006) found “audio-vocal” brainstem pontine neurons active both in perception and action in the Lombard effect, where FC cues are mediated to affect motor acts.
Hence, subcortical auditory-motor relays, bi-functional neurons, and FC-direction distinction exist; response amplitude and latency here foretell later processing and motor responses.
Cortical Evidence: Middle Latency Responses
Of the auditory MLRs, the Na and Nb components, at the primary auditory cortex (A1), occur at ca 20–28 and 38–46 ms; frequency-deviance detection alters their amplitude (Shiga et al., Citation2015). FC direction deviance detection was not found in human A1 (Cornella, Leung, Grimm, & Escera, Citation2013), but high-level rat A1 SSA implies more than simple frequency deviance detection (Nelken, Yaron, Polterovich, & Hershenhoren, Citation2013), and ferret A1 neuron clusters show FC direction bias (Nelken & Versnel, Citation2000). MLR SSA may be a correlate of human MMN (Grimm, Escera, & Nelken, Citation2016).
Cortical Evidence: Later Latency Responses
Some LLRs reveal FC direction bias, mostly of EEG amplitude, not latency. The P1 component (at 50 ms latency) during FC direction discrimination in discrete tones is larger in rise vs fall (Noguchi, Fujiwara, & Hamano, Citation2015). Unattended deviant-direction rising glides (vs falling) elicit earlier the N100m component (85 vs 93 ms; Pardo & Sams, Citation1993); rising glides elicit larger N100 (at 115 ms; Maiste & Picton, Citation1989) and larger N100-P200 (Wang, Tan, & Martin, Citation2013). Deviant frequency-rise elicits larger MMN (vs fall; unattended discrete tones; at ~140 ms for ~tritone; Peter, McArthur, & Thompson, Citation2010).
Anatomical Evidence
Is a midbrain auditory-motor relay of responses to FC and its direction feasible? The IC’s dependence on the cortex in FC processing was tested in animals.
Lesioning the auditory cortex leaves discrimination of frequency intact, that of frequency glide direction deteriorated, and that of pure tones frequency patterns severely hindered (felines: Kelly, Citation1973; rodents: Ohl, Wetzel, Wagner, Rech, & Scheich, Citation1999). Comparison of new cues to retained traces is affected by missing input from the auditory cortex. Cortical influence on IC SSA is debated. IC neurons exhibiting SSA exist in an area innervated heavily by cortico-fugal projections; the auditory cortex, though, modulates IC SSA, rather than generates it (Anderson & Malmierca, Citation2013). Similar evidence was found in the thalamic medial geniculate body (MGB) (Antunes & Malmierca, Citation2011). Cortical predictions based on recent past input, routed downward to face new cues, may decode FC and its direction (Malmierca, Anderson, & Antunes, Citation2015). If so, notwithstanding the reported role of the cortex in fine FC direction decoding, swift motor response to novel FC direction cues could be dispatched directly from the midbrain auditory hub.
The Present Study – An Overview
Following our previous work, we set to further study effects of FC and its direction on motor activity, to verify their specificity to the frequency parameter, and to better understand the neural routes involved by closely monitoring the effects’ time-course. Narrowing the IOI to 250 ms, we aimed to induce action at a post-sound-onset latency that will elucidate the arrival time of FC information, by relay, to endpoints of the motor system, and thus the processing levels at which such relay may occur. We recorded tap timing, muscle activity, and finger acceleration. Four test conditions presented increments/decrements in FRQ/INT; the control presented uniform beeps.
Methods
Participants
Our 23 participants were excelling music students, or professional musicians, with at least 10 years of intense occupation in musicFootnote1 (12 male; mean age = 25.7 y, StD = 4.4 y; handedness: 19 R, 2 L, 2 Amb). We chose to employ musicians due to their proficiency in synchronization, which we predicted will yield small variance and a possibility to reliably ascribe taps to “their” beeps at a fast tempo. The study was approved by the Ethics Committee of the Faculty of Social Sciences of the Hebrew University of Jerusalem. The participants signed an informed consent form; they were paid 60 NIS (roughly 15 USD).
Sample Size
In our previous study (Boasson & Granot, Citation2012), standard deviation of ITIs in the control condition was 1.64 ms, and the effect of “melodic direction” on first tap after change was 2.1 ms. We expected the effect to shrink now by half due to the doubled tapping pace. Our sample size nears Van Belle’s (Citation2011) sample size rule-of-thumb (Δ = 1.05/1.64, n = (16/Δ2)/2 [within subject] ~ = 20) and is comparable to that in our previous study (n = 21).
Procedure
The experiments were held at the Neurobiology Lab of Prof. Yifat Prut, at the Hadassah medical school, in the Ein Kerem campus of the Hebrew University of Jerusalem. Subjects were seated, chair-height adjusted to convenience, the forearm of their dominant hand placed on a custom-made tapping-board. To prevent excess movement, the forearm was secured to the board by adjustable straps, without causing discomfort. The subjects tapped with their index finger on a small lense recessed off the board surface; board resonance was attenuated. A proximeter within the board recorded the taps. An accelerometer was worn as a ring on the finger’s middle joint.
Four surface electromyography [EMG] electrodes were placed on the [cleansed] skin overlying two antagonist pairs of muscles: the (distal) index-finger flexor and extensor (flexor digitorium superfacialis and extensor indicis proprius, the main tapping muscles), and the (proximal) biceps and triceps. An elastic band kept the electrodes from shifting. Subjects were asked to keep the following posture: rounded tapping finger, near right angle at the elbow, upper arm close to torso, seating upright.
Experiment Design
Participants were asked to synchronize finger tapping to isochronous sequences of pure-tone beeps (heard over headphones), ignoring changes. Beep pace was 4 Hz (i.e., IOI = 250 ms).
Sequences comprised of four continuous segments, at randomized lengths (see ):
Figure 1. Stimulus design. Change was presented either in intensity or frequency (not both). It was either an increment followed by a decrement or vice versa. A control condition presented no change. See details in the text.
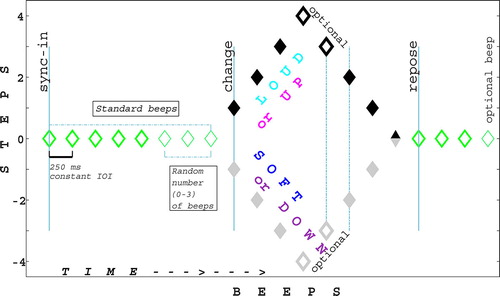
a) “Sync-in” segment: 5–8 identical beeps at standard intensity and frequency.
b + c) “Change” segments: 3–4 steps of increments/decrements of intensity/frequency (b), followed by direction reversal and exact backtracking (c); total: either 6 or 8 beeps.
d) “Repose” segment: 3–4 identical beeps at standard intensity and frequency.
Total sequence length was 14–20 beeps (3.3–4.8 s).
Cases of increments-then-decrements were tagged Loud in INT / Up in FRQ; opposite cases were tagged Soft / Down (L, U; S, D). Randomly, one-third of the sequences included 4 steps instead of 3 in each direction, making the direction reversal moment unpredictable. In the Repose segment a 4th beat was added randomly in one-third of the sequences to keep alertness at sequence end. Control (Ctrl) sequences of randomized length (14–20 beeps) presented identical standard beeps.
In each block, 37 sequences were presented, randomly intermixed: nine of each of the four conditions, and one Ctrl. Silence of 3.7 s interspersed successive sequences. Block duration was ~4.5 min, followed by 30 s silence. Ten blocks were presented in all, consisting of 90 trials of each test condition and 10 Ctrl trials; the total duration was ~49.5 min.
This design ensured subjects’ naïvity at the outset of each sequence as to the ordinal location, parameter and direction of the first change, and the exact moment of direction reversal.
Stimulus Specifications
Stimuli were produced by Matlab software, delivered over a soundcard (Roland UA-25 EX cakewalk) and over Roland RH-5 headphones.
Each beep was of constant frequency, its duration 50 ms; 5 ms ramps of linear rise and decay framed 40 ms of constant amplitude. Standard frequency was 500 Hz. Standard intensity was 70 dB at ear, measured with both headphones’ earpads clamped on the microphone of the measuring device (QUEST technologies, model 2400 sound level meter) before the experiment.
Intensity conditions: Intensity-change steps were 5 dB. Range: 50–90 dB. Frequency was kept equal (see General Discussion, §Caveats, §§Pitch warp).
Frequency conditions: Frequency-change steps were 28.9% each (3^3∕13, triple step of the Bohlen-Pierce [B-P] scale [Mathews, Pierce, Reeves, & Roberts, Citation1988]). This interval, ca midpoint between the Western major third and perfect fourth, was chosen to minimize biases and expectations related to the Western tonal system. Affording cross-reference to future studies, we used the B-P system in our previous study too (single/double steps, Boasson & Granot, Citation2012). As we halved now the IOI, possibly shrinking the effect, we chose to increase step-size to triple.
Frequency range was 181–1378 Hz. Intensity was corrected to be perceptually equal across the various frequencies (Suzuki et al., Citation2003 [setting ISO 226:2003]).
Data Acquisition and Processing
Data from the proximeter, accelerometer and EMG were acquired on a multi-channel data acquisition (DAQ) system (AlphaLab, AlphaOmega, Nazareth, Israel), sampled at 10 kHz. Pre-amplified electrodes were used (MotionLab Systems, MA-411-002, gain X20, 15 Hz – 3.5 kHz); signals were further amplified (gain ×100, total gain ×2000) and digitized online. Stimulus was delivered (split) to both subject and the DAQ, thus synchronized with the signals acquired. EMG signal processing consisted of mean-subtraction, rectification, filtering and averaging. A BP filter of 70–400 Hz (Butterworth, 6), then an LP filter at 36 Hz (Butterworth, 8) were applied.
Event Categorization
In each sequence, six taps were analyzed: the three following first change, and the three following direction change. In segments comprising 4 taps, the 4th was not analyzed. Missing taps, or taps later than 120 ms post-sound, were excluded (less than 7%).
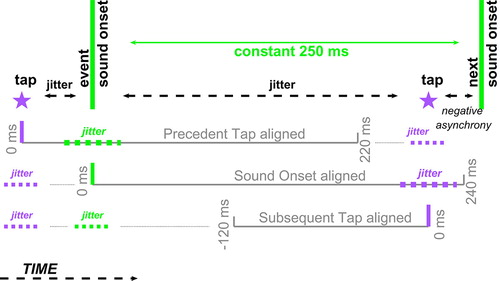
Our analysis refers to Beats and Taps, using several data alignments (cf § Acceleration and EMG data alignments, and ). In Sound-Onset aligned analysis, the time span from onset of the first nonstandard beep in a sequence until the next beep is defined as Beat 1 (B1). In the Timing results, and in Subsequent-Tap aligned analysis, Tap 1 (T1) is the tap just preceding the second nonstandard beep, B2; T4 is tapped after change-direction reversal is heard, near B5. In Precedent-Tap aligned analysis, T0 is the tap just preceding B1; successive taps are correspondingly numbered. In sequences in which change segments comprised 4 beeps each, the taps analyzed as T3 and T4 were in fact interspersed by an extra beep (and its tap).
Figure 2. Acceleration and EMG data alignments. Three alignments were used to shed light on the relation between the yielded (jittery) human action and the isochronous stimulus. Two of them are aligned to tap moments – either the precedent or the subsequent tap, and one is aligned to the beep onset. Note the jitter inherent in each.
Taps to beeps of standard frequency and intensity where change may have been presented but has not, served as control (Ctrl). This category included taps to beeps 6, 7, and 8 of the Sync-in segment when allotted by the randomization in the test conditions, and beeps 6 and later in the Ctrl sequence. The first five Sync-in beeps and the Repose beeps were not included in Ctrl.
General Statistical Procedures
Repeated measures (rm) ANOVAs were conducted (IBM SPSS Statistics 19), as detailed further on. We compared (a) each of the four test conditions (L, S, U, D) vs baseline; (b) the two within-parameter pairs (Intensity: L vs S, Frequency: U vs D); and (c) two grouping contrasts (by change direction [increase vs decrease]: L&U vs S&D, and by parameter: L&S vs U&D). Results were corrected within each rmAnova for multiple comparisons (8 in each rmANOVA) by the Benjamini and Hochberg (Citation1995) procedure. Experiment-wise multiple testing was controlled for, as detailed in the Supplementary Material [hence: SpMtrl], Sheet G. Where Mauchly’s Test of Sphericity indicated violation of the assumption of sphericity, Greenhouse-Geisser correction was used. Details of F, p, and partial-Eta-squared, as output by SPSS, are supplied in the tables, as well as generalized Eta squared (Lakens, Citation2013). From on, the full statistical tables, due to their hefty size, are given in the SpMtrl; in-text tables only cite partial-Eta-squared values.
For computing effect sizes (ES) of contrasts we used two Matlab functions: mes1way.m and mes.m of the measures of effect size toolbox (Hentchke, Citation2011/2015), employing mdbysd – mean difference divided by the standard deviation of the difference score, and its oneway equivalent – psibysd. Confidence intervals are specified when contrasts are discussed in the text.
Statistical Procedures and Approach for Tap Timing Data
An inherent duality exists between an isochronuous stimulus and the jittery timing of human action. Therefore, tap timing data was examined in two complimentary ways:
Negative Mean Asynchrony (NMA): each subject’s mean tap-to-beep asynchrony in the Ctrl beeps served as a baseline for comparison with the experimental conditions;
InterTap Intervals (ITI): deviations from the “ideal” inter-tap interval (250 ms, = IOI).
Note that NMA ignores the role of the specific timing of the previous tap, while ITI ignores the relation to the beep. Separate ANOVAs were conducted for NMA and for ITI data.
Acceleration and EMG Data Alignments
Data, binned to 20 ms segments, were aligned in three ways (see outline in ):
to the tap following a beep, hence Subsequent Tap alignment (−120 to 0 ms [0 = touchdown]): an alignment encompassing the finger-flexing action;
from the tap aimed at a beep (tapped in fact just before it, due to NMA), hence PrecedentTap alignment (0 to 220 ms [0 = touchdown]): an alignment encompassing the finger-lifting action and initiation of the next flexion (excluding its tap);
to Sound Onset (0 to 240 ms [0 = onset]).
Mean action in Ctrl beats/taps, by alignments, is given in the SpMtrl, sheet A.
Statistical Procedures for Acceleration and EMG Data
Acceleration
Data were normalized across subjects by relating each subject’s data under the four conditions to his/her average subsequent-tap aligned activation in the control beats.
Our acceleration data are directional: acceleration away from the tapping board yields negative values (cf SpMtrl, sheet A). In Figures 5–7, deviation from baseline is presented; note therefore, that, relative to baseline, both attenuated finger-lifts and animated taps are of positive (= less negative) values, and interpretation depends on which action phase is inspected. Also due to this reason, in the two alignments encompassing both beat-halves (those to Precedent Tap and to Sound Onset), separate rmANOVAs were run for acceleration data for first/second beat-halves in each segment. See further about the statistical analysis of the acceleration data.
EMG
Data were normalized by Z scoring, for each subject, within each experiment block (thus also correcting for baseline drifts). The proximal muscle pair, biceps and triceps, under all alignments, yielded almost no deviations from baseline and will not be discussed here.
Both
Data were averaged within subject, for each time bin, in each condition. For each segment, a rmANOVA was run with factors Beats, Time bins, and Conditions. Tests compared the conditions Loud, Soft, Up, Down, and baseline (BL).
Further analysis, of beat-to-beat change, was carried out; key findings will be mentioned. For fuller details, as well as tables and figures of that complimentary analysis, see SpMtrl, sheet F.
Results
Tap Timing
Controls Timing Results
To assess the test conditions’ effects, we first inspected behavior in the Ctrl condition. Each subject tapped ~457 Ctrl taps. Mean NMA across subjects was 19.87 ms (StD = 9.5 ms, StE = 2 ms, range of personal means: 41→3 ms; mean of within-subject-StDs = 16.53 ms, mean of within-subject-StE = 0.77 ms). These results agree with literature on NMA (Repp, Citation2005), and on its variance at lower limits of “tappable” IOI (Zendel, Ross, & Fujioka, Citation2011, their ; cf. Peters, Citation1989). Our subjects’ small NMA and its small variance, typical of expert musicians (as in Zendel et al., Citation2011), enabled us reliable ascription of taps to “their” beeps, despite the rapid pace.
General Notes
Timing results for the change segments are depicted in , for NMA and ITI. Two analysis types were conducted. Analysis 1 views data over the six beats and five conditions (four test conditions: Loud, Soft, Up, Down; and BL: 0 for [deviations from] NMA, and 250 for ITI; statistics: cf ). Analysis 2 refers to the beats as a pair of triplets, probing (a) broader timing trends, over triplets, (b) grouped similar conditions between and across triplets, and (c) effect of ordinal position after change onset within triplets (statistics: cf ).
Figure 3. Timing results – deviations from personal negative mean asynchrony (NMA) and from the “ideal” 250 ms inter-tap interval (ITI) under the test conditions. Standard error of the mean is given. Baselines appear as green lines. Statistically significant differences from NMA and from inter-onset interval (IOI, 250 ms) are noted by stars; significant differences between condition pairs within parameter by diamonds. Panes titled TRU present the data. Panes titled EXC present the post-hoc manipulation (see text), switching condition-pairs within parameter in segment 2.
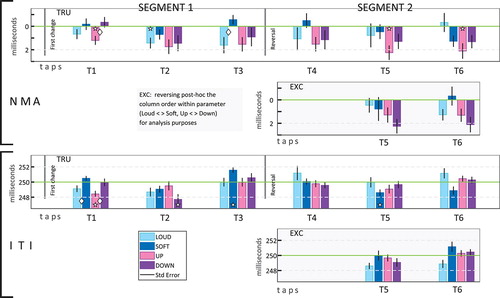
Table 1. Tap timing, Analysis 1. Main results of the rmANOVAs and statistically significant contrasts (also for the NMA interaction, which was just short of statistical significance). P values are corrected for multiple comparisons. Asterisks mark strong significance. Marginal results are grayed and italicized. For effect size calculation see text. For contrasts, timing gaps are given. For larger NMA (earlier taps), negative values are given. L, S, U, D = Loud, Soft, Up, Down.
Analysis 1
A rmANOVA was run, with factors TAP (x6) and Condition (x5: L, S, U, D, BL) (see results in the long panels of , and in ). A main effect of Tap on ITI is seen. Pairs of consecutive taps (only) were analyzed, to check developments across conditions. ITIs of T2 and T5 were shorter than those of T3 and T6, respectively (and shorter than BL [post hoc]: T2: p = .008, T5: marginal p = .052). A main effect of Condition on NMA is seen. Up yields earlier taps (−1.2 ms) than BL.
The Tap * Condition interaction in ITIs is significant. At T1, Up is short; at T2, Down is (T1: UvBL: ES = .79, 95% CI: [.36, 1.23]; UvD: ES = .74 [.31, 1.18]; T2: DvBL: ES = .69 [.26, 1.13]; DvU: marginal, corrected p = .055, ES = .58 [.15, 1.01]). At T1 as well, Loud is shorter than Soft.
In NMA, the interaction nears significance (p = .058)Footnote2. Up exerts an effect first, its taps at T1 earlier than both Down and BL. At T2 and T3, Loud is tapped early (vs BL, then vs Soft); at T5 and T6 Up is early again (vs BL). The Tap * Condition interaction being more significant in ITI (vs NMA) could be due to the Up-Down switch between T1 and T2, expressed only in ITI.
Summary of Analysis 1
In INT, timing deviations diverge, correlating with change in stimulus intensity: INT rise elicits earlier taps and vice versa (an ITI “trim” [< IOI] at T2 in Soft is “offset” [> IOI] at T3). In FRQ, though, both conditions elicit enlarged NMAs and shorter ITIs. An ITI trim in Up at T1 is “amended” (~ = IOI) at T2, but not “offset”; in Down a similar effect is shifted one tap later. Enlarged NMAs, under both FRQ conditions (more so Up), persist until T6.
Analysis 2
The segments of change, b + c, are “beat triplets” (ignoring added 4th beats) of contrasting change-direction. We probed the effects of segment membership, of tap location within segment, and of the test conditions, on tap timing. RmANOVAs were run, separately for NMA and ITI, with factors Segment (x2), Location (x3), and Condition (x5) (see , ).
Figure 4. Analysis 2 – results of factors Location and Condition. RmANOVAs for NMA and ITI, with factors Segment (x2), Location in segment (x3) and Conditions (x5) were run. Note: EXC only treats locations 2&3; for the factor Location, they are identical for TRU and EXC. TRU data are shown by diamonds, EXC by squares. All ANOVAs are significant. Significant contrasts (lines) and significance vs baseline (rings) appear in red (TRU) and orange (EXC). Error bars depict standard error of the mean. See the Location*Condition interaction in the SpMtrl, sheet B1.
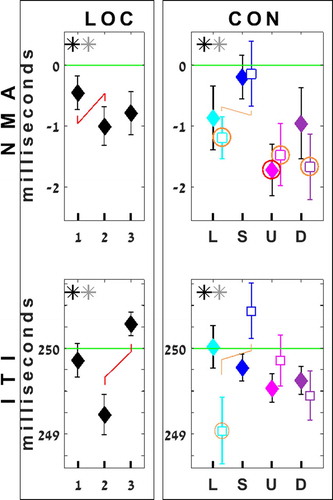
We also set to compare responses to segments 1v2 within uniform conditions (e.g., increment only), and to compare between such uniform conditions over both segments. To that end, we ran the same rmANOVAs, exchanging (hence: EXC) in Segment_2 the columns within the INT/FRQ pairs (L↔S, U↔D). The “new” Up thus consists only of frequency increments, etc. However, T4 had to be omitted, since no ITIs elicited by a uniform condition existed at that ordinal location. EXC rmANOVAs thus included locations 2 and 3 only. Unexchanged Analysis 2 is hence tagged TRU.
The TRU/EXC versions aid together in weighing effects of change in stimulus-value vs the effects of its absolute value, by the different range-relations they feature between segments and between increments and decrements. details the step-sets in the two versions. See results in (partial display), statistics (ηp2 only) in (for a full table cf SpMtrl). TRU data are as in Analysis 1 (, long panels); the EXC version concerns T5 and T6 (Figure 3, short panels).
Table 2. Analysis 2, Step-sets in TRU vs EXC. Different gaps between segments and between within-parameter stimuli in TRU vs EXC aid to assess the effects of change vs absolute values. In Segment_2, one third of the trials were further from standard (due to a 4th beep in Segment_1). The steps for increments are specified; decrements were a mirror set.
Table 3. Tap timing, Analysis 2. RmANOVAs results, with factors Segment (x2), ordinal Location of the tap in the segment (Location: TRU: x3; EXC: x2), and Condition (x5). Non-significant results in gray, italicized. Partial Eta squared values only are given. For the full table: cf SpMtrl, Sheet B2.
In both NMA and ITI, under both TRU and EXC, no main effect of Segment is seen. Thus, across Locations and Conditions, a segment of uni-directional change preceded by stability did not differ in overall tap-timing from one preceded by a segment of change in an opposing direction; nor were timing delays/advances accumulated from Segment_1 to Segment_2.
Under TRU, a main effect of Location is shown in both NMA and ITI. Location_2 (across Segments and Conditions) is tapped earliest [NMA]Footnote3, significantly earlier than Location_1; it also elicits the shortest ITIs, significantly shorter than Location_3. Post-hoc tests show Location_2’s NMA is larger than BL (p = .005, ES = .66), its ITIs shorter than IOI (p = .003, ES = .69). See , left. No interaction of Segment and Location is shown; across conditions, inter-Location “hierarchy”, as well as specific Locations, do not differ between Segments.
A main effect of Condition is shown [NMA/ITI, TRU/EXC]: Test conditions elicit timing deviations over the 6-beat span (, right). In EXC NMA, juxtaposing responses to “one sort” stimuli (e.g., increase only) from two separate contexts, Loud, Up and Down are earlier than BL; Loud is earlier than Soft too. Hence, escalation of NMA is found to an increase in INT or FRQ, but also to a decrease in FRQ. In TRU NMA, as seen in Analysis 1 before, only Up is earlier than BL.
In EXC ITI, Up and Down do not differ, whereas Loud tends to be shorter than BL and Soft. In both EXC NMA and EXC ITI, then, INT increments elicit significantly earlier taps than decrements, at closer absolute value ranges than in TRU (EXC vs TRU: 1.666 vs 3.333 steps gap).
The interaction between Segment and Condition is significant for TRU ITI. Soft’s ITIs in Segment_2 (where intensity rises) are shorter than in Segment_1 (where it drops). Within Segment_2, Soft’s ITIs are shorter than Loud’s (decrementing here), despite Loud’s higher absolute intensity range (2.666 steps gap). Further, this interaction is not significant for EXC NMA or EXC ITI, despite the 2.666 steps gap between Segments within condition. By several findings, then, a rise in intensity (the change), more than high intensity (per se), elicits early taps (cf § Discussion §§ Caveats §§§ Absolute values).
The interaction between Location and Condition is significant in TRU NMA,Footnote4 EXC NMA and EXC ITI (cf SpMtrl, Sheet B1). One aspect compares a given condition between Locations. In TRU NMA, Soft and Down yield earlier taps (wider NMAs) in Location_2 than at Location_1. In EXC NMA, a decrease in intensity (the “new” Soft) elicits later taps at Location_3 than at Location_2. This delay is reflected also in the [EXC] ITIs, longer at Location_3 than at Location_2 for Soft (+ 1.9 ms) and Down (+ 2.1 ms).
A second aspect compares between conditions at a given location. In TRU NMA, Up is early (vs BL) in each Location. In EXC NMA, an early tap (vs BL) is shown at both Locations 2 and 3 for Loud, Up and Down; at Location_3, Soft is later than Loud. In EXC ITI, at Location_2, Loud and Down’s ITIs are shorter than BL, and at Location_3 Soft’s ITIs are longer than both BL and Loud.
As for the triple interaction, in TRU NMA at Location_3, Soft is tapped earlier in Segment_2 (B6, the 3rd loudening beep) than in Segment_1 (B3, the softest beep). Down is earlier at B2 than at B1, and Soft is earlier at B2 than at B3. Other findings by this interaction are common with Analysis 1.
Summary of Analysis 2
Location and Condition show main effects. Condition’s interactions with Location and with Segment are significant. Under our test conditions, urged timing (NMA escalation and ITI shortening) peaks at Location_2. Only for Up is a response seen already at Location_1.
FRQ vs INT. In TRU ITI, FRQ conditions are overall marginally shorter than BL, as the ITI trim in B1 (Up) and B2 (Down) is not offset until Repose sets. INT ITI symmetry over Segment borderline (response and stimulus-change correlate) nulls TRU Condition effects for Loud and Soft.
INT. In INT, tap timing correlates to change in stimulus intensity. Thus, (a) in EXC Condition, INT increments elicit shorter ITIs (by 1.3 ms) than decrements; (b) in the Condition*Segment interaction, TRU ITI shows differences between Segments in Soft (significant) and Loud (marginal), and within each Segment between Loud and Soft; and (c) in the Condition*Location interaction, both EXC NMA and EXC ITI show at Location_3 a significant Loud vs Soft difference.
FRQ. Up is tapped early at each Location. Down’s effect, less robust, begins only at B2. FRQ increments tend to elicit larger NMAs than decrements.
Acceleration and EMG Data
We performed two analysis types. One probed the difference between activity under each test condition and the baseline activity under Ctrl; its results are presented below. In the other, within test conditions, each time bins was tested vs its parallel in the directly preceding beat; thus, (a) beat-to-beat unfoldings were probed, and (b) insight was added on response to first change, exposing the actual sequence from the last (specific) control beat (i.e., not a general baseline) to the (specific) directly following B1. Results of the second analysis are presented in the SpMtrl, Sheet F. Overall, the analyses corroborate each other’s results.
RmANOVAs were run separately for each change-segment (b, c), with factors Beats (x3), Time Bins [Tbn] (n differs between alignments), and Conditions (x5). Our focus was mainly the factor Conditions: over a segment as a whole (Condition), over specific beats (Condition*Beat) and in specific phases of action (Condition*Beat*Tbn). Eight comparisons were made: a–d) change under each condition vs baseline (or change vs the previous beat within each condition); e–f) contrasts within parameters (LvS, UvD); g–h) grouping contrasts (by parameter [L&S v U&D] and direction [L&U v S&D]).
– show results for the accelerometer [Accel, panel I] and EMG results (the finger flexor [Flx, panel II], and finger extensor [Ext, panel III]). The differences from baseline under the test conditions (not the action profiles proper) in the different alignments are shown.
Figure 5. Subsequent Tap data alignment – deviations from baseline under the four test conditions. Each data point stands for a 20 ms time bin of the epoch −120 ms to 0 (touchdown). Only the accelerometer results and those of the two distal antagonist muscles are shown. The figure format here will be iterated in –; it is explained here. Segment-wide results – in boxes at the outset of each segment: conditions (circles, for L, S, U, D), cond. contrasts (bi-color “diamonds”, for LvS, UvD), and groupings (asterisk pairs, for L&U v S&D, INT v FRQ). Beat*Condition interactions – at inset bottom: conditions (triangles, for L, S, U, D), and cond. contrasts as before. Triple interactions (Beat*Condition*Tbn) – on the conditions’ lines (squares), or at the insets’ tops for condition contrasts (bi-color stars).
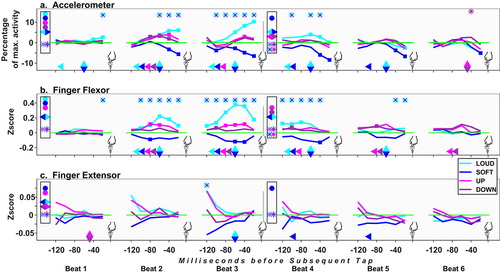
Figure 6. Precedent Tap data alignment: deviations from control under the four test conditions. See symbols explanation in ’s caption. Depiction of the accelerometer’s results (top panel) is split to beat halves, both at each segment’s outset and along the sequence; see above in the chapter statistical procedures for acceleration and EMG data.
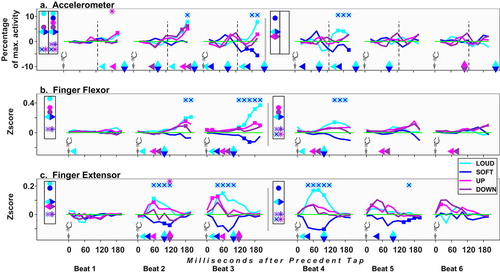
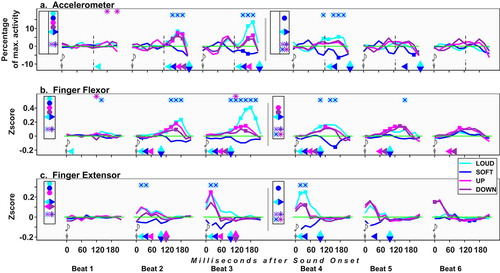
Figure 7. Sound Onset data alignment: deviations from control under the four test conditions. See about symbols in the caption for . Here too, accelerometer results are split by beat halves; see details in statistical procedures.
In the figures, we depict segment-wide conditions-effects in boxes at each segment’s outset, Condition*Beat interactions under each beat, triple interactions at specific bins by squares on the lines, and intra-parameter contrasts (LvS, UvD) by asterisks above.
Subsequent Tap Aligned Data
Aligning to action ensuing an event (cf , and SpMtrl, sheet A) reveals the action’s profile (not its event-related timing). We probed the 120 ms preceding taps (cf ; statistics cf , and SpMtrl, Sheet C). Hereafter, phrases as “Up rises” mean activity-rise is evoked by Up.
Table 4. Alignment to Subsequent Tap, comparison vs baseline. Separate rmANOVAs for each index (Accelerometer, Flexor and Extensor), for each segment, were run, with factors Beats (x3), Time bins (Tbn, x6) and Conditions (x5). Non-significant results are grayed out and italicized. Full details (df, F, p, gnrlzd. η2): cf SpMtrl Sheet C.
Accel
Response patterns to INT differ from those to FRQ (see , panel I). In INT, deviations from BL are positively correlated with change in auditory intensity: loudening elicits increased activity, softening elicits a decrease; gradual return to BL is seen at Segment_2, during backtracking. In FRQ, increments and decrements elicit increase at Segment_1, mainly in B2; return to BL is completed by B4 (no significant effect at change direction reversal). As for early effect latencies, Loud rises more than both BL and Soft already at B1 (at tbns close to the tap).
EMG
This alignment exposes the tap action at the Flx (cf panel II). The finger-lifter, Ext, is less active at this action-phase (cf panel III, note its different scale). The response pattern at the Flx corresponds roughly to that in acceleration: in INT it correlates with change in auditory intensity (from B2), whereas in FRQ a rise in activity is elicited by both rise and fall, mostly at B2 and B3. Notably, Flx activity under both FRQ directions remains aroused up to Segment_2’s end. Up tends to yield higher activity than Down at B2 and B3.
In the Ext, in INT, Loud is more active than Soft at Segment_1, a gap peaking at B3. In FRQ, Up is more active than BL and Down at Segment_1, as early as B1 (UvD: p = .001, ES = .80 [.32, 1.26]).
Interim Summary for Subsequent-Tap Data Alignment
Activity under INT conditions correlates with the auditory change, whereas in FRQ both FC directions elicit increase. In FRQ, finger acceleration rises at B2, then wanes; the Flx, though, stays roused from B2 on, maybe due to concurrent (subtler) arousal in the antagonist muscle [Ext]. Up tends to top Down (though both rise) in B2 & B3 [Flx]. These response patterns for INT and FRQ recur in all alignments; in reporting results henceforth, we focus only on additional findings.
As for early latency, in this alignment, at B1 effects are mostly in INT: Loud yields more activity, by several indices. Except for the Ext at B1, FRQ affects action dynamics only from B2.
Precedent Tap Aligned Data
This alignment elucidates action profile from touch-down onwards. Note: Touchdowns anticipate their auditory targets, due to NMA; thus, B1 begins here with T0. A 220 ms post-tap epoch was studied. Results: cf , statistics: cf and SpMtrl, Sheet D. In the accelerometer analysis, due to noisy touchdown data, tbn1 is omitted. In the current and the next alignment, separate rmANOVAs were run for acceleration data of first or second beat-halves (cf §Statistical procedures); results are depicted in separate symbol sets in – (top panel).
Table 5. Alignment to Precedent Tap, comparison vs baseline. For the accelerometer beats were split due to the data being directional – movement toward/away from the tapping board; see text. Of the two halves, the later’s results are given, reflecting the tapping action. Non-significant results are grayed and italicized. Full statistics: cf SpMtrl Sheet D.
Accel
Up rises already at B1 (UvC ES = .71 [.25, 1.17], UvD ES = .43 [0, .86]), mostly in the two last tbns (from 180 ms post-tap; ES: tbn10 UvC = .69 [.26, 1.13], UvD = .64 [.21, 1.08]; tbn11 UvC = .82 [.39, 1.25], UvD = .54 [.11, .98]). At B2, Down tends to be more active than Up, though both rise.
EMG
The Ext’s activity is here exposed. At B2, Up and Loud rise 60 ms post-tap. At B1, only Loud rises, in the Flx. In Segment_2, Up and Down remain aroused in both muscles.
Interim Summary for Precedent Tap Data Alignment
At B1, Loud and Up rise. Up rises already 180 ms post-tap (~160 ms post-sound-onset [hence pOnst]); this rise is hinted yet 40 ms earlier (cf comp. vs the previous beat, SpMtrl, sheet F). Down rises late in B2, from 100 ms post-tap (~330 ms pOnst). Up is more than Down at B3 [Ext]. Loud’s effect latency is similar to Up’s (cf B1 [Flx], comp. vs the previous beat).
Sound Onset Aligned Data
Chronometrically true to the delivery of auditory information, this alignment reflects action timing rather than profile. It relates to viewing tap-timing by NMA deviations, adding insight inbetween taps. Results: cf , statistics cf and SpMtrl, Sheet E.
Table 6. Alignment to Sound Onset, comparison vs baseline. As related in Table 5 and in the text, for acceleration data only results of beats’ later half are given. Non-significant results are grayed and italicized. Full statistics: cf SpMtrl Sheet E.
Accel
At B1, Loud is above BL; Up is above Down at 180 ms pOnst.
EMG
At the outset of B2 [Ext], Loud and Up rise while Soft drops. In the ensuing tap (end of B2), Loud and Up rise, trailed by Down [Flx]. Up rises more than Down at B2 [Ext] & B3 [Flx, Ext]. In Segment_2, only FRQ conditions remain aroused.
Interim Summary for Sound Onset Data Alignment
A rise is seen in Up already at B1, 160 ms pOnst [Accel.]; its presage is hinted in the Flx some 40 ms earlier (cf analysis vs previous beat, SpMtrl, sheet F). Up’s effect is distinct from Down’s, agreeing with NMA data.
General Discussion
Main Findings
Our study examined short latency effects of auditory FC on motor behavior in humans, asking (a) whether FC influences motor acts within a brief latency; if so, (b) does FC direction make a difference?; and (c) is response to frequency change distinct from response to other auditory changes (here namely intensity)? Our subjects’ task was to synchronize tapping to isochronous auditory beep sequences presenting changes. We found the answers to all three questions positive. FC did influence the motor act, yielding early tap triggering; the effect commenced within 160 ms post-change – a brief latency indeed. Moreover, FC direction did make a difference: though the general response outline to both FRQ conditions was similar, response patterns to Up and Down were distinct, mostly in initial stages following presentation of FC. At Beat_1 (B1), Up was tapped to before Down (larger NMA, shorter ITI); at B2, ITI was shorter in Down. Enlarged NMA throughout the sequence, seen in both, was more robust in Up.
Further, we did find responses to INT/FRQ change qualitatively distinct. In response to INT conditions, finger acceleration, EMG amplitude and NMA correlated with change in auditory intensity; ITI was inversely correlated (i.e., in response to an increase of intensity, taps anticipated beeps more, with shorter ITIs, and larger acceleration and EMG amplitude; opposite responses were elicited to intensity decrease). Initial activity alterations in INT were mainly of action profile. In contrast, both increase and decrease in FRQ elicited enlarged NMA and early action triggering, retained until Repose set off; aroused action profile peaked at B2, then subsided.
Intensity vs Frequency, Increments vs Decrements
Our results, showing that NMA, ITI, EMG, and finger acceleration responses to INT bifurcated, correlated with the change in stimulus intensity, whereas those to FRQ were shared by both rise and fall, suggest that response to FRQ is specific, not a general one to auditory change.
Response differed between INT and FRQ in some of the indices already at B1, where significantly early tap timing and action triggering were seen in Up, whereas significant increase in action profile was seen in Loud. Therefore, in our task, divergent processing of INT and FRQ seems to have occurred in latencies shorter than 160 ms (cf introduction). Revealing behavioral, motor expressions of this divergence, we add an angle to the study of processing of INT vs FRQ.
The difference in response between parameters recalls Stevens (Citation1957) prothetic vs metathetic percepts. Response to Up may activate “fresh” neuron populations (a substitutive mechanism), yielding an earlier tap, whereas response to Loud may activate more strongly populations already engaged (an additive mechanism), yielding a more energetic action.
In our FRQ results, response to Down was later than to Up, but almost as robust, whereas in Kohn, Lifshitz, and Litchfield (Citation1978), for change in steps, responses to both directions were similar in amplitude, response-time dispersion and latency (see above; note they used continuous-tone stimuli). Responses to the initial INT change in our discrete beeps concur with Kohn, Lifshitz, and Litchfield (Citation1980), who found much weaker cortical response and wider response-time dispersion to INT decrements, and with the slower response-time elicited to softer stimuli following stronger warning signals (review: Nissen, Citation1977). An EMG study of response-time to INT change found the epoch from stimulus-onset to action, not action itself, to be extended in response to decrements (Spehar & Kolesarić, Citation2010). We find INT decrements first alter action profile, not its latency; however, our regularly-timed, preplanned tapping task differs from a response-time task.
Past research has suggested cross-effects in perception of changes in INT, FRQ, and tempo, due to experience with sound in the environment and in speech. Boltz (Citation2011) found that higher melody register, melodic ascent and increasing loudness led to judgment of melodic tempo as being faster; lower register, descent and decreasing loudness led to the opposite judgment. She reasoned such associations, or joint representation, as stemming from covariations of such changes in the general auditory environment. Indeed, we did find a response of speeded action to rising frequency; however, falling frequency yielded the same response tendency, only later. Further, whereas increments and decrements yielded in our experiment contrasting responses when presented in the intensity domain, they yielded unidirectional responses in the frequency domain.
Latency and Routes
Latency
Evolutionary pressure to react fast to vital cues, as FC and its direction, may favor swift processing via short neural routes in yielded responses. We probed whether brief-latency effects of FC and its direction on motor actions existed; such effects could suggest early, perhaps subcortical, mediation from auditory to motor pathways, less dependent on higher functions.
We found at B1 an effect of FRQ change on NMA and ITI. The effect was direction-specific: Up was tapped to earlier than Down (B1, UvD, NMA: ES = 1.17 [.74, 1.60]), and earlier than baseline. This behavioral outcome occurred ~230 ms pOnst. Evidence at still earlier latency, captured by physiological indices, was exposed in various data alignments. A robust increase of acceleration was found ~160 ms pOnst in Up; seen in alignments to Sound and to the Precedent Tap, it implies early action triggering. Yet earlier, ~120 ms pOnst, near statistical significance, an advantage of Up over Down in the flexor seems to bring about Up’s acceleration advantage.
In INT, at short-latency, Loud was more active in the two last time bins of B1. Aligned to Subsequent Tap, this finding suggests altered action profile. It is ~50 ms later than Up’s effect.
At the outset of B2, the effects of INT and FRQ developed further: ~290 ms pOnst [Ext], Loud and Up yielded a rise; Soft yielded a decay. Only at mid B2 (~350 ms pOnst) was Down significantly affected – rising, as Loud and Up. Down’s ITI at B2 was 2.3 ms shorter than at B1 (ES = .55 [.11, .99]). Taps’ profile, altered at B1 mostly by INT, was affected at B2 by FRQ too.
The considerable latency difference between responses to Up and Down (160 vs 350 ms) may be task-dependent. Perhaps the response to Down just “missed” T1’s dispatch and was then robustly expressed only toward T2 (with some hints at T1’s finger-lift).
Routes
Latency as short as 160 ms still allows information-travel by an ear-cortex-muscle loop. Several cortical components in this time-range, mentioned above as biased by FC direction, may be involved. An amplitude advantage for Up in the cortical P1 is seen already ~50 ms pOnst (Noguchi et al., Citation2015); a FC-direction latency bias in the N100m is seen at 85 ms pOnst (Pardo & Sams, Citation1993). Also the N100 amplitude bias for FRQ rise, at 115 ms pOnst (Maiste & Picton, Citation1989), could be involved in our effect at 160 ms, if 45 extra ms suffice for its mediation to distal muscles. MMN to FC direction at ~150 ms (to “our” Δ, Ruusuvirta & Astikainen, Citation2012), would cast a motor effect only later. Our next study, probing in parallel motor response-time and EEG to FC, may shed light on possible correlations between these components and motor response. SSA-release, though we did not directly test it, could have been evoked by the sequences’ first deviant beep. Whereas SSA-release to FRQ deviance per se could have occurred as “low” as IC level, FC direction bias in SSA-release, by research thus far, may have occurred only cortically.
The effect latencies yielded in our study, not short enough to rule out cortical mediation, leave the ear-cortex-muscle route the more probable one. However, our subjects’ task required action in a specific time relative to sound onset; physiological effects were primarily seen during that action. In the “inter-action” time between finger lifting and lowering – ~80–120 ms pOnst – EMG and acceleration amplitude were low and variability high, blurring possible effects. Thus, shorter latencies for arrival-to-muscle of information about FC and its direction could perhaps be found in other tasks. Also, the plausibility of cortical involvement in our results does not rule out the existence of a subcortical “direct” path, which may function in parallel with the “upper” path. As detailed in our introduction, several traits of processing at the IC seem to imply that “ground is ready” for direct auditory-motor mediation at that level: attested reflex pathways, bi-modal neurons, differential FFR and varied SSA. In our next study we monitor brainstem response too.
Looming
Our stimulus, and our subjects’ responses to it, can be viewed also through the prism of “loomingness”: our Loud and Up conditions could have been perceived as looming, vs Soft and Down, perceived perhaps as less endangering, receding. Perception of a stimulus as looming could, by the research mentioned, elicit earlier action. Our results for INT, and, importantly, the early advantage in FRQ of Up over Down, fit such an interpretation. Loomingness, however, can not account for the enlarged NMA retained under both FRQ conditions at later sequence stages.
Surprise Account
Though our subjects were surprised by the moment of first change, a surprise effect alone cannot account for the results. First, surprise often impedes ongoing action (Horstmann, Citation2006), whereas our Up and (less so) Loud conditions elicited early taps. Further, the effect’s direction-specificity (at symmetrical Δs) defies surprise models which base effect-size predictions on the Δ from the preceding “routine” stimuli. Moreover, such models cannot predict the difference, from early on post change onset, between the patterns of response to the INT and FRQ parameters.
Comparison to Relatable Research
The work of Ammirante et al. (Citation2011), Ammirante & Thompson (Citation2012) is the closest to ours, in topic (FC effects), and in paradigm (despite major methodological differences). Whereas they found longer ITIs following contour-change, we, in a post-hoc t-test, did not find FRQ ITIs of T4 (following contour change) to differ from those of T2, 3, 5, and 6 (our contour preserving instances), or from baseline. Their finding of shorter ITIs on descending contour-preserving tones is partly replicated in our present results. That condition, in Ammirante et al.’s (Citation2011) study, parallels our T2 Down and T5 Up, in which, as seen in Analysis 2 EXC, shorter ITIs were indeed elicited. ITIs of our T3 Down and T6 Up, still contour-preserving, do not concur, though. It seems to us, that shorter ITIs elicited by frequency decrements are specific to a certain time window. Further, our short ITI of T1 Up is absent altogether in their study, due to their discarding of the first five unpaced taps, and due to their tap pacing at 500 ms IOI, twice slower than ours.
An interesting similarity is seen in the effect extent (in ITI terms) attained under the two tapping tasks – self-maintained ITI vs ITI constrained by a pacing stimulus, and 500 ms vs 250 ms IOI. Ammirante et al.’s paradigm elicited ITI modulations of up to 2%; in ours they were up to 1.5%. In NMA terms we found the effect was up to 13.5% (!) of the general average (2.7 ms deviation from the 20 ms NMA), and up to 3 times the mean of personal standard error.
Caveats
Absolute Values vs Value Change
Our results may be confounded with effects of higher vs lower absolute stimulus values. Our second variant (EXC) of Analysis 2 of the timing results controls partly for such confound (see to Analysis 2 and ). Revealing effects of INT and FRQ change in partially over-lapping ranges implies that, e.g., a frequency rise, not higher frequency per se, casts an effect.
Previous research adds supporting evidence. As for frequency, Ruusuvirta and Astikainen (Citation2012) found stronger MMN response to higher-than-standard than lower-than-standard frequency-deviant tones under an oddball condition, but not when tones were equi-probable; this rebuts an absolute frequency confound, attesting a FC direction bias in deviance processing. Pardo and Sams (Citation1993) found a larger N100m to rising (vs falling) glides, independently of the center frequency. MMN to FC-direction deviants among tone pairs which varied randomly in absolute-frequency means shows that FC-direction extraction is independent of that confound too (Saarinen, Paavilainen, Schröger, Tervaniemi, & Näätänen, Citation1992).
As for intensity, Białuńska, Dalla Bella, and Jaśkowski (Citation2011) found an effect of SPL on sensorimotor times in a simple response-time task (tighter latency at higher SPL), but not in a quasi-tapping task (closer to ours). In sum, change in INT or FRQ seems to yield effects independently of absolute parameter values. Our next study controls explicitly for that confound.
Relation between Step-sizes in the Two Parameters
The relation between the step-sizes in our INT and FRQ sequences may have influenced results. In an MMN study, Giard et al. (Citation1995) presented change in various parameters (INT, FRQ, and duration); marked inter-parameter MMN-latency differences were equated in a follow-up session by changing stimuli values. However, both the values’ absolute range and the relative difference between standards and deviants were adjusted; inference is therefore hindered.
Using a different INT Δ in our study may have yielded, perhaps, responses relating differently to effect latencies or activity modulations attained with our FRQ Δ. However, the patterns of response to increments vs decrements shown within INT/FRQ, and the difference thereof between the parameters, are plausibly independent from the relation between deltas.
Pitch Warp
Though perceived pitch is warped by intensity change, we did not adjust the frequency of nonstandard beeps in the INT conditions. An increase of 5 dB (as our intensity steps) in discrete tones at 500 Hz (our standard frequency) yields a perceived pitch roughly 0.3% lower than its F0 (albeit with large inter-subject variance; Verschuure & Van Meeteren, Citation1975). Parallel changes in our FRQ conditions were two magnitudes wider. The acute perceived pitch warp to intensity rise of the Doppler effect is limited to continuous stimuli, unlike ours (McBeath & Neuhoff, Citation2002).
Subject Population
We chose musicians as subjects, due to their proficiency in synchronization tasks; this could have affected results, or their magnitude, limiting generalization to the wider population. By Liang et al. (Citation2016), FC detection elicits earlier N100 (at 122 ms) and larger P200 (at 226 ms) in musicians. In our earlier tapping study (Boasson & Granot, Citation2012), though, musicians, vs non-musicians, showed lesser effect of FC direction on ITI, perhaps due to superior tempo adherence in dynamic auditory environments. Also, to reduce biases of musical conventions and conscious attempts to “fit”, we asked the subjects in both our studies (Boasson & Granot, Citation2012, and the current study) to continue tapping “no matter what” and maintain utmost accuracy. This instruction, our subjects’ unique skills, and the involvement of rhythmic entrainment, may have in fact suppressed FC effects.
Task Appropriateness
The test conditions in our study modulated preplanned muscle action; they did not evoke action. However, our tapping task did yield non-verbal, rapid response to unpredictable changes, low action variance, in a defined time window. In future studies we shall explore other motor responses.
Summary
The evidence of a short-latency effect of Up and its dissociation from Down corroborates and extends findings from our previous study, revealing yet tighter travel latencies of upwards FC information from ear to muscles. To our knowledge this evidence is novel.
We hypothesized that an “express” ear-to-muscle route able to transmit FC information may exist, as rapid responses may be advantageous, even crucial. A potential “early” locus of auditory to motor mediation anteceding the cortex seems the inferior colliculus, where FC processing is advanced; descending motor ways could perhaps be “contacted” in its vicinity with an ear-to-muscle “message”. However, a latency of ca 160 ms from ear to muscle, as we show, does not preclude the ear-cortex-muscle loop. Finding a rapid asymmetrical effect of FC on motor action, is still intriguing, even if the “full” loop is at work.
The latencies we found may be task-dependent: effects were seen clearly when variance was low, namely when action was regular; for both the Acc and Flx that was in the later part of beats. Earlier effects could perhaps surface if a task’s peak action occurred in earlier time frames.
To study embodied sound perception, which may possibly be relevant to higher functions as music or speech, we employed innovatively methods from other fields, using physiological indices – finger acceleration and EMG. In our next study, underway, we trace simultaneously both the auditory ear-to-cortex processing timeline (by ABR and EEG), and the arrival of FC information to the muscles (by action indices).
Supplemental Material
Download Zip (515.5 KB)Acknowledgments
This experiment is part of the doctoral research of the first author. We thank the members of the doctoral committee Prof. Merav Ahissar, Prof. Zohar Eitan and Prof. Israel Nelken for their guidance. We thank Prof. Prut, head of the Neurobiology Lab at Hadassah medical school in the Ein Kerem campus of the Hebrew University of Jerusalem, for her cooperation, guidance, advice, and permission to use the lab. We thank Dan Valsky for his help in signal processing, and Dr. Nori Jacobi for his advice and encouragement.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Notes
1. The musicians were either professional musicians of the Jerusalem Symphony Orchestra, or students of advanced degrees at the Jerusalem Music Academy. However, the number of years of music education is an approximation, as it was not directly asked. In our next study, underway, the musician subjects, at similar ages, from the same millieu, reported in average 17.7 years of music practice (StD 5.9 y).
2. After experiment-wise correction, p = .082.
3. In the Results section, italicized square brackets denote relevant analyses and indices.
4. The p value for this interaction, .038, turns after experiment-wise correction to marginal (.056).
References
- Ammirante, P., & Thompson, W. F. (2012). Continuation tapping to triggered melodies: Motor resonance effects of melodic motion. Experimental Brain Research, 216, 51–60.
- Ammirante, P., Thompson, W. F., & Russo, F. (2011). Ideomotor effects of pitch on continuation tapping. The Journal of Experimental Psychology, 64(2), 381–393.
- Anderson, L. A., & Malmierca, M. S. (2013). The effect of auditory cortex deactivation on stimulus‐specific adaptation in the inferior colliculus of the rat. European Journal of Neuroscience, 37(1), 52–62.
- Antunes, F. M., & Malmierca, M. S. (2011). Effect of auditory cortex deactivation on stimulus-specific adaptation in the medial geniculate body. Journal of Neuroscience, 31(47), 17306–17316.
- Arlinger, S., & Jerlvall, L. (1981). Early auditory electric responses to fast amplitude and frequency tone glides. Electroencephalography and Clinical Neurophysiology, 51(6), 624–631.
- Ausmus, D. M., & Clarke, J. A. (2014). Mother knows best: Functionally referential alarm calling in white-tailed ptarmigan. Animal Cognition, 17, 671–679.
- Baumgartner, R., Reed, D. K., Tóth, B., Best, V., Majdak, P., Colburn, H. S., & Shinn-Cunningham, B. (2017). Asymmetries in behavioral and neural responses to spectral cues demonstrate the generality of auditory looming bias. Proceedings of the National Academy of Sciences, 114(36), 9743–9748.
- BBC Earth. (n.d.). Drongo bird tricks meerkats. Retrieved from https://www.youtube.com/watch?v=tEYCjJqr21A
- Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 289–300. Retrieved from https://www.jstor.org/stable/2346101
- Białuńska, A., Dalla Bella, S., & Jaśkowski, P. (2011). Increasing stimulus intensity does not affect sensorimotor synchronization. Psychological Research, 75, 43–53.
- Boasson, A. D., & Granot, R. (2012, July 23–28). Melodic direction’s effect on tapping. In E. Cambouropoulos, C. Tsougras, P. Mavromatis, & K. Pastiadis (Eds.), Proceedings of the 12th International Conference on Music Perception and Cognition (ICMPC) and the 8th Triennial Conference of the European Society for the Cognitive Sciences of Music (pp. 110–119). Thessaloniki, Greece. Retrieved from http://icmpc-escom2012.web.auth.gr/files/papers/110_Proc.pdf
- Boltz, M. G. (2011). Illusory tempo changes due to musical characteristics. Music Perception, 28(4), 367–386.
- Cacciaglia, R., Escera, C., Slabu, L., Grimm, S., Sanjuán, A., Ventura-Campos, N., & Ávila, C. (2015). Involvement of the human midbrain and thalamus in auditory deviance detection. Neuropsychologia, 68, 51–58.
- Clément, S., Demany, L., & Semal, C. (1999). Memory for pitch versus memory for loudness. The Journal of the Acoustical Society of America, 106(5), 2805–2811.
- Cornella, M., Leung, S., Grimm, S., & Escera, C. (2013). Regularity encoding and deviance detection of frequency modulated sweeps: Human middle-and long-latency auditory evoked potentials. Psychophysiology, 50(12), 1275–1281.
- Cousineau, M., Demany, L., & Pressnitzer, D. (2009). What makes a melody: The perceptual singularity of pitch sequences. The Journal of the Acoustical Society of America, 126(6), 3179–3187.
- Demany, L., Carcagno, S., & Semal, C. (2013). The perceptual enhancement of tones by frequency shifts. Hearing Research, 298, 10–16.
- Demany, L., & Ramos, C. (2005). On the binding of successive sounds: Perceiving shifts in nonperceived pitches. The Journal of the Acoustical Society of America, 117(2), 833–841.
- Demany, L., & Semal, C. (2018). Automatic frequency-shift detection in the auditory system: A review of psychophysical findings. Neuroscience, 389, 30–40.
- DiGiovanni, J. J., & Schlauch, R. S. (2007). Mechanisms responsible for differences in perceived duration for rising-intensity and falling-intensity sounds. Ecological Psychology, 19(3), 239–264.
- Duque, D., Wang, X., Nieto-Diego, J., Krumbholz, K., & Malmierca, M. S. (2016). Neurons in the inferior colliculus of the rat show stimulus-specific adaptation for frequency, but not for intensity. Scientific Reports, 6, 1.
- Escera, C., & Malmierca, M. S. (2014). The auditory novelty system: An attempt to integrate human and animal research. Psychophysiology, 51(2), 111–123.
- Fernald, A. (1992a). Human maternal vocalizations to infants as biologically relevant signals: An evolutionary perspective. In J. H. Barkow, L. Cosmides, & J. Tooby (Eds.), The adapted mind: Evolutionary psychology and the generation of culture (pp. 391–428). NY: Oxford University Press.
- Fernald, A. (1992b). Meaningful melodies in mothers’ speech to infants. In H. Papoušek, U. Jürgens, & M. Papoušek (Eds.), Nonverbal vocal communication: Comparative and developmental approaches (pp. 262–282). NY: Cambridge University Press.
- Freiberg, K., Tually, K., & Crassini, B. (2001). Use of an auditory looming task to test infants’ sensitivity to sound pressure level as an auditory distance cue. British Journal of Developmental Psychology, 19(1), 1–10.
- Galbraith, G. C., Chae, B. C., Cooper, J. R., Gindi, M. M., Ho, T. N., Kim, B. S., … Lunde, S. E. (2000). Brainstem frequency-following response and simple motor reaction time. International Journal of Psychophysiology, 36(1), 35–44.
- Galbraith, G. C., Gutterson, R. P., Levy, D. S., Mussey, J. L., Sabatasso, F. A., & Wasserman, R. I. (2004). Correlated brain stem and cortical evoked responses to auditory tone change. Neuroreport, 15(17), 2613–2616.
- Ghazanfar, A. A., & Maier, J. X. (2009). Rhesus monkeys (Macaca mulatta) hear rising frequency sounds as looming. Behavioral Neuroscience, 123(4), 822.
- Giard, M. H., Lavikahen, J., Reinikainen, K., Perrin, F., Bertrand, O., Pernier, J., & Näätänen, R. (1995). Separate representation of stimulus frequency, intensity, and duration in auditory sensory memory: An event-related potential and dipole-model analysis. Journal of Cognitive Neuroscience, 7(2), 133–143.
- Gordon, M., & O’neill, W. E. (2000). An extralemniscal component of the mustached bat inferior colliculus selective for direction and rate of linear frequency modulations. Journal of Comparative Neurology, 426, 165–181.
- Grieser, D. L., & Kuhl, P. K. (1988). Maternal speech to infants in a tonal language: Support for universal prosodic features in motherese. Developmental Psychology, 24(1), 14–20.
- Grimm, S., & Escera, C. (2012). Auditory deviance detection revisited: Evidence for a hierarchical novelty system. International Journal of Psychophysiology, 85(1), 88–92.
- Grimm, S., Escera, C., & Nelken, I. (2016). Early indices of deviance detection in humans and animal models. Biological Psychology, 116, 23–27.
- Haff, T. M., & Magrath, R. D. (2013). Eavesdropping on the neighbours: Fledglings learn to respond to heterospecific alarm calls. Animal Behaviour, 85, 411–418.
- Hage, S. R., Jiang, T., Berquist, S. W., Feng, J., & Metzner, W. (2013). Ambient noise induces independent shifts in call frequency and amplitude within the Lombard effect in echolocating bats. PNAS, 110(10), 4063–4068.
- Hage, S. R., Jürgens, U., & Ehret, G. (2006). Audio-vocal interaction in the pontine brainstem during self-initiated vocalization in the squirrel monkey. European Journal of Neuroscience, 23, 3297–3308.
- Hentchke, H. (2011/2015). Measures of effect size toolbox (mes.m). Matlab central file exchange. Retrieved from http://www.mathworks.com/matlabcentral/fileexchange/32398-measures-of-effect-size-toolbox/content/mes.m
- Horstmann, G. (2006). Latency and duration of the action interruption in surprise. Cognition & Emotion, 20(2), 242–273.
- Kelly, J. B. (1973). The effects of insular and temporal lesions in cats on two types of auditory pattern discrimination. Brain Research, 62(1), 71–87.
- Kohn, M., Lifshitz, K., & Litchfield, D. (1978). Averaged evoked potentials and frequency modulation. Electroencephalography and Clinical Neurophysiology, 45(2), 236–243.
- Kohn, M., Lifshitz, K., & Litchfield, D. (1980). Average evoked potentials and amplitude modulation. Electroencephalography and Clinical Neurophysiology, 50(1), 134–140.
- Krishnan, A., & Parkinson, J. (2000). Human frequency-following response: Representation of tonal sweeps. Audiology and Neurotology, 5(6), 312–321.
- Krishnan, A., Xu, Y., Gandour, J. T., & Cariani, P. A. (2004). Human frequency-following response: Representation of pitch contours in Chinese tones. Hearing Research, 189, 1–12.
- Kuo, R. I., & Wu, G. K. (2012). The generation of direction selectivity in the auditory system. Neuron, 73(5), 1016–1027.
- Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863.
- Larson, C. R., Sun, J., & Hain, T. C. (2007). Effects of simultaneous perturbations of voice pitch and loudness feedback on voice F0 and amplitude control. Journal of the Acoustical Society of America, 121(5), 2862–2872.
- Liang, C., Earl, B., Thompson, I., Whitaker, K., Cahn, S., Xiang, J., … Zhang, F. (2016). Musicians are better than non-musicians in frequency change detection: Behavioral and electrophysio-logical evidence. Frontiers in Neuroscience, 10. doi:10.3389/fnins.2016.00464
- Maier, J. X., & Ghazanfar, A. A. (2004, August). Auditory and multisensory perception of looming signals by rhesus monkeys: A naturalistic behaviour research. 5. Neurowissenschaftliche Nachwuchskonferenz Tübingen (NeNa’04). Retrieved from http://hdl.handle.net/11858/00-001M-0000-0013-D87B-1
- Maiste, A., & Picton, T. (1989). Human auditory evoked potentials to frequency-modulated tones. Ear and Hearing, 10(3), 153–160.
- Malmierca, M. S., Anderson, L. A., & Antunes, F. M. (2015). The cortical modulation of stimulus-specific adaptation in the auditory midbrain and thalamus: A potential neuronal correlate for predictive coding. Frontiers in Systems Neuroscience, 9, 19.
- Malmierca, M. S., Niño-Aguillón, B. E., Nieto-Diego, J., Porteros, Á., Pérez-González, D., & Escera, C. (2019). Pattern-sensitive neurons reveal encoding of complex auditory regularities in the rat inferior colliculus. NeuroImage, 184, 889–900.
- Manser, M. B., Seyfarth, R. M., & Cheney, D. L. (2002). Suricate alarm calls signal predator class and urgency. Trends in Cognitive Sciences, 6(2), 55–57.
- Mathews, M. V., Pierce, J. R., Reeves, A., & Roberts, L. A. (1988). Theoretical and experimental explorations of the Bohlen–Pierce scale. The Journal of the Acoustical Society of America, 84(4), 1214–1222.
- McBeath, M. K., & Neuhoff, J. G. (2002). The Doppler effect is not what you think it is: Dramatic pitch change due to dynamic intensity change. Psychonomic Bulletin & Review, 9(2), 306–313.
- Miller, C. T., Miller, J., Gil-da-Costa, R., & Hauser, M. D. (2001). Selective phonotaxis by cotton-top tamarins (Saguinus oedipus). Behaviour, 138, 811–826.
- Morton, E. S. (1977). On the occurrence and significance of motivation-structural rules in some bird and animal sounds. The American Naturalist, 111(981), 855–869.
- Nelken, I., & Versnel, H. (2000). Responses to linear and logarithmic frequency‐ modulated sweeps in ferret primary auditory cortex. European Journal of Neuroscience, 12(2), 549–562.
- Nelken, I., Yaron, A., Polterovich, A., & Hershenhoren, I. (2013). Stimulus-specific adaptation beyond pure tones. In Basic aspects of hearing (pp. 411–418). New York: Springer. doi:10.1007/978-1-4614-1590-9_45
- Neuhoff, J. G. (2016). Looming sounds are perceived as faster than receding sounds. Cognitive Research: Principles and Implications, 1(1), 15.
- Nissen, M. J. (1977). Stimulus intensity and information processing. Perception & Psychophysics, 22(4), 338–352.
- Noguchi, Y., Fujiwara, M., & Hamano, S. (2015). Temporal evolution of neural activity underlying auditory discrimination of frequency increase and decrease. Brain Topography, 28(3), 437–444.
- Ohala, J. J. (1984). An ethological perspective on common cross-language utilization of F0 of voice. Phonetica, 41, 1–16. Germany: Karger.
- Ohl, F. W., Wetzel, W., Wagner, T., Rech, A., & Scheich, H. (1999). Bilateral ablation of auditory cortex in Mongolian gerbil affects discrimination of frequency modulated tones but not of pure tones. Learning & Memory, 6(4), 347–362. Retrieved from http://learnmem.cshlp.org/content/6/4/347.full.pdf
- Pardo, P. J., Mäkelä, J. P., & Sams, M. (1999). Hemispheric differences in processing tone frequency and amplitude modulations. Neuroreport, 10(14), 3081–3086.
- Pardo, P. J., & Sams, M. (1993). Human auditory cortex responses to rising versus falling glides. Neuroscience Letters, 159, 43–45. Elsevier Scientific Publishers Ireland Ltd.
- Peter, V., McArthur, G., & Thompson, W. F. (2010). Effect of deviance direction and calculation method on duration and frequency mismatch negativity (MMN). Neuroscience Letters, 482(1), 71–75.
- Peters, M. (1989). The relationship between variability of intertap intervals and interval duration. Psychological Research, 51, 38–42.
- Pisanski, K., Cartei, V., McGettigan, C., Raine, J., & Reby, D. (2016). Review – Voice modulation: A window into the origins of human vocal control? Trends in Cognitive Sciences, 20, 304–318.
- Plack, C. J., & Carlyon, R. P. (1995). Loudness perception and intensity coding. In B. C. Moore (Ed.), Hearing (pp. 123–160). Academic Press. doi:10.1016/b978-012505626-7/50006-6
- Repp, B. (2005). Sensorimotor synchronization: A review of the tapping literature. Psychonomic Bulletin and Review, 12(6), 969–992. [ See p.3, top right, for 1:1 tapping rate limits].
- Ruch, H., Zürcher, Y., & Burkart, J. M. (2018). The function and mechanism of vocal accommodation in humans and other primates. Biological Reviews, 93(2), 996–1013.
- Ruusuvirta, T. T., & Astikainen, P. (2012). Mismatch negativity of higher amplitude for melodic ascendance than descendance. Neuroreport, 23(4), 220–223.
- Ryan, M. J. (1980, July 25). Female mate choice in a neotropical frog. Science, New Series, 209(4455), 523–555.
- Saarinen, J., Paavilainen, P., Schröger, E., Tervaniemi, M., & Näätänen, R. (1992). Representation of abstract attributes of auditory stimuli in the human brain. NeuroReport, 3(12), 1149–1151.
- Saenz, M., & Langers, D. R. (2014). Tonotopic mapping of human auditory cortex. Hearing Research, 307, 42–52.
- Shiga, T., Althen, H., Cornella, M., Zarnowiec, K., Yabe, H., & Escera, C. (2015). Deviance-related responses along the auditory hierarchy: Combined FFR, MLR and MMN evidence. PloS One, 10(9), e0136794.
- Slugocki, C., Bosnyak, D., & Trainor, L. J. (2017). Simultaneously-evoked auditory potentials (SEAP): A new method for concurrent measurement of cortical and subcortical auditory-evoked activity. Hearing Research, 345, 30–42.
- Spehar, B., & Kolesarić, V. (2010). The effects of stimulus context on components of simple reaction time. Review of Psychology, 17(1), 59–67. Retrieved from https://hrcak.srce.hr/70662
- Stevens, S. S. (1957). On the psychophysical law. Psychological Review, 64(3), 153–181.
- Suzuki, Y., Mellert, V., Richter, U., Møller, H., Nielsen, L., Hellman, R., … Takeshima, H. (2003). Precise and full-range determination of two-dimensional equal loudness contours. Japan: Tohoku University. Retrieved from http://www.mp3-tech.org/programmer/docs/IS-01Y-E.pdf
- Väljamäe, A. (2009). Auditorily-induced illusory self-motion: A review. Brain Research Reviews, 61(2), 240–255.
- Van Belle, G. (2011). Sample size. In Statistical rules of thumb (Vol. 699). John Wiley & Sons. Retrieved from https://www.stat.washington.edu/NRCSE/research/struts/chapter2.pdf
- Verschuure, J., & Van Meeteren, A. A. (1975). The effect of intensity on pitch. Acta Acustica United with Acustica, 32(1), 33–44.
- Wang, W. J., Tan, C. T., & Martin, B. A. (2013, June). Auditory evoked responses to a frequency glide following a static pure tone. Proceedings of Meetings on Acoustics ICA2013, 19(1), 050122. ASA.
- Zendel, R., Ross, B., & Fujioka, T. (2011). The effects of stimulus rate and tapping rate on tapping performance. Music Perception, 29(1), 65–78. University of California Press.
