ABSTRACT
Packaging of food products is one of the most important stages of the food supply chain. Nano-size materials for packing food substances with appropriate properties result in better packaging performance and longer food shelf-life. In this review, the application of ZnO nano-size in active packaging of foods is discussed to identify gaps in applications for food packaging and safety. First, the crystal structures and morphologies of modified ZnO nanoparticles (ZnO NPs) are presented, and their synergistic effects on antimicrobial activities are discussed. This review also provides an overview of antimicrobial packaging containing ZnO NPs with a focus on preparation methods, antimicrobial mechanisms, and recent progress in packaging applications. The generation of reactive oxygen species (ROS) is the primary antimicrobial mechanism, which can be varied depending on morphology and size. Generally, ZnO NPs can inactivate fungi or Gram-positive and Gram-negative bacteria growth, which reduce the risk of cross-contamination, thereby extending the shelf life of products. Notably, the health concerns and hazards regarding the safety and migration of ZnO NPs application are also elaborated. Unintentional migration, inhalation, skin penetration, and ingestion may result in human health hazards. Therefore, to provide safety regulations, further investigations such as case by case study are recommended.
Introduction
Nanotechnology is being exploited in various fields of science since Richard Feynman introduced it at 1959, and nanoscience has spread out its root in a wide range of applications such as electronic, optical or magnetic devices, biology, medicine, energy, defense, pharmaceutical areas, food, and agriculture industries.[1] Nanomaterials are commonly referred to the materials that possess nanometer-scale, at least in one dimension ranging from approximately 1 nm to 100 nm. Various nanomaterials have been generally considered as zero-dimensional (e.g., nanoparticles (NPs): quantum dots, nanoclusters and fullerenes), one-dimensional (e.g., nanotubes and nanorods), two-dimensional (e.g., thin films), and three-dimensional (e.g., nanocomposites and nanofibers) nanomaterials.[Citation2] Such materials are frequently used in various fields owing to unique mesoscopic properties such as highly reactive materials, appropriate particles size, high surface area, and high ductility and durability.[Citation3,Citation4]
There is a wide range of nanomaterials used in industry, which ZnO NPs are considered as a multifunctional one because ZnO NPs exhibit high antimicrobial efficacy, near-UV emission, piezoelectricity, optical transparency, and electrical conductivity.[Citation5] ZnO NPs are also used in corrosion-protective drug delivery systems, cosmetics, food packaging materials, and biosensors owing to their excellent biocompatibility. The crucial parameters for antibacterial activity of ZnO NPs are dependent on phase, crystallite size, lattice constants, crystal orientation, surface defects, and size.[Citation1] ZnO has been considered as a GRAS (generally recognized as safe) material by the US Food and Drug Administration (21 CFR 182.8991). As such, ZnO NPs are frequently used in food packaging as an active antimicrobial agent.
As reported by the World Health Organization (WHO), over 200 diseases ranging from cancers to diarrhea are caused by hazardous food that contains chemical substances or microorganisms, such as bacteria, viruses, and parasites. It has been estimated that nearly 600 million people (almost 10% of the global population) become ill after the intake of contaminated food, resulting in 420,000 deaths every year (WHO, 2017). Due to such food-borne pathogenic microorganisms, the scientific community has focused research on materials for active packaging (AP) and intelligent packaging (IP), which are upcoming technologies specially designed to prevent the growth of microbes in foods and preserve their safety, quality, and freshness.[Citation6–12] Antimicrobial packaging materials are used to inhibit and control microbial growth, retain moisture, ensure safety, resist liquid or gas penetration, and control the shelf life.[Citation13,Citation14] Such antimicrobial agents are classified as either organic compounds, such as quaternary ammonium salts, halogenated compounds, phenols, chitosan, and chitin, or inorganic materials, including metals and metal oxides.[Citation15] Metals and metal oxides have always been important owing to their excellent antimicrobial activity and high stability of the compounds in this atmosphere. Notably, metal oxide particles do not need necessarily enter the cells to induce toxicity; the particles react even when they come in contact with bacterial cell walls or the abdomen of a crustacean. Accordingly, several modifications to the organism-particle contact surface have been reported to enhance the solubility of metal particles or develop extracellular ROS that damages cell membranes. Recently, with the advent of nanotechnology, inorganic materials like TiO2, ZnO, MgO, and CaO have drawn immense research attention due to their stabilities and resistances under harsh processing conditions and good biocidal behavior against food-borne pathogens despite some are commercially available.[Citation16–19]
There are three plausible scenarios to application of nanotechnology in food packaging such as embedding into food packaging materials, directs-contact, and adding in during food processing. The acceptability and successful execution of nanotechnology in food packaging are dependent on the consumer’s feedback and marketing results. Packaging containing nanomaterials tend to enhance conventional packaging systems regarding to protection and preservation (keep the food inside against microorganism and mechanical stress), communication and market-oriented (provide information regarding to quality and preparatory guidelines), and containment (distribution and handling).
This review provides an overview of antimicrobial food packaging using ZnO NPs with a focus on synthesizing methods, crystal structures, morphology properties, and biocidal mechanisms. To provide a profound understanding of ZnO NPs performance in food packaging applications as well as their antimicrobial and antifungal mechanism, recent progress and future prospects with respect to safety and migration concerns are elaborately discussed.
Zinc oxide nanomaterials
Synthesis techniques
ZnO NPs are commercially synthesized by the following two methods: physical vapor deposition (PVD) processing and mechanochemical processing (MCP). MCP is a facile process in which the starting materials, zinc chloride (ZnCl2) and sodium carbonate (Na2CO3) are ball-milled together to produce zinc carbonate (ZnCO3) and sodium chloride (NaCl).[Citation20] During the process of ball-milling, the ball-milling initiates a low-temperature chemical reaction, resulting in ball powder collision. Next, a chemical exchange reaction occurs due to local heat and pressure at the interfaces. The ball-milled ZnO NPs measure around 20–30 nm. The simplicity and low cost of MCP make it suitable for large-scale ZnO NP production. In PVD processes, at high temperatures, vapors are generated when the solid precursor is subjected to plasma arc energy. Thus, when the precursors are introduced to the plasma, supersaturation and particle nucleation are achieved as a consequence of the plasma arc energy, decomposing the precursors into atoms. Further, upon cooling by expansion through a nozzle or using a gas mixture, these atoms condense to form particles.[Citation21,Citation22] The ZnO NPs produced by this method have an average size of 8–75 nm.[Citation23,Citation24] presents different synthetic techniques, size, and morphologies of ZnO nanomaterials employed previously.
Table 1. ZnO nanomaterials of different size and morphology synthesized by various methods
Recent eco-friendly synthesis techniques will be discussed here to illustrate various techniques for several morphological and structural modifications and apply for antibiocidal systems. Ecofriendly techniques have been developed to prevent the employment of toxic and hazardous chemicals. Thus, green methods using biosources, such as plants, fungi, bacteria, and algae, have been developed.[Citation37–45] Agarwal et al. (2017) reported a broad view towards green synthesis and characterization techniques of ZnO NPs from different biological sources (plant, bacteria, fungus and algae).[Citation46] Synthesis of ZnO NPs used different bio-sources are summarized in . Previous researches have shown that the antibacterial activities of the ZnO NPs are greatly influenced by the particle size, powder concentration, and their photocatalytic properties. Additionally, the photocatalytic behavior of ZnO NPs complements their antimicrobial efficiencies.[Citation47–49]
Table 2. ZnO nanostructure synthesis from various biosources
Crystal structure
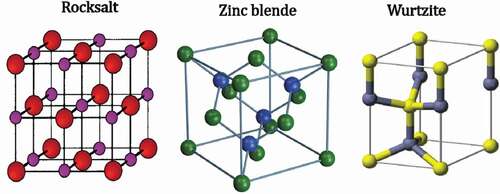
Zinc oxides are non-toxic n-type II–VI semiconductors with a wide and direct band gap of 3.37 eV that usually crystallize in either a hexagonal wurtzite or cubic zinc blende structures. The zinc blende structure consists of anions surrounded by four cations at the corners of a tetrahedron, whereas in the wurtzite structure the cations are surrounded by four anions at the corners of the tetrahedron. The wide band gap is formed as a consequence of the tetrahedral coordination forming sp[Citation3] covalent bonds and the ionic character of the structure. In addition to the wurtzite and zinc blende structures, a rock salt (or Rochelle salt) can be formed; these three structures are shown in . Wurtzite is the most thermally stable and common structure, and the other two forms occur under special conditions. Wurtzite consists of two interpenetrating hexagonal close-packed (hcp) sub-lattices individually containing one type of atom displaced along the threefold c-axis. The atomic terminations facilitate the formation of various novel nanostructures, which broadens the application of ZnO. The crystal lattice growth and morphology are influenced by kinetic parameters, like temperature and pH. Such parameters play a vital role in the growth of facets and hence the crystal structure. Thus, a desired crystallinity and overall surface morphology can be obtained by controlling nucleation.[Citation50–53]
Morphology
ZnO is a multifunctional potential candidate for various applications, well established due to its inherent ability to be stabilized in various nanoscale morphologies, such as combs, rods, wires, flowers, rings, helixes/springs, tetrapods, belts, cages, and prismatic crystals. Concentration and surface area play a vital role in the antibacterial activity of ZnO NPs, while particle shape and crystallinity do not show any significant contribution.[Citation50] Previous reports on the microwave-assisted synthesis of ZnO NPs with varying pH of the precursor solution demonstrated that layered basic zinc acetate (LBZA) was formed at pH 6, whereas at pH 8 and 10, uniform ZnO NPs crystallized. A novel technique to synthesize LBZA via microwave irradiation was adopted.[Citation51] ZnO nanorods/nanowires grown on polycrystalline alumina substrates using Au catalysts had uniform diameters and lengths but were not evenly distributed. The Au catalyst was the key factor determining the diameter of the nanorods, which was observed on the tips of the nanorods. Similarly, aligned nanorods were grown using ZnO nanorod epitaxial growth on ZnO crystal substrates with Sn as catalyst ().[Citation52] Herein, the substrate determined the epitaxial orientation for aligned growth, while the direction of nanorod growth was determined by the Sn catalyst. Using microwave solvothermal synthesis, Wojnarowicz et al. (2016) showed that the water concentration in the precursor solution directly influenced the size of ZnO NPs. Hence, various dimensionally confined morphologies can be formed depending on the synthesis technique.[Citation53]
Figure 2. Different structures of ZnO: (a) flower, (b) rods, (c,d) wires. Images reproduced with permission from reference.[Citation54].
![Figure 2. Different structures of ZnO: (a) flower, (b) rods, (c,d) wires. Images reproduced with permission from reference.[Citation54].](/cms/asset/0b5d14e5-07f1-4147-b41c-5942c154eb2a/lfri_a_1737709_f0002_b.gif)
In addition, hierarchical nanostructures are being developed as an advanced design method for producing novel materials using nanostructures as building blocks. Different surfactants were used to stabilize ZnO NPs homogeneously on cotton fibers with controlled shapes using ultrasound irradiation for antimicrobial activity by El-Nahhal et al. (2017).[Citation55] Functionalized surface coatings of Al-doped ZnO nanostructures were sputter-deposited with various sputtering powers onto poly(lactic acid) (PLA) films.[Citation56] The development of strategies that involve the synthesis of nanomaterials with unique properties resulting from the combined effect of structural and morphological modifications has attracted much interest. For example, Verrier et al. (2017) comprehensively investigated the influence of pH on the morphology and doping properties of ZnO nanowires (NWs) grown using chemical bath deposition using zinc nitrate Zn(NO3)2, hexamethylenetetramine (HMTA), and aluminum nitrate Al(NO3)3.[Citation57] For a fixed concentration of Al(NO3)3 by varying the pH, various ZnO nanostructures, such as nanopencils and nanoneedles, were obtained, which provided a comprehensive idea about the influence of post-deposition annealing conditions and the pH effect of the synthesis medium on the shaping of ZnO nanostructures and the process of extrinsic doping. For effective integration of dopants in ZnO, apart from adding Al-based precursors to the aqueous solution, it is necessary to carefully select the pH and the post-deposition annealing conditions.
Antimicrobial activity and its mechanism
In general, when the particle size decreases its antibacterial activity increases. The antibacterial activity of ZnO is a consequence of visible light irradiation and its direct contact with the microbial cell wall resulting in destruction of bacterial cell integrity, release of antimicrobial Zn2+ ions, and generation of ROS.[Citation58,Citation59] In addition, ZnO NPs (70 nm) suspended in solutions at various concentrations were evaluated against Escherichia coli (0157:H7), and increased inhibition on the growth of E. coli was observed with increasing ZnO NPs concentrations.[Citation60] Sawai et al. (1998) observed that ZnO had superior antibacterial activity for Staphylococcus aureus compared to MgO and CaO.[Citation61] Jin et al. (2009) demonstrated that ZnO NPs showed superior antibacterial activity against Listeria monocytogenes and Salmonella enteritidis in liquid culture media and egg white when different forms of ZnO, such as powder, film, polyvinylpyrrolidone (PVP)-capped, and coating, were evaluated. Evaluated the bacterial growth rate (Pseudomonas spp.) using the colony count method, and it was observed that ZnO nanoparticles reduced the growth rate and damage to the bacterial cell membrane.[Citation62] Another study by Li et al. (2011) substantiated that ZnO NPs showed better antimicrobial activity against food-borne pathogens, such as Bacillus cereus, E. coli, Staphylococcus aureus, and S. enteritidis, when compared with bulk ZnO powder.[Citation63] Recently, poly(butylene succinate) (PBS)/ZnO composite was shown to have considerable antibacterial activity against E. coli and S. aureus.[Citation64] Using a green synthesis method for preparing ZnO NPs as using Cassia fistula plant extract as a fuel for solution combustion synthesis. As such, Suresh et al., (2015) evaluated the NPs against Gram-positive and Gram-negative bacteria, and the NPs showed inhibition against pathogenic bacterial strains.[Citation65] Essential oils have been shown to be potential antimicrobial agents in PBS films, but their strong odors limit their use with food products. The inhibitory action of carvacrol oil against S. aureus and E. coli was analyzed and reported by Wiburanawong et al. (2014), in which the authors noticed that its acceptance was limited due to its bad odor.[Citation66] However, ZnO was introduced to this PBS film to minimize the bad odor, and the end product was found to exhibit better antibacterial activity with no odor from volatiles detected.[Citation67–69]
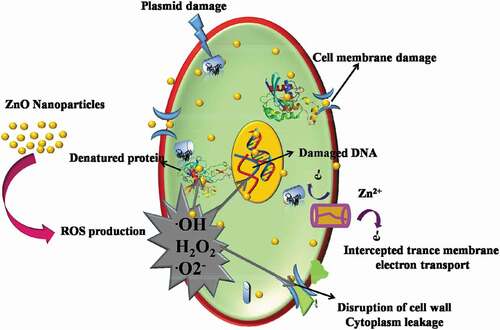
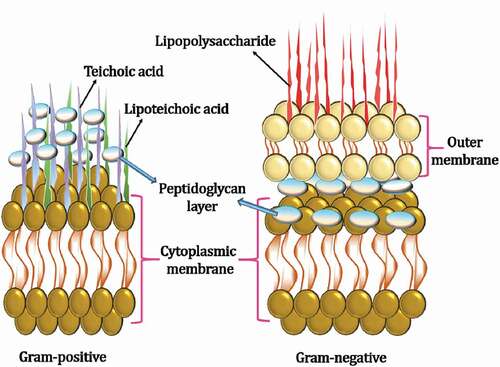
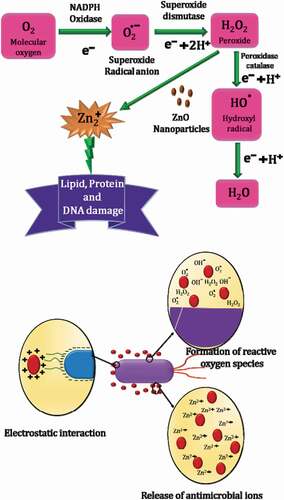
shows that the oxidative stress generated by ZnO NPs has a large effect on their antibacterial activity. Formation of Zn2+ ions from ZnO inhibited the actions of respiratory enzymes by interacting with them. The ability of ZnO NPs to damage the cell membrane and generate ROS has already been demonstrated. Thus, irreversible damage to the bacterial cell membrane, DNA, and mitochondria occurs by absorbing the Zn2+ ions, leading to ROS formation and free radicals that cause oxidative stress and inhibit the action of respiratory enzymes and finally cell death. A schematic representation of the destruction of bacteria by ZnO NPs is shown in . Acinetobacter baumannii, an opportunistic pathogen that causes human pneumonia and meningitis, was used to analyze the effectiveness of ZnO in combination with conventional antibiotics, such as ciprofloxacin and ceftazidime.[Citation70] From these studies, it was found that for both ciprofloxacin and ceftazidime, ZnO NPs in a sub-inhibitory concentration exhibited enhanced antibacterial activity. This was ascribed to the increased uptake of antibiotics and change in the morphology of bacterial cells due to the presence of ZnO NPs. Sarwar et al. (2016) examined the antibacterial activity of ZnO NPs against Vibrio cholera (a causative agent of severe watery diarrhea).[Citation71] Depolarization of cell membranes, deformation of cellular architecture, and increased fluidity and protein leakage were found to be induced by the ZnO NPs. DNA damage and ROS production were also observed. Hence, the combination of ZnO NPs and antibiotics was suggested as an alternative treatment for bacterial infections. The synergistic effect of ZnO NPs and the photosensitizer crystal violet (CV) against bacterial cells was investigated under white light, showing lethal photosensitization activity against Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria. The antimicrobial behavior of ZnO NPs against various Gram-negative and Gram-positive bacteria depends on the interfacial potential between them. When bacteria with negative surface potentials interact with the positively charged ZnO surface, the production of reactive oxygen is enhanced by mechanical stress, resulting in membrane depolarization. In comparison with Gram-positive bacteria, a higher negative potential is exhibited by Gram-negative bacteria owing to the existence of an additional lipopolysaccharide layer with a negative potential (). The cell membrane of S. aureus that was less negatively charged than the cell membrane of E. coli was also found to be a reason for the enhanced resistance against ZnO NPs activity in E. coli.[Citation72] Gordon et al. (2011) reported that negatively charged free radicals, such as peroxide ions, superoxides, and hydroxyl radicals, can penetrate more effectively to cause cell death and damage in S. aureus, even at lower concentrations than required for E. coli.[Citation73] Similarly, to illustrate the differences in antibacterial activity of ZnO towards Gram-positive and Gram-negative bacteria, different mechanisms have been proposed. However, more studies are required to explain the exact reason for the sensitivity of these bacterial cells to ZnO NPs.
Further, surface area, particle size, and synergistic activity with other antimicrobial agents influence the antimicrobial activity of ZnO NPs. Since the particle size has a large impact on the functional activity of nanoparticles, ZnO NPs with smaller particle size exhibit improved antibacterial activity against S. aureus and E. coli.[Citation74] This may be ascribed to the increased surface area-to-volume ratio of ZnO NPs, leading to increased reactivity, since H2O2 generation strongly depends on the surface area.[Citation75] As the surface area increases with decreasing particle size, greater number of ROS are generated on smaller ZnO particle surfaces. schematiclly shows the role of ZnO NPs in the generation of ROS.
In addition, theoretical studies have shown that smaller particles should have enhanced toxicity for fungi and bacteria. However, this toxicity can also be ascribed to various other factors, such as particle morphology, surface chemistry, concentration of the microorganism, and light intensity.[Citation76] Consequently, control of such external factors should also be included in the studies to clearly understand the effect of particle size on the toxicity of ZnO NPs. In addition, the synergistic antimicrobial effects of ZnO when combined with other antimicrobial agents have attracted considerable interest.
Antimicrobial food packaging
Antimicrobial packaging is the packaging system that inhibits, inactivates or kills pathogens and spoilage microorganisms contaminating foods.[Citation77] Antimicrobial food packaging is particularly designed to control microorganism growth for shelf-life extension, maintaining quality, and safety insurance of food products. The antimicrobial function can be achieved by adding antimicrobial agents into the packaging system for indirect food contact or introducing antimicrobial agent into polymers for food contact items.[Citation78] Methods of preparing antimicrobial packaging can be divided into different types as follows:
Preparation methods
Solvent casting
Owing to better mixing efficacy and lower required temperature compared with melt casting method, solution casting method can be used for polymer composite preparation.[Citation79] Appropriate mixing method results in higher tendency to metal particles, thereby better particles dispersion. Off-gassing process is a challenge regarding to solution casting method, leading to the solvent loss and solvent recovery limitation. In this system, well-dispersion could be achieved upon degree of particle disbursement can be optimized. Such a method is widely used for optical films, medical films sheet (electronic applications), and engineering plastic.[Citation79]
Coating method
Polymer coating can provide materials with advanced performance through printing and painting techniques. It can be prepared by coating inorganic compounds on organic substrate. Coating method tends to stabilize the substrate materials to prevent adverse side-interactions with environmental factors or improve the surface properties such as conductivity, magnetic, adsorption, and optical perfectness. The coating mechanism is based on the interaction of performed polymers with inorganic cores or adsorption of monomers on the surfaces using a direct polymerization. The chemical tendency between materials involved in coating process is critical in both methods. Appropriate interface adhesion between coatings and substrate is also exploited for high-quality materials. Depending on the coating methods, some advanced properties can be achieved such as mechanical strength, barrier properties, chemical and abrasion resistance as well as biocompatibility and wettability. Coating method is commonly used in packaging materials like coating antimicrobial agents on polymers. In general, coating antimicrobial agents can be released from packaging materials to direct contact to suppress microbial growth. Low-density polyethylene (LDPE), chitosan, and methyl cellulose are widely used as antimicrobial packaging coatings. In addition, coating methods can be also used for packaged liquid food. As such, the ZnO NPs were applied in various attempts to achieve antimicrobial coating packaging nanocomposite.[Citation80–82]
Extrusion method
Melt extrusion method was firstly used to preparation of lead pipes at the end of the eighteenth century. As such, this method has been frequently used in food preparation industry, plastic, and rubber to fabricate various materials such as bags, sheets, and pipes. Extrusion is a technique to prepare the uniform shape and density materials through blending raw materials under controlled temperature and pressure through a heated barrel. In addition, hot melting process converts and compacts the blends such as granular materials or powders into a uniform shape. Accordingly, polymer resins are melted and then reshaped into new products with different shapes and sizes such as bags, sheets, and pipes through compacting polymers or active substances including additive or plasticizers by controlling pressure, temperature, screw speed, and feeding rate. Extrusion, the major manufacturer method for plastic, is widely used in pharmacology, food industry, and plastic manufacturer owing to solvent-free, time-effective, and non-ambient process. Several studies were utilized the extrusion method to prepare antimicrobial packaging containing ZnO NPs.[Citation64,Citation83,Citation84]
Injection molding
Injection method as a manufacturing process can be used for thermoplastic and thermosetting products such as plastics, metals by heating the materials to a fluid state and injecting into molds. There are three operation steps involved with injection methods: (i) heating materials to plasticizing or injecting units to flow under pressure, (ii) allowing melting materials to solidify in the mold, and (iii) ejecting the molded products. For example, the molting metals or plastics can be injected into the molds with internal cavities to solidify parts and reshape the under a pressurized condition. Accordingly, molds may include single cavity, several cavities or dissimilar cavities, in which cavities can be connected to flow channels or runner, which can let the melts to flow to cavities. Such a method can be applied for preparation a wider products such as automotive body parts, plastic trinkets, cell phone cases, toys, containers, and water bottles.[Citation85–87]
Antimicrobial food packaging materials incorporated ZnO NPs can be divided into two types: bio-based nanocomposite and petroleum-based nanocomposite. The representative samples of antimicrobial food packaging are summarized in .
Table 3. Antimicrobial food packaging
Bio-based nanocomposite films
Numerous research attempts have been directed to develop the antimicrobials packaging films because such nanocomposite films were prepared from bio-based materials such as gelatin, chitosan, carboxymethyl cellulose (CMC), PLA, PBS, which are environmental friendly, non-toxic, sustainable, and degradable.[Citation88–92,Citation99] The bio-based polymer is a biodegradable plastic that degrades due to the action of living organisms such as microbes and fungi. A bioplastic can be defined as a polymer that is manufactured into a commercial product from natural sources or renewable resources. Bio-based polymers tend to replace petroleum-based polymers or may obviate serious issues caused by the overuse of petroleum-based polymers such as water and soil pollution, human health hazards, and overdependence on petroleum. It can be also claimed that current drawbacks of bio-based polymers, both in the economic and performance, preclude their rapid adoption at least in the near future. The biobased polymer evaluation is performed on six parameters (cost, recyclability, mechanical properties, ease of processing, barrier properties, and biodegradability), and a scoring system for this evaluation was developed, where the score of 5 denotes the best performance and 1 denotes the worst. Biopolymers are not restricted to packaging application, they are being also used in biomedical applications such as pharmaceutical, medical device coatings, and resorbable implants that require biocompatibility and nontoxicity. Biobased polymers are being used in the packaging industry in subsegments, whose biodegradation is of importance and oxygen permeability is of lower priority. Such an application includes compostable waste bags and packaging film for foods with a short shelf life as well as compostable pouches, plates, and cutlery. Biopolymer with good degradability also renders it appropriate for deployment in the agriculture and forestry industry as mulch film, nets, and trappings. Biopolymer high potential to be deployed into the medical industry. These plastics present better processability, lower toxicity profile than those of petroleum-based polymers.[Citation99] Several researches investigated the effects of ZnO NPs on bio-based nanocomposite films for extending shelf life of food products as follows.
Ejaz et al. (2018) prepared bovine skin gelatin (BSG) composite films incorporated with 2% zinc oxide nanorods (ZnO NRs) (<100 nm) and clove essential oil (CEO) using a solvent casting method, and applied for shrimp packaging. The refrigerated storage test showed that the BSG/CEO/ZnO NRs composite films with 50% CEO exhibited maximum antibacterial activity against L. monocytogenes and Salmonella Typhimurium. This result indicated that the developed BSG/CEO/ZnO NRs film could be utilized as active packaging for peeled shrimp.[Citation88] Amjadi et al. (2019) also prepared bio-nanocomposite packaging from gelatin containing nanofiber (10%) and ZnO NPs (30 nm and 5%) using solution casting method. The bio-nanocomposite exhibited strong antimicrobial activity against food-borne microorganisms such as S. aureus, E. coli, and Pseudomonas aeruginosa. Chicken fillet and cheese were used as food modeling tests in which wrapping the active bio-nanocomposite film remarkably reduced the number of inoculation bacteria in chicken fillet and cheese samples. The bio-nanocomposite also maintained the organoleptic properties of chicken fillet and cheese during storage.[Citation89]
Saekow et al. (2019) prepared a CMC coating material containing ZnO NPs (0%, 5%, 10%, 20% and 40% w/v) and pineapple extracts to evaluate antifungal activity in persimmon and tomato fruits. The coatings significantly reduced the number of black spots and fungal proliferation (Alternaria alternata) in the fruits. The organoleptic evaluation proved that CMC containing ZnO NPs effectively maintained the quality of fruits during storage in which the size of ZnO NPs was 25–55 nm.[Citation82] Mohammadi et al. (2019) fabricated packaging bio-nanocomposite derived from CMC, okra mucilage (OM), and ZnO NPs (0.5%) to extend the shelf life of chicken breast meat during storage at 4°C. The antimicrobial efficacy of bio-nanocomposite was investigated using total viable counts (TVC), S. aureus counts, and Lactic Acid Bacteria (LAB) count of chicken breast during storage. The film containing ZnO NPs exhibited the large inhibition zone against microorganisms as well as the lowest chemical and organoleptic changes in the chicken breast during storage.[Citation90]
Youssef et al. (2016) prepared a novel chitosan/CMC/ZnO bio-nanocomposite film by solution casting method, and the effects of different ZnO NPs contents (2%, 4%, and 8 wt%) on the shelf life of Egyptian soft white cheese were investigated. The bio-nanocomposites exhibited good antibacterial activity against bacteria (S. aureus, P. aeruginosa, and E. coli) and fungi (Candida albicans). As such, the antibacterial activity of the composite film increased with increasing ZnO NPs content. In addition, the storage tests (7°C for 30 days) indicated that the bionanocomposite packaging films enhanced the shelf life of white soft cheese.[Citation91] Noshirvani et al. (2017) also fabricated carboxymethyl cellulose-chitosan-oleicacid (CMC-CH-OL) containing ZnO NPs (<25 nm) in different concentrations ranging 0.5%, 1% and 2% to extend the sliced wheat bread. To provide the maximum contact between bread and active film, the CMC-CH-OL containing ZnO NPs was coated on the bread surface. The ZnO NPs and oleic acid significantly reduced the water vapor transmission rate (WVTR) of film. This active packaging maintained the quality of bread including microbial and staling properties in which this active packaging reduced the yeast and mold number. Further, Differential Scanning Calorimetry (DSC) analysis presented that this active packaging delayed the staling rate of bread.[Citation92] Indumathi et al. (2019) also prepared a biodegradable film including chitosan (CS)-cellulose acetate phthalate (CAP) containing ZnO NPs (2%, 5% and 7.5% with 30 nm) using solution casing method. The results reported that CS-CAP containing 5% ZnO NPs showed the optimal level of mechanical, thermal, and UV-protective properties. In addition, CS-CAP containing 5% ZnO NPs exhibited strong antimicrobial activity against E. coli and S. aureus and extended the shelf life of black grapefruit up to 9 days.[Citation93]
Petchwattana et al. (2016) prepared PBS/ZnO composite films for food packaging applications. The composite films containing difference ZnO concentration (2 to 10 wt%) were prepared using a blown film extruder. The size of ZnO was maintained at 10 nm. The antimicrobial activity reported that minimum ZnO NPs content required to inhibit E. coli and S. aureus was 6 wt.% with the inhibition zone of 1.31 and 1.25 cm, respectively. The tensile test showed that introducing ZnO into PBS film resulted in an increase in the tensile strength but a decrease in the elongation at break.[Citation64]
Haydary-Majd et al. (2019) developed the PLA/ZnO NPs containing essential oil including Zataria multiflora Boiss and Menthe piperita using solution casting method. The nanocomposite film showed strong antimicrobial efficacy against five common foodborne microorganisms such as E. coli, Salmonella enterica, Pseudomonas aeruginosa, Bacillus cereusand, and S. aureus. PLA/ZnO NPs containing essential oils significantly maintained the shelf life of Otolithesruber fish during storage. They also reported that Zn2+ migration was lower than standard limit (National Institute of Health for food contact materials).[Citation94]
Baek and Song (2018) prepared Gracilaria vermiculophylla extract (GVE) films containing ZnO NPs for smoked salmon packaging. Gracilari avermiculophylla (GV) was extracted by modifying method, and GVE films containing different concentrations of ZnO NPs (1%, 3% and 5%) and plasticizers (glycerol and sorbitol) were prepared using film-forming solution. With increasing ZnO NPs in GVE films, the antibacterial activity of the films against pathogenic bacteria (L. monocytogenes and Salmonella Typhimurium) increased, and light-blocking property was improved. Further, the storage test indicated that the smoked salmon packed with the GVE film containing 3% ZnO NPs showed the strong antibacterial activity against pathogenic bacteria and lower degree of lipid oxidation compared with control, indicating that this nanocomposite could be applied as an active food packaging material for smoked salmon.[Citation95] Kumar et al. (2019) prepared agar-ZnO nanocomposite films using solution casting method and applied as an active packaging material for extending shelf life of green grape. In this study, the ZnO NPs were synthesized using Mimusopselengi fruit extract in polyhedral shapes (mostly hexagonal) with the size of 14–48 nm (average size of 24.75 ± 0.78 nm). Introducing ZnO NPs into composite films improved thermal stability and elongation at break of the composite films, whereas decreased tensile strength and transparency. Storage test indicated that grapes packaged in nanocomposite films containing 2% (w/w) and 4% (w/w) of ZnO NPs showed fresh appearance up to 14 and 21 days in the ambient condition, respectively. Therefore, the agar-ZnO nanocomposite films enhanced the postharvest life of fresh fruits like green grapes.[Citation96]
Jafarzadeh et al. (2018) investigated the application of antimicrobial active packaging film containing semolina flour, ZnO (rod type, 50–100 nm in diameter and 0.5–2 μm in length) and nano kaolin for mozzarella cheese storage. The semolina-based nanocomposite containing different ratios of ZnO NPs and kaolin was prepared using solution casting method. As such, introducing ZnO NPs into nanocomposite films exhibited strong antimicrobial activity against bacteria (E. coli and S. aureus), yeast C. albicans, and mold Aspergillus niger (A. niger). The long-term storage test (72 days) indicated that mozzarella cheese packed with semolina-based films containing ZnO NPs inhibited the growth of microorganisms and maintained the sensory properties of cheese.[Citation97]
Petroleum-based nanocomposites
Petroleum-based nanocomposites are being continuously developed as antimicrobial food packaging because petroleum-based materials possess high thermal stability, high tensile strength, appropriate flexibility, conventional processability (blow-film extrusion or cast film).[Citation100,Citation101] The petroleum-based polymer industry represents the major end use of many petrochemical monomers such as ethylene, styrene, and vinyl chloride. Petroleum-based materials have greatly affected our lifestyle because they are not expensive. The major use of the plastics is in the packaging field. Finding an alternative (biobased polymers) for the petroleum-based plastics is impartial because conventional plastics are unsustainable (due to environmental problems) and consume 65% more energy, and emits 30–80% higher greenhouse gases compared with bioplastics. Some petroleum-based materials such as LDPE, polypropylene (PP), and polyurethane (PU) are commonly used to prepare antimicrobial food packaging incorporated with ZnO NPs.
Emamifar and Mohammadizadeh (2016) prepared LDPE/ZnO NPs nanocomposites for extending shelf life of fresh strawberries. The nanocomposite films with difference ZnO NPs contents (1%, 3% and 5%) and the nanocomposite film with 3% ZnO NPs and 10% polyethylene-grafted maleic anhydride (PE-g-MA) were fabricated using twin-screw extruder. TEM analysis indicated that nanoparticles are well dispersed in the LDPE containing PE-g-MA with aggregation ranging from 10 to 20 nm. With increasing the ZnO nanoparticle up to 5%, the antimicrobial activity of the film increased. Further, all packaging films containing ZnO NPs maintained the microbial number of fresh strawberries below the level (5 log CFU/g) that can keep the shelf life during storage (16 days).[Citation83]
Polat et al. (2017) fabricated PP nanocomposite containing ZnO NPs (70 nm) using blown film method. The different contents of ZnO NPs (0.5%, 1%, 3% and 5%) were incorporated into PP to extend the shelf life of fresh lemon juice. The nanocomposite showed higher barrier, mechanical, and antimicrobial properties. Further, the storage test proved that PP containing ZnO NPs significantly extended the shelf life of fresh juice lemon. In this study, masterbach was prepared using twin-screw extruder, and migration rate increased with increment of nanoparticles content in film matrix.[Citation84]
An environmental-friendly nanocomposite film was developed from Mahua oil-based PU/chitosan containing ZnO NPs (1%, 3% and 5% with 30 nm) using solution casting method to extend the shelf life of carrot. The introducing ZnO NPs improved barrier properties, hydrophobicity, and antibacterial properties of film. This nanocomposite showed strong inhibition zone against E. coli and S. aureus. Further, wrapping the sliced carrot with this active packaging extended the shelf life up to 9 days.[Citation93] In this study, Mahua oil-based polyurethane was synthesized using epoxidation method followed by hydroxylation. summarizes the patents on the material structure application of active antimicrobial packaging
Table 4. Patents on the material structure application of active antimicrobial packaging
Safety issues
Migration
The ZnO NPs can improve the mechanical, barrier, and antimicrobial properties of packaging nanocomposites. On the other hand, nanoparticles are prone to migrate through packaging in/onto the food items, which can be attributed to nanomaterial characteristics (concentration, size, shape, and dispersion), environmental factors (mechanical stress, temperature, etc.), food condition (pH and composition), polymer properties (viscosity and structure) and contact duration. In general, there is no comprehensive protocol or standard to investigate the migration in the packaging nanocomposites because migration is dependent on aforementioned parameters. Therefore, to minimize the migration and its impacts on the human body and food quality, packaging nanocomposite should be evaluated case by case.
Migration is described as an unintentional or unexpected transformation of organic or inorganic substances contact into food materials. Migration in the food is categorized into three main types as follows: (i) Overall Migration Limit (OML) measures the total amount of all non-volatile substances that can migrate into food, (ii) Specific Migration Limit (SML) is a concentration of the specific substance in food simulant, which is evaluated using advanced detecting assays, and (iii) maximum permitted quantity (QM) measures the level of the residual substance in food contact materials. The OML is commonly used for inertness of the substances, which can be applied for polymers based on the 60 mg/kg of food (or food simulant) or 10 mg/dm2 expressed on a contact area. The articles No (EU) 10/2011 from Plastics Regulation and No (EU) 2016/1416 from European Commission published Commission Regulation have imposed the 5 to 25 mg zinc per kg food (25 to 5 mg/kg food) for food contact items based on the SML consideration. In addition, National Institute of Health for food contact materials imposed the 40 mg/day on zinc daily consumption for human body.[Citation102] Accordingly, Aristizabal-Gil et al. (2019) recorded that the alginate nanocomposite containing 5 g/L ZnO NPs or ZnO/CaO NPs crossed the maximum migration limit, whereas, the nanocomposite containing 0.5 g/L ZnO NPs remained in the approvable level of migration.[Citation103] Bumbudsanpharoke et al (2019) evaluated the migration of Zn2+ from LDPE-ZnO nanocomposite films, in which the level of migrated Zn2+ (3.5 mg L − 1) was lower than the specific migration limit based on European Plastics Regulation. Thus, the migrated level was considered as non-toxic level for human health.[Citation3] Heydari-Majd et al., (2019) reported that the level of migrated zinc in fish fillet wrapped by PLA bionanocomposite increased slightly during storage time. Whereas, the level of migrated Zn2+ increased in the presence of essential oil in the nanocomposite despite the migrated level was still below the maximum migration limit based on National Institute of Health for food contact materials.[Citation94]
Toxicity of ZnO nanoparticles
ZnO nanoparticles either directly diffuse into the cell or intrude by endocytosis, thereby inducing the generation of reactive oxygen species (ROS) and oxidative stress, causing damage to the biological system. The size of ZnO NPs is another factor that needs to be considered as the large surface areas results in high surface reactivity. The biological impacts of ZnO NPs include lipid peroxidation, protein impairments, organelle dysfunctions, inflammation, and DNA damage. Generally, during metabolism, the cells are inherently endowed with antioxidants to normalize the generated ROS.[Citation104] However, in the presence of ZnO NPs, the ROS generation exceeds the normal capacity of cellular antioxidant machinery. Moreover, such ZnO NPs behave as pro-oxidant molecules to induce oxidative stress either by generating ROS or by inhibiting antioxidant molecules. The neurotoxicity of ZnO NPs arises from the high generation of ROS by disturbing metal-ion homeostasis due to an intracellular increase in dissolved free zinc ions. This high concentration of Zn2+ ions primarily contributes to elevated levels of zinc ions, leading to ZnO NP-induced cytotoxicity and hence, oxidative stress and inflammation.[Citation105]
Cytotoxicity severity is dependent on the physicochemical properties of the NPs, such as chemical composition, size, shape, aggregation, surface chemistry, and surface energy. The physicochemical characteristics of NPs, such as being sufficiently small to penetrate the blood–brain barrier and their large surface area can promote their neurotoxicity.[Citation106] Various studies have shown that ZnO NPs with various shapes, such as rods and spheres, probably cause neurotoxicity due to their ability to access the brain. Moreover, the olfactory brain route has also been reported as a potential route for the transfer of ZnO NPs to the brain. However, information regarding the neurotoxic effects of ZnO NPs is scarce. Therefore, understanding the effects of nanomaterial exposure, including neurotoxicity, is important for ensuring the safety of nanomaterials, particularly when the particles are used in biomedical applications.[Citation107]
The intrusion of ZnO NPs at the intracellular level disrupts metal homeostasis, eventually leading to the modification of enzymatic activity. Translational and transcriptional processes occur due to the perinuclear localization of these ZnO NPs. Several mRNA-stabilizing enzymes have metal-responsive domains that get activated in the presence of metal ions, thereby disturbing transcriptome. Metal nanoparticles like gold are capable of directly interacting with DNA to alter the gene expression profile of cells by inducing oxidative stress.[Citation108] It has been reported that foods packed using ZnO NPs contain ZnO NPs 100 times higher than the recommended values. Therefore, toxicity and safety measures while employing ZnO NPs for active food packaging are equally important, as ZnO NPs can reach the organs via inhalation, parental routes, and ingestion.[Citation109]
Impact of nanomaterials on human health and safety
The development of nanoscience and nanomaterials is steadily progressing. Developing solutions for the many limitations of nanomaterials is extremely important. Reports show that nanomaterials enter organisms in three different ways: inhalation, skin penetration, and ingestion. NPs are capable of crossing cellular barriers, resulting in inflammatory reactions and oxidative damage. In the food packaging industry, consumers are indirectly prone to nanomaterial consumption due to the migration of the materials from the packaging to the food. In addition, workers could suffer from inhalation and skin penetration. Personal protection, e.g., the use of masks with filters, glasses, and gloves, is necessary and highly recommended for these workers. Thus, better understanding of the transmission of these nanomaterials is necessary, beginning from their detachment and migration to when they enter the human body. In addition, after entering the body, the reaction between different organs and the NPs, their metabolism, and methods to eliminate them from the body need to be understood in detail while the literature contains very few reports of such issues. Notably, in some cases, the NPs embedded in packaging films positively influence and prevent the migration of chemicals into the food. In this regard, nanoclays slowed the migration of 5-chloro-2-(2,4-dichlorophenoxy)phenol (triclosan), trans,trans-1,4-diphenyl-1,3-butadiene (DPBD), and caprolactam from the polyamide matrix by up to six times. Risk assessments of a few nanomaterials in the food packaging industry have been reported (e.g., TiO2, Ag NPs, and carbon NPs/nanotubes), which showed that they could be taken into circulation from the gastrointestinal tract. The physicochemical properties, size of the NPs, and physiological state of the organs play a vital role in all these associated processes. Upon reaching the blood circulation system, the liver and spleen further distribute the nanomaterials, and if the NPs have positively charged surfaces and are hydrophilic in nature, the circulation time increases. In this case, it was reported that all organs are at risk, including the brain, testes/reproductive system, and fetuses in utero, where remains of the chemical components have been found. Specifically, detailed knowledge of the lifetime of the NPs and their impact on secondary organs due to chronic exposure after accumulation is poorly understood and requires extensive further research. Likewise, the effects of other emergent NPs for food packaging are being investigated, including ZnO NPs and fullerenes.[Citation110]
Currently, it is necessary to evaluate the safety of NPs used in food packaging for their potential impact on consumers (with respect to food safety and quality), as it is yet another threat. Several research groups are actively investigating the lifetime and the deposition of NPs from the packaging materials and their negative impact on the safety and quality of the packed food. Chaudhry et al. (2008) and Bradley et al. (2011) have demonstrated that the increased surface area of NPs greatly affects the biological and physicochemical properties compared to bulk particles.[Citation111,Citation112] Thus, to analyze the risk of NPs, three basic strategies are essential: in vivo and in vitro assays and physicochemical characterization.[Citation113] Espitia et al. (2012) examined ZnO NPs after consumer exposure and demonstrated that they are toxic towards eukaryotic (healthy) cells and carcinogenic ones.[Citation20] In addition, as in-vivo experiments are ethically questionable, expensive, and slow when performed without compromising the efficiency and reliability of the risk assessment, in-vitro assays have been widely reported.[Citation20] Owing to the correspondingly larger specific surface areas and reactivity, nanomaterials are considered to have more adverse effects on organisms than microscale materials. Cytotoxic effects of ZnO NPs on different cell types have been reported, including human kidney cells, human lung epithelium cells, and human bronchial epithelium cells.[Citation113,Citation114] The cytotoxicity effect of ZnO NPs on healthy cells was attributed to elevated oxidative DNA damage and oxidative stress. However, the effects of ZnO on human cells are still not well understood. In addition to these issues, several reports have suggested that ZnO NPs do not enter normal cells or penetrate the human or animal skin.[Citation20,Citation115]
The required level of zinc is naturally obtained from diet in which most human body takes the required zinc from natural food sources in the zinc oxide form. Zinc trace is a crucial factor for a wide range of biological and chemical activities in body such as immune function, wound healing, blood clotting, and thyroid function. Recommended dietary allowances (RDAs) for Zinc 0–6 months old 2 mg for male and female, 7–12 months old 3 mg for male and female, 1–3 years old 3 mg for male and female, 4–8 years old 5 mg for male and female, 9–13 years old 8 mg for male and female, 14–18 years old male 11 mg and female 9 mg, 19+ years old 11 mg for male, 8 mg for female and 11 mg for Pregnancy. However, higher concentration of zinc is prone to be harmful to human health. Zinc toxicity has consisting two steps: (i) acute and (ii) chronic form. Inhalation is the main way to enter the zinc oxide into human body, which can lead to lung damage through welding activities (metal fume fever). Reduction of copper-containing enzyme and marker of cupper status depends on the high zinc intakes of 60 mg/day for up to 10 weeks.[Citation116] The doses of zinc used in the Age-Related Eye Disease Study excessive amount (80 mg per day of zinc in the form of zinc oxide for 6.3 years) of zinc intakes significant increase in hospitalizations for genitourinary causes and adversely affect a urinary system of physiology. Zinc oxide also can enter to the human body through skin. Accordingly, cosmetic products are prone to facilitate this entrance because of frequent application of zinc oxide in the cosmetic products.
Future prospects and challenges
As mentioned earlier, owing to the presence of high surface to volume ratio, nanomaterials are capable to enhance the packaging performance regarding the shelf life of fresh food products. As such, numerous attempts have been conducted to prepare antimicrobial packaging based on the ZnO NPs because of excellent properties. The progressive trend in application of antimicrobial packaging nanocomposites in the food industry illustrates the matter of such systems in the upcoming years.
The next generation of packaging can be aligned with polymeric nanocomposite containing active agents, in which ZnO NPs could play a key role because of strong antimicrobial activities. Accordingly, addition of appropriate amount of ZnO NPs can provide the sufficient antimicrobial activity in the packaging. It is reported that the lower of 5% nanofillers in the biopolymers can give excellent properties to packaging. In addition, ZnO NPs can improve other properties of packaging such as mechanical, barrier and thermal. Compared with some nanoparticles such as silver, titanium oxide, ZnO NPs are inexpensive, which can receive much more attention in the packaging industry.[Citation84] Thus, the application of packaging nanocomposite containing ZnO nanofillers possesses strong application potential for perishable or short shelf-life products such as fresh juice,[Citation84] vegetable or fruit and meat-based products.[Citation82,Citation117] The multifunctional properties can be expected from ZnO NPs such as antimicrobial activity as the primary property as well as light blocking, barrier, and thermal as the secondary properties.[Citation118] Packaging disposal, as a main waste in landfill, is still a big challenge in the waste management so that active packaging bionanocompsoite can not only prolong the shelf life of fresh food but also reduce the food packaging disposal. Accordingly, antimicrobial bio-packaging containing ZnO NPs, particularly, synthetic biopolymers such as PLA, polycarprolactone, polyglycol acid, polyvinyl alcohol, PBS, and polybutylene adipate terephthalate could be precisely investigated. Besides, natural biopolymers (cellulose, gelatin, chitosan, starch, etc.) are environmentally compatible materials, which could be frequently investigated to achieve excellent antimicrobial packaging based on ZnO NPs.[Citation119]
Despite, ZnO NPs tend to enhance biocidal efficacy in the packaging materials, there are still concerns regarding the migration and toxicity. The available information regarding the ZnO trace in the food packaging and their impacts on human body are not still sufficient. The ZnO NPs are described as a low toxicity and biocompatible material, which human body needs Zn[Citation2] trace.[Citation120] It has been highly recommended to consider the six main factors dealing with nanomaterial migration from food contact materials including migration rate, mechanism of migration, how to analyze the trace, how to predict migration modeling, appropriate food simulant, and risk of the migration.[Citation121] Such factors tend to enlighten all scenarios involved in migration, safety, and possibility to mitigate or address the side-effects.
There are no global safety regulations and considerations regarding to food contacts nanomaterials. Because physiochemical properties of macro-scale and nano-scale materials are different, it is strongly required to consider new regulation for antimicrobial nano-agents, particularly, ZnO NPs as a frequently used biocidal material. The safety considerations tend to change the purchasing behavior based on the safety and security. The important considerations required to make a comprehensive regulation in nanomaterial application are as follows: (i) It has been recommended that to achieve a reliable migration detection protocol, analytical methods should be conducted such as sp-ICP-MS and TEM-EDX characterization can detect the nanomaterial trace in the low amount because of high accurate detection. (ii) As the chemicals used in the food simulants are not chemically same with food ingredient, thus it is necessary to use the chemicals and procedures with the maximum similarity with food content materials. (iii) Nanomaterials used in food contact items have not particularly considered regarding to adverse impacts on human body. Care must be taken to consider human body entrance pathways such as inhalation, skin penetration, and ingestion. Therefore, the comprehensive regulation can guild the workers, manufacturers, consumers to minimize the risk.
The global market for antimicrobial packaging is continuously increasing, which was 6.51 million tons in 2015 to 10.09 million tons by 2024 (11 billion USD). The nano zinc oxide has also received much attention, and it has been predicted that its mark reaches 7,677 million USD by 2022 from 2,099 million USD in 2015. It implies that there is an increasing trend to use of antimicrobial packaging, in which ZnO NPs as an inexpensive and human body compatible biocidal can play a key role. The antimicrobial packaging can prolong the shelf life of fresh products, which can meet the demands for fresh produces. Antimicrobial packaging in general and packaging nanocomposite containing antimicrobial agents, in particular, are potentially applicable to extend the shelf life of food items and keep their quality. However, to achieve expected results and feasibility of antimicrobial packaging containing ZnO NPs, some issues should be addressed. ZnO NPs are frequently used in wide applications because of strong antimicrobial activities, but their antimicrobial efficacy reduces upon incorporating in the packaging matrix. It might be attributed to covering the bulk ZnO NPs by polymer segments, while ZnO NPs need to be in direct contact with food items to extend the shelf life. Therefore, it may limit ZnO NPs applications for wider range of foodstuffs.
Further, to make an advanced packaging nanocomposite, dispersion of nanofiller in its matrix is an important factor. Imperfectness dispersion of ZnO NPs is prone to drop in polymer performance, thereby weak properties in packaging materials. As such, achieving a balance between properties required for packaging containing ZnO NPs is still a challenge in industry. As mentioned earlier, 5% nanoparticles can provide an approvable biocidal activity, but because of poor interaction and agglomeration of ZnO NPs in higher concentrations, application of the ZnO NPs limits to their concentration.[Citation122] Consumer-acceptability is the last consideration prior to feasibility and applicability of packaging materials. Despite there is a continuous demand for fresh products, the safety is also important as much as freshness is. As such, migration and side-impacts of ZnO NPs are likely considered by consumers to identify the possible safety concerns. There are various reports on compatibility and safeness of ZnO NPs, but care must be taken to keep the migration below the hazardous level and satisfy consumer concerns regarding the safety.[Citation123] The nanoparticles are widely applied in the packaging materials to achieve advanced properties such as antimicrobial activities. As such, antimicrobial packaging systems, particularly based on Ag, TiO2 and ZnO, are still limited to their cost. The metal-based nanoparticles and scaling up the packaging nanocomposites require cutting-edge technologies, which may enhance the final cost, thereby reducing the market-acceptability.[Citation124]
Conclusion
The great attempts are being directed to maintain the quality of fresh food items and prevent foodborne diseases, in which packaging nanocomposite can meet the antimicrobial packaging requirements. ZnO NPs can be synthesized based on various methods, which can provide advanced ZnO NPs with different morphological and structural properties. Biocidal efficacy of ZnO NPs against gram-positive and gram-negative bacteria as well as fungi is the major characteristic in antimicrobial packaging systems. Accordingly, ZnO NPs can be incorporated into packaging materials using solution casting and extrusion methods or can be coated on the packaging material surface to achieve the antimicrobial packaging. Such systems can provide ZnO NPs with antimicrobial properties based on ROS generation, cellular metabolism destabilization, and cell rupture. ZnO NPs as an active antimicrobial agent are frequently used to prepare bio-based or petroleum-based packaging materials to make human body compatible, cost-effective, and strong biocidal packaging systems. Care must be taken to consider the migration and safety concerns to minimize the adverse impacts of ZnO NPs on the packaged food and human body. The progressive trend in antimicrobial packaging can be aligned based on the ZnO NPs to meet the intense demands for fresh products in the market.
Additional information
Funding
References
- Sharma, C.; Dhiman, R.; Rokana, N.; Panwar, H. Nanotechnology: An Untapped Resource for Food Packaging. Front. Microbiol. 2017, 8, 1735. DOI: https://doi.org/10.3389/fmicb.2017.01735.
- Bratovcic, A.; Odobaši, ´. C. A.; Cati´, C. S.; Šestan, I. Application of Polymer Nanocomposite Materials in Food Packaging. Croat. J. Food Sci. Technol. 2015, 7, 86–94. DOI: https://doi.org/10.17508/CJFST.2015.7.2.06.
- Bumbudsanpharoke, N.; Ko, S. Nano-food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J. Food Sci. 2015, 80, R910–R923. DOI: https://doi.org/10.1111/1750-3841.12861.
- Trujillo, L. E.; Ávalos, R.; Granda, S.; Guerra, L. S.; País-Chanfrau, J. M. Nanotechnology Applications for Food and Bioprocessing Industries. Biol. Med. 2016, 8, 289. DOI: https://doi.org/10.4172/0974-8369.1000289.
- Mihindukulasuriya, S. D. F.; Lim, L. T. Nanotechnology Development in Food Packaging: A Review. Trends Food Sci. Tech. 2014, 40, 149–167. DOI: https://doi.org/10.1016/j.tifs.2014.09.009.
- Nopwinyuwong, A.; Trevanich, S.; Suppakul, P. Development of a Novel Colorimetric Indicator Label for Monitoring Freshness of Intermediate-moisture Dessert Spoilage. Talanta. 2010, 81(3), 1126–1132. DOI: https://doi.org/10.1016/j.talanta.2010.02.008.
- Robertson, G. L.;. Food Packaging: Principles and Practice, 3rd ed.; CRC Press: Boca Raton, 2012.
- Suppakul, P.;. Intelligent Packaging. Part VII: Trends in Frozen Food Packing. In Handbook of Frozen Food Processing and Packaging, 2nd ed.; Sun, D.W., Ed.; CRC Press: Boca Raton, 2012; pp 837–860.
- Goodburn, C.; Wallace, C. A. The Microbiological Efficacy of Decontamination Methodologies for Fresh Produce: A Review. Food Control. 2013, 32(2), 418–427. DOI: https://doi.org/10.1016/j.foodcont.2012.12.012.
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a Food Spoilage Indicator for Monitoring Freshness of Skinless Chicken Breast. Talanta. 2014, 130, 547–554. DOI: https://doi.org/10.1016/j.talanta.2014.07.048.
- Galstyan, V.; Bhandari, M.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors. 2018, 6(2), 16. DOI: https://doi.org/10.3390/chemosensors6020016.
- Hoseinnejad, M.; Jafari, S. M.; Katouzian, I. Inorganic and Metal Nanoparticles and Their Antimicrobial Activity in Food Packaging Applications. Crit. Rev. Microbiol. 2018, 44(2), 161–181. DOI: https://doi.org/10.1080/1040841X.2017.1332001.
- Cha, D. S.; Chinnan, M. S. Biopolymer-based Antimicrobial Packaging: A Review. Crit. Rev. Food Sci. Nutr. 2004, 44(4), 223–237. DOI: https://doi.org/10.1080/10408690490464276.
- Sangsuwan, J.; Rattanapanone, N.; Rachtanapun, P. Effect of Chitosan/methyl Cellulose Films on Microbial and Quality Characteristics of Fresh-cut Cantaloupe and Pineapple. Postharvest Biol. Technol. 2008, 49(3), 403–410. DOI: https://doi.org/10.1016/j.postharvbio.2008.02.014.
- Hosseinnejad, M.; Jafari, S. M. Evaluation of Different Factors Affecting Antimicrobial Properties of Chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. DOI: https://doi.org/10.1016/j.ijbiomac.2016.01.022.
- Anbukkarasi, V.; Srinivasan, R.; Elangovan, N. Antimicrobial Activity of Green Synthesized Zinc Oxide Nanoparticles from Emblica Officinalis. Int. J. Pharm. Sci. Rev. Res. 2015, 33(2), 110–115.
- Barthomeuf, M.; Raymond, P.; Castel, X.; LeGendre, L.; Denis, M.; Pissavin, C. Bactericidal Efficiency of UV-active TiO2 Thin Films on Adhesion and Viability of Food-borne Bacteria. Iowa State University Library. 2015, 26, 195–199. DOI: https://doi.org/10.31274/safepork-180809-300.
- Prabhu, Y. T.; Rao, K. V.; Kumari, B. S.; Pavani, T. Decoration of Magnesium Oxide Nanoparticles on O-MWCNTs and Its Antibacterial Studies. Rend Lincei. 2015, 26(3), 263–270. DOI: https://doi.org/10.1007/s12210-015-0417-2.
- Hameed, A. S. H.; Karthikeyan, C.; Ahamed, A. P.; Thajuddin, N.; Alharbi, N. S.; Alharbi, S. A.; Ravi, G. In Vitro Antibacterial Activity of ZnO and Nd Doped ZnO Nanoparticles against ESBL Producing Escherichia Coli and Klebsiella Pneumoniae. Sci. Rep. 2016, 6, 24312. DOI: https://doi.org/10.1038/srep24312.
- Espitia, P. J. P.; Soares, N. D. F. F.; Dos Reis Coimbra, J. S.; De Andrade, N. J.; Cruz, R. S.; Medeiros, E. A. A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioproc. Tech. 2012, 5(5), 1447–1464. DOI: https://doi.org/10.1007/s11947-012-0797-6.
- Swihart, M. T.;. Vapor-phase Synthesis of Nanoparticles. Curr. Opin. Colloid Interface Sci. 2003, 8(1), 127–133. DOI: https://doi.org/10.1016/S1359-0294(03)00007-4.
- Lee, G. J.; Choi, E. H.; Nam, S. H.; Lee, J. S.; Boo, J. H.; Oh, S. D.; Choi, S. H.; Cho, J. H.; Yoon, M. Y. Optical Sensing Properties of ZnO Nanoparticles Prepared by Spray Pyrolysis. J. Nanosci. Nanotechnol. 2019, 19(2), 1048–1051. DOI: https://doi.org/10.1166/jnn.2019.15918.
- Casey, P.; Hannick, R.; Hill, A. Nanoparticle Technologies and Applications. Nanostruct Control Mater. 2006, 1–31, Woodhead Publising. DOI: https://doi.org/10.1533/9781845691189.1.
- Hessien, M.; Da’na, E.; Kawther, A. L.; Khalaf, M. M. Nano ZnO (Hexagonal Wurtzite) of Different Shapes under Various Conditions: Fabrication and Characterization. Mater. Res. Express. 2019, 6(8), 085057. DOI: https://doi.org/10.1088/2053-1591/ab1c21.
- Soni, A.; Mavani, K. R. Controlling Porosity and Ultraviolet Photoresponse of Crystallographically Oriented ZnO Nanostructures Grown by Pulsed Laser Deposition. Scr. Mater. 2019, 162, 24–27. DOI: https://doi.org/10.1016/j.scriptamat.2018.10.026.
- Chen, X.; Shen, Y.; Zhou, P.; Zhao, S.; Zhong, X.; Li, T.; Han, C.; Wei, D.; Meng, D. NO2 Sensing Properties of One-pot-synthesized ZnO Nanowires with Pd Functionalization. Sens. Actuators B Chem. 2019, 280, 151–161. DOI: https://doi.org/10.1016/j.snb.2018.10.063.
- Qin, G.; Sun, X.; Xiao, Y.; Liu, F. Rational Fabrication of Plasmonic Responsive N-Ag-TiO2-ZnO Nanocages for Photocatalysis under Visible Light. J. Alloys Compd. 2019, 772, 885–899. DOI: https://doi.org/10.1016/j.jallcom.2018.09.190.
- Dash, P.; Manna, A.; Mishra, N. C.; Varma, S. Synthesis and Characterization of Aligned ZnO Nanorods for Visible Light Photocatalysis. Physica E Low Dimens Syst Nanostruct. 2019, 107, 38–46. DOI: https://doi.org/10.1016/j.physe.2018.11.007.
- Goel, S.; Sinha, N.; Kumar, B. 3D Hierarchical Ho-doped ZnO Micro-flowers Assembled with Nanosheets: A High Temperature Ferroelectric Material. Phys E: Low-dimensional Syst Nanostruct. 2019, 105, 29–40. DOI: https://doi.org/10.1016/j.physe.2018.09.002.
- Chen, R.; Wan, Y.; Wu, W.; Yang, C.; He, J. H.; Cheng, J.; Jetter, R.; Ko, K. F.; Chen, Y. A Lotus Effect-inspired Flexible and Breathable Membrane with Hierarchical Electrospinning Micro/nanofibers and ZnO Nanowires. Mater. Des. 2019, 162, 246–248. DOI: https://doi.org/10.1016/j.matdes.2018.11.041.
- Anbuvannan, M.; Ramesh, M.; Manikandan, E.; Srinivasan, R. Vitex Negundo Leaf Extract Mediated Synthesis of ZnO Nanoplates and Its Antibacterial and Photocatalytic Activities. Asian J. Nanosci. Mater. 2019, 2, 99–110. DOI: https://doi.org/10.26655/AJNANOMAT.2019.1.7.
- Mao, Y.; Li, Y.; Zou, Y.; Shen, X.; Zhu, L.; Liao, G. Solvothermal Synthesis and Photocatalytic Properties of ZnO Micro/nanostructures. Ceram. Int. 2019, 45(2), 1724–1729. DOI: https://doi.org/10.1016/j.ceramint.2018.10.054.
- Zhang, Y.; Liu, M.; Ren, W.; Ye, Z. G. Well-ordered ZnO Nanotube Arrays and Networks Grown by Atomic Layer Deposition. Appl. Surf. Sci. 2015, 340, 120–125. DOI: https://doi.org/10.1016/j.apsusc.2015.02.176.
- Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. High-sensitivity NO2 Gas Sensors Based on Flower-like and Tube-like ZnO Nanomaterials. Sens. Actuators. B Chem. 2011, 157(2), 565–574. DOI: https://doi.org/10.1016/j.snb.2011.05.023.
- Galstyan, V.; Comini, E.; Baratto, C.; Ponzoni, A.; Bontempi, E.; Brisotto, M.; Bontempi, E.; Brisotto, M.; Fagliaa, G.; Sberveglieri, G. Synthesis of Self-assembled Chain-like ZnO Nanostructures on Stiff and Flexible Substrates. Cryst. Eng. Comm. 2013, 15(15), 2881–2887. DOI: https://doi.org/10.1039/C3CE27011D.
- Galstyan, V.; Comini, E.; Kholmanov, I.; Ponzoni, A.; Sberveglieri, V.; Poli, N.; Faglia, G.; Sberveglieri, G. A Composite Structure Based on Reduced Graphene Oxide and Metal Oxide Nanomaterials for Chemical Sensors. Beilstein. J. Nanotechnol. 2016, 7, 1421. DOI: https://doi.org/10.3762/bjnano.7.133.
- Anbuvannan, M.; Ramesh, M.; Viruthagiri, G.; Shanmugam, N.; Kannadasan, N. Anisochilus Carnosus Leaf Extract Mediated Synthesis of Zinc Oxide Nanoparticles for Antibacterial and Photocatalytic Activities. Mater. Sci. Semicond. Process. 2015, 39, 621–628. DOI: https://doi.org/10.1016/j.mssp.2015.06.005.
- Fu, L.; Fu, Z. Plectranthus Amboinicus Leaf Extract–assisted Biosynthesis of ZnO Nanoparticles and Their Photocatalytic Activity. Ceram. Int. 2015, 41(2), 2492–2496. DOI: https://doi.org/10.1016/j.ceramint.2014.10.069.
- Ambika, S.; Sundrarajan, M. Green Biosynthesis of ZnO Nanoparticles Using Vitex Negundo L. Extract: Spectroscopic Investigation of Interaction between ZnO Nanoparticles and Human Serum Albumin. J. Photochem. Photobiol. B. 2015, 149, 143–148. DOI: https://doi.org/10.1016/j.jphotobiol.2015.05.004.
- Ambika, S.; Sundrarajan, M. Antibacterial Behaviour of Vitex Negundo Extract Assisted ZnO Nanoparticles against Pathogenic Bacteria. J. Photochem. Photobiol. B. 2013, 146, 52–57. DOI: https://doi.org/10.1016/j.jphotobiol.2015.02.020.
- Otari, S. V.; Patil, R. M.; Nadaf, N. H.; Ghosh, S. J.; Pawar, S. H. Green Biosynthesis of Silver Nanoparticles from an Actinobacteria Rhodococcu Ssp. Mater. Lett. 2012, 72, 92–94. DOI: https://doi.org/10.1016/j.matlet.2011.12.109.
- Azizi, S.; Ahmad, M. B.; Namvar, F.; Mohamad, R. Green Biosynthesis and Characterization of Zinc Oxide Nanoparticles Using Brown Marine Macroalga Sargassum Muticum Aqueous Extract. Mater. Lett. 2014, 116, 275–277. DOI: https://doi.org/10.1016/j.matlet.2013.11.038.
- Nagarajan, S.; Kuppusamy, K. A. Extracellular Synthesis of Zinc Oxide Nanoparticle Using Seaweeds of Gulf of Mannar, India. J. Nanobiotechnology. 2013, 11(1), 39. DOI: https://doi.org/10.1186/1477-3155-11-39.
- Rao, M. D.; Gautam, P. Synthesis and Characterization of ZnO Nanoflowers Using Chlamydomonas Reinhardtii: A Green Approach. Environ. Prog. Sustain. Energy. 2016, 35(4), 1020–1026. DOI: https://doi.org/10.1002/ep.12315.
- Pavani, K. V.; Kumar, N. S.; Sangameswaran, B. B. Synthesis of Lead Nanoparticles by Aspergillus Species. Pol. J. Microbiol. 2012, 61(1), 61–63. DOI: https://doi.org/10.1166/jnn.2019.15918.
- Agarwal, H.; Kumar, S. V.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide nanoparticles–An Eco-friendly Approach. Resour Efficie Technol. 2017, 3(4), 406–413. DOI: https://doi.org/10.1016/j.reffit.2017.03.002.
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial Activity of ZnO Nanoparticle on Gram-positive and Gram-negative Bacteria. Afr. J. Microbiol. Res. 2011, 5(12), 1368–1373. DOI: https://doi.org/10.5897/ajmr10.159.
- Babitha, N.; Priya, L. S.; Christy, S. R.; Manikandan, A.; Dinesh, A.; Durka, M.; Arunadevi, S. Enhanced Antibacterial Activity and Photo-Catalytic Properties of ZnO Nanoparticles: Pedalium Murex Plant Extract-Assisted Synthesis. J. Nanosci. Nanotechnol. 2019, 19(5), 2888–2894. DOI: https://doi.org/10.1166/jnn.2019.16023.
- He, W.; Kim, H. K.; Wamer, W. G.; Melka, D.; Callahan, J. H.; Yin, J. J. Photogenerated Charge Carriers and Reactive Oxygen Species in ZnO/Au Hybrid Nanostructures with Enhanced Photocatalytic and Antibacterial Activity. J. Am. Chem. Soc. 2013, 136(2), 750–757. DOI: https://doi.org/10.1021/ja410800y.
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N. H. M.; Ann, L. C.; Bakhori, S. K. M.; Bakhori, M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7(3), 219–242. DOI: https://doi.org/10.1007/s40820-015-0040-x.
- Chauhan, D. S.; Gopal, C. S. A.; Kumar, D.; Mahato, N.; Quraishi, M. A.; Cho, M. H. Microwave Induced Facile Synthesis and Characterization of ZnO Nanoparticles as Efficient Antibacterial Agents. Mater. Disc. 2018, 11, 19–25. DOI: https://doi.org/10.1016/j.md.2018.05.001.
- Gao, P. X.; Ding, Y.; Wang, Z. L. Crystallographic Orientation-aligned ZnO Nanorods Grown by a Tin Catalyst. Nano Lett. 2003, 3(9), 1315–1320. DOI: https://doi.org/10.1021/nl034548q.
- Wojnarowicz, J.; Opalinska, A.; Chudoba, T.; Gierlotka, S.; Mukhovskyi, R.; Pietrzykowska, E.; Sobczak, K.; Lojkowski, W. Effect of Water Content in Ethylene Glycol Solvent on the Size of ZnO Nanoparticles Prepared Using Microwave Solvothermal Synthesis. J. Nanomater. 2016, 1. DOI: https://doi.org/10.1155/2016/2789871.
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—from Synthesis to Application: A Review. Materials. 2014, 7(4), 2833–2881. DOI: https://doi.org/10.3390/ma7042833.
- El-Nahhal, I. M.; Elmanama, A. A.; El Ashgar, N. M.; Amara, N.; Selmane, M.; Chehimi, M. M. Stabilization of Nano-structured ZnO Particles onto the Surface of Cotton Fibers Using Different Surfactants and Their Antimicrobial Activity. Ultrason. Sonochem. 2017, 38, 478–487. DOI: https://doi.org/10.1016/j.ultsonch.2017.03.050.
- Valerini, D.; Tammaro, L.; Di Benedetto, F.; Vigliotta, G.; Capodieci, L.; Terzi, R.; Rizzo, A. Aluminum-doped Zinc Oxide Coatings on Polylactic Acid Films for Antimicrobial Food Packaging. Thin Solid Films. 2018, 645, 187–192. DOI: https://doi.org/10.1016/j.tsf.2017.10.038.
- Verrier, C.; Appert, E.; Chaix-Pluchery, O.; Rapenne, L.; Rafhay, Q.; Kaminski-Cachopo, A.; Consonni, V. Effects of the pH on the Formation and Doping Mechanisms of ZnO Nanowires Using Aluminum Nitrate and Ammonia. Inorg. Chem. 2017, 56(21), 13111–13122. DOI: https://doi.org/10.1021/acs.inorgchem.7b01916.
- Yamamoto, O.;. Influence of Particle Size on the Antibacterial Activity of Zinc Oxide. Int. J. Inorg. Mater. 2001, 3(7), 643–646. DOI: https://doi.org/10.1016/s1466-6049(01)00197-0.
- Jones, N.; Ray, B.; Ranjit, K. T.; Manna, A. C. Antibacterial Activity of ZnO Nanoparticle Suspensions on a Broad Spectrum of Microorganisms. FEMS Microbiol. Lett. 2008, 279(1), 71–76. DOI: https://doi.org/10.1111/j.1574-6968.2007.01012.x.
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z. Q.; Lin, M. Antibacterial Activities of Zinc Oxide Nanoparticles against Escherichia coli O157: H7. J. Appl. Microbiol. 2009, 107(4), 1193–1201. DOI: https://doi.org/10.1111/j.1365-2672.2009.04303.x.
- Sawai, J.; Shoji, S.; Igarashi, H.; Hashimoto, A.; Kokugan, T.; Shimizu, M.; Kojima, H. Hydrogen Peroxide as an Antibacterial Factor in Zinc Oxide Powder Slurry. J. Ferment. Bioeng. 1998, 86(5), 521–522. DOI: https://doi.org/10.1016/s0922-338x(98)80165-7.
- Jin, T.; Sun, D.; Su, J. Y.; Zhang, H.; Sue, H. J. Antimicrobial Efficacy of Zinc Oxide Quantum Dots against Listeria Monocytogenes, Salmonella Enteritidis, and Escherichia coli O157: H7. J. Food Sci. 2009, 74(1), M46–M52. DOI: https://doi.org/10.1016/j.foodcont.2008.02.009.
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO Nanoparticles to Escherichia coli: Mechanism and the Influence of Medium Components. Environ. Sci. Technol. 2011, 45(5), 1977–1983. DOI: https://doi.org/10.1021/es102624t.
- Petchwattana, N.; Covavisaruch, S.; Wibooranawong, S.; Naknaen, P. Antimicrobial Food Packaging Prepared from Poly (Butylene Succinate) and Zinc Oxide. Measurement. 2016, 93, 442–448. DOI: https://doi.org/10.1016/j.measurement.2016.07.048.
- Suresh, D.; Nethravathi, P. C.; Lingaraju, K.; Rajanaika, H.; Sharma, S. C.; Nagabhushana, H. EGCG Assisted Green Synthesis of ZnO Nanopowders: Photodegradative, Antimicrobial and Antioxidant Activities. Spectrochim Acta A Mol Biomol Spectrosc. 2015, 136, 1467–1474. DOI: https://doi.org/10.1016/j.saa.2014.10.038.
- Wiburanawong, S.; Petchwattana, N.; Covavisaruch, S. Carvacrol as an Antimicrobial Agent for Poly (Butylene Succinate): Tensile Properties and Antimicrobial Activity Observations. Adv. Mater. Res. 2014, 931–932, 111–115. www.scientific.net/amr.931-932.111.
- Li, X.; Li, W.; Jiang, Y.; Ding, Y.; Yun, J.; Tang, Y.; Zhang, P. Effect of Nano ZnO Coated Active Packaging on Quality of Fresh-cut ‘Fuji’ Apple. Int. J. Food Sci. Technol. 2011, 46(9), 1947–1955. DOI: https://doi.org/10.1111/j.1365-2621.2011.02706.x.
- Murariu, M.; Doumbia, A.; Bonnaud, L.; Dechief, A. L.; Paint, Y.; Ferreira, M.; Campagne, C.; Devaux, E.; Dubois, P. High-performance polylactide/ZnO Nanocomposites Designed for Films and Fibers with Special End-use Properties. Biomacromolecules. 2011, 12(5), 1762–1771. DOI: https://doi.org/10.1021/bm2001445.
- Zaman, H. U.; Hun, P. D.; Khan, R. A.; Yoon, K. B. Morphology, Mechanical, and Crystallization Behaviors of Micro-and nano-ZnO Filled Polypropylene Composites. J. Reinf. Plast. Comp. 2012, 31(5), 323–329. DOI: https://doi.org/10.1177/0731684411436126.
- Ghasemi, F.; Jalal, R. Antimicrobial Action of Zinc Oxide Nanoparticles in Combination with Ciprofloxacin and Ceftazidime against Multidrug-resistant Acinetobacter Baumannii. J. Glob. Antimicrob. Resist. 2016, 6, 118–122. DOI: https://doi.org/10.1016/j.jgar.2016.04.007.
- Sarwar, S.; Chakraborti, S.; Bera, S.; Sheikh, I. A.; Hoque, K. M.; Chakrabarti, P. The Antimicrobial Activity of ZnO Nanoparticles against Vibrio Cholerae: Variation in Response Depends on Biotype. Nanomed. 2016, 12(6), 1499–1509. DOI: https://doi.org/10.1016/j.nano.2016.02.006.
- Sonohara, R.; Muramatsu, N.; Ohshima, H.; Kondo, T. Difference in Surface Properties between Escherichia coli and Staphylococcus aureus as Revealed by Electrophoretic Mobility Measurements. Biophys. Chem. 1995, 55(3), 273–277. DOI: https://doi.org/10.1016/0301-4622(95)00004-h.
- Gordon, T.; Perlstein, B.; Houbara, O.; Felner, I.; Banin, E.; Margel, S. Synthesis and Characterization of Zinc/iron Oxide Composite Nanoparticles and Their Antibacterial Properties. Colloids Surf. A. 2011, 374(1–3), 1–8. DOI: https://doi.org/10.1016/j.colsurfa.2010.10.015.
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the Antibacterial Behaviour of Suspensions of ZnO Nanoparticles (Zno Nanofluids). J. Nanopart. Res. 2007, 9(3), 479–489. DOI: https://doi.org/10.1007/s11051-006-9150-1.
- Ohira, T.; Yamamoto, O.; Iida, Y.; Nakagawa, Z. E. Antibacterial Activity of ZnO Powder with Crystallographic Orientation. J. Mater. Sci. Mater. Med. 2008, 19(3), 1407–1412. DOI: https://doi.org/10.1007/s10856-007-3246-8.
- Adams, L. K.; Lyon, D. Y.; Alvarez, P. J. Comparative Eco-toxicity of Nanoscale TiO2, SiO2, and ZnO Water Suspensions. Water Res. 2006, 40(19), 3527–3532. DOI: https://doi.org/10.1016/j.watres.2006.08.004.
- Han, J. H.;. Antimicrobial Packaging Systems. In Innovations Antimicrobial in Food Packaging, 2nd ed.; Han, J. H., Ed.; Academic Press, Elsevier: New York, 2005; pp 80–107.
- Appendini, P.; Hotchkiss, J. H. Review of Antimicrobial Food Packaging. Innov. Food Sci. Emerg. Technol. 2002, 3(2), 113–126. DOI: https://doi.org/10.1016/S1466-8564(02)00012-7.
- Siemann, U. Solvent Cast Technology–a Versatile Tool for Thin Film Production. In Scattering Methods and the Properties of Polymer Materials.; Norbert, S., Bernd. S., Eds.; Springer: Berlin, Heidelberg, 2005; pp 1–14. DOI: https://doi.org/10.1007/b107336.
- Li, X.; Xing, Y.; Jiang, Y.; Ding, Y.; Li, W. Antimicrobial Activities of ZnO Powder‐coated PVC Film to Inactivate Food Pathogens. Inter. J. Food Sci. Technol. 2009, 44(11), 2161–2168. DOI: https://doi.org/10.1111/j.1365-2621.2009.02055.x.
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc Oxide Nanoparticle Composite Coating for Active Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. DOI: https://doi.org/10.1016/j.ifset.2016.10.010.
- Saekow, M.; Naradisorn, M.; Tongdeesoontorn, W.; Hamauzu, Y. Effect of Carboxymethyl Cellulose Coating Containing ZnO-nanoparticles for Prolonging Shelf Life of Persimmon and Tomato Fruit. J. Food Sci. Agri. Technol. 2019, 5, 41–48.
- Emamifar, A.; Mohammadizadeh, M. Preparation and Application of LDPE/ZnO Nanocomposites for Extending Shelf Life of Fresh Strawberries. Food Technol. Biotechnol. 2015, 53(4), 488–495. DOI: https://doi.org/10.17113/ftb.53.04.15.3817.
- Polat, S.; Fenercioglu, H.; UnalTurhan, E.; Guclu, M. Effects of Nanoparticle Ratio on Structural, Migration Properties of Polypropylene Films and Preservation Quality of Lemon Juice. J. Food Process. Preserv. 2018, 42(4), 13541. DOI: https://doi.org/10.1111/jfpp.13541.
- Simões, D. N.; Pittol, M.; Tomacheski, D.; Ribeiro, V. F.; Santana, R. M. C. Thermoplastic Elastomers Containing Zinc Oxide as Antimicrobial Additive under Thermal Accelerated Ageing. Mater. Res. 2017, 20, 325–330. DOI: https://doi.org/10.1590/1980-5373-mr-2016-0790.
- Altan, M.; Yildirim, H. Effects of Compatibilizers on Mechanical and Antibacterial Properties of Injection Molded nano-ZnO Filled Polypropylene. J. Compos. Mater. 2012, 46(25), 3189–3199. DOI: https://doi.org/10.1177/0021998312436999.
- Altan, M.; Yildirim, H. Comparison of Antibacterial Properties of Nano TiO2 and ZnO Particle Filled Polymers. Acta Phys. Pol. A. 2014, 125, 645–647. DOI: https://doi.org/10.12693/APhysPolA.125.645.
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S. H.; Roufegarinejad, L.; Hamishehkar, H. Application of Reinforced ZnO Nanoparticle-Incorporated Gelatin Bionanocomposite Film with Chitosan Nanofiber for Packaging of Chicken Fillet and Cheese as Food Models. Food Bioprocess. Technol. 2019, 1–15. DOI: https://doi.org/10.1007/s11947-019-02286-y.
- Ejaz, M.; Arfat, Y. A.; Mulla, M.; Ahmed, J. Zinc Oxide Nanorods/clove Essential Oil Incorporated Type B Gelatin Composite Films and Its Applicability for Shrimp Packaging. Food Packag. Shelf Life. 2018, 15, 113–121. DOI: https://doi.org/10.1016/j.fpsl.2017.12.004.
- Mohammadi, H.; Kamkar, A.; Misaghi, A.; Zunabovic-Pichler, M.; Fatehi, S. Nanocomposite Films with CMC, Okra Mucilage, and ZnO Nanoparticles: Extending the Shelf-life of Chicken Breast Meat. Food Packag. Shelf Life. 2019, 21, 100330. DOI: https://doi.org/10.1016/j.fpsl.2019.100330.
- Youssef, A. M.; El-Sayed, S. M.; El-Sayed, H. S.; Salama, H. H.; Dufresne, A. Enhancement of Egyptian Soft White Cheese Shelf Life Using a Novel Chitosan/carboxymethyl Cellulose/zinc Oxide Bionanocomposite Film. Carbohydr. Polym. 2016, 151, 9–19. DOI: https://doi.org/10.1016/j.carbpol.2016.05.023.
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R. R.; Hashemi, M. Novel Active Packaging Based on Carboxymethyl cellulose-chitosan-ZnO NPs Nanocomposite for Increasing the Shelf Life of Bread. Food Packag. Shelf Life. 2017, 11, 106–114. DOI: https://doi.org/10.1016/j.fpsl.2017.01.010.
- Indumathi, M. P.; Sarojini, K. S.; Rajarajeswari, G. R. Antimicrobial and Biodegradable Chitosan/cellulose Acetate phthalate/ZnO Nano Composite Films with Optimal Oxygen Permeability and Hydrophobicity for Extending the Shelf Life of Black Grape Fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. DOI: https://doi.org/10.1016/j.ijbiomac.2019.03.171.
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M. A.; Hosseini, M. A New Active Nanocomposite Film Based on PLA/ZnO Nanoparticle/essential Oils for the Preservation of Refrigerated Otolithesruber Fillets. Food Packag. Shelf Life. 2019, 19, 94–103. DOI: https://doi.org/10.1016/j.fpsl.2018.12.002.
- Baek, S. K.; Song, K. B. Development of Gracilariavermiculophylla Extract Films Containing Zinc Oxide Nanoparticles and Their Application in Smoked Salmon Packaging. LWT. 2018, 89, 269–275. DOI: https://doi.org/10.1016/j.lwt.2017.10.064.
- Kumar, S.; Boro, J. C.; Ray, D.; Mukherjee, A.; Dutta, J. Bionanocomposite Films of Agar Incorporated with ZnO Nanoparticles as an Active Packaging Material for Shelf Life Extension of Green Grape. Heliyon. 2019, 5(6), 01867. DOI: https://doi.org/10.1016/j.heliyon.2019.e01867.
- Jafarzadeh, S.; Rhim, J. W.; Alias, A. K.; Ariffin, F.; Mahmud, S. Application of Antimicrobial Active Packaging Film Made of Semolina Flour, Nano Zinc Oxide and Nano‐kaolin to Maintain the Quality of Low‐moisture Mozzarella Cheese during Low‐temperature Storage. J. Sci. Food Agri. 2019, 99(6), 2716–2725. DOI: https://doi.org/10.1002/jsfa.9439.
- Indumathi, M. P.; Rajarajeswari, G. R. Mahua Oil-based Polyurethane/chitosan/nano ZnO Composite Films for Biodegradable Food Packaging Applications. Int. J. Biol. Macromol. 2019, 124, 163–174. DOI: https://doi.org/10.1016/j.ijbiomac.
- Meraldo, A.;. Introduction to Bio-based Polymers. In Multilayer Flexible Packaging; 2nd ed.: Wagner, J. R., Ed.; Academic Press, Elsevier: New York, 2016; pp 47–52. DOI:https://doi.org/10.1016/B978-0-323-37100-1.00004-1.
- Billham, M.; Clarke, A. H.; Garrett, G.; Mcnally, G. M.; Murphy, W. R. The Effect of Extrusion Processing Conditions on the Properties of Blown and Cast Polyolefin Packaging Films. Dev. Chem. Eng. Mineral Process. 2003, 11(1‐2), 137–146. DOI: https://doi.org/10.1002/apj.5500110214.
- Rhim, J. W.; Kim, Y. T. Biopolymer-based Composite Packaging Materials with Nanoparticles. In Innovations in Food Packaging; 2nd ed.; Han, J. H., Ed.; Academic Press, Elsevier: New York, 2014; pp 413–442. DOI:https://doi.org/10.1016/B978-0-12-394601-0.00017-5.
- https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/
- Aristizabal-Gil, M. V.; Santiago-Toro, S.; Sanchez, L. T.; Pinzon, M. I.; Gutierrez, J. A.; Villa, C. C. ZnO and ZnO/CaO Nanoparticles in Alginate Films. Synthesis, Mechanical Characterization, Barrier Properties and Release Kinetics. LWT. 2019, 102(7), 108217. DOI: https://doi.org/10.1016/j.lwt.2019.05.115.
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective Toxicity of ZnO Nanoparticles toward Gram-positive Bacteria and Cancer Cells by Apoptosis through Lipid Peroxidation. Nanomedicine. 2011, 7(2), 184–192. DOI: https://doi.org/10.1016/j.nano.2010.10.001.
- Deng, X.; Luan, Q.; Chen, W.; Wang, Y.; Wu, M.; Zhang, H.; Jiao, Z. Nanosized Zinc Oxide Particles Induce Neural Stem Cell Apoptosis. Nanotechnology. 2009, 20(11), 115101. DOI: https://doi.org/10.1088/0957-4484/20/11/115101.
- Heng, B. C.; Zhao, X.; Tan, E. C.; Khamis, N.; Assodani, A.; Xiong, S.; Rued, C.; Ng, K. W.; Loo, J. S. C. Evaluation of the Cytotoxic and Inflammatory Potential of Differentially Shaped Zinc Oxide Nanoparticles. Arch. Toxicol. 2011, 85(12), 1517–1528. DOI: https://doi.org/10.1007/s00204-011-0722-1.
- Hanley, C.; Thurber, A.; Hanna, C.; Punnoose, A.; Zhang, J.; Wingett, D. G. The Influences of Cell Type and ZnO Nanoparticle Size on Immune Cell Cytotoxicity and Cytokine Induction. Nanoscale. Res. Lett. 2009, 4(12), 1409. DOI: https://doi.org/10.1007/s11671-009-9413-8.
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; et al. Decreased Dissolution of ZnO by Iron Doping Yields Nanoparticles with Reduced Toxicity in the Rodent Lung and Zebrafish Embryos. ACS Nano. 2011, 5(2), 1223–1235. DOI: https://doi.org/10.1021/nn1028482.
- Huang, Y.; Mei, L.; Chen, X.; Wang, Q. Recent Developments in Food Packaging Based on Nanomaterials. Nanomaterials. 2018, 8(10), 830. DOI: https://doi.org/10.3390/nano8100830.
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food Packaging Based on Polymer Nanomaterials. Prog Polym. Sci. 2011, 36(12), 1766–1782. DOI: https://doi.org/10.1016/j.tsf.2010.08.073.
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and Implications of Nanotechnologies for the Food Sector. Food Addit. Contam. 2008, 25(3), 241–258. DOI: https://doi.org/10.1080/02652030701744538.
- Bradley, E. L.; Castle, L.; Chaudhry, Q. Applications of Nanomaterials in Food Packaging with a Consideration of Opportunities for Developing Countries. Trends Food Sci. Technol. 2011, 22(11), 604–610. DOI: https://doi.org/10.1016/j.tifs.2011.01.002.
- Pujalté, I.; Passagne, I.; Brouillaud, B.; Tréguer, M.; Durand, E.; Ohayon-Courtès, C.; L’Azou, B. Cytotoxicity and Oxidative Stress Induced by Different Metallic Nanoparticles on Human Kidney Cells. Part. Fibre. Toxicol. 2011, 8(1), 1–16. DOI: https://doi.org/10.1186/1743-8977-8-10.
- Heng, B. C.; Zhao, X.; Xiong, S.; Ng, K. W.; Boey, F. Y. C.; Loo, J. S. C. Toxicity of Zinc Oxide (Zno) Nanoparticles on Human Bronchial Epithelial Cells (BEAS-2B) Is Accentuated by Oxidative Stress. Food Chem. Toxicol. 2010, 48(6), 1762–1766. DOI: https://doi.org/10.1016/j.fct.2010.04.023.
- Nohynek, G. J.; Antignac, E.; Re, T.; Toutain, H. Safety Assessment of Personal Care Products/cosmetics and Their Ingredients. Toxicol. Appl. Pharmacol. 2010, 243(2), 239–259. DOI: https://doi.org/10.1016/j.taap.2009.12.001.
- Trumbo, P.; Yates, A. A.; Schlicker, S.; Poos, M. Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. J. Acad. Nutr. Diet. 2001, 101(3), 294. DOI: https://doi.org/10.1016/S0002-8223(01)00078-5.
- Suo, B.; Li, H.; Wang, Y.; Li, Z.; Pan, Z.; Ai, Z. Effects of ZnO Nanoparticle‐coated Packaging Film on Pork Meat Quality during Cold Storage. J. Sci. Food Agric. 2017, 97(7), 2023–2029. DOI: https://doi.org/10.1002/jsfa.8003.
- Youssef, A. M.;. Polymer Nanocomposites as a New Trend for Packaging Applications. Polym. Plast. Technol. Eng. 2013, 52(7), 635–660. DOI: https://doi.org/10.1080/03602559.2012.762673.
- Shankar, S.; Rhim, J. W. Bionanocomposite Films for Food Packaging Applications. Ref. Mod. Food Sci. 2018, 1–10. DOI: https://doi.org/10.1016/B978-0-08-100596-5.21875-1.
- Zhang, Y. R.; Nayak, T.; Hong, H.; Cai, W. Biomedical Applications of Zinc Oxide Nanomaterials. Curr. Mol. Med. 2013, 13(10), 1633–1645. DOI: https://doi.org/10.2174/1566524013666131111130058.
- Jokar, M.; Pedersen, G. A.; Loeschner, K. Six Open Questions about the Migration of Engineered Nano-objects from Polymer-based Food-contact Materials: A Review. Food Addit. Contam. Part A. 2017, 34(3), 434–450. DOI: https://doi.org/10.1080/19440049.2016.1271462.
- Honarvar, Z.; Hadian, Z.; Mashayekh, M. Nanocomposites in Food Packaging Applications and Their Risk Assessment for Health. Electron. Physician. 2016, 8(6), 2531. DOI: https://doi.org/10.19082/2531.
- Wahab, R.; Mishra, A.; Yun, S. I.; Kim, Y. S.; Shin, H. S. Antibacterial Activity of ZnO Nanoparticles Prepared via Non-hydrolytic Solution Route. Appl. Microbiol. Biotechnol. 2010, 87(5), 1917–1925. DOI: https://doi.org/10.1007/s00253-010-2692-2.
- Zare, Y.;. Study of Nanoparticles Aggregation/agglomeration in Polymer Particulate Nanocomposites by Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2016, 84, 158–164. DOI: https://doi.org/10.1016/j.compositesa.2016.01.020.