ABSTRACT
To address the issues related to high perishability and limited shelf life of food proteins from muscle origin, different innovative processing, and preservation techniques, as well as analytical methodological approaches have been developed to meet environmental challenges and consumer demand for food of high quality and sustainable production supported by circular economy principles. This development has been enhanced and increased during the ongoing age of the fourth industrial revolution (Industry 4.0), which has been gaining momentum since 2015, coming up with a range of automated and digitized technologies. This review provides an updated overview of the recently developed thermal and nonthermal processing and preservation technologies, along with selected advanced analytical techniques used in the industry of muscle foods. Particular attention has been given to Industry 4.0 technologies and their role in achieving smart production with high automation and digitalization rates. As in other industry sectors, technology breakthroughs are reshaping the food industry, including the muscle food sector. Emerging technologies, such as pulsed electric field, high-pressure processing, ohmic heating, nanotechnology, advanced mass spectrometry and hyperspectral imaging sensors are among the key elements in the current food revolution 4.0. Although Industry 4.0 offers countless possibilities, more studies are still needed to capture its full potential and further harness its technologies to solve current challenges and move forward toward Industry 5.0.
Introduction
Nowadays, food security is facing major challenges posed by resource depletion, climate change, loss of biodiversity, current geopolitical issues, as well as the growing world population, which is expected to reach nearly 10 billion people by 2050. These contemporary issues present formidable societal and environmental problems that can be addressed only by deep-structural changes and ambitious socio-economic initiatives. The challenge of feeding all people requires innovative strategies and new multicultural rethinking and paradigms toward more sustainable use of the available natural and human resources, to ensure food and nutrition safety.[Citation1–6]
From the standpoint of human nutrition, muscle foods (such as chicken, lamb, beef, hog, ham, and fish) are among the most significant food commodities.[Citation7] Consumption of muscle foods has been increasing in recent years due to their high-quality proteins, vitamins, and minerals.[Citation8,Citation9] However, muscle foods are very perishable and their quality decays quickly unless adequately prepared, packed, and kept under refrigerated conditions or other approved preservative strategies.[Citation10,Citation11] The deteriorations and degradations are caused mostly by high fat and moisture contents, making them prone to biological factors, such as protein degradation, lipid oxidation, or putrefactions, which are interceded by microbial and endogenous enzymes, leading to a shortened shelf life.[Citation12,Citation13] As a result, a variety of preservation and processing procedures have been developed over the years. Because of their availability and simplicity, traditional thermal treatments, such as broiling, frying, roasting, and grilling have been frequently utilized for centuries.[Citation8,Citation14,Citation15] Thermal treatments are used to prepare food and improve sensory characteristics and digestibility, inactivate germs and assure food safety, and increase shelf life.[Citation16] Nevertheless, applying high heat loads may result in degradations and negative impacts on the sensory and nutritional quality characteristics of the treated foods, particularly in the case of muscle food items, which are recognized for their high susceptibility to heat treatments.[Citation10,Citation17] Furthermore, the ever-increasing customer demand for fresh, safe, nutritious, and healthful food, as well as unique consumption patterns (e.g., minimally processed foods) have pushed the food sector to create novel and revolutionary thermal processing processes.[Citation16,Citation18,Citation19] For example, different research has focused on the application of microwave,[Citation20–22] radio frequency,[Citation23,Citation24] ohmic heating,[Citation25,Citation26] and infrared processing [Citation18,Citation27] approaches in different areas of food processing and manufacturing.
More innovative preservation procedures have arisen in recent years to fulfil consumer expectations for high-quality products with prolonged shelf life, greater safety, and increased process efficiency. Several studies have shown that such preservation methods are energy-efficient and allow for the inactivation of microbes and enzyme activity in food items while maintaining sensory quality characteristics.[Citation28–33] With these considerations in mind, a variety of nonthermal treatments, such as high-pressure processing,[Citation34,Citation35] pulsed electric field,[Citation36,Citation37] ultrasound,[Citation38] modified atmosphere packaging,[Citation39,Citation40] and cold plasma [Citation41] have gained in popularity in recent years. Certain of these emerging technologies can be used for assisting the traditional conservative approaches such as freezing [Citation42] or other preservative technologies.[Citation43]
Food quality, safety, and authenticity are important issues that have attracted much attention in recent years from the industry, the scientific community, and consumers. Traditional analytical methods used in food analysis are characterized by several challenges, encouraging the development of novel tools and instruments.[Citation44–47] Moreover, the outbreak of the COVID-19 pandemic has highlighted the need for the development of analytical techniques that reduce human contact with food products.[Citation48] Analytical techniques, especially hyperspectral imaging [Citation49–51] and mass spectrometric fingerprinting,[Citation52–54] are two promising approaches that have been extensively studied to fight against food fraud and ensure food authenticity in a rapid and non-targeted manner.
Some of the aforementioned preservative, processing, and analytical approaches are currently in use in the food industry, while others are still in progress and need refinement. The advent of the fourth industrial revolution (Industry 4.0) technologies could accelerate the transition of these technologies from the laboratory to the industry. Industry 4.0 has recently emerged with the convergence and interaction of biological, physical, and digital worlds in which automation, digitalization, and networking play a crucial role.[Citation55–57] Industry 4.0 has been gaining momentum, being an incentive to address critical global challenges and to achieve sustainable development.[Citation58–60] The major Industry 4.0 technology clusters that are more relevant to the food industry are Artificial Intelligence (AI), smart sensors, autonomous robotics, the Internet of Things (IoT), big data, blockchain, additive technologies, and advanced nano-biotechnology, among others.[Citation6,Citation61–63]
Several review papers dealing with either thermal/nonthermal processing techniques[Citation10,Citation28,Citation64] or analytical methodologies[Citation65–67] have recently been published. However, a comprehensive review that covers a wide range of processing, preservation, and analytical technologies in muscle foods in the age of Industry 4.0 is not available. To the best of our knowledge, this work is the first to raise awareness of the importance of considering a wide range of emerging technologies simultaneously, meeting the key principle of Industry 4.0. Thus, this manuscript will highlight the main Industry 4.0 technologies and show how to harness these technologies, especially those associated with preservation/processing and analytical techniques, to address current challenges in the muscle food industry. This manuscript will first give a general overview of key technologies associated with Industry 4.0. Emerging technology breakthroughs in processing, preservation, and analytical techniques will be then discussed. Finally, future perspectives will be highlighted and briefly explained.
Industry 4.0 in the food field
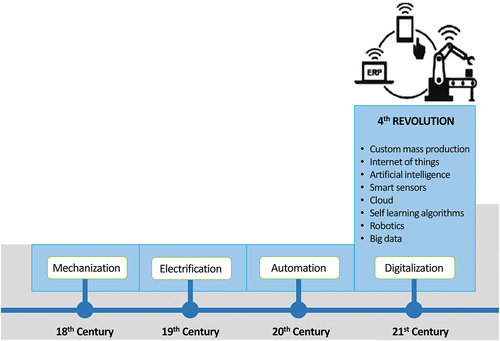
All industrial revolutions have characteristics that allow them to be classified as revolutions rather than simply evolutionary changes in the industry (). Industry 4.0 is occurring because of advancements in many technologies, such as smart sensors, additive manufacturing, robots, IoT, blockchain, AI, and other information and communication technologies.[Citation61,Citation68,Citation69] These technologies have enabled custom mass production with increased productivity, flexibility, and efficiency.[Citation70,Citation71] Several publications argued that Industry 4.0 technologies could help to achieve several United Nations Sustainable Development Goals.[Citation71] By adopting new technologies and harnessing Industry 4.0 technologies, a digital transformation of both manufacturing/production and consumption is currently taking place.[Citation62,Citation69,Citation72]
The implementation of Industry 4.0 principles offers several possibilities in the meat and muscle-based food industry. One example is the significant advancement in several spectral fingerprinting techniques used for online measurement of composition and quality predictions, safety, and authenticity of muscle foods. Miniaturization of spectral techniques has driven the development of portable and hand-held devices in recent years.[Citation46,Citation73–75] Moreover, smartphones are more and more used as promising biosensors for non-invasive, portable food quality assessment.[Citation76] Additive manufacturing or 3D printing constitutes another example of technologies that have boomed in the age of Industry 4.0. Although it is still at the conceptual stage, 3D food printing offers numerous possibilities for the development of tailored animal protein-based products, such as meat and other muscle food products.[Citation77,Citation78]
Robotic technology is advancing in all fields, including the muscle food industry. For instance, robots can be used in cutting and packaging in meat processing industries, or in collecting data such as temperature, relative humidity, and ammonia concentrations in poultry barns.[Citation79] Misimi et al.[Citation80] developed a novel robotic 3D vision-guided concept for chicken fillet harvesting, while a similar intelligent robot was recently designed for half-ship cutting.[Citation81] A detailed overview of the possibilities and limitations of implementing different robotic technologies in the food sector was provided.[Citation82] More recently, several intelligent robotic systems used for carcass cutting, deboning, and other relevant automation in abattoirs were reviewed in detail.[Citation83]
The food industry has seen unprecedented digital shifts due to the COVID-19 pandemic, which has put enormous pressure on food supply chains, with consumers being at the centre of this transformation. There has been an increased focus on food sustainability with a surge in demand for food availability, convenience, and traceability. Blockchain technology, which is a decentralized, distributed data structure and public digital ledger, has recently been suggested for food traceability and enhancement of sustainable operations.[Citation74,Citation84] This technology provides information on the entire history of a product as it travels along the whole supply chain. For instance, it can be applied to trace fish lots, back and forth, throughout the entire fisheries value chain.[Citation85,Citation86]
Recent advances in muscle food processing methods
With the ever-growing global population, technology has an essential role today in assisting the food industry to progress toward more sustainable production and consumption systems.[Citation87] In fact, the recent technological advances and innovations that emerged in the age of Industry 4.0 have enabled a digital transformation of food production systems to meet new food production requirements, including among others less resource and energy consumption and less waste, better food quality and safety, and more diversity and convenience to consumers. Advanced engineering research has resulted in the emergence of a large variety of “green technology” based on thermal and nonthermal processing techniques in the food industry. In the muscle food sector, several innovative technologies including ohmic heating (OH), radio frequency (RF), pulsed electric field (PEF), cold plasma (CP), high-pressure processing (HPP), as well as ultrasound technologies have been under exponential development and some of them are already adopted by certain food sectors.
Thermal processing
Thermal processing can be used in muscle food products for several purposes such as cooking, thawing, extraction, pasteurization, sterilization, enzyme inactivation and microbial decontamination.[Citation17,Citation88] Conventional methods used for cooking muscle food involve the use of hot water or steam, leading to surface overheating with slow heat conduction while waiting for the interior to reach the suitable temperature.[Citation89–91] To overcome these shortcomings, intensive research has been conducted in the field of OH, RF, and microwave cooking of muscle foods ().
Table 1. Effects of different thermal treatments on various muscle foods.
The OH is based on the production of heat as a result of the electrical resistance of a material to the flow of electric current.[Citation110,Citation111] In contrast to traditional cooking approaches, OH cooking has the benefits of shortening cooking time, providing regular temperature distribution and higher heating yields. In addition, ohmic-cooked muscle food has a much more constant appearance, better gelation characteristics, less cooking loss, softer texture, and more satisfactory tenderness compared to traditional cooking methods.[Citation26,Citation111–113] Therefore, OH can be successfully integrated with a design of Industry 4.0 driving the development of environmentally friendly technologies.[Citation114] Due to the growing demand for rapid thawing methods, the potential of OH was investigated as an alternative method in meat and fish products.[Citation115,Citation116] For instance, ohmic thawing is a promising alternative thawing method for minced beef, providing a shorter process time and less water loss compared to conventional thawing methods. Besides, OH was applied to loosen the connection between the shell and meat, improving the peelability of shrimps (Pandalus borealis).[Citation97] In another recent study, the application of OH at 120 voltages for 5 min was found to be suitable to process green mussel meat, achieving higher nutritional quality and lower loss compared to conventional cooking methods, such as steaming and boiling.[Citation117]
As OH, RF heating is a volumetric heating technology that may be employed in many applications in the food industry. However, thawing frozen products is one of the main applications of RF in muscle foods.[Citation89,Citation103,Citation118] The impact of RF on the thawing process was explained by ionic displacement and polar molecules in frozen samples that induce dipole rotation, converting the energy of the electromagnetic wave to heat, leading to thawing.[Citation89,Citation118,Citation119] For example, Bedane et al.[Citation103] showed that RF thawing could be performed on beef blocks using a moving conveyor belt. According to the authors, the movement and the rotation of frozen lean beef meat block can promote the redistribution of the electromagnetic field and improve the heating uniformity. They also explored the effects of different processing parameters and conditions on the heating uniformity of samples under static and moving situations on the conveyor belt. The results indicated that RF at a frequency, power, and conveyor belt speed equal to 27.12 MHz, 6 kW and 3 m/hr, respectively could moderately improve the heating uniformity. Despite the limited applications of RF in the muscle food industry presently, it is believed that the arrival of Industry 4.0 technologies would accelerate the processes and RF technology optimization. For example, computer modelling and simulation of RF heating could be one of the main research directions due to the rapid increases in computation power and improvements in commercial software.[Citation120,Citation121]
The implantation of microwaves, which are electromagnetic waves having frequencies ranging between 300 MHz and 300 GHz corresponding to wavelengths ranging from 1 m to 1 mm, has revolutionized the way food is processed and prepared both commercially and domestically.[Citation91,Citation122,Citation123] Microwave cooking can be considered an alternative and efficient method to minimize time, preserve nutritional quality, reduce cost and save energy due to volumetric heating. However, microwave heating was traditionally associated with several issues, including uneven heating/cooking, creating hot and cold spots, inability to brown food, and excessive drying of foods. In addition, the penetration depth of microwaves is affected by the dielectric properties of food, which in turn are affected by the moisture content. It should be highlighted that the penetration depth of microwaves into foods depends among others on frequencies, noticing that lower frequency can penetrate deeper into food. That is why frequency at MHz level (penetration depths are large) is used for industry processing, whereas frequency at GHz level (penetration depths are small) is used for household microwave ovens. It should be also noticed that the penetration depth of microwaves varies with other factors, such as temperature and salt contents, etc.[Citation124,Citation125]
Compared with OH and RF, microwave heating is more suitable for household applications. Recent advances in microwave technologies, such as the use of solid-state generators or coupling microwaves with other emerging processing technologies [Citation126] could expand the application range of microwaves. For example, the use of solid-state generators as alternative power sources to conventional magnetrons could be considered a techno-economically promising solution that has the potential to control frequency and power, increase repeatability and reproducibility, reduce energy consumption, and overcome the non-uniform heating of traditional microwaves. Such advanced applications (i.e., the use of solid-state and/or coupled technology) could pave the way for the development of smart cooking/kitchen and smart processing systems,[Citation127] which is in line with Industry 4.0 principles.
Nonthermal processing
PEF and CP are related to high-voltage pulsed technologies, and both employ a similar food treatment experimental setup (). The PEF system is comprised of a pulsed power source and metal electrodes in a treatment chamber.[Citation37] The high voltage and ground electrodes are both solid for PEF, while only the ground electrode is solid for CP. The high voltage electrode of CP is a hollow metal pipe with a needle tip. This hollow pipe is filled with a working gas and a strong electric field or voltage triggers the breakdown of gas molecules. The PEF and plasma jet ground electrodes are coupled to a high-voltage pulsed power supply through a current-limiting resistor.
Figure 2. Schematic assembly (a) Pulsed electric field (PEF) treatment, (b) Cold plasma (CP) treatment.
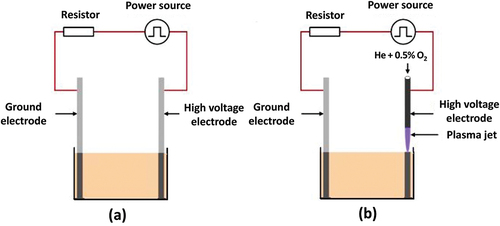
Meat tissue is a single layer of longitudinal muscle cells, and a PEF treatment cut the muscles across the fibre direction. PEF meat treatment is done in either a batch or continuous treatment chamber. The batch chamber is filled with fluids depending on the number and kind of treated meat pieces. The treatment of cross-contamination makes this approach undesirable in meat processing. Other possibilities for continuous treatment of meat parts might include conveying belts, cartridges or pistons to transport slightly compressed meat pieces via electrode systems. Cell electroporation, in PEF-treatment, increases membrane permeability to normally non-permeable molecules, allowing molecular transport and improving meat tenderization.[Citation128,Citation129] Scanning electron microscopy of the treated food demonstrated that PEF-treated meat displayed pore development in connective tissue.[Citation130]
The synergistic effect of PEF may expedite proteolysis, resulting in increased meat tenderization on frozen samples. Faridnia et al.[Citation131] stored beef muscle samples (4°C) before applying moderate PEF treatments with varying electric field strength and frequency (0.2–0.6 kV/cm, 1–50 Hz, 20 s). They found no significant variations in colour stability, pH, cooking losses, or protein profile in the meat. However, Ma et al.[Citation132] discovered that samples of cooked lamb meat chops treated to lengthy storage duration and frozen-thawed pre-treatment before PEF resulted in substantial increases in volatile chemicals owing to lipid and protein oxidation. Therefore, building a thawing phase before PEF treatment is critical to achieving a good meat product.
PEF-treated meat showed better mass transfer during drying and better water-binding during cooking due to increased micro diffusion of brine and water-binding chemicals. Khan et al.[Citation133] revealed that high PEF (10 kV, 200 Hz and 20 s) treatments may severely impact beef quality compared to low PEF treatments (2.5 kV, 200 Hz and 20 s). With another research, the function of the most critical process parameters (150 vs. 300 and 450 vs. 600) and (0.60 vs. 1.20 kV/cm) in assessing the influence of PEF on the critical technical qualities of chicken meat was investigated.[Citation134] The authors found no effects on pH or brightness or yellowness. However, there was a considerable tenderizing impact of PEF treatment on beef[Citation129,Citation135]; and a meta-analysis found that PEF increased beef tenderization by 20%.[Citation136] In addition, a PEF combined with mechanical pressing offers a platform for the extraction of functional compounds from meat wastes.[Citation137] Ghosh et al.[Citation138] demonstrated that PEF may be utilized to produce protein-rich functional products from biorefinery waste. This technique should encourage farmers and meat processors to recycle trash. Zhou et al.[Citation139] demonstrated that PEF extraction is quicker and yields more protein than standard approaches.
PEF methods used for post-mortem tenderization to enhance meat quality have shown modest promise in limited research with muscle foods. However, optimal processing parameters seem to vary amongst muscles. Moreover, not many research discusses the customization of PEF treatments and their impact on the various quality features of meat.[Citation140] As a result, the variable influence on meat texture across various muscles must be addressed, and further research is needed to figure out how PEF affects muscle structure. More examples of the application of PEF on muscle foods can be found in .
Table 2. Effects of pulsed electric field (PEF) and cold plasma (CP) treatments on various muscle foods.
CP has been tested in chicken,[Citation145–148] beef,[Citation149,Citation150] and pork.[Citation151–153] A 300-second CP exposure provided a maximum decrease of 1.5 logs bacterial load using a high oxygen atmosphere, but utilizing air or high nitrogen atmospheres resulted in lesser antibacterial effectiveness.[Citation154] Moutiq et al.[Citation146] detected a 2-log CFU/g decrease in natural chicken microflora after 5 minutes of treatment and 24 hours of storage at 100 kV for 1, 3, and 5 minutes. Wang et al.[Citation155] tested the impact of voltage and time on in-package raw chicken meat. After 24 hours at 4°C, CP treatment with 55, 65, or even 80 kV for 3 minutes did not affect raw chicken breast meat microbial populations. Pérez-Andrés et al.[Citation156] found that CP treatment altered functional capabilities depending on the protein’s natural structure and nature. Roh et al.[Citation148] found that CP treatment results in homogeneous microbial inactivation in stacked or non-stacked meat cube samples regardless of sample location or surface-to-volume ratio.
HPP is another promising technique that has been widely used to get rid of microorganisms and undesirable enzymes and extend the shelf life of muscle food products. The efficiency of HPP in destroying spoilage microorganisms and deactivating food enzymes can be influenced by various factors including process parameters, type and growth period of microorganisms, and the type of food being processed. HPP enhances the water holding ability of muscle foods, thus keeping the food fresh for a long time. Pressure levels between 100 to 600 MPa are generally used to extend the shelf life of muscle food products.[Citation157] The common mechanism of microbial destruction by HPP includes many aspects, such as modification in the cell wall, and cell membrane, as well as protein and enzyme function.[Citation158] When HPP is used at commercial levels (), pressure ranges from 400 to 600 MPa with an extreme temperature of 15°C.[Citation174]
Table 3. Effects of high-pressure processing (HPP) and ultrasound (US) technology on various muscle foods.
Previous studies showed that high-pressure levels (>100 MPa) completely deactivate microorganisms while moderate or low levels of pressure (10–50 MPa) only decrease the reproduction and growth rate of microorganisms. Microbial deactivation by HPP can also be effected by different parameters involving water activity, temperature, pH, concentrations of sugar and salt as well as the time of process implementation.[Citation28,Citation31,Citation175] A comprehensive overview of the effect of HPP on the physical, chemical, microbial, and nutritional quality attributes of crab meat was carried out in a recent study.[Citation35] In another recent study, the application of HPP treatment at 600 Mpa for 8 min could be efficient for reducing L. monocytogenes in dry-cured sausage and loin.[Citation171] More applications of HPP in muscle foods and other food products have been reviewed thoroughly in recent papers.[Citation175,Citation176]
Ultrasound is the energy produced through mechanical waves of vibration frequencies higher than 20,000 cycles/sec, which is beyond the hearing capacity of humans. Ultrasound is a newly developed nonthermal green eco-friendly technology that increases the efficiency of food handling procedures in the food industry.[Citation177] It can be used in combination with pressure (manosonication) and with temperature (thermosonication) giving more efficiency in food handling and processing.[Citation178] In addition, the technique can be applied in combination with other existing and novel technologies including HPP, microwave, supercritical CO2, and enzymatic extraction, among others.[Citation179,Citation180]
Ultrasound techniques can be classified into two categories (i.e., high-intensity ultrasound and low-intensity ultrasound) according to the frequency and intensity ranges.[Citation177,Citation179,Citation181] Ultrasound technology inactivated microorganisms by producing cavitation in the liquid media containing the sample, generating free radicals (e.g., H2O2 and their hydroxyl radicals) that destruct the cell membrane.[Citation177,Citation180] The antimicrobial activity of high-intensity ultrasound could be influenced by several parameters such as temperature, type of microorganism, contact time with microorganism, and quantity and composition of treated food.[Citation182] The main applications of ultrasound in muscle foods include thawing, tenderization, curing, and decontamination.[Citation181,Citation182]
The application of CP and ultrasound technologies is still very limited in food processing in general and in the muscle food industry in particular. However, with the considerable advances offered by Industry 4.0 technologies, it is expected that these technologies could be implemented in the food industry in near future.
Impact of emerging technologies on the protein digestibility of muscle food
Muscle foods are an important source of dietary proteins and can fulfil the protein requirement of the human body.[Citation183] As discussed before, muscle foods are subjected to different processing methods, both thermal and nonthermal, before they are ready for human consumption. Salting, smoking, curing, marinating, drying, and chilling are among the most used treatments before and during manufacturing processes. These nonthermal processing methods have the potential to affect the digestibility of meat proteins and have been widely studied.[Citation183] Meat and meat products are also subjected to various thermal processes such as oven roasting, frying, braising, broiling or stewing, either by the manufacturer or by the consumer. While the effects of thermal processing on the digestibility of muscle proteins have been widely studied,[Citation17] little information is available on the effect of emerging technologies on the digestibility of muscle proteins. Only recently a review [Citation184] was published highlighting the effects of emerging technologies on the digestibility of meat and seafood proteins.
Several emerging technologies are studied for various applications in the meat industry. Nonthermal technologies such as PEF, ultrasonication, HPP, and shockwave technology are widely applied to fresh and processed meat products, inducing various beneficial effects such as improved tenderization and microbial quality.[Citation141,Citation185,Citation186] Thermal-based emerging technologies including OH, or re-emerging technologies (e.g., sous-vide) are becoming popular and widely studied as alternative processing methods for the development of minimally processed foods.[Citation187] Limited literature is available on the effect of emerging technologies on the digestibility of meat and seafood proteins and needs scientific attention. shows the underlying mechanisms for different emerging technologies, which improve the digestibility of muscle proteins.
Figure 3. Mechanisms of the pulsed electric field, high-pressure, ultrasound, and shockwaves for improving the digestibility of muscle proteins.
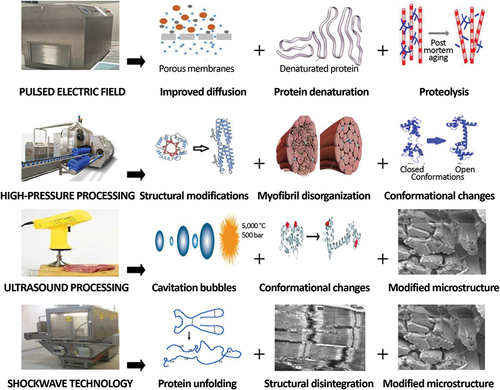
Overall, a positive effect of PEF processing has been reported on the digestibility of beef and venison proteins. Baht et al.[Citation142,Citation188] reported a positive effect of PEF treatment on the digestibility of beef Longissimus dorsi and Biceps femoris, respectively, during in vitro gastrointestinal digestion. A similar effect of PEF treatment was also observed on the protein digestibility of deer Longissimus dorsi during in vitro gastrointestinal digestion.[Citation189] High-intensity treatments (10 kV, 20 or 90 Hz, 20 µs) were more effective in improving the digestibility of the proteins. While these studies used uncooked meat proteins, a positive effect of PEF treatment was also confirmed on the protein digestibility of cooked beef and venison muscles.[Citation190,Citation191]
Whereas Alahakoon et al.[Citation192] did not observe any effect of PEF treatment (0.7–1.5 kV/cm) on the protein digestibility of sous-vide processed beef brisket, Chian et al.[Citation193] reported an increase of 18 to 31% in the protein digestibility of PEF-treated beef Longissimus thoracis (1.0–1.25 kV/cm). This positive effect on protein digestibility was attributed to various protein structural modifications and microstructural changes induced in the food matrices by PEF treatments.
Most of the studies have reported a positive impact of HPP on the digestibility of muscle proteins. Kaur et al.[Citation194] observed faster hydrolysis of the beef proteins treated with HPP (175 and 600 MPa) during in vitro gastrointestinal digestion. The effect was more pronounced for the samples treated at 600 MPa. This positive effect was attributed to pressure-induced structural and microstructural modifications and other related changes such as protein solubilisation and denaturation. A significant increase was reported by Xue et al.[Citation195] in the protein digestibility of cooked rabbit meat batters treated with HPP (100–300 MPa, 9 min, 25°C) during in vitro simulated digestion. An increase of 6.13% and 61.31% was recorded for HPP-processed samples during gastric and intestinal digestion, respectively. Cepero-Betancourt et al.[Citation196] observed a positive effect of HPP processing (200–500 MPa, 5 min) on the digestion of abalone muscle proteins during in vitro gastrointestinal digestion. The effect was independent of the magnitude of the applied pressure and was attributed to pressure-induced unfolding of the proteins. Rakotondramavo et al.[Citation197] observed a significantly higher digestibility and digestion rate for HPP-treated cooked ham compared to raw meat after in vitro gastrointestinal digestion.
Like PEF and HPP, ultrasonication seems to have a positive effect on the digestibility of muscle proteins and induce conformational changes which lead to the exposure of enzyme cleavage sites.[Citation184] Dong et al.[Citation198] reported an increasing trend for protein digestibility with processing time (20 kHz, 400 W, 0–20 min) and significantly higher values were observed for the shrimp samples processed for 20 min. Bagarinao et al.[Citation199] found similar results that are a significantly higher protein digestibility for ultrasound processed (20 kHz, 464 W, 5 min) abalone (Haliotis iris) samples after in vitro gastrointestinal digestion.
Recently, a positive impact of shockwave technology, an emerging technology that uses mechanical high-pressure pulses produced by high-voltage electrical discharge, was reported on the digestibility of muscle proteins. Chian et al.[Citation200,Citation201] studied the effect of shockwave processing (0.57 Hz, 35 kV, 18 μF, 11 kJ/pulse) on the digestibility of beef brisket proteins using in vitro gastrointestinal and in vitro gastric digestion models, respectively. Both these studies reported a positive and significant effect of the shockwave processing on the protein digestibility of the beef samples. This positive effect was attributed to various structural and microstructural changes induced by shockwave processing.
Current trends and advancements in muscle food preservation techniques
Traditionally, ice, drying, smoking, fermentation, or salting have been widely used as preservative treatments to maintain the quality of food.[Citation202] More advanced techniques have emerged in recent years as a result of the implementation of Industry 4.0 technologies. In the following sections, current trends and advancements in muscle food preservation techniques are discussed.
Freezing-based techniques
Freezing, one of the most ancient preservation techniques, firstly used cryogenic freezing, plate contact freezing, and air blast freezing.[Citation203] Nevertheless, these techniques sometimes present poor freezing rates and can trigger cellular damage due to ice crystals and protein denaturation. Thus undesirable effects such as changes in texture, water-holding capacity (WHC), color, etc. are taken place.[Citation42,Citation204] Considering these inconveniences, new trends include other advanced techniques such as high-pressure freezing (HPF), electrically assisted freezing (EF), magnetically aided freezing (MF), ultrasound-assisted freezing (UAF), microwave-assisted freezing (MAF), osmo-dehydro-freezing (ODF) and antifreeze protein (AFP).[Citation42,Citation205] Some examples of the application of freezing-based techniques are tabulated in . The HPF began to be used in the late 1990s although it has not been extensively applied to food matrices yet. In the literature, a few examples of muscle foods (i.e., abalone, pink salmon, or tuna) are available [Citation211,Citation223] whereas a higher number of applications focused on the use of high-pressure pretreatment before freezing.[Citation206,Citation208] The principles of HPF differ in small particularities though they all can reduce quality loss because of the formation of small ice crystals uniformly distributed.[Citation224,Citation225] The EF, MF, or the combination of both referred to as electromagnetically freezing (EMF) are known to modify the molecular structure of water and have been used since the early 2000s.[Citation214,Citation226] The EF might break and debilitate the hydrogen bonds of water molecules leading to a less ordered structure or contrarily, reorganize the water molecules thus reducing the free energy. The MF directly affects water by organizing water molecules, increasing hydrogen bonding, and weakening the van der Waals bonding force.[Citation212,Citation226,Citation227] Recently, different works have applied this technology to muscle foods, but further studies are needed to prove its effectiveness.[Citation212,Citation214] UAF uses a frequency between 20 and 100 kHz in food processing. UAF can accelerate the freezing rate due to the enhancement of ice nucleation, increasing the rate of mass and heat transfer and controlling the size and formation of ice crystals.[Citation228,Citation229]
Table 4. Freezing-based techniques applied to muscle food products.
Although several studies focused on fruits and vegetables, UAF application has been improved in the last few years on muscle food products.[Citation230] Among these studies, most of them used ultrasound-assisted immersion freezing (UIF), which uses a liquid medium for ultrasound transmission thus significantly shortening freezing time.[Citation217,Citation231] MAF is still one of the less applied techniques, based on the use of 2.45 GHz microwave irradiation to better maintain the structure of the food while freezing.[Citation232] MAF has been applied to fresh pork tenderloins while radiofrequency waves have been used in fresh rainbow trout fish.[Citation233,Citation234] However, further efforts need to be made towards the development of this technique. Regarding ODF, its application is still very limited and no applications on muscle food products have been found in the literature.[Citation235]
Packaging and other non-thermal treatments
Packaging, such as modified atmosphere packaging (MAP), is known to increase perishable food safety and shelf life. Together with other non-thermal treatments such as the use of natural preservatives or the application of nanotechnology can prevent microbes and enzyme activity in muscle foods maintaining sensory quality characteristics.[Citation32,Citation42,Citation236]
Natural preservatives
The use of natural preservatives is one of the most recent trends among consumers and the scientific community. This increment is mainly due to the consumer´s concern about synthetic additives thus changing to natural strategies to extend the shelf-life of food products.[Citation202,Citation237] Among the different properties of these natural preservatives, antioxidant and antimicrobial activities have been highlighted for their combined use to prevent contamination and the loss of the sensory quality characteristics of muscle foods. Therefore, the compounds most used can be classified according to their origin: vegetal (e.g., essential oils and plant extracts), animal (such as chitosan, lysozyme) or microbial (bacteriocins) sources.[Citation237–240] In this section, those compounds that are fundamentally used directly on the food product are addressed. However, the same compounds can be included in edible films and coating for packaging purposes. Some of the most recent studies using natural preservatives in muscle foods are compiled in .
Table 5. Packaging and other non-thermal treatments applied to muscle food products.
Regarding natural additives of vegetal origin, plant extracts and essential oils are the most used as food preservation techniques. Oregano, thyme, or rosemary have been successfully used to preserve not only muscle foods but also other such plants (e.g., orange, pomegranate). Essential oils (EOs) and phenolic compounds, together with terpenes and alkaloids are secondary metabolites present in plants which usually present antioxidant and antimicrobial activities, among others.[Citation237,Citation263,Citation264] Therefore, natural additives are an alternative to synthetic preservatives, although more research is still needed towards the industrial application of these molecules.
Edible films and coatings
Edible films and coatings are considered primary packaging systems made from edible ingredients that have been proposed as alternative methods for food preservation.[Citation265] In recent years, they have been increasingly studied owing to different advantages over synthetic materials used for food packaging, namely high efficacy in retarding food degradation, extended shelf life, lack of toxicity, as well as an eco-friendly character.[Citation249Citation266].
The main difference between films and coatings is related to their application procedure. Edible films are prepared separately as solid sheets and then used to cover the surface of the food, whereas coatings are formed directly onto the food surfaces.Citation266,Citation267] Most the edible materials are formed from natural biopolymers from animal-derived compounds (chitin, chitosan), plant-derived (cellulose, starch, pectin,), seaweed-derived (agar, alginate, carrageenan) as well as microbial-derived (pullulan, xanthan gum). In addition, lipid compounds such as oil, resins or waxes have been used and protein-based films (gelatine, collagen and milk, soy or whey proteins) are being widely investigated as well.[Citation236,Citation265] As happened with plant extracts and EOs, edible coatings and films act as antioxidant and antimicrobial agents due to the bioactive molecules. In recent years, different studies have tested the efficacy of these materials in different muscle food products (). The successful results obtained with these materials have led to this trend having considerable relevance in the preservation of muscle food and continues to be further explored.
Recent development and application of analytical techniques for muscle foods
Spectroscopic and hyperspectral sensors
Advanced analytical tools have been developed over the years giving the possibility to realize simple and rapid measurements with or without sample contact in the specific fringe of the electromagnetic spectrum, Vis (Visible), UV (Ultraviolet), MIR (Mid-infrared), NIR (near infrared), Raman, and fluorescence. Each spectral range is associated with specific chemical and physical information about the molecular content of the studied sample. For example, the NIR (780–2,500 nm) and MIR (2,500–25,000 nm) infrared ranges are associated with the absorption spectrum of organic molecules[Citation268] with fundamentals vibrations observed in the MIR and combinations/overtones vibrations observed in the NIR range, respectively. Raman spectroscopy is based on the inelastic scattering of light observed after radiation with monochromatic light of an organic molecule.[Citation269] Fluorescence can be considered as the emission of lower energy light by a fluorophore after excitation by UV (200–400 nm) or Vis (400–700 nm) light.
Hyperspectral imaging (I), also called chemical or spectral imaging, can be considered one of the most recent and disruptive innovations or development in the field of spectroscopy sensors and was proposed for the first time for airborne detection and mapping.[Citation270] Food products, especially muscle foods are generally anisotropic and can have high local heterogeneity in physical properties (e.g. size and shape) and chemical composition (e.g., fat, protein, and collagen) making it challenging to control and optimize the quality of final food product. TIHSI technique is very smart since it gives the possibility to both record spectral and spatial information of the analyzed sample, allowing addressing this heterogeneity challengesIhe HSI data analysis is generally associated with multivariate or chemometrics techniques (e.g., principal components analysis, partial least squares analysis and artificial neural network) to build predictive models or to visualize quality treats variations based on distribution maps of muscle food products (e.g., fish, chicken, red meats).[Citation271–273] HSI sensors to evaluate the quality of treats (e.g., sensory properties, grade, and nutrition properties) of muscle food products has been the subject of different research/review papers and book chapters.[Citation48,Citation75,Citation274] Therefore, its interest in the field of the implementation of this technique in muscle food industry 4.0 is no longer to be proved.
An HSI sensor generally contains four elements, including a “brain” (a computer with appropriate software), a sample stage, a “vision” system (CDD hyperspectral camera), and an illumination source (e.g. tungsten-halogen, UV lamp). The illumination source should be chosen carefully depending on the application, to provide illumination homogeneity and prevent heating effects.[Citation275] When using UV lamps, a cooling system must be included in the system. For example, in the study of Zhuang et al.[Citation276] two DC cooling fans (12 V, 5.4 W) were used in the HSI analysis of meat pork.
HSI sensors generate 3D data or hypercube including two spatial dimensions (length: X and width: Y), and one spectral dimension (I) (). The HSI sensors can provide images using three configurations: reflection, transmission, and interactance. In general, in muscle product analysis, the reflectance mode is the most used probably because it is the most convenient and informative.[Citation277] Depending on the image acquisition procedure, four techniques can be used to record 3D HSI, the whiskbroom (i.e. point scan), pushbroom (i.e. line scanning), tunable filter (or staring), and snapshot systems. The selection of the mode to use in the muscle food industry is affected by different factors, such as the application targeted (e.g. out-line, at-line, in-line), and the device cost and its robustness.
Figure 4. Different sensing and image acquisition modes that can be used for muscle foods analysis by hyperspectral imaging.
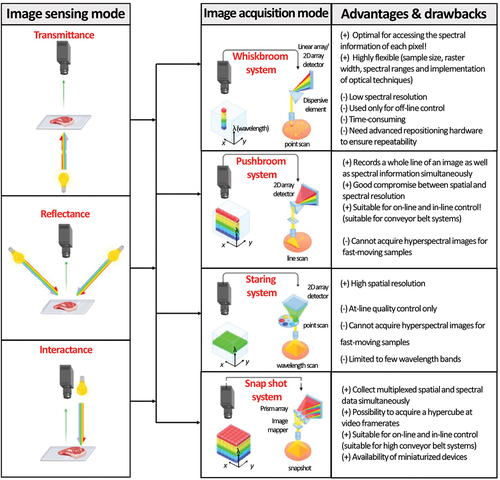
The simplest approach is called staring, in which an image plane is collected at one waveband after another with a fixed image field of view. In this configuration, the system generally uses filters (band-pass filters, a circular-variable filter, a liquid-crystal tunable filter or an acousto-optical tunable filter),[Citation278] giving the possibility to record simultaneously both spatial dimensions (X and Y) while the spectral dimension is acquired sequentially.[Citation279] However, this system is not well adapted to process monitoring because acquisition is quite slow and the sample needs to be in a fixed position which makes it poorly compatible with the high production rate that is generally required by the muscle food industry. This acquisition technique is more adapted for multispectral (MSI) systems. The MSI system proved its effectiveness in predicting different properties of muscle foods (e.g. microbial spoilage, muscle breed discrimination, sensory properties, chemical composition, and adulteration detection).[Citation280–287]
The whiskbroom or point-scanning mode gives the possibility to record the full spectrum on a single pixel of the sample image. Therefore, the three dimensions (λ, X, and Y) are recorded separately and the sample is moved to give the possibility to the HSI camera to map the entire pixels of the sample image. With this mode, an optical grating, prism or a similar element is required to achieve light dispersion. However, since a double scan (i.e. spatial and spectral) is required, the acquisition time is a barrier to its implementation on a food processing line. This acquisition mode has mainly been used in microscopy analysis of muscle foods. For example, microspectroscopy was used to evaluate the thermal denaturation of proteins in the muscle fibre and connective tissue of bovine muscles[Citation288] and to study the effects of high-pressure treatment on the muscle structure of salmon (Salmo salar).[Citation34]
The push broom can be considered an upgrade of the point-scanning system. The principle is based on line-scan acquisition in which each line contains full-spectrum information for every spatial pixel. Thus, the spectral dimension and one spatial dimension are acquired simultaneously, while the second spatial dimension is acquired sequentially. Similar to whiskbroom instruments, a dispersing element is used in the spectrograph. However, as an entire line of pixels is recorded at once, a two-dimensional dispersing element and a two-dimensional detector array are required. As this method does not require the changing of filters and only requires the sample to be moved in one direction (the direction of the second spatial dimension), it is well suited to implementation for non-destructive evaluation of quality control during processing. This mode is the most commonly used for online applications in muscle food analysis.
This technique was investigated to predict or monitor chemical composition (e.g. moisture, total fat, protein, and biogenic amine) and sensory properties of different muscle foods (fish, chicken breast, minced pork, fresh minced beef, lamb cuts, and mutton).[Citation289–291] Recent advances in optical components and data analytics have revolutionized imaging spectroscopy, allowing the emergence of a variety of specialized research and industrial platforms. For instance, a range of advanced cameras (called HySpex) has been developed by a Norwegian research and development organization to be used for various applications, including quality control and inspection of fish. This system can be used for sorting raw material passing through the production lines based on different qualities, or even fish species in real time.[Citation292]
The most recent HSI sensors are called single shot or snapshot HSI.[Citation293] This state-of-the-art technology has been developed recently taking advantage of the advancements that Industry 4.0 has provided (e.g., the availability of a larger amount of spatial resolution). These sensors are capable to record both spatial and spectral information in all the object area in one shot without scanning. This system provides advantages such as collecting HSI images at video frame rate making the sensors more appropriate for real-time applications, ultra-portability or miniaturization. The acquisition rate (e.g. 150 frames per second permits) of these devices permits to have a fixed platform and detector and therefore more robustness of the acquisition system. This technology is developed by different companies; for example, an HSI camera presenting 50 bands in the 450–850 nm range, a frame rate higher than 15 Hz, a resolution of 250 × 250 pixels, with small dimensions (29 × 29 × 49 mm) is proposed by Cubert Company.
In the past few years, snapshot HSI systems have gained attention in the research area of muscle food processing. For example, Ma et al.[Citation294] used a single shot camera (SPM-EVM-VIS, Interuniversity Microelectronics Centre, Leuven, Belgium) with 16 bands (465, 474, 485, 496, 510, 522, 534, 546, 548, 562, 578, 586, 600, 608, 624, and 630 nm) and a rate of 340 fps to evaluate protein content of processed pork meat (freezing, thawing, salting and drying). The prediction models, combining Back Propagation with Neural Network and HSI absorbance spectra, gave good cross-validation results for the protein content of the different processed samples (R2CV = 0.8318 and RMSECV = 8.38 mg/g). In another study,[Citation295] two snapshot HSI cameras were used in the NIR (25 wavelengths between 673–957 nm) and VIS (16 wavelengths from 467–639 nm) range to discriminate between three red meat species (pork, beef, and lamb). The exposure times of the snapshot were 2 ms and 3.9 ms for the NIR and VIS cameras, respectively. The combination of the HSI image features and a new chemometrics method (3D-CNN: deep 3D convolution neural network) gave discrimination models with good overall accuracy for both NIR (96.9%) and VIS systems (97.1%). However, more research is still needed to fully exploit the potential of the snapshot acquisition mode in more food applications.[Citation51]
The rapid development in imaging and spectroscopic technologies including both hardware and software [Citation291] has been spurred by Industry 4.0, extending the possibilities and providing innovative technologies in emerging applications. Recently, many publications have argued that Industry 4.0 technologies, such as smart sensors based on HSI and spectroscopy can enhance food traceability [Citation296] and food quality.[Citation63] Such smart technologies are connected by networks to software and can help the muscle food industry to move to the next level by enabling real-time monitoring and reducing measurement time. For example, the role of these sensors and digitalization in the move towards smart farming in the livestock industry was reviewed recently by Fuentes et al.[Citation297] In the processing industry of meat, these smart sensors can be used to optimize inventory use by checking the confirmation of the carcass, missing parts, size, and presence or absence of defects, thus sorting meat products into various categories according to their properties.[Citation79]
Advanced mass spectrometry
In recent years, mass spectrometry (MS) methods have been developed for the high throughput non-targeted analysis of muscle foods.[Citation52] Among them, ambient mass spectrometry (AMS) techniques deserve special mention for their innovative approaches and powerful performances. AMS covers a family of techniques that allow the generation of ions under ambient conditions after minimal sample preparation. One of the advantages of AMS techniques is its ability to quickly reveal the food chemical profile[Citation298] that can be used to set up non-targeted methods as recommended by the European Community and the United States Pharmacopeial Convention to successfully face food frauds.[Citation299,Citation300] A graphical representation of the most recent AMS techniques applied to muscle food analysis is reported in . Since no chromatography separation is performed, ambient sources are usually coupled to high-resolution mass spectrometry to obtain very precise information related to the encountered metabolites and to facilitate the identification of the examined muscle food.
Figure 5. Schematics of ambient mass spectrometry techniques that have been applied to authentication of muscle foods. a) Desorption electrospray ionization (DESI-MS)[Citation301]; b) Easy ambient sonic-spray ionization (Easi)[Citation302]; c) Direct analysis in real-time (Dart)[Citation303]; d) Rapid evaporative ionization mass spectrometry (Reims)[Citation304]; e) MasSpec pen[Citation304]; f) Liquid extraction surface analysis (Lesa)[Citation305]; and g) Sheath-flow probe electrospray ionization (sfPesi).[Citation306] Adapted with permission from the publishers (Wiley, Royal Society of Chemistry, ACS, AAAS, and Elsevier).
![Figure 5. Schematics of ambient mass spectrometry techniques that have been applied to authentication of muscle foods. a) Desorption electrospray ionization (DESI-MS)[Citation301]; b) Easy ambient sonic-spray ionization (Easi)[Citation302]; c) Direct analysis in real-time (Dart)[Citation303]; d) Rapid evaporative ionization mass spectrometry (Reims)[Citation304]; e) MasSpec pen[Citation304]; f) Liquid extraction surface analysis (Lesa)[Citation305]; and g) Sheath-flow probe electrospray ionization (sfPesi).[Citation306] Adapted with permission from the publishers (Wiley, Royal Society of Chemistry, ACS, AAAS, and Elsevier).](/cms/asset/e4f348ae-9677-439e-bc49-40ac1b1e73c2/lfri_a_2149776_f0005_oc.jpg)
Desorption electrospray ionization-mass spectrometry (DESI-MS) is one of the most famous and well-established AMS techniques, developed by Cooks and co-workers in 2004.[Citation301] DESI is characterized by a nitrogen-assisted charged solvent that, on hitting the sample surface, desorbs and ionizes the analytes that are then revealed by MS. Although it is not extensively applied to food authentication, it has been recently tested for the screening of paralytic shellfish toxins in clams [Citation307] ().
Haddad et al.[Citation308] created a voltage-free, easy ambient sonic spray ionization (EASI) MS method () able to efficiently desorb and ionize analytes directly from the sample surface. While the technique has been extensively used for the authentication of different types of honey, oil, emulsifiers and propolis,[Citation309] thermal imprints of salmon fillets were screened by EASI-high resolution MS (HRMS) to monitor the impacts of the fish-raising regime on the triacylglycerol composition of salmon fat.[Citation310]
Another well-established and commercially available AMS technique called direct analysis in real-time mass spectrometry (DART-HRMS), has been recently applied for the rapid authentication of thawed-frozen fish [Citation311,Citation312] (). Specifically, DART-MS successfully revealed the freshness of salmon collected from a local market and analyzed at the purchase time and after a few days of storage under refrigerated conditions.[Citation312] In the same vein, Massaro et al.[Citation311] combined DART-HRMS data for the rapid assessment of fish freshness, opening new avenues for the development of species-independent approaches for the differentiation of fresh and thawed-frozen fish.
Rapid evaporative ionization coupled with mass spectrometry (REIMS-) is, nowadays, one of the most widely used AMS techniques in food authentication. REIMS is characterized by the point heating of a sample using a soldering iron, a laser beam or an electronic surgical knife. The aerosol is pulled using a Venturi pump through the tubing, from where part of the sample gas flow is diverted into a mass spectrometer via an impact heater.[Citation313] While Rigano et al.[Citation314] used this technique for the rapid authentication of Mediterranean sea fish species, Song et al.[Citation315] applied it for the discrimination of salmon and rainbow trout. Moreover, efficiency and times of analysis for REIMS-MS and polymerase chain reaction (PCR) were compared for the detection of mislabelled fish species such as cod, coley, haddock, pollock and whiting. The REIMS-MS showed promising performances.[Citation316] REIMS-HRMS is also able to quickly and reliably screen for meat adulteration (2.5% of protein-based adulterants) [Citation317] as well as the fraudulent addition of offals to meats.[Citation318] In the same manner, REIMS-HRMS measured the chemical fingerprints of meat, revealing the characteristic ionic features related to species, geographical origin, breed, types of strip loin sections and their tenderness.[Citation313,Citation319,Citation320]
The same research group compared the power of REIMS-HRMS and DART-MS in determining distinct production systems for poultry. REIMS-HRMS showed >90% accuracy in differentiating organic and conventional poultry, while DART-HRMS showed a predictive ability of >99%.[Citation321] Notably, Abigail et al.[Citation322] implemented the MasSpec Pen technology, a handheld device connected to a high-resolution mass spectrometer that uses a water droplet for gentle desorption and ionization of the sample () for rapid authentication of muscle foods. Different meat and fish types, including grain-fed beef, grass-fed beef, venison, cod, halibut, Atlantic salmon, sockeye salmon, and steelhead trout, were successfully differentiated.
Liquid extraction surface analysis mass spectrometry (LESA-MS – ) is another innovative technology that combines micro-liquid sample extraction with nano-electrospray mass spectrometry.[Citation323] In 2015, a LESA-MS method was developed for the authentication of processed meat products by detecting heat-stable peptide markers. Since skeletal muscle proteins are species-specific, Montowska et al.[Citation324] exploited the potential use of these muscle protein markers for meat authentication. After suitable digestion, peptidic ions derived from myofibrillar and sarcoplasmic proteins were detected and correlated to meat species for the authentication of sausages and minced meat.
Hiraoka et al.[Citation325] developed a point analysis technique for food by using sheath-flow probe electrospray ionization/mass spectrometry (sfPESI/MS). An acupuncture needle, inserted into a fine plastic capillary filled with solvent, was placed on the food surface (). This enabled the solvent preloaded in the plastic capillary to rapidly extract the analytes on the sample surface. After sampling, the probe was moved up to the highest position and a high voltage (HV) was applied. After lifting the probe to the default position, the analytes were transferred into the mass spectrometer via a self-aspirating electrospray source.
Other advanced techniques
Food safety analysis involves well-established techniques such as gas chromatography (GC), MS, ultrahigh performance liquid chromatography (UHPLC), quantitative real-time polymerase chain reaction (qPCR) as well as enzyme-linked immunosorbent assay (ELISA).[Citation326] In addition, some traditional methods are still used for determining muscle food freshness [Citation327,Citation328] including chemical measurements (meat pH, total volatile base nitrogen (TVB-N), and 2,3,5-triphenyltetrazolium chloride (TTC)), microbiological measurements and sensory evaluations. These last two approaches, however, can be very long (bacterial cultures), and dependent on the human factor (high expertise, judgment deviations due to fatigue and subjectivity). Finally, the fact that sensory analysis cannot be used for online measurement is also a significant caveat. There are, however, several studies reported in the literature that constitute promising and innovative applications to improve muscle foods analysis throughout the food chain, providing more efficient alternatives to conventional detection techniques. Among those technologies, there are sensors and immunoassays designed to measure specific analytes, like a gold nanoparticles (NP) sensor for histamine.[Citation329] This biogenic amine can easily be produced in certain fishes, fermented foods and beverages under deficient manipulation, causing many intoxications.[Citation330] Recent and promising advances in the design and development of NP-based sensors, with colorimetric and electrochemical detection, focusing on sensors for assessing food safety, mainly for the detection of chemical (pesticides, heavy metals) and biological contaminants (bacterial pathogens and natural toxins), were reviewed by Bülbül et al.[Citation331]
Within the same subject, Chen et al.[Citation332] fabricated and implemented a novel and low-cost colorimetric sensor array, with a specific calorific fingerprint to volatile compounds. This sensor uses chemically responsive dyes printed on a C2 reverse silica-gel flat plate, to evaluate chicken freshness. In addition, they proposed a novel algorithm, namely AdaBoost – OLDA (orthogonal linear discriminant analysis coupled with adaptive boosting) for sensors data classification and compared it with two classical classification algorithms – linear discriminant analysis (LDA) and back propagation artificial neural network (BP-ANN). Sionek et al.[Citation333] explored the potential of biosensor technology to assess the quality of pork meat, significantly improving meat quality assessment while reducing simultaneously the cost of analysis in meat plants and slaughterhouses. They hypothesized that the biosensors used to measure triglycerides, lactic acid and glucose could be effectively applied to measure these metabolites in natural meat drip loss and that the results could be related to the technological quality of meat.
Choi et al.[Citation334] reviewed paper-based nucleic acid testing (NAT) as alternative to laborious, expensive and time-consuming conventional assays, presenting substantially higher sensitivity and specificity than immunoassays. Non-destructive techniques such as the electronic tongue[Citation335] electronic nose (E-Nose),[Citation336] computer vision (CV),[Citation337] spectroscopic techniques,[Citation338] and artificial tactile (AT) sensory technologies [Citation339] have been proposed for meat and freshness assessment. Nonetheless, since they are used only to detect freshness information parameters (e.g., odour, colour, and rubbery state) these technologies cannot be used to carry out a comprehensive assessment of muscle food freshness or spoilage. Furthermore, they are relatively expensive, time-consuming, labour-intensive, and in addition, require trained professionals to operate specialized instrumentation. This makes these technologies unsuitable for a point-of-need food safety inspection, especially in low- and middle-income countries, where insufficient equipment and facilities preclude modern methods of detection and therefore foodborne illnesses are more prevalent. In this context, the development of simple, cost-effective, and robust analytical devices for muscle-based food safety monitoring are mandatory to create effective prevention and control strategies. Among these, (e.g., three-dimensional paper-based microfluidic and lateral flow test strips devices), microfluidic chip-based devices (e.g., poly(methyl methacrylate) (PMMA), polydimethylsiloxane (PDMS)-based chips), which have significant impact due to their high performance, rapidly gained popularity for use in quality control and food safety.[Citation334,Citation340] Regarding this, Pang et al.[Citation341] developed a self-priming polydimethylsiloxane (PDMS)/paper hybrid microfluidic chip (SPH chip) with mixed-dye-loaded loop-mediated isothermal amplification (LAMP) for multiplex foodborne pathogens detection, using Staphylococcus aureus (SA) and Vibrio parahaemolyticus (VP) for method verification. In turn, Shih et al.[Citation342] developed a simple paper-based ELISA (colorimetric) platform as an innovative point-of-care diagnostic tool to rapidly detect E. coli, and possibly other pathogens, in contaminated foods. This technique is easier to perform, less time-consuming, and less expensive than conventional methods.
Smartphone-based analytical techniques have also become popular for health-related and food safety monitoring.[Citation343] A food allergen testing platform with a specially designed optical attachment to image and analyze immunoassays performed in microwells was built by Coskun et al.[Citation344] The smartphone camera was used to acquire the transmission images of the assay. Liu et al.[Citation345] implemented a rapid and cost-effective 3D printed smartphone-based platform (SBP) for a point-of-need food safety inspection, which employs aptamer-conjugated AuNPs as the colourimetric indicator, and a battery-powered optosensing accessory attached to the camera of a smartphone for transmission images capture.
These emerging technologies offer great potential to meet ASSURED criteria recommended by World Health Organization (WHO), which are affordable, sensitive, specific, user-friendly, rapid, robust, equipment-free and deliverable to end-users. Moreover, they can facilitate the active screening of food contaminants and toxicants, constituting powerful alternatives to conventional benchtop detection technologies [Citation346] and thus significantly improving the current worldwide food safety control system.
Perspectives and future directions
The food industry had experienced radical changes over the past century and more so over the last six years due to the arrival of Industry 4.0 technologies that have transformed almost every food industry sector, including the muscle food industry. While the first industrial revolution was defined by the mechanization of production, the second industrial revolution enabled mass production and the third one allowed automated production. Concerning the ongoing Industry 4.0, it has highlighted the need for multidisciplinary approaches and connectivity between various domains, particularly, physical, biological, and digital fields.
In this work, the recent applications of Industry 4.0 technologies in muscle food processing/preservation and analysis are enlightened, confirming the potential of emerging technologies (e.g., ohmic and radiofrequency heating, pulsed electric field, cold plasma, high-pressure processing, emerging freezing-based techniques, ultrasound, spectroscopic sensors, advanced mass spectrometry, etc.) as drivers toward more sustainable and healthier food production and consumption. Innovations in different domains of muscle food preservation and processing have led to better sensory and nutritional (especially digestibility) quality of treated products with a longer shelf life compared to those treated with traditional preservation techniques. In addition, energy use and production costs can be reduced and greater production capacity can be achieved with novel techniques, providing environmental and economic sustainability. Moreover, these emerging techniques can be used to extract bioactive compounds from food waste and by-products, thus bringing substantial added value for both the consumer and the producer.[Citation37,Citation137,Citation175,Citation347]
The incorporation of robotics in the muscle food sector (such as performing manual operations in meat processing plants) offers countless possibilities that could be enhanced by the current rapid development of smart sensors. Such sensors enable real-time remote monitoring of quality, safety, authenticity and other relevant parameters directly inline of production. The assessment of muscle food quality and other related parameters have traditionally been determined using physico-chemical destructive and time-consuming techniques, while the advent of emerging analytical methods (e.g., hyperspectral imaging sensors, portable and smartphone-based techniques) has revolutionized the methods of analysing food products.
Despite the accelerated development in many muscle food processing/preservation and analysis areas, new requirements have been introduced by the disruptive technologies of Industry 4.0 and some challenges are still to be addressed. Overall, the muscle food industry is complex and challenging as it is influenced by multiple elements. For example, the heterogeneity of muscle food products in terms of shape and size makes it difficult to handle (during processing/preservation, analysis) and even much more difficult to automate using robots. Although the investigation of the potential of nonthermal treatments is among the most focused research areas, more research is still needed to understand the exact mechanism of action of these techniques, and their impact on treated food, and especially to demonstrate the safety of processed products. It should be highlighted that most emerging technologies have not yet crossed the barriers of the laboratory scale because of the high cost and lack of adaptability to an industrial environment. Another factor that hinders the wider acceptance of Industry 4.0 technologies is the technical and technological skill gap, which is one of the key barriers to the adoption of new technologies. Chapman et al.[Citation6] pointed out the necessity of training courses to face disruptions due to Industry 4.0 technologies.
Overall, the adoption of new technologies can seem like a daunting task, and the uptake of these technologies is still slow in the food industry compared to other sectors, which might be due to the silo mentality that still exists among the researchers in the food industry.[Citation348,Citation349] Finally, various public and private policies in different countries are one of the main obstacles to the implementation of emerging technologies. Therefore, discussion and close collaboration between government agencies to establish common standards are indispensable to take full profit from the current Industry 4.0 technologies, ushering in the next wave of technological advances and innovations that will move the world towards Industry 5.0.
Final remarks
This review provided a comprehensive overview of recent applications of Industry 4.0 technologies in the muscle food sector, with a special focus on processing/preservation methods and analytical techniques. Most of the topics discussed in this review paper were previously reviewed in more detail in other publications. However, to the best of our knowledge, this work is the first to raise awareness of the importance of simultaneously considering a wide range of emerging technologies that address the key principle of Industry 4.0, namely the convergence between various areas of science, especially physical, biological, and digital disciplines.
This review showed that emerging technologies, such as novel thermal and nonthermal processing, smart spectroscopic sensors, and other high throughput analysis (e.g., advanced spectrometry) have significant potential for applications to muscle food products despite the very different characteristics (such as shapes and sizes) of these food products. However, additional research and extensive collaboration between different players in the food supply chain as well as close policy coordination among countries are still needed to overcome various barriers that are currently hindering the wider implementation of emerging technology breakthroughs in the food industry. While the capture of the full potential of Industry 4.0 technologies and innovations is probably still a distant future in the muscle food industry, automation and digitalization are likely to only grow in prominence in the coming years.
CRediT authorship contribution statement
Abdo Hassoun: Conceptualization, methodology, writing – original draft preparation, revision, editing. Shahida Anusha Siddiqui, Slim Smaoui, İlknur Ucak, Rai Naveed Arshad, Zuhaib F. Bhat, Hina F. Bhat, María Carpena, Miguel A. Prieto, Abderrahmane Aït-Kaddour, Jorge A.M. Pereira, Carmela Zacometti, Alessandra Tata, Salam A. Ibrahim, Fatih Ozogul: writing – original draft preparation, revision. José S. Câmara: writing – original draft preparation, revision, supervision, Review & Editing. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors acknowledge FCT and Madeira 14-2020 program to the Portuguese Mass Spectrometry Network (RNEM) through PROEQUIPRAM program, M14-20 M1420-01-0145-FEDER-000008).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Boyacι-Gündüz, C. P.; Ibrahim, S. A.; Wei, O. C.; Galanakis, C. M. Transformation of the Food Sector : Security and Resilience During the COVID-19 Pandemic. Foods. 2021, 10, 497. DOI: 10.3390/foods10030497.
- Galanakis, C. M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods. 2020, 9, 523. DOI: 10.3390/foods9040523.
- Chowdhury, R. B.; Moore, G. A.; Weatherley, A. J.; Arora, M. Key Sustainability Challenges for the Global Phosphorus Resource, Their Implications for Global Food Security, and Options for Mitigation. J. Clean. Prod. 2017, 140, 945–963. DOI: 10.1016/J.JCLEPRO.2016.07.012.
- El Bilali, H. Research on Agro-Food Sustainability Transitions: A Systematic Review of Research Themes and an Analysis of Research Gaps. J. Clean. Prod. 2019, 221, 353–364. DOI: 10.1016/J.JCLEPRO.2019.02.232.
- Rowan, N. J.; Galanakis, C. M. Unlocking Challenges and Opportunities Presented by COVID-19 Pandemic for Cross-Cutting Disruption in Agri-Food and Green Deal Innovations: Quo Vadis? Sci. Total Environ. 2020, 748, 141362. DOI: 10.1016/J.SCITOTENV.2020.141362.
- Chapman, J.; Power, A.; Netzel, M. E.; Sultanbawa, Y.; Smyth, H. E.; Truong, V. K.; Cozzolino, D. Challenges and Opportunities of the Fourth Revolution: A Brief Insight into the Future of Food. Crit. Rev. Food Sci. Nutr. 2021, 62, 2845–2853. DOI: 10.1080/10408398.2020.1863328.
- Pu, H.; Kamruzzaman, M.; Sun, D. -W. Selection of Feature Wavelengths for Developing Multispectral Imaging Systems for Quality, Safety and Authenticity of Muscle Foods-A Review. Trends Food Sci. Technol. 2015, 45, 86–104. DOI: 10.1016/J.TIFS.2015.05.006.
- Sobral, M. M. C.; Cunha, S. C.; Faria, M. A.; Ferreira, I. M. P. L. V. O. Domestic Cooking of Muscle Foods: Impact on Composition of Nutrients and Contaminants. Compr. Rev. Food Sci. Food Saf. 2018, 17, 309–333. DOI: 10.1111/1541-4337.12327.
- Chen, Y. -N.; Sun, D. -W.; Cheng, J. -H.; Gao, W. -H. Recent Advances for Rapid Identification of Chemical Information of Muscle Foods by Hyperspectral Imaging Analysis. Food Eng. Rev. 2016, 8, 336–350. DOI: 10.1007/s12393-016-9139-1.
- Hassoun, A.; Ojha, S.; Tiwari, B.; Rustad, T.; Nilsen, H.; Heia, K.; Cozzolino, D.; El-Din Bekhit, A.; Biancolillo, A.; Wold, J. P. Monitoring Thermal and Non-Thermal Treatments During Processing of Muscle Foods: A Comprehensive Review of Recent Technological Advances. Appl. Sci. 2020, 10, 6802. DOI: 10.3390/app10196802.
- Hassoun, A.; Måge, I.; Schmidt, W. F.; Temiz, H. T.; Li, L.; Kim, H. -Y.; Nilsen, H.; Biancolillo, A.; Aït-Kaddour, A.; Sikorski, M., et al. Fraud in Animal Origin Food Products: Advances in Emerging Spectroscopic Detection Methods Over the Past Five Years. Foods. 2020, 9, 1069. DOI: 10.3390/foods9081069.
- Hematyar, N.; Rustad, T.; Sampels, S.; Kastrup Dalsgaard, T. Relationship Between Lipid and Protein Oxidation in Fish. Aquac. Res. 2019, 50, 1393–1403. DOI: 10.1111/are.14012.
- Rathod, N. B.; Ranveer, R. C.; Benjakul, S.; Kim, S.; Pagarkar, A. U.; Patange, S.; Ozogul, F. Recent Developments of Natural Antimicrobials and Antioxidants on Fish and Fishery Food Products. Compr. Rev. Food Sci. Food Saf. 2021, 1541–4337.12787. DOI:10.1111/1541-4337.12787.
- Luo, J.; Taylor, C.; Nebl, T.; Ng, K.; Bennett, L. E. Effects of Macro-Nutrient, Micro-Nutrient Composition and Cooking Conditions on in vitro Digestibility of Meat and Aquatic Dietary Proteins. Food Chem. 2018, 254, 292–301. DOI: 10.1016/J.FOODCHEM.2018.01.164.
- Hassoun, A.; Heia, K.; Lindberg, S.; Nilsen, H. Spectroscopic Techniques for Monitoring Thermal Treatments in Fish and Other Seafood : A Review of Recent Developments and Applications. Foods. 2020, 9, 767. DOI: 10.3390/foods9060767.
- Kubo, M. T.; Siguemoto, É. S.; Funcia, E. S.; Augusto, P. E.; Curet, S.; Boillereaux, L.; Sastry, S. K.; Gut, J. A. Non-Thermal Effects of Microwave and Ohmic Processing on Microbial and Enzyme Inactivation: A Critical Review. Curr. Opin. Food Sci. 2020, 35, 36–48. DOI: 10.1016/j.cofs.2020.01.004.
- Bhat, Z. F.; Morton, J. D.; Bekhit, A. E. A.; Kumar, S.; Bhat, H. F. Thermal Processing Implications on the Digestibility of Meat, Fish and Seafood Proteins. Compr. Rev. Food Sci. Food Saf. 2021, 1541–4337.12802. DOI:10.1111/1541-4337.12802.
- Rastogi, N. K. Recent Trends and Developments in Infrared Heating in Food Processing. Crit. Rev. Food Sci. Nutr. 2012, 52, 737–760. DOI: 10.1080/10408398.2010.508138.
- Troy, D. J.; Ojha, K. S.; Kerry, J. P.; Tiwari, B. K. Sustainable and Consumer-Friendly Emerging Technologies for Application Within the Meat Industry: An Overview. Meat Sci. 2016, 120, 2–9. DOI: 10.1016/J.MEATSCI.2016.04.002.
- Jiang, H.; Liu, Z.; Wang, S. Microwave Processing: Effects and Impacts on Food Components. Crit. Rev. Food Sci. Nutr. 2018, 58, 2476–2489. DOI: 10.1080/10408398.2017.1319322.
- Guo, Q.; Sun, D. -W.; Cheng, J. -H.; Han, Z. Microwave Processing Techniques and Their Recent Applications in the Food Industry. Trends Food Sci. Technol. 2017, 67, 236–247. DOI: 10.1016/J.TIFS.2017.07.007.
- Guzik, P.; Kulawik, P.; Zając, M.; Migdał, W. Microwave Applications in the Food Industry: An Overview of Recent Developments. Crit. Rev. Food Sci. Nutr. 2022, 62, 7989–8008. DOI: 10.1080/10408398.2021.1922871.
- Zhang, L.; Lan, R.; Zhang, B.; Erdogdu, F.; Wang, S. A Comprehensive Review on Recent Developments of Radio Frequency Treatment for Pasteurizing Agricultural Products. Crit. Rev. Food Sci. Nutr. 2021, 61, 380–394. DOI: 10.1080/10408398.2020.1733929.
- Zhang, Y.; Li, S.; Jin, S.; Li, F.; Tang, J.; Jiao, Y. Radio Frequency Tempering Multiple Layers of Frozen Tilapia Fillets: The Temperature Distribution, Energy Consumption, and Quality. Innov. Food Sci. Emerg. Technol. 2021, 68, 102603. DOI: 10.1016/j.ifset.2021.102603.
- Makroo, H. A.; Rastogi, N. K.; Srivastava, B. Ohmic Heating Assisted Inactivation of Enzymes and Microorganisms in Foods: A Review. Trends Food Sci. Technol. 2020, 97, 451–465. DOI: 10.1016/j.tifs.2020.01.015.
- Alkanan, Z. T.; Altemimi, A. B.; Al-Hilphy, A. R. S.; Watson, D. G.; Pratap-Singh, A.; Alkanan, Z. T.; Altemimi, A. B.; Al-Hilphy, A. R. S.; Watson, D. G. Ohmic Heating in the Food Industry: Developments in Concepts and Applications During 2013–2020. Appl. Sci. 2021, 11, 2507. DOI: 10.3390/APP11062507.
- Aboud, S. A.; Altemimi, A. B.; Al-HiIphy, A. R. S.; Yi-Chen, L.; Cacciola, F. A Comprehensive Review on Infrared Heating Applications in Food Processing. Molecules. 2019, 24, 1–20. DOI: 10.3390/molecules24224125.
- Zhao, Y. M.; de Alba, M.; Sun, D. W.; Tiwari, B. Principles and Recent Applications of Novel Non-Thermal Processing Technologies for the Fish Industry—a Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 728–742. DOI: 10.1080/10408398.2018.1495613.
- Olatunde, O. O.; Benjakul, S. Nonthermal Processes for Shelf-Life Extension of Seafoods: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 892–904. DOI: 10.1111/1541-4337.12354.
- Ali, A.; Wei, S.; Liu, Z.; Fan, X.; Sun, Q.; Xia, Q.; Liu, S.; Hao, J.; Deng, C. Non-Thermal Processing Technologies for the Recovery of Bioactive Compounds from Marine By-Products. LWT. 2021, 147, 111549. DOI: 10.1016/J.LWT.2021.111549.
- Chakka, A. K.; Sriraksha, M. S.; Ravishankar, C. N. Sustainability of Emerging Green Non-Thermal Technologies in the Food Industry with Food Safety Perspective: A Review. LWT. 2021, 151, 112140. DOI: 10.1016/J.LWT.2021.112140.
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M. P.; Marchesseau, S. Potentialities and Limits of Some Non-Thermal Technologies to Improve Sustainability of Food Processing. Front Nutr. 2019, 5. DOI: 10.3389/fnut.2018.00130.
- Rosario, D. K. A.; Rodrigues, B. L.; Bernardes, P. C.; Conte-Junior, C. A. Principles and Applications of Non-Thermal Technologies and Alternative Chemical Compounds in Meat and Fish. Crit. Rev. Food Sci. Nutr. 2021, 61, 1163–1183. DOI: 10.1080/10408398.2020.1754755.
- Renaud, C.; de Lamballerie, M.; Guyon, C.; Astruc, T.; Venien, A.; Pottier, L. Effects of High-Pressure Treatment on the Muscle Structure of Salmon (Salmo Salar). Food Chem. 2022, 367, 130721. DOI: 10.1016/J.FOODCHEM.2021.130721.
- Lian, F.; De Conto, E.; Del Grippo, V.; Harrison, S. M.; Fagan, J.; Lyng, J. G.; Brunton, N. P. High-Pressure Processing for the Production of Added-Value Claw Meat from Edible Crab (Cancer Pagurus). Foods. 2021, 10, 955. DOI: 10.3390/foods10050955.
- Cropotova, J.; Tappi, S.; Genovese, J.; Rocculi, P.; Laghi, L.; Dalla Rosa, M.; Rustad, T. Study of the Influence of Pulsed Electric Field Pre-Treatment on Quality Parameters of Sea Bass During Brine Salting. Innov. Food Sci. Emerg. Technol. 2021, 70, 102706. DOI: 10.1016/J.IFSET.2021.102706.
- Arshad, R. N.; Abdul-Malek, Z.; Roobab, U.; Munir, M. A.; Naderipour, A.; Qureshi, M. I.; El-Din Bekhit, A.; Liu, Z. W.; Aadil, R. M. Pulsed Electric Field: A Potential Alternative Towards a Sustainable Food Processing. Trends Food Sci. Technol. 2021, 111, 43–54. DOI: 10.1016/j.tifs.2021.02.041.
- Ojha, K. S.; Aznar, R.; O’Donnell, C.; Tiwari, B. K. Ultrasound Technology for the Extraction of Biologically Active Molecules from Plant, Animal and Marine Sources. TrAc - Trends Anal. Chem. 2020, 122, 115663. DOI: 10.1016/j.trac.2019.115663.
- Hassoun, A.; Karoui, R. Monitoring Changes in Whiting (Merlangius Merlangus) Fillets Stored Under Modified Atmosphere Packaging by Front Face Fluorescence Spectroscopy and Instrumental Techniques. Food Chem. 2016, 200, 343–353. DOI: 10.1016/j.foodchem.2016.01.028.
- Bouletis, A. D.; Arvanitoyannis, I. S.; Hadjichristodoulou, C. Application of Modified Atmosphere Packaging on Aquacultured Fish and Fish Products: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2263–2285. DOI: 10.1080/10408398.2013.862202.
- Asl, P. J.; Rajulapati, V.; Gavahian, M.; Kapusta, I.; Putnik, P.; Khaneghah, A. M.; Marszałek, K. Non-Thermal Plasma Technique for Preservation of Raw or Fresh Foods: A Review. Food Control. 2022, 134, 108560. DOI: 10.1016/J.FOODCONT.2021.108560.
- Zhan, X.; Sun, D. W.; Zhu, Z.; Wang, Q. J. Improving the Quality and Safety of Frozen Muscle Foods by Emerging Freezing Technologies: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2925–2938. DOI: 10.1080/10408398.2017.1345854.
- Esmaeilian, S.; Rotabakk, B. T.; Lerfall, J.; Jakobsen, A. N.; Abel, N.; Sivertsvik, M.; Olsen, A. The Use of Soluble Gas Stabilization Technology on Food – a Review. Trends Food Sci. Technol. 2021, 118, 154–166. DOI: 10.1016/J.TIFS.2021.09.015.
- Artavia, G.; Cortés-Herrera, C.; Granados-Chinchilla, F. Selected Instrumental Techniques Applied in Food and Feed: Quality, Safety and Adulteration Analysis. Foods. 2021, 10, 1081. DOI: 10.3390/FOODS10051081.
- Hassoun, A.; Karoui, R. Quality Evaluation of Fish and Other Seafood by Traditional and Nondestructive Instrumental Methods: Advantages and Limitations. Crit. Rev. Food Sci. Nutr. 2017, 57, 1976–1998. DOI: 10.1080/10408398.2015.1047926.
- Delgado-Pando, G.; Allen, P.; Troy, D. J.; McDonnell, C. K. Objective Carcass Measurement Technologies: Latest Developments and Future Trends. Trends Food Sci. Technol. 2021, 111, 771–782. DOI: 10.1016/j.tifs.2020.12.016.
- Wu, D.; Zhang, M.; Chen, H.; Bhandari, B. Freshness Monitoring Technology of Fish Products in Intelligent Packaging. Crit. Rev. Food Sci. Nutr. 2021, 61, 1279–1292. DOI: 10.1080/10408398.2020.1757615.
- Khaled, A. Y.; Parrish, C. A.; Adedeji, A. Emerging Nondestructive Approaches for Meat Quality and Safety Evaluation—a Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3438–3463. DOI: 10.1111/1541-4337.12781.
- Liu, Y.; Pu, H.; Sun, D. W. Hyperspectral Imaging Technique for Evaluating Food Quality and Safety During Various Processes: A Review of Recent Applications. Trends Food Sci. Technol. 2017, 69, 25–35. DOI: 10.1016/j.tifs.2017.08.013.
- Temiz, H. T.; Ulaş, B. A Review of Recent Studies Employing Hyperspectral Imaging for the Determination of Food Adulteration. Photochem. 2021, 1, 125–146. DOI: 10.3390/PHOTOCHEM1020008.
- Saha, D.; Manickavasagan, A. Machine Learning Techniques for Analysis of Hyperspectral Images to Determine Quality of Food Products: A Review. Curr. Res. Food Sci. 2021, 4, 28–44. DOI: 10.1016/J.CRFS.2021.01.002.
- Domínguez, I.; Garrido Frenich, A.; Romero-González, R. Mass Spectrometry Approaches to Ensure Food Safety. Anal. Methods. 2020, 12, 1148–1162. DOI: 10.1039/C9AY02681A.
- Freitas, J.; Silva, P.; Perestrelo, R.; Vaz-Pires, P.; Câmara, J. S. Improved Approach Based on MALDI-TOF MS for Establishment of the Fish Mucus Protein Pattern for Geographic Discrimination of Sparus Aurata. Food Chem. 2022, 372, 131237. DOI: 10.1016/j.foodchem.2021.131237.
- Man, K. Y.; Chan, C. O.; Tang, H. H.; Dong, N. P.; Capozzi, F.; Wong, K. H.; Kwok, K. W. H.; Chan, H. M.; Mok, D. K. W. Mass Spectrometry-Based Untargeted Metabolomics Approach for Differentiation of Beef of Different Geographic Origins. Food Chem. 2021, 338, 127847. DOI: 10.1016/j.foodchem.2020.127847.
- Mhlanga, D. Artificial Intelligence in the Industry 4.0, and Its Impact on Poverty, Innovation, Infrastructure Development, and the Sustainable Development Goals: Lessons from Emerging Economies? Sustain. 2021, 13, 5788. DOI: 10.3390/su13115788.
- Maynard, A. D. Navigating the Fourth Industrial Revolution. Nat. Nanotechnol. 2015, 10, 1005–1006. DOI: 10.1038/nnano.2015.286.
- Sima, V.; Gheorghe, I. G.; Subić, J.; Nancu, D. Influences of the Industry 4.0 Revolution on the Human Capital Development and Consumer Behavior: A Systematic Review. Sustain. 2020, 12, 4035. DOI: 10.3390/SU12104035.
- Hassoun, A.; Aït-Kaddour, A.; Abu-Mahfouz, A. M.; Rathod, N. B.; Bader, F.; Barba, F. J.; Cropotova, J.; Galanakis, C. M.; Jambrak, A. R.; Lorenzo, M., et al. The Fourth Industrial Revolution in the Food Industry — Part I: Industry 4.0 Technologies. Crit. Rev. Food Sci. Nutr. 2022, 1–17. DOI:10.1080/10408398.2022.2034735.
- Hassoun, A.; Bekhit, A. E.; Jambrak, A. R.; Regenstein, J. M.; Chemat, F.; Morton, J. D.; Gudjónsdóttir, M.; Carpena, M.; Prieto, A.; Varela, P., et al. The Fourth Industrial Revolution in the Food Industry — Part II : Emerging Food Trends Trends. Crit. Rev. Food Sci. Nutr. 2022, 1–31. DOI:10.1080/10408398.2022.2106472.
- Hassoun, A.; Cropotova, J.; Trif, M.; Rusu, A. V.; Bobi, O.; Nayik, G. A.; Jagdale, Y. D.; Saeed, F.; Afzaal, M.; Regenstein, J. M. Consumer Acceptance of New Food Trends Resulting from the Fourth Industrial Revolution Technologies: A Narrative Review of Literature and Future Perspectives. Front Nutr. 2022, 9, 972154. DOI: 10.3389/fnut.2022.972154.
- Jambrak, A. R.; Nutrizio, M.; Djekić, I.; Pleslić, S.; Chemat, F. Internet of Nonthermal Food Processing Technologies (Iontp): Food Industry 4.0 and Sustainability. Appl. Sci. 2021, 11, 686. DOI: 10.3390/app11020686.
- Liu, Y.; Ma, X.; Shu, L.; Hancke, G. P.; Abu-Mahfouz, A. M. From Industry 4.0 to Agriculture 4.0: Current Status, Enabling Technologies, and Research Challenges. IEEE Trans. Ind. Informatics. 2021, 17, 4322–4334. DOI: 10.1109/TII.2020.3003910.
- Hassoun, A.; Jagtap, S.; Garcia-Garcia, G.; Trollman, H.; Pateiro, M.; Lorenzo, M.; Trif, M.; Vasile, A.; Muhammad, R.; Simat, V., et al. Taylor and Francis Custom Citation. J. Food Eng. 2023, 337, 111216. DOI: 10.1016/j.jfoodeng.2022.111216.
- Hernández-Hernández, H. M.; Moreno-Vilet, L.; Villanueva-Rodríguez, S. J. Current Status of Emerging Food Processing Technologies in Latin America: Novel Non-Thermal Processing. Innov. Food Sci. Emerg. Technol. 2019, 58, 102233. DOI: 10.1016/j.ifset.2019.102233.
- Hassoun, A.; Sahar, A.; Lakhal, L.; Aït-Kaddour, A. Fluorescence Spectroscopy as a Rapid and Non-Destructive Method for Monitoring Quality and Authenticity of Fish and Meat Products: Impact of Different Preservation Conditions. LWT. 2019, 103, 279–292. DOI: 10.1016/j.lwt.2019.01.021.
- Feng, C. -H.; Makino, Y.; Oshita, S.; García Martín, J. F. Hyperspectral Imaging and Multispectral Imaging as the Novel Techniques for Detecting Defects in Raw and Processed Meat Products: Current State-Of-The-Art Research Advances. Food Control. 2018, 84, 165–176. DOI: 10.1016/J.FOODCONT.2017.07.013.
- Fu, X.; Chen, J. A Review of Hyperspectral Imaging for Chicken Meat Safety and Quality Evaluation: Application, Hardware, and Software. Compr. Rev. Food Sci. Food Saf. 2019, 18, 535–547. DOI: 10.1111/1541-4337.12428.
- Jagtap, S.; Bader, F.; Garcia-Garcia, G.; Trollman, H.; Fadiji, T.; Salonitis, K. Food Logistics 4.0: Opportunities and Challenges. Logistics. 2021, 5, 2. DOI: 10.3390/LOGISTICS5010002.
- Aceto, G.; Persico, V.; Pescapé, A. A Survey on Information and Communication Technologies for Industry 4.0: State-Of-The-Art, Taxonomies, Perspectives, and Challenges. IEEE Commun. Surv. Tutorials. 2019, 21, 3467–3501. DOI: 10.1109/COMST.2019.2938259.
- Rojko, A. Industry 4.0 Concept: Background and Overview. Int. J. Interact. Mob. Technol. 2017, 11, 77–90. DOI: 10.3991/ijim.v11i5.7072.
- Bai, C.; Dallasega, P.; Orzes, G.; Sarkis, J. Industry 4.0 Technologies Assessment: A Sustainability Perspective. Int. J. Prod. Econ. 2020, 229, 107776. DOI: 10.1016/J.IJPE.2020.107776.
- Manavalan, E.; Jayakrishna, K. A Review of Internet of Things (IoT) Embedded Sustainable Supply Chain for Industry 4.0 Requirements. Comput. Ind. Eng. 2019, 127, 925–953. DOI: 10.1016/J.CIE.2018.11.030.
- Müller-Maatsch, J.; Bertani, F. R.; Mencattini, A.; Gerardino, A.; Martinelli, E.; Weesepoel, Y.; van Ruth, S. The Spectral Treasure House of Miniaturized Instruments for Food Safety, Quality and Authenticity Applications: A Perspective. Trends Food Sci. Technol. 2021, 110, 841–848. DOI: 10.1016/j.tifs.2021.01.091.
- Misra, N. N.; Dixit, Y.; Al-Mallahi, A.; Bhullar, M. S.; Upadhyay, R.; Martynenko, A. IoT, Big Data and Artificial Intelligence in Agriculture and Food Industry. IEEE Internet Things J. 2022, 9. DOI: 10.1109/jiot.2020.2998584.
- McVey, C.; Elliott, C. T.; Cannavan, A.; Kelly, S. D.; Petchkongkaew, A.; Haughey, S. A. Portable Spectroscopy for High Throughput Food Authenticity Screening: Advancements in Technology and Integration into Digital Traceability Systems. Trends Food Sci. Technol. 2021, 118, 777–790. DOI: 10.1016/J.TIFS.2021.11.003.
- Kalinowska, K.; Wojnowski, W.; Tobiszewski, M. Smartphones as Tools for Equitable Food Quality Assessment. Trends Food Sci. Technol. 2021, 111, 271–279. DOI: 10.1016/J.TIFS.2021.02.068.
- Bhat, Z. F.; Morton, J. D.; Kumar, S.; Bhat, H. F.; Aadil, R. M.; Bekhit, A.E. -D.A. 3D Printing: Development of Animal Products and Special Foods. Trends Food Sci. Technol. 2021, 118, 87–105. DOI: 10.1016/J.TIFS.2021.09.020.
- Portanguen, S.; Tournayre, P.; Sicard, J.; Astruc, T.; Mirade, P. S. Toward the Design of Functional Foods and Biobased Products by 3D Printing: A Review. Trends Food Sci. Technol. 2019, 86, 188–198. DOI: 10.1016/j.tifs.2019.02.023.
- Barbut, S. Meat Industry 4.0: A Distant Future? Anim. Front. 2020, 10, 38–47. DOI: 10.1093/af/vfaa038.
- Misimi, E.; Øye, E. R.; Eilertsen, A.; Mathiassen, J. R.; Åsebø, O. B.; Gjerstad, T.; Buljo, J.; Skotheim, Ø. GRIBBOT - Robotic 3D Vision-Guided Harvesting of Chicken Fillets. Comput. Electron. Agric. 2016, 121, 84–100. DOI: 10.1016/j.compag.2015.11.021.
- Mu, S.; Qin, H.; Wei, J.; Wen, Q.; Liu, S.; Wang, S.; Xu, S. Robotic 3D Vision-Guided System for Half-Sheep Cutting Robot. Math. Probl. Eng. 2020, 2020. DOI: 10.1155/2020/1520686.
- Khan, Z. H.; Khalid, A.; Iqbal, J. Towards Realizing Robotic Potential in Future Intelligent Food Manufacturing Systems. Innov. Food Sci. Emerg. Technol. 2018, 48, 11–24. DOI: 10.1016/J.IFSET.2018.05.011.
- de Medeiros Esper, I.; From, P. J.; Mason, A. Robotisation and Intelligent Systems in Abattoirs. Trends Food Sci. Technol. 2021, 108, 214–222. DOI: 10.1016/J.TIFS.2020.11.005.
- Esmaeilian, B.; Sarkis, J.; Lewis, K.; Behdad, S. Blockchain for the Future of Sustainable Supply Chain Management in Industry 4.0. Resour. Conserv. Recycl. 2020, 163, 105064. DOI: 10.1016/J.RESCONREC.2020.105064.
- Lennon Olsen, T.; Tomlin, B. Industry 4.0: Opportunities and Challenges for Operations Management. SSRN Electron. J. 2019, 1–20. DOI: 10.2139/ssrn.3365733.
- Oliveira, J.; Lima, J. E.; da Silva, D.; Kuprych, V.; Faria, P. M.; Teixeira, C.; Ferreira Cruz, E.; Rosado da Cruz, A. M. Traceability System for Quality Monitoring in the Fishery and Aquaculture Value Chain. J. Agric. Food Res. 2021, 5, 100169. DOI: 10.1016/J.JAFR.2021.100169.
- Lorenzo, J. M.; Munekata, P. E. S., and Barba, F. J., Eds. Sustainable Production Technology in Food; Amsterdam: Academic Press, 2021.
- Jia, W.; Zhang, R.; Liu, L.; Zhu, Z.; Mo, H.; Xu, M.; Shi, L.; Zhang, H. Proteomics Analysis to Investigate the Impact of Diversified Thermal Processing on Meat Tenderness in Hengshan Goat Meat. Meat Sci. 2022, 183, 108655. DOI: 10.1016/J.MEATSCI.2021.108655.
- Llave, Y.; Erdogdu, F. Radio Frequency Processing and Recent Advances on Thawing and Tempering of Frozen Food Products. Crit. Rev. Food Sci. Nutr. 2020, 62, 598–618. DOI: 10.1080/10408398.2020.1823815.
- Hassoun, A.; Aït-Kaddour, A.; Sahar, A.; Cozzolino, D. Monitoring Thermal Treatments Applied to Meat Using Traditional Methods and Spectroscopic Techniques: A Review of Advances Over the Last Decade. Food Bioprocess Technol. 2021, 14, 195–208. DOI: 10.1007/s11947-020-02510-0.
- Suleman, R.; Wang, Z.; Aadil, R. M.; Hui, T.; Hopkins, D. L.; Zhang, D. Effect of Cooking on the Nutritive Quality, Sensory Properties and Safety of Lamb Meat: Current Challenges and Future Prospects. Meat Sci. 2020, 167, 108172. DOI: 10.1016/j.meatsci.2020.108172.
- Inmanee, P.; Kamonpatana, P.; Pirak, T. Ohmic Heating Effects on Listeria Monocytogenes Inactivation, and Chemical, Physical, and Sensory Characteristic Alterations for Vacuum Packaged Sausage During Post Pasteurization. LWT. 2019, 108, 183–189. DOI: 10.1016/J.LWT.2019.03.027.
- Hradecky, J.; Kludska, E.; Belkova, B.; Wagner, M.; Hajslova, J. Ohmic Heating: A Promising Technology to Reduce Furan Formation in Sterilized Vegetable and Vegetable/Meat Baby Foods. Innov. Food Sci. Emerg. Technol. 2017, 43, 1–6. DOI: 10.1016/J.IFSET.2017.07.018.
- Llave, Y.; Udo, T.; Fukuoka, M.; Sakai, N. Ohmic Heating of Beef at 20 kHz and Analysis of Electrical Conductivity at Low and High Frequencies. J. Food Eng. 2018, 228, 91–101. DOI: 10.1016/J.JFOODENG.2018.02.019.
- Sengun, I. Y.; Icier, F.; Kor, G. Effects of Combined Ohmic–Infrared Cooking Treatment on Microbiological Inactivation of Meatballs. J. Food Process. Eng. 2017, 40, 1–11. DOI: 10.1111/jfpe.12309.
- Külcü, D. B.; Gürbüz, Ü. Use of Ohmic Heating System in Meat Thawing and Its Effects on Microbiological Quality. MANAS J. Eng. 2018, 6, 129–142.
- Dang, T. T.; Feyissa, A. H.; Gringer, N.; Jessen, F.; Olsen, K.; Bøknæs, N.; Orlien, V. Effects of High Pressure and Ohmic Heating on Shell Loosening, Thermal and Structural Properties of Shrimp (Pandalus Borealis). Innov. Food Sci. Emerg. Technol. 2020, 59, 102246. DOI: 10.1016/j.ifset.2019.102246.
- Llave, Y.; Morinaga, K.; Fukuoka, M.; Sakai, N. Characterization of Ohmic Heating and Sous-Vide Treatment of Scallops: Analysis of Electrical Conductivity and the Effect of Thermal Protein Denaturation on Quality Attribute Changes. Innov. Food Sci. Emerg. Technol. 2018, 50, 112–123. DOI: 10.1016/J.IFSET.2018.09.007.
- Muñoz, I.; Serra, X.; Guàrdia, M. D.; Fartdinov, D.; Arnau, J.; Picouet, P. A.; Gou, P. Radio Frequency Cooking of Pork Hams Followed with Conventional Steam Cooking. LWT. 2020, 123, 109104. DOI: 10.1016/j.lwt.2020.109104.
- Bedane, T. F.; Altin, O.; Erol, B.; Marra, F.; Erdogdu, F. Thawing of Frozen Food Products in a Staggered Through-Field Electrode Radio Frequency System: A Case Study for Frozen Chicken Breast Meat with Effects on Drip Loss and Texture. Innov. Food Sci. Emerg. Technol. 2018, 50, 139–147. DOI: 10.1016/J.IFSET.2018.09.001.
- Wang, X.; Wang, L.; Yang, K.; Wu, D.; Ma, J.; Wang, S.; Zhang, Y.; Sun, W. Radio Frequency Heating Improves Water Retention of Pork Myofibrillar Protein Gel: An Analysis from Water Distribution and Structure. Food Chem. 2021, 350, 129265. DOI: 10.1016/J.FOODCHEM.2021.129265.
- Uemura, K.; Kanafusa, S.; Takahashi, C.; Kobayashi, I. Development of a Radio Frequency Heating System for Sterilization of Vacuum-Packed Fish in Water. Biosci. Biotechnol., Biochem. 2017, 81, 762–767. DOI: 10.1080/09168451.2017.1280660.
- Bedane, T. F.; Chen, L.; Marra, F.; Wang, S. Experimental Study of Radio Frequency (RF) Thawing of Foods with Movement on Conveyor Belt. J. Food Eng. 2017, 201, 17–25. DOI: 10.1016/J.JFOODENG.2017.01.010.
- Zhu, Y.; Li, F.; Tang, J.; Wang, T. T.; Jiao, Y. Effects of Radio Frequency, Air and Water Tempering, and Different End-Point Tempering Temperatures on Pork Quality. J. Food Process. Eng. 2019, 42, e13026. DOI: 10.1111/JFPE.13026.
- Taşkıran, M.; Olum, E. Changes in Chicken Meat Proteins During Microwave and Electric Oven Cooking. J. Food Process Preserv. 2020, 44, e14324. DOI: 10.1111/jfpp.14324.
- Wang, X.; Muhoza, B.; Wang, X.; Feng, T.; Xia, S.; Zhang, X. Comparison Between Microwave and Traditional Water Bath Cooking on Saltiness Perception, Water Distribution and Microstructure of Grass Crap Meat. Food. Res. Int. 2019, 125, 108521. DOI: 10.1016/j.foodres.2019.108521.
- Wang, X.; Wang, X.; Muhoza, B.; Feng, T.; Xia, S.; Zhang, X. Microwave Combined with Conduction Heating Effects on the Tenderness, Water Distribution, and Microstructure of Pork Belly. Innov. Food Sci. Emerg. Technol. 2020, 62, 102344. DOI: 10.1016/J.IFSET.2020.102344.
- Póltorak, A.; Wyrwisz, J.; Moczkowska, M.; Marcinkowska-Lesiak, M.; Stelmasiak, A.; Rafalska, U.; Wierzbicka, A.; Sun, D. W. Microwave Vs. Convection Heating of Bovine Gluteus Medius Muscle: Impact on Selected Physical Properties of Final Product and Cooking Yield. Int. J. Food Sci. Technol. 2015, 50, 958–965. DOI: 10.1111/ijfs.12729.
- Li, S.; Tang, S.; Yan, L.; Li, R. Effects of Microwave Heating on Physicochemical Properties, Microstructure and Volatile Profiles of Yak Meat. J. Appl. Anim. Res. 2019, 47, 262–272. DOI: 10.1080/09712119.2019.1624553.
- Lafarga, T.; Queralt, A. V.; Bobo, G.; Abadias, M.; Aguiló-Aguayo, I. Thermal Processing Technologies. In Food Formulation: Novel Ingredients and Processing Techniques; Pathania, S. and Tiwari, B.K., Eds.; Hoboken, New Jersey, U.S.: John Wiley & Sons, Ltd, 2021; pp. 165–181.
- Tian, X.; Shao, L.; Yu, Q.; Li, W. S. X.; Dai, R. Comparative Analysis of Quality Uniformity of Ohmic and Water Bath Heating Treated Pork Batter with Different Fat Content. J. Food Process Preserv. 2020, 44, 1–11. DOI: 10.1111/jfpp.14377.
- Rodrigues, R. M.; Avelar, Z.; Machado, L.; Pereira, R. N.; Vicente, A. A. Electric Field Effects on Proteins – Novel Perspectives on Food and Potential Health Implications. Food. Res. Int. 2020, 137, 109709. DOI: 10.1016/J.FOODRES.2020.109709.
- Tian, X.; Liu, Y.; Yu, Q.; Shao, L.; Li, X.; Dai, R. Label Free-Based Proteomic Analysis of Escherichia Coli O157:H7 Subjected to Ohmic Heating. Food. Res. Int. 2020, 128, 108815. DOI: 10.1016/J.FOODRES.2019.108815.
- Ferreira-Santos, P.; Miranda, S. M.; Belo, I.; Spigno, G.; Teixeira, J. A.; Rocha, C. M. R. Sequential Multi-Stage Extraction of Biocompounds from Spirulina Platensis: Combined Effect of Ohmic Heating and Enzymatic Treatment. Innov. Food Sci. Emerg. Technol. 2021, 71, 102707. DOI: 10.1016/J.IFSET.2021.102707.
- Backi, C. J. Methods for (Industrial) Thawing of Fish Blocks: A Review. J. Food Process. Eng. 2018, 41, e12598. DOI: 10.1111/jfpe.12598.
- Cevik, M.; Icier, F. Comparison of Quality Attributes of Minced Beef Samples Thawed by Ohmic and Conventional Methods. J. Food Process Preserv. 2021, 45, e15122. DOI: 10.1111/JFPP.15122.
- Rajasekaran, B.; Subbiah, B.; Stephen, N. M.; Nagarajan, M.; Muniasamy, S. Design, Fabrication, and Validation of Ohmic Heater to Process Green Mussel Meat. J. Food Process Preserv. 2021, 45, e15511. DOI: 10.1111/JFPP.15511.
- Cai, L.; Cao, M.; Regenstein, J.; Cao, A. Recent Advances in Food Thawing Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 953–970. DOI: 10.1111/1541-4337.12458.
- Wu, X. F.; Zhang, M.; Adhikari, B.; Sun, J. Recent Developments in Novel Freezing and Thawing Technologies Applied to Foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3620–3631. DOI: 10.1080/10408398.2015.1132670.
- Jiao, Y.; Tang, J.; Wang, Y.; Koral, T. L. Radio-Frequency Applications for Food Processing and Safety. Annu. Rev. Food Sci. Technol. 2018, 9, 105–127. DOI: 10.1146/annurev-food-041715-033038.
- Zhou, X.; Wang, S. Recent Developments in Radio Frequency Drying of Food and Agricultural Products: A Review. Dry. Technol. 2019, 37, 271–286. DOI: 10.1080/07373937.2018.1452255.
- Meijer, G. W.; Lähteenmäki, L.; Stadler, R. H.; Weiss, J. Issues Surrounding Consumer Trust and Acceptance of Existing and Emerging Food Processing Technologies. Crit. Rev. Food Sci. Nutr. 2021, 61, 97–115. DOI: 10.1080/10408398.2020.1718597.
- Dong, X.; Wang, J.; Raghavan, V. Impact of Microwave Processing on the Secondary Structure, in-Vitro Protein Digestibility and Allergenicity of Shrimp (Litopenaeus Vannamei) Proteins. Food Chem. 2021, 337, 127811. DOI: 10.1016/j.foodchem.2020.127811.
- Tang, J. Unlocking Potentials of Microwaves for Food Safety and Quality. J. Food Sci. 2015, 80, E1776–1793. DOI: 10.1111/1750-3841.12959.
- Zia, S.; Khan, M. R.; Shabbir, M. A.; Aslam Maan, A.; Khan, M. K. I.; Nadeem, M.; Khalil, A. A.; Din, A.; Aadil, R. M. An Inclusive Overview of Advanced Thermal and Nonthermal Extraction Techniques for Bioactive Compounds in Food and Food-Related Matrices. Food Rev. Int. 2020, 38, 1166–1196. DOI: 10.1080/87559129.2020.1772283.
- Chizoba Ekezie, F. -G.; Sun, D. -W.; Han, Z.; Cheng, J. -H. Microwave-Assisted Food Processing Technologies for Enhancing Product Quality and Process Efficiency: A Review of Recent Developments. Trends Food Sci. Technol. 2017, 67, 58–69. DOI: 10.1016/J.TIFS.2017.05.014.
- Atuonwu, J. C.; Tassou, S. A. Quality Assurance in Microwave Food Processing and the Enabling Potentials of Solid-State Power Generators: A Review. J. Food Eng. 2018, 234, 1–15. DOI: 10.1016/J.JFOODENG.2018.04.009.
- Arshad, R. N.; Abdul-Malek, Z.; Munir, A.; Buntat, Z.; Ahmad, M. H.; Jusoh, Y. M. M.; Bekhit, A. E. D.; Roobab, U.; Manzoor, M. F.; Aadil, R. M. Electrical Systems for Pulsed Electric Field Applications in the Food Industry: An Engineering Perspective. Trends Food Sci. Technol. 2020, 104, 1–13. DOI: 10.1016/j.tifs.2020.07.008.
- Bekhit, A.E. -D.A.; Carne, A.; van de Ven, R.; Hopkins, D. L. Effect of Repeated Pulsed Electric Field Treatment on the Quality of Hot-Boned Beef Loins and Topsides. Meat Sci. 2016, 111, 139–146. DOI: 10.1016/j.meatsci.2015.09.001.
- Alahakoon, A. U.; Oey, I.; Bremer, P.; Silcock, P. Optimisation of Sous Vide Processing Parameters for Pulsed Electric Fields Treated Beef Briskets. Food Bioprocess Technol. 2018, 11, 2055–2066. DOI: 10.1007/s11947-018-2155-9.
- Faridnia, F.; Ma, Q. L.; Bremer, P. J.; Burritt, D. J.; Hamid, N.; Oey, I. Effect of Freezing as Pre-Treatment Prior to Pulsed Electric Field Processing on Quality Traits of Beef Muscles. Innov. Food Sci. Emerg. Technol. 2015, 29, 31–40. DOI: 10.1016/j.ifset.2014.09.007.
- Ma, Q.; Hamid, N.; Oey, I.; Kantono, K.; Faridnia, F.; Yoo, M.; Farouk, M. Effect of Chilled and Freezing Pre-Treatments Prior to Pulsed Electric Field Processing on Volatile Profile and Sensory Attributes of Cooked Lamb Meats. Innov. Food Sci. Emerg. Technol. 2016, 37, 359–374. DOI: 10.1016/J.IFSET.2016.04.009.
- Khan, A. A.; Randhawa, M. A.; Carne, A.; Ahmed, I. A. M.; Barr, D.; Reid, M.; Bekhit, A.E. -D.A. Effect of Low and High Pulsed Electric Field on the Quality and Nutritional Minerals in Cold Boned Beef M. Longissimus Et Lumborum. Innov. Food Sci. Emerg. Technol. 2017, 41, 135–143. DOI: 10.1016/j.ifset.2017.03.002.
- Baldi, G.; D’Elia, F.; Soglia, F.; Tappi, S.; Petracci, M.; Rocculi, P. Exploring the Effect of Pulsed Electric Fields on the Technological Properties of Chicken Meat. Foods. 2021, 10, 241. DOI: 10.3390/foods10020241.
- Mok, J. H.; Her, J. -Y.; Kang, T.; Hoptowit, R.; Jun, S. Effects of Pulsed Electric Field (PEF) and Oscillating Magnetic Field (OMF) Combination Technology on the Extension of Supercooling for Chicken Breasts. J. Food Eng. 2017, 196, 27–35. DOI: 10.1016/j.jfoodeng.2016.10.002.
- Warner, R. D.; McDonnell, C. K.; Bekhit, A. E. D.; Claus, J.; Vaskoska, R.; Sikes, A.; Dunshea, F. R.; Ha, M. Systematic Review of Emerging and Innovative Technologies for Meat Tenderisation. Meat Sci. 2017, 132, 72–89. DOI: 10.1016/j.meatsci.2017.04.241.
- Arshad, R. N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M. I.; Khan, N.; Ahmad, M. H.; Liu, Z. W.; Aadil, R. M. Effective Valorization of Food Wastes and By-Products Through Pulsed Electric Field: A Systematic Review. J. Food Process. Eng. 2021, 44, 1–14. DOI: 10.1111/jfpe.13629.
- Ghosh, S.; Gillis, A.; Sheviryov, J.; Levkov, K.; Golberg, A. Towards Waste Meat Biorefinery: Extraction of Proteins from Waste Chicken Meat with Non-Thermal Pulsed Electric Fields and Mechanical Pressing. J. Clean. Prod. 2019, 208, 220–231. DOI: 10.1016/j.jclepro.2018.10.037.
- Zhou, Y.; He, Q.; Zhou, D. Optimization Extraction of Protein from Mussel by High-Intensity Pulsed Electric Fields. J. Food Process Preserv. 2017, 41, e12962. DOI: 10.1111/jfpp.12962.
- Gómez, B.; Munekata, P. E. S.; Gavahian, M.; Barba, F. J.; Martí-Quijal, F. J.; Bolumar, T.; Campagnol, P. C. B.; Tomasevic, I.; Lorenzo, J. M. Application of Pulsed Electric Fields in Meat and Fish Processing Industries: An Overview. Food. Res. Int. 2019, 123, 95–105. DOI: 10.1016/j.foodres.2019.04.047.
- Bhat, Z. F.; Morton, J. D.; Mason, S. L.; Bekhit, A.E. -D.A. Pulsed Electric Field Operates Enzymatically by Causing Early Activation of Calpains in Beef During Ageing. Meat Sci. 2019, 153, 144–151. DOI: 10.1016/j.meatsci.2019.03.018.
- Bhat, Z. F.; Morton, J. D.; Mason, S. L.; Bekhit, A.E. -D.A. Pulsed Electric Field Improved Protein Digestion of Beef During in-Vitro Gastrointestinal Simulation. LWT. 2019, 102, 45–51. DOI: 10.1016/j.lwt.2018.12.013.
- Khan, A. A.; Randhawa, M. A.; Carne, A.; Ahmed, I. A. M.; Al-Juhaimi, F. Y.; Barr, D.; Reid, M.; Bekhit, A.E. -D.A. Effect of Low and High Pulsed Electric Field Processing on Macro and Micro Minerals in Beef and Chicken. Innov. Food Sci. Emerg. Technol. 2018, 45, 273–279. DOI: 10.1016/j.ifset.2017.11.012.
- Kantono, K.; Hamid, N.; Ma, Q.; Oey, I.; Farouk, M. Changes in the Physicochemical Properties of Chilled and Frozen-Thawed Lamb Cuts Subjected to Pulsed Electric Field Processing. Food. Res. Int. 2021, 141, 110092. DOI: 10.1016/j.foodres.2020.110092.
- Wang, J.; Zhuang, H.; Hinton, A., Jr; Zhang, J. Influence of In-Package Cold Plasma Treatment on Microbiological Shelf Life and Appearance of Fresh Chicken Breast Fillets. Food Microbiol. 2016, 60, 142–146. DOI: 10.1016/j.fm.2016.07.007.
- Moutiq, R.; Misra, N. N.; Mendonca, A.; Keener, K. In-Package Decontamination of Chicken Breast Using Cold Plasma Technology: Microbial, Quality and Storage Studies. Meat Sci. 2020, 159, 107942. DOI: 10.1016/j.meatsci.2019.107942.
- Zhuang, H.; Rothrock, M. J., Jr; Hiett, K. L.; Lawrence, K. C.; Gamble, G. R.; Bowker, B. C.; Keener, K. M. In-Package Air Cold Plasma Treatment of Chicken Breast Meat: Treatment Time Effect. J. Food Qual. 2019, 2019, 1–7. DOI: 10.1155/2019/1837351.
- Roh, S. H.; Lee, S. Y.; Park, H. H.; Lee, E. S.; Min, S. C. Effects of the Treatment Parameters on the Efficacy of the Inactivation of Salmonella Contaminating Boiled Chicken Breast by In-Package Atmospheric Cold Plasma Treatment. Int. J. Food Microbiol. 2019, 293, 24–33. DOI: 10.1016/j.ijfoodmicro.2018.12.016.
- Gök, V.; Aktop, S.; Özkan, M.; Tomar, O. The Effects of Atmospheric Cold Plasma on Inactivation of Listeria Monocytogenes and Staphylococcus Aureus and Some Quality Characteristics of Pastırma—a Dry-Cured Beef Product. Innov. Food Sci. Emerg. Technol. 2019, 56, 102188. DOI: 10.1016/j.ifset.2019.102188.
- Wang, X.; Wang, Z.; Zhuang, H.; Nasiru, M. M.; Yuan, Y.; Zhang, J.; Yan, W. Changes in Color, Myoglobin, and Lipid Oxidation in Beef Patties Treated by Dielectric Barrier Discharge Cold Plasma During Storage. Meat Sci. 2021, 176, 108456. DOI: 10.1016/j.meatsci.2021.108456.
- Huang, M.; Wang, J.; Zhuang, H.; Yan, W.; Zhao, J.; Zhang, J. Effect of In-Package High Voltage Dielectric Barrier Discharge on Microbiological, Color and Oxidation Properties of Pork in Modified Atmosphere Packaging During Storage. Meat Sci. 2019, 149, 107–113. DOI: 10.1016/j.meatsci.2018.11.016.
- Yadav, B.; Spinelli, A. C.; Govindan, B. N.; Tsui, Y. Y.; McMullen, L. M.; Roopesh, M. S. Cold Plasma Treatment of Ready-To-Eat Ham: Influence of Process Conditions and Storage on Inactivation of Listeria Innocua. Food. Res. Int. 2019, 123, 276–285. DOI: 10.1016/j.foodres.2019.04.065.
- Luo, J.; Nasiru, M. M.; Yan, W.; Zhuang, H.; Zhou, G.; Zhang, J. Effects of Dielectric Barrier Discharge Cold Plasma Treatment on the Structure and Binding Capacity of Aroma Compounds of Myofibrillar Proteins from Dry-Cured Bacon. LWT. 2020, 117, 108606. DOI: 10.1016/j.lwt.2019.108606.
- Han, L.; Ziuzina, D.; Heslin, C.; Boehm, D.; Patange, A.; Sango, D. M.; Valdramidis, V. P.; Cullen, P. J.; Bourke, P. Controlling Microbial Safety Challenges of Meat Using High Voltage Atmospheric Cold Plasma. Front. Microbiol. 2016, 7, 977. DOI: 10.3389/fmicb.2016.00977.
- Wang, J. M.; Zhuang, H.; Lawrence, K.; Zhang, J. H. Disinfection of Chicken Fillets in Packages with Atmospheric Cold Plasma: Effects of Treatment Voltage and Time. J. Appl. Microbiol. 2018, 124, 1212–1219. DOI: 10.1111/jam.13637.
- Pérez-Andrés, J. M.; Álvarez, C.; Cullen, P. J.; Tiwari, B. K. Effect of Cold Plasma on the Techno-Functional Properties of Animal Protein Food Ingredients. Innov. Food Sci. Emerg. Technol. 2019, 58, 102205. DOI: 10.1016/j.ifset.2019.102205.
- Truong, B. Q.; Buckow, R.; Stathopoulos, C. E.; Nguyen, M. H. Advances in High-Pressure Processing of Fish Muscles. Food Eng. Rev. 2015, 7, 109–129. DOI: 10.1007/s12393-014-9084-9.
- Maldonado, J. A.; Schaffner, D. W.; Cuitinõ, A. M.; Karwe, M. V. In situ Studies of Microbial Inactivation During High Pressure Processing. High Press. Res. 2016, 36, 79–89. DOI: 10.1080/08957959.2015.1111887.
- Kang, D.; Jiang, Y.; Xing, L.; Zhou, G.; Zhang, W. Inactivation of Escherichia Coli O157:H7 and Bacillus Cereus by Power Ultrasound During the Curing Processing in Brining Liquid and Beef. Food. Res. Int. 2017, 102, 717–727. DOI: 10.1016/J.FOODRES.2017.09.062.
- Ojha, K. S.; Harrison, S. M.; Brunton, N. P.; Kerry, J. P.; Tiwari, B. K. Statistical Approaches to Access the Effect of Lactobacillus Sakei Culture and Ultrasound Frequency on Fatty Acid Profile of Beef Jerky. J. Food Compos. Anal. 2017, 57, 1–7. DOI: 10.1016/J.JFCA.2016.12.007.
- Mikš-Krajnik, M.; James Feng, L. X.; Bang, W. S.; Yuk, H. G. Inactivation of Listeria Monocytogenes and Natural Microbiota on Raw Salmon Fillets Using Acidic Electrolyzed Water, Ultraviolet Light Or/And Ultrasounds. Food Control. 2017, 74, 54–60. DOI: 10.1016/J.FOODCONT.2016.11.033.
- Condón-Abanto, S.; Arroyo, C.; Álvarez, I.; Brunton, N.; Whyte, P.; Lyng, J. G. An Assessment of the Application of Ultrasound in the Processing of Ready-To-Eat Whole Brown Crab (Cancer Pagurus). Ultrason. Sonochem. 2018, 40, 497–504. DOI: 10.1016/J.ULTSONCH.2017.07.044.
- Piñon, M. I.; Alarcon-Rojo, A. D.; Renteria, A. L.; Mendez, G.; Janacua-Vidales, H. Reduction of Microorganisms in Marinated Poultry Breast Using Oregano Essential Oil and Power Ultrasound. Acta Aliment. 2015, 44, 527–533. DOI: 10.1556/066.2015.44.0024.
- Caraveo, O.; Alarcon-Rojo, A. D.; Renteria, A.; Santellano, E.; Paniwnyk, L. Physicochemical and Microbiological Characteristics of Beef Treated with High-Intensity Ultrasound and Stored at 4 °C. J. Sci. Food Agric. 2015, 95, 2487–2493. DOI: 10.1002/JSFA.6979.
- Silva, F. V. M. Use of Power Ultrasound to Enhance the Thermal Inactivation of Clostridium Perfringens Spores in Beef Slurry. Int. J. Food Microbiol. 2015, 206, 17–23. DOI: 10.1016/J.IJFOODMICRO.2015.04.013.
- Pedrós-Garrido, S.; Condón-Abanto, S.; Beltrán, J. A.; Lyng, J. G.; Brunton, N. P.; Bolton, D.; Whyte, P. Assessment of High Intensity Ultrasound for Surface Decontamination of Salmon (S. Salar), Mackerel (S. Scombrus), Cod (G. Morhua) and Hake (M. Merluccius) Fillets, and Its Impact on Fish Quality. Innov. Food Sci. Emerg. Technol. 2017, 41, 64–70. DOI: 10.1016/j.ifset.2017.02.006.
- Chuang, S.; Sheen, S.; Sommers, C. H.; Sheen, L. Y. Modeling the Reduction of Salmonella and Listeria Monocytogenes in Ground Chicken Meat by High Pressure Processing and Trans-Cinnamaldehyde. LWT. 2021, 139, 110601. DOI: 10.1016/J.LWT.2020.110601.
- Cava, R.; García-Parra, J.; Ladero, L. Effect of High Hydrostatic Pressure Processing and Storage Temperature on Food Safety, Microbial Counts, Colour and Oxidative Changes of a Traditional Dry-Cured Sausage. LWT. 2020, 128, 109462. DOI: 10.1016/J.LWT.2020.109462.
- Jia, G.; Orlien, V.; Liu, H.; Sun, A. Effect of High Pressure Processing of Pork (Longissimus Dorsi) on Changes of Protein Structure and Water Loss During Frozen Storage. LWT. 2021, 135, 110084. DOI: 10.1016/J.LWT.2020.110084.
- Katsaros, G.; Taoukis, P. Microbial Control by High Pressure Processing for Shelf-Life Extension of Packed Meat Products in the Cold Chain: Modeling and Case Studies. Appl. Sci. 2021, 11, 1317. DOI: 10.3390/APP11031317.
- Cava, R.; Higuero, N.; Ladero, L. High-Pressure Processing and Storage Temperature on Listeria Monocytogenes, Microbial Counts and Oxidative Changes of Two Traditional Dry-Cured Meat Products. Meat Sci. 2021, 171, 108273. DOI: 10.1016/J.MEATSCI.2020.108273.
- Martillanes, S.; Rocha-Pimienta, J.; Llera-Oyola, J.; Gil, M. V.; Ayuso-Yuste, M. C.; García-Parra, J.; Delgado-Adámez, J. Control of Listeria Monocytogenes in Sliced Dry-Cured Iberian Ham by High Pressure Processing in Combination with an Eco-Friendly Packaging Based on Chitosan, Nisin and Phytochemicals from Rice Bran. Food Control. 2021, 124, 107933. DOI: 10.1016/J.FOODCONT.2021.107933.
- Luo, H.; Sheng, Z.; Guo, C.; Jia, R.; Yang, W. Quality Attributes Enhancement of Ready-To-Eat Hairtail Fish Balls by High-Pressure Processing. LWT. 2021, 147, 111658. DOI: 10.1016/J.LWT.2021.111658.
- Jalarama Reddy, K.; Jayathilakan, K.; Chauhan, O. P.; Pandey, M. C.; Radhakrishna, K. Effect of High-Pressure Processing on Physico-Chemical and Microbial Quality Characteristics of Chevon (Capra Aegagrus Hircus). Food Bioprocess Technol. 2015, 8, 2347–2358. DOI: 10.1007/S11947-015-1617-6.
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K. H.; Guyon, C.; Stübler, A. S.; de Lamballerie, M.; Hertel, C.; Brüggemann, D. A. High-Pressure Processing of Meat: Molecular Impacts and Industrial Applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 332–368. DOI: 10.1111/1541-4337.12670.
- Agregán, R.; Munekata, P. E. S.; Zhang, W.; Zhang, J.; Pérez-Santaescolástica, C.; Lorenzo, J. M. High-Pressure Processing in Inactivation of Salmonella Spp. in Food Products. Trends Food Sci. Technol. 2021, 107, 31–37. DOI: 10.1016/J.TIFS.2020.11.025.
- Bhargava, N.; Mor, R. S.; Kumar, K.; Sharanagat, V. S. Advances in Application of Ultrasound in Food Processing: A Review. Ultrason. Sonochem. 2021, 70, 105293. DOI: 10.1016/J.ULTSONCH.2020.105293.
- Condón-Abanto, S.; Arroyo, C.; Álvarez, I.; Condón, S.; Lyng, J. G. Application of Ultrasound in Combination with Heat and Pressure for the Inactivation of Spore Forming Bacteria Isolated from Edible Crab (Cancer Pagurus). Int. J. Food Microbiol. 2016, 223, 9–16. DOI: 10.1016/J.IJFOODMICRO.2016.02.001.
- Singla, M.; Sit, N. Application of Ultrasound in Combination with Other Technologies in Food Processing: A Review. Ultrason. Sonochem. 2021, 73, 105506. DOI: 10.1016/J.ULTSONCH.2021.105506.
- Zhou, C.; Okonkwo, C. E.; Inyinbor, A. A.; Yagoub, A. E. G. A.; Olaniran, A. F. Ultrasound, Infrared and Its Assisted Technology, a Promising Tool in Physical Food Processing: A Review of Recent Developments. Crit. Rev. Food Sci. Nutr. 2021, 1–25. DOI: 10.1080/10408398.2021.1966379.
- Strieder, M. M.; Silva, E. K.; Meireles, M. A. A. Advances and Innovations Associated with the Use of Acoustic Energy in Food Processing: An Updated Review. Innov. Food Sci. Emerg. Technol. 2021, 74, 102863. DOI: 10.1016/J.IFSET.2021.102863.
- Alarcon-Rojo, A. D.; Carrillo-Lopez, L. M.; Reyes-Villagrana, R.; Huerta-Jiménez, M.; Garcia-Galicia, I. A. Ultrasound and Meat Quality: A Review. Ultrason. Sonochem. 2019, 55, 369–382. DOI: 10.1016/J.ULTSONCH.2018.09.016.
- Bhat, Z. F.; Morton, J. D.; Bekhit, A. E. D. A.; Kumar, S.; Bhat, H. F. Non-Thermal Processing Has an Impact on the Digestibility of the Muscle Proteins. Crit. Rev. Food Sci. Nutr. 2021, 62, 7773–7800. DOI: 10.1080/10408398.2021.1918629.
- Bhat, Z. F.; Morton, J. D.; Bekhit, A.E. -D.A.; Kumar, S.; Bhat, H. F. Emerging Processing Technologies for Improved Digestibility of Muscle Proteins. Trends Food Sci. Technol. 2021, 110, 226–239. DOI: 10.1016/j.tifs.2021.02.010.
- Bhat, Z. F.; Morton, J. D.; Mason, S. L.; Bekhit, A. E. D. A. The Application of Pulsed Electric Field as a Sodium Reducing Strategy for Meat Products. Food Chem. 2020, 306, 125622. DOI: 10.1016/J.FOODCHEM.2019.125622.
- Bhat, Z. F.; Morton, J. D.; Mason, S. L.; Bekhit, A. E. D. A. Does Pulsed Electric Field Have a Potential to Improve the Quality of Beef from Older Animals and How? Innov. Food Sci. Emerg. Technol. 2019, 56, 102194. DOI: 10.1016/J.IFSET.2019.102194.
- Bhat, Z. F.; Morton, J. D.; Zhang, X.; Mason, S. L.; Bekhit, A. E. D. A. Sous-Vide Cooking Improves the Quality and in-Vitro Digestibility of Semitendinosus from Culled Dairy Cows. Food. Res. Int. 2020, 127, 108708. DOI: 10.1016/J.FOODRES.2019.108708.
- Bhat, Z. F.; Morton, J. D.; Mason, S. L.; Bekhit, A. E. D. A. Pulsed Electric Field: Role in Protein Digestion of Beef Biceps Femoris. Innov. Food Sci. Emerg. Technol. 2018, 50, 132–138. DOI: 10.1016/J.IFSET.2018.09.006.
- Bhat, Z. F.; Morton, J. D.; Mason, S. L.; Bekhit, A. E. D. A.; Mungure, T. E. Pulsed Electric Field: Effect on in-Vitro Simulated Gastrointestinal Protein Digestion of Deer Longissimus Dorsi. Food. Res. Int. 2019, 120, 793–799. DOI: 10.1016/J.FOODRES.2018.11.040.
- Bhat, Z. F.; Morton, J. D.; Mason, S. L.; Jayawardena, S. R.; Bekhit, A. E. D. A. Pulsed Electric Field: A New Way to Improve Digestibility of Cooked Beef. Meat Sci. 2019, 155, 79–84. DOI: 10.1016/J.MEATSCI.2019.05.005.
- Bhat, Z. F.; Morton, J. D.; Mason, S. L.; Jayawardena, S. R.; Mungure, T.; Bekhit, A. E. D. A. Cooking Does Not Impair the Impact of Pulsed Electric Field on the Protein Digestion of Venison (Cervus Elaphus) During in vitro Gastrointestinal Digestion. Int. J. Food Sci. Technol. 2020, 56, 3026–3033. DOI: 10.1111/IJFS.14946.
- Alahakoon, A. U.; Oey, I.; Bremer, P.; Silcock, P. Process Optimisation of Pulsed Electric Fields Pre-Treatment to Reduce the Sous Vide Processing Time of Beef Briskets. Int. J. Food Sci. Technol. 2019, 54, 823–834. DOI: 10.1111/ijfs.14002.
- Chian, F. M.; Kaur, L.; Oey, I.; Astruc, T.; Hodgkinson, S.; Boland, M. Effect of Pulsed Electric Fields (PEF) on the Ultrastructure and in vitro Protein Digestibility of Bovine Longissimus Thoracis. LWT. 2019, 103, 253–259. DOI: 10.1016/J.LWT.2019.01.005.
- Kaur, L.; Astruc, T.; Vénien, A.; Loison, O.; Cui, J.; Irastorza, M.; Boland, M. High Pressure Processing of Meat: Effects on Ultrastructure and Protein Digestibility. Food Funct. 2016, 7, 2389–2397. DOI: 10.1039/C5FO01496D.
- Xue, S.; Wang, C.; Kim, Y. H. B.; Bian, G.; Han, M.; Xu, X.; Zhou, G. Application of High-Pressure Treatment Improves the in vitro Protein Digestibility of Gel-Based Meat Product. Food Chem. 2020, 306, 125602. DOI: 10.1016/J.FOODCHEM.2019.125602.
- Cepero-Betancourt, Y.; Opazo-Navarrete, M.; Janssen, A. E. M.; Tabilo-Munizaga, G.; Pérez-Won, M. Effects of High Hydrostatic Pressure (HHP) on Protein Structure and Digestibility of Red Abalone (Haliotis Rufescens) Muscle. Innov. Food Sci. Emerg. Technol. 2020, 60, 102282. DOI: 10.1016/J.IFSET.2019.102282.
- Rakotondramavo, A.; Rabesona, H.; Brou, C.; de Lamballerie, M.; Pottier, L. Ham Processing: Effects of Tumbling, Cooking and High Pressure on Proteins. Eur. Food Res. Technol. 2019, 245, 273–284. DOI: 10.1007/s00217-018-3159-4.
- Dong, X.; Wang, J.; Raghavan, V. Effects of High-Intensity Ultrasound Processing on the Physiochemical and Allergenic Properties of Shrimp. Innov. Food Sci. Emerg. Technol. 2020, 65, 102441. DOI: 10.1016/j.ifset.2020.102441.
- Bagarinao, N. C.; Kaur, L.; Boland, M. Effects of Ultrasound Treatments on Tenderness and in vitro Protein Digestibility of New Zealand Abalone, Haliotis Iris. Foods. 2020, 9, 1122. DOI: 10.3390/FOODS9081122.
- Chian, F. M.; Kaur, L.; Astruc, T.; Vénien, A.; Loison, O.; Stübler, A. -S.; Aganovic, K.; Hodgkinson, S.; Boland, M.; Shockwave Processing and Sous Vide Cooking Improve Sensorial and Nutritional Qualities of Beef. In Proceedings of the Food Structure Digestion and Health congress; Rotorua, New Zealand, 2019.
- Chian, F. M.; Kaur, L.; Astruc, T.; Venien, A.; Loison, O.; Stubler, A. -S.; Aganovic, K.; Hodgkinson, S.; Boland, M. The Effect of Shockwave Processing on Muscle Protein Structure and Digestibility in vitro. In Proceedings of the 64th International Congress of Meat Science and Technology; Melbourne, Australia, 2018.
- Wang, L. The Storage and Preservation of Seafood. Encycl. Food Secur. Sustain. 2018, 1, 619–624.
- Duangkhamchan, W.; Phomphai, A.; Wanna, R.; Wiset, L.; Laohavanich, J.; Ronsse, F.; Pieters, J. G. Infrared Heating as a Disinfestation Method Against Sitophilus Oryzae and Its Effect on Textural and Cooking Properties of Milled Rice. Food Bioprocess Technol. 2017, 10, 284–295. DOI: 10.1007/s11947-016-1813-z.
- Cheng, L.; Sun, D. W.; Zhu, Z.; Zhang, Z. Effects of High Pressure Freezing (HPF) on Denaturation of Natural Actomyosin Extracted from Prawn (Metapenaeus Ensis). Food Chem. 2017, 229, 252–259. DOI: 10.1016/j.foodchem.2017.02.048.
- Cheng, L.; Sun, D. W.; Zhu, Z.; Zhang, Z. Emerging Techniques for Assisting and Accelerating Food Freezing Processes: A Review of Recent Research Progresses. Crit. Rev. Food Sci. Nutr. 2017, 57, 769–781. DOI: 10.1080/10408398.2015.1004569.
- Cartagena, L.; Puértolas, E.; Martínez de Marañón, I. Impact of Different Air Blast Freezing Conditions on the Physicochemical Quality of Albacore (Thunnus Alalunga) Pretreated by High Pressure Processing. LWT. 2021, 145. DOI: 10.1016/j.lwt.2021.111538.
- Truong, B. Q.; Buckow, R.; Nguyen, M. H.; Stathopoulos, C. E. High Pressure Processing of Barramundi (Lates Calcarifer) Muscle Before Freezing: The Effects on Selected Physicochemical Properties During Frozen Storage. J. Food Eng. 2016, 169, 72–78. DOI: 10.1016/j.jfoodeng.2015.08.020.
- Ekonomou, S. I.; Bulut, S.; Karatzas, K. A. G.; Boziaris, I. S. Inactivation of Listeria Monocytogenes in Raw and Hot Smoked Trout Fillets by High Hydrostatic Pressure Processing Combined with Liquid Smoke and Freezing. Innov. Food Sci. Emerg. Technol. 2020, 64, 102427. DOI: 10.1016/j.ifset.2020.102427.
- Năstase, G.; Lyu, C.; Ukpai, G.; Şerban, A.; Rubinsky, B. Isochoric and Isobaric Freezing of Fish Muscle. Biochem. Biophys. Res. Commun. 2017, 485, 279–283. DOI: 10.1016/j.bbrc.2017.02.091.
- Tironi, V.; De Lamballerie, M.; Le-Bail, A. Quality Changes During the Frozen Storage of Sea Bass (Dicentrarchus Labrax) Muscle After Pressure Shift Freezing and Pressure Assisted Thawing. Innov. Food Sci. Emerg. Technol. 2010, 11, 565–573. DOI: 10.1016/j.ifset.2010.05.001.
- Boziaris, I. S.; Parlapani, F. F.; Mireles DeWitt, C. A. High Pressure Processing at Ultra-Low Temperatures: Inactivation of Foodborne Bacterial Pathogens and Quality Changes in Frozen Fish Fillets. Innov. Food Sci. Emerg. Technol. 2021, 74, 102811. DOI: 10.1016/j.ifset.2021.102811.
- Abie, S. M.; Münch, D.; Egelandsdal, B.; Bjerke, F.; Wergeland, I.; Martinsen, Ø. G. Combined 0.2 T Static Magnetic Field and 20 kHz, 2 V/Cm Square Wave Electric Field Do Not Affect Supercooling and Freezing Time of Saline Solution and Meat Samples. J. Food Eng. 2021, 311, 110710. DOI: 10.1016/j.jfoodeng.2021.110710.
- Tang, J.; Shao, S.; Tian, C. Effects of the Magnetic Field on the Freezing Parameters of the Pork. Int. J. Refrig. 2019, 107, 31–38. DOI: 10.1016/j.ijrefrig.2019.07.019.
- Otero, L.; Pérez-Mateos, M.; Rodríguez, A. C.; Sanz, P. D. Electromagnetic Freezing: Effects of Weak Oscillating Magnetic Fields on Crab Sticks. J. Food Eng. 2017, 200, 87–94. DOI: 10.1016/j.jfoodeng.2016.12.018.
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A. Effect of Freezing Under Electrostatic Field on the Quality of Lamb Meat. Innov. Food Sci. Emerg. Technol. 2016, 37, 68–73. DOI: 10.1016/j.ifset.2016.07.028.
- Ma, X.; Mei, J.; Xie, J. Effects of Multi-Frequency Ultrasound on the Freezing Rates, Quality Properties and Structural Characteristics of Cultured Large Yellow Croaker (Larimichthys Crocea). Ultrason. Sonochem. 2021, 76, 105657. DOI: 10.1016/j.ultsonch.2021.105657.
- Zhang, C.; Sun, Q.; Chen, Q.; Kong, B.; Diao, X. Effects of Ultrasound-Assisted Immersion Freezing on the Muscle Quality and Physicochemical Properties of Chicken Breast. Int. J. Refrig. 2020, 117, 247–255. DOI: 10.1016/j.ijrefrig.2020.05.006.
- Zhang, C.; Li, X. A.; Wang, H.; Xia, X.; Kong, B. Ultrasound-Assisted Immersion Freezing Reduces the Structure and Gel Property Deterioration of Myofibrillar Protein from Chicken Breast. Ultrason. Sonochem. 2020, 67, 105137. DOI: 10.1016/j.ultsonch.2020.105137.
- Chen, X.; Liu, H.; Li, X.; Wei, Y.; Li, J. Effect of Ultrasonic-Assisted Immersion Freezing and Quick-Freezing on Quality of Sea Bass During Frozen Storage. LWT. 2022, 154, 112737. DOI: 10.1016/j.lwt.2021.112737.
- Sun, Q.; Sun, F.; Xia, X.; Xu, H.; Kong, B. The Comparison of Ultrasound-Assisted Immersion Freezing, Air Freezing and Immersion Freezing on the Muscle Quality and Physicochemical Properties of Common Carp (Cyprinus Carpio) During Freezing Storage. Ultrason. Sonochem. 2019, 51, 281–291. DOI: 10.1016/j.ultsonch.2018.10.006.
- Sun, Q.; Chen, Q.; Xia, X.; Kong, B.; Diao, X. Effects of Ultrasound-Assisted Freezing at Different Power Levels on the Structure and Thermal Stability of Common Carp (Cyprinus Carpio) Proteins. Ultrason. Sonochem. 2019, 54, 311–320. DOI: 10.1016/j.ultsonch.2019.01.026.
- Zhang, M.; Haili, N.; Chen, Q.; Xia, X.; Kong, B. Influence of Ultrasound-Assisted Immersion Freezing on the Freezing Rate and Quality of Porcine Longissimus Muscles. Meat Sci. 2018, 136, 1–8. DOI: 10.1016/j.meatsci.2017.10.005.
- Hong, G. P.; Choi, M. J. Comparison of the Quality Characteristics of Abalone Processed by High-Pressure Sub-Zero Temperature and Pressure-Shift Freezing. Innov. Food Sci. Emerg. Technol. 2016, 33, 19–25. DOI: 10.1016/j.ifset.2015.12.024.
- Cheng, L.; Zhu, Z.; Sun, D. W. Impacts of High Pressure Assisted Freezing on the Denaturation of Polyphenol Oxidase. Food Chem. 2021, 335, 127485. DOI: 10.1016/j.foodchem.2020.127485.
- Zhu, Z.; Li, T.; Sun, D. W. Pressure-Related Cooling and Freezing Techniques for the Food Industry: Fundamentals and Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 2793–2808. DOI: 10.1080/10408398.2020.1841729.
- Otero, L.; Rodríguez, A. C.; Pérez-Mateos, M.; Sanz, P. D. Effects of Magnetic Fields on Freezing: Application to Biological Products. Compr. Rev. Food Sci. Food Saf. 2016, 15, 646–667. DOI: 10.1111/1541-4337.12202.
- Mok, J. H.; Choi, W.; Park, S. H.; Lee, S. H.; Jun, S. Emerging Pulsed Electric Field (PEF) and Static Magnetic Field (SMF) Combination Technology for Food Freezing. Int. J. Refrig. 2015, 50, 137–145. DOI: 10.1016/j.ijrefrig.2014.10.025.
- Ma, L.; Huang, L.; Liu, Q.; Xu, S.; Wen, Z.; Qin, S.; Li, T.; Feng, Y. Positive Effects of Applying Endophytic Bacteria in Eggplant-Sedum Intercropping System on Cd Phytoremediation and Vegetable Production in Cadmium Polluted Greenhouse. J. Environ. Sci. (China). 2022, 115, 383–391. DOI: 10.1016/j.jes.2021.08.005.
- Qiu, L.; Zhang, M.; Chitrakar, B.; Bhandari, B. Application of Power Ultrasound in Freezing and Thawing Processes: Effect on Process Efficiency and Product Quality. Ultrason. Sonochem. 2020, 68, 105230. DOI: 10.1016/j.ultsonch.2020.105230.
- Sun, Q.; Zhao, X.; Zhang, C.; Xia, X.; Sun, F.; Kong, B. Ultrasound-Assisted Immersion Freezing Accelerates the Freezing Process and Improves the Quality of Common Carp (Cyprinus Carpio) at Different Power Levels. LWT. 2019, 108, 106–112. DOI: 10.1016/j.lwt.2019.03.042.
- Astráin-Redín, L.; Abad, J.; Rieder, A.; Kirkhus, B.; Raso, J.; Cebrián, G.; Álvarez, I. Direct Contact Ultrasound Assisted Freezing of Chicken Breast Samples. Ultrason. Sonochem. 2021, 70, 105319. DOI: 10.1016/j.ultsonch.2020.105319.
- Kumar, P.; Chevallier, S.; Xanthakis, E.; Jury, V. Effect of Innovative Microwave Assisted Freezing (MAF) on the Quality Attributes of Apples and Potatoes. Food Chem. 2020, 309, 125594. DOI: 10.1016/j.foodchem.2019.125594.
- Hafezparast-Moadab, N.; Hamdami, N.; Dalvi-Isfahan, M.; Farahnaky, A. Effects of Radiofrequency-Assisted Freezing on Microstructure and Quality of Rainbow Trout (Oncorhynchus Mykiss) Fillet. Innov. Food Sci. Emerg. Technol. 2018, 47, 81–87. DOI: 10.1016/j.ifset.2017.12.012.
- Xanthakis, E.; Le-Bail, A.; Ramaswamy, H. Development of an Innovative Microwave Assisted Food Freezing Process. Innov. Food Sci. Emerg. Technol. 2014, 26, 176–181. DOI: 10.1016/j.ifset.2014.04.003.
- Goula, A. M.; Lazarides, H. N. Modeling of Mass and Heat Transfer During Combined Processes of Osmotic Dehydration and Freezing (Osmo-Dehydro-Freezing). Chem. Eng. Sci. 2012, 82, 52–61. DOI: 10.1016/j.ces.2012.07.023.
- Hassoun, A.; Carpena, M.; Prieto, M. A.; Simal-Gandara, J.; Özogul, F.; Özogul, Y.; Çoban, Ö. E.; Guðjónsdóttir, M.; Barba, F. J.; Marti-Quijal, F. J., et al. Use of Spectroscopic Techniques to Monitor Changes in Food Quality During Application of Natural Preservatives: A Review. Antioxidants. 2020, 9, 882. DOI: 10.3390/ANTIOX9090882.
- Baptista, R. C.; Horita, C. N.; Sant’Ana, A. S. Natural Products with Preservative Properties for Enhancing the Microbiological Safety and Extending the Shelf-Life of Seafood: A Review. Food. Res. Int. 2020, 127, 108762. DOI: 10.1016/j.foodres.2019.108762.
- Mei, J.; Ma, X.; Xie, J. Review on Natural Preservatives for Extending Fish Shelf Life. Foods. 2019, 8, 490. DOI: 10.3390/foods8100490.
- Gokoglu, N. Novel Natural Food Preservatives and Applications in Seafood Preservation: A Review. J. Sci. Food Agric. 2019, 99, 2068–2077. DOI: 10.1002/jsfa.9416.
- Inanli, A. G.; Tümerkan, E. T. A.; Abed, N. E.; Regenstein, J. M.; Özogul, F. The Impact of Chitosan on Seafood Quality and Human Health: A Review. Trends Food Sci. Technol. 2020, 97, 404–416. DOI: 10.1016/j.tifs.2020.01.029.
- Karoui, R.; Hassoun, A. Efficiency of Rosemary and Basil Essential Oils on the Shelf-Life Extension of Atlantic Mackerel (Scomber Scombrus) Fillets Stored at 2°C. J. AOAC Int. 2017, 100, 335–344. DOI: 10.5740/jaoacint.16-0410.
- Ozogul, Y.; Yuvka, İ.; Ucar, Y.; Durmus, M.; Kösker, A. R.; Öz, M.; Ozogul, F. Evaluation of Effects of Nanoemulsion Based on Herb Essential Oils (Rosemary, Laurel, Thyme and Sage) on Sensory, Chemical and Microbiological Quality of Rainbow Trout (Oncorhynchus Mykiss) Fillets During Ice Storage. LWT - Food Sci. Technol. 2017, 75, 677–684. DOI: 10.1016/j.lwt.2016.10.009.
- Alparslan, Y.; Yapici, H. H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality Assessment of Shrimps Preserved with Orange Leaf Essential Oil Incorporated Gelatin. LWT - Food Sci. Technol. 2016, 72, 457–466. DOI: 10.1016/j.lwt.2016.04.066.
- de Carvalho, F. A. L.; Lorenzo, J. M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M. A. Effect of Guarana (Paullinia Cupana) Seed and Pitanga (Eugenia Uniflora L.) Leaf Extracts on Lamb Burgers with Fat Replacement by Chia Oil Emulsion During Shelf Life Storage at 2 °C. Food. Res. Int. 2019, 125, 108554. DOI: 10.1016/j.foodres.2019.108554.
- Jayawardana, B. C.; Warnasooriya, V. B.; Thotawattage, G. H.; Dharmasena, V. A. K. I.; Liyanage, R. Black and Green Tea (Camellia Sinensis L.) Extracts as Natural Antioxidants in Uncured Pork Sausages. J. Food Process Preserv. 2019, 43, 1–8. DOI: 10.1111/jfpp.13870.
- Koné, A. P.; Desjardins, Y.; Gosselin, A.; Cinq-Mars, D.; Guay, F.; Saucier, L. Plant Extracts and Essential Oil Product as Feed Additives to Control Rabbit Meat Microbial Quality. Meat Sci. 2019, 150, 111–121. DOI: 10.1016/j.meatsci.2018.12.013.
- Gómez-Estaca, J.; López-Caballero, M. E.; Martínez-Bartolomé, M. Á.; de Lacey, A. M. L.; Gómez-Guillen, M. C.; Montero, M. P. The Effect of the Combined Use of High Pressure Treatment and Antimicrobial Edible Film on the Quality of Salmon Carpaccio. Int. J. Food Microbiol. 2018, 283, 28–36. DOI: 10.1016/j.ijfoodmicro.2018.06.015.
- Balti, R.; Ben Mansour, M.; Zayoud, N.; Le Balc’h, R.; Brodu, N.; Arhaliass, A.; Massé, A. Active Exopolysaccharides Based Edible Coatings Enriched with Red Seaweed (Gracilaria Gracilis) Extract to Improve Shrimp Preservation During Refrigerated Storage. Food Biosci. 2020, 34, 100522. DOI: 10.1016/j.fbio.2019.100522.
- Abdel-Naeem, H. H. S.; Sallam, K. I.; Malak, N. M. L. Improvement of the Microbial Quality, Antioxidant Activity, Phenolic and Flavonoid Contents, and Shelf Life of Smoked Herring (Clupea Harengus) During Frozen Storage by Using Chitosan Edible Coating. Food Control. 2021, 130, 108317. DOI: 10.1016/j.foodcont.2021.108317.
- Qian, Y. F.; Cheng, Y.; Ye, J. X.; Zhao, Y.; Xie, J.; Yang, S. P. Targeting Shrimp Spoiler Shewanella Putrefaciens: Application of ε-Polylysine and Oregano Essential Oil in Pacific White Shrimp Preservation. Food Control. 2021, 123, 107702. DOI: 10.1016/j.foodcont.2020.107702.
- Wu, T.; Ge, Y.; Li, Y.; Xiang, Y.; Jiang, Y.; Hu, Y. Quality Enhancement of Large Yellow Croaker Treated with Edible Coatings Based on Chitosan and Lysozyme. Int. J. Biol. Macromol. 2018, 120, 1072–1079. DOI: 10.1016/j.ijbiomac.2018.08.188.
- Farsanipour, A.; Khodanazary, A.; Hosseini, S. M. Effect of Chitosan-Whey Protein Isolated Coatings Incorporated with Tarragon Artemisia Dracunculus Essential Oil on the Quality of Scomberoides Commersonnianus Fillets at Refrigerated Condition. Int. J. Biol. Macromol. 2020, 155, 766–771. DOI: 10.1016/j.ijbiomac.2020.03.228.
- Cardoso, G. P.; Dutra, M. P.; Fontes, P. R.; Ramos, A. D. L. S.; Gomide, L. A. D. M.; Ramos, E. M. Selection of a Chitosan Gelatin-Based Edible Coating for Color Preservation of Beef in Retail Display. Meat Sci. 2016, 114, 85–94. DOI: 10.1016/j.meatsci.2015.12.012.
- Farajzadeh, F.; Motamedzadegan, A.; Shahidi, S. A.; Hamzeh, S. The Effect of Chitosan-Gelatin Coating on the Quality of Shrimp (Litopenaeus Vannamei) Under Refrigerated Condition. Food Control. 2016, 67, 163–170. DOI: 10.1016/j.foodcont.2016.02.040.
- Lekjing, S. A Chitosan-Based Coating with or Without Clove Oil Extends the Shelf Life of Cooked Pork Sausages in Refrigerated Storage. Meat Sci. 2016, 111, 192–197. DOI: 10.1016/j.meatsci.2015.10.003.
- Wu, C.; Li, Y.; Wang, L.; Hu, Y.; Chen, J.; Liu, D.; Ye, X. Efficacy of Chitosan-Gallic Acid Coating on Shelf Life Extension of Refrigerated Pacific Mackerel Fillets. Food Bioprocess Technol. 2016, 9, 675–685. DOI: 10.1007/s11947-015-1659-9.
- Kang, H. J.; Jo, C.; Kwon, J. H.; Kim, J. H.; Chung, H. J.; Byun, M. W. Effect of a Pectin-Based Edible Coating Containing Green Tea Powder on the Quality of Irradiated Pork Patty. Food Control. 2007, 18, 430–435. DOI: 10.1016/j.foodcont.2005.11.010.
- Feng, X.; Ng, V. K.; Mikš-Krajnik, M.; Yang, H. Effects of Fish Gelatin and Tea Polyphenol Coating on the Spoilage and Degradation of Myofibril in Fish Fillet During Cold Storage. Food Bioprocess Technol. 2017, 10, 89–102. DOI: 10.1007/s11947-016-1798-7.
- Feng, X.; Bansal, N.; Yang, H. Fish Gelatin Combined with Chitosan Coating Inhibits Myofibril Degradation of Golden Pomfret (Trachinotus Blochii) Fillet During Cold Storage. Food Chem. 2016, 200, 283–292. DOI: 10.1016/j.foodchem.2016.01.030.
- Hosseini, S. F.; Rezaei, M.; Zandi, M.; Ghavi, F. F. Effect of Fish Gelatin Coating Enriched with Oregano Essential Oil on the Quality of Refrigerated Rainbow Trout Fillet. J. Aquat. Food Prod. Technol. 2016, 25, 835–842. DOI: 10.1080/10498850.2014.943917.
- Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Effects of Edible Coatings Based on Ultrasound-Treated Whey Proteins in Quality Attributes of Frozen Atlantic Salmon (Salmo Salar). Innov. Food Sci. Emerg. Technol. 2012, 14, 92–98. DOI: 10.1016/j.ifset.2011.12.003.
- Shokri, S.; Ehsani, A. Efficacy of Whey Protein Coating Incorporated with Lactoperoxidase and α-Tocopherol in Shelf Life Extension of Pike-Perch Fillets During Refrigeration. LWT - Food Sci. Technol. 2017, 85, 225–231. DOI: 10.1016/j.lwt.2017.07.026.
- Hassoun, A.; Emir Çoban, Ö. Essential Oils for Antimicrobial and Antioxidant Applications in Fish and Other Seafood Products. Trends Food Sci. Technol. 2017, 68, 26–36. DOI: 10.1016/j.tifs.2017.07.016.
- El-Saber Batiha, G.; Hussein, D. E.; Algammal, A. M.; George, T. T.; Jeandet, P.; Al-Snafi, A. E.; Tiwari, A.; Pagnossa, J. P.; Lima, C. M.; Thorat, N. D., et al. Application of Natural Antimicrobials in Food Preservation: Recent Views. Food Control. 2021, 126, 108066. DOI: 10.1016/j.foodcont.2021.108066.
- Mohamed, S. A. A.; El-Sakhawy, M.; El-Sakhawy, M. A. M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. DOI: 10.1016/j.carbpol.2020.116178.
- Maciel, V. B. V.; Contini, L. R. F.; Yoshida, C. M. P.; Venturini, A. C. Application of Edible Biopolymer Coatings on Meats, Poultry, and Seafood. In Biopolymer Membranes and Films, De Moraes, M., Da Silva, C. and Vieira, R.; Eds.; Amsterdam: Elsevier. 2020; Vol. 18. pp. 515–533.
- Dehghani, S.; Hosseini, S. V.; Regenstein, J. M. Edible Films and Coatings in Seafood Preservation: A Review. Food Chem. 2018, 240, 505–513. DOI: 10.1016/J.FOODCHEM.2017.07.034.
- Loudiyi, M.; Temiz, H. T.; Sahar, A.; Haseeb Ahmad, M.; Boukria, O.; Hassoun, A.; Aït-Kaddour, A. Spectroscopic Techniques for Monitoring Changes in the Quality of Milk and Other Dairy Products During Processing and Storage. Crit. Rev. Food Sci. Nutr. 2022, 62, 3063–3087. DOI: 10.1080/10408398.2020.1862754.
- Yang, Z. -J.; Zhao, Q.; He, J. Boosting Magnetic Field Enhancement with Radiative Couplings of Magnetic Modes in Dielectric Nanostructures. Opt. Express. 2017, 25, 15927. DOI: 10.1364/OE.25.015927.
- Goetz, A. F. H.; Vane, G.; Solomon, J. E.; Rock, B. N. Imaging Spectrometry for Earth Remote Sensing. Science. 1985, 228, 1147–1153. DOI: 10.1126/science.228.4704.1147.
- Kamruzzaman, M.; Makino, Y.; Oshita, S.; Liu, S. Assessment of Visible Near-Infrared Hyperspectral Imaging as a Tool for Detection of Horsemeat Adulteration in Minced Beef. Food Bioprocess Technol. 2015, 8, 1054–1062. DOI: 10.1007/s11947-015-1470-7.
- Wu, D.; Sun, D. -W. Potential of Time Series-Hyperspectral Imaging (TS-HSI) for Non-Invasive Determination of Microbial Spoilage of Salmon Flesh. Talanta. 2013, 111, 39–46. DOI: 10.1016/J.TALANTA.2013.03.041.
- Xiong, Z.; Sun, D. -W.; Pu, H.; Xie, A.; Han, Z.; Luo, M. Non-Destructive Prediction of Thiobarbituric acid Reactive Substances (TBARS) Value for Freshness Evaluation of Chicken Meat Using Hyperspectral Imaging. Food Chem. 2015, 179, 175–181. DOI: 10.1016/j.foodchem.2015.01.116.
- Wang, B.; Sun, J.; Xia, L.; Liu, J.; Wang, Z.; Li, P.; Guo, Y.; Sun, X. The Applications of Hyperspectral Imaging Technology for Agricultural Products Quality Analysis: A Review. Food Rev. Int. 2021, 1–20. DOI: 10.1080/87559129.2021.1929297.
- Amigo, J. M.; Grassi, S. Chapter 1.2 - Configuration of Hyperspectral and Multispectral Imaging Systems. In Hyperspectral Imaging; Amigo, J.M., Ed.; Data Handling in Science and Technology; Amsterdam: Elsevier, 2020; Vol. 32, pp. 17–34.
- Zhuang, Q.; Peng, Y.; Yang, D.; Wang, Y.; Zhao, R.; Chao, K.; Guo, Q. Detection of Frozen Pork Freshness by Fluorescence Hyperspectral Image. J. Food Eng. 2022, 316, 110840. DOI: 10.1016/J.JFOODENG.2021.110840.
- Elmasry, G.; Kamruzzaman, M.; Sun, D. -W.; Allen, P. Principles and Applications of Hyperspectral Imaging in Quality Evaluation of Agro-Food Products: A Review. Crit. Rev. Food Sci. Nutr. 2012, 52, 999–1023. DOI: 10.1080/10408398.2010.543495.
- Yang, Q. Broadband High-Spectral-Resolution Ultraviolet-Visible Coherent-Dispersion Imaging Spectrometer. Opt. Express. 2018, 26, 20777–20791. DOI: 10.1364/OE.26.020777.
- ElMasry, G.; Sun, D. -W. CHAPTER 1 - Principles of Hyperspectral Imaging Technology. In Hyperspectral Imaging for Food Quality Analysis and Control; Sun, D.-W., Ed.; Amsteradm: Academic Press, 2010; pp. 3–43.
- Aït-Kaddour, A.; Jacquot, S.; Micol, D.; Listrat, A. Discrimination of Beef Muscle Based on Visible-Near Infrared Multi-Spectral Features: Textural and Spectral Analysis. Int. J. Food. Prop. 2017, 20, 1391–1403. DOI: 10.1080/10942912.2016.1210163.
- Aït-Kaddour, A.; Andueza, D.; Dubost, A.; Roger, J. -M.; Hocquette, J. -F.; Listrat, A. Visible and Near-Infrared Multispectral Features in Conjunction with Artificial Neural Network and Partial Least Squares for Predicting Biochemical and Micro-Structural Features of Beef Muscles. Foods. 2020, 9, 1254. DOI: 10.3390/foods9091254.
- Alshejari, A.; Kodogiannis, V. S. An Intelligent Decision Support System for the Detection of Meat Spoilage Using Multispectral Images. Neural Comput. Appl. 2017, 28, 3903–3920. DOI: 10.1007/s00521-016-2296-6.
- Ma, F.; Qin, H.; Shi, K.; Zhou, C.; Chen, C.; Hu, X.; Zheng, L. Feasibility of Combining Spectra with Texture Data of Multispectral Imaging to Predict Heme and Non-Heme Iron Contents in Pork Sausages. Food Chem. 2016, 190, 142–149. DOI: 10.1016/j.foodchem.2015.05.084.
- Spyrelli, E. D.; Ozcan, O.; Mohareb, F.; Panagou, E. Z.; Nychas, G. -J.E. Spoilage Assessment of Chicken Breast Fillets by Means of Fourier Transform Infrared Spectroscopy and Multispectral Image Analysis. Curr. Res. Food Sci. 2021, 4, 121–131. DOI: 10.1016/j.crfs.2021.02.007.
- Alonso, R.; Picon, A.; Rodríguez, B.; Gaya, P.; Fernández-García, E.; Nuñez, M. Microbiological, Chemical, and Sensory Characteristics of Hispánico Cheese Manufactured Using Frozen High Pressure Treated Curds Made from Raw Ovine Milk. Int. Dairy. J. 2011, 21, 484–492. DOI: 10.1016/j.idairyj.2011.02.008.
- Tsakanikas, P.; Karnavas, A.; Panagou, E. Z.; Nychas, G. J. A Machine Learning Workflow for Raw Food Spectroscopic Classification in a Future Industry. Sci. Rep. 2020, 10, 1–11. DOI: 10.1038/s41598-020-68156-2.
- Ropodi, A. I.; Panagou, E. Z.; Nychas, G. -J.E. Rapid Detection of Frozen-Then-Thawed Minced Beef Using Multispectral Imaging and Fourier Transform Infrared Spectroscopy. Meat Sci. 2018, 135, 142–147. DOI: 10.1016/J.MEATSCI.2017.09.016.
- Vaskoska, R.; Vénien, A.; Ha, M.; White, J. D.; Unnithan, R. R.; Astruc, T.; Warner, R. D. Thermal Denaturation of Proteins in the Muscle Fibre and Connective Tissue from Bovine Muscles Composed of Type I (Masseter) or Type II (Cutaneous Trunci) Fibres: DSC and FTIR Microspectroscopy Study. Food Chem. 2021, 343, 128544. DOI: 10.1016/j.foodchem.2020.128544.
- Antequera, T.; Caballero, D.; Grassi, S.; Uttaro, B.; Perez-Palacios, T. Evaluation of Fresh Meat Quality by Hyperspectral Imaging (HSI), Nuclear Magnetic Resonance (NMR) and Magnetic Resonance Imaging (MRI): A Review. Meat Sci. 2021, 172, 108340. DOI: 10.1016/j.meatsci.2020.108340.
- Fan, N.; Liu, G.; Wan, G.; Ban, J.; Yuan, R.; Sun, Y.; Li, Y. A Combination of Near-Infrared Hyperspectral Imaging with Two-Dimensional Correlation Analysis for Monitoring the Content of Biogenic Amines in Mutton. Int. J. Food Sci. Technol. 2021, 56, 3066–3075. DOI: 10.1111/ijfs.14950.
- Özdoğan, G.; Lin, X.; Sun, D. W. Rapid and Noninvasive Sensory Analyses of Food Products by Hyperspectral Imaging: Recent Application Developments. Trends Food Sci. Technol. 2021, 111, 151–165. DOI: 10.1016/j.tifs.2021.02.044.
- Norsk Elektro Optikk AS HySpex. https://www.hyspex.com/hyspex-custom-solutions/fish-quality-analyzer/ (accessed Dec 8, 2021).
- Geelen, B.; Blanch, C.; Gonzalez, P.; Tack, N.; Lambrechts, A. A Tiny VIS-NIR Snapshot Multispectral Camera. Proceedings Volume 9374, Advanced Fabrication Technologies for Micro/Nano Optics and Photonics VIII; 937414, 2015. DOI: 10.1117/12.2077583.
- Ma, J.; Sun, D. -W.; Pu, H.; Wei, Q.; Wang, X. Protein Content Evaluation of Processed Pork Meats Based on a Novel Single Shot (Snapshot) Hyperspectral Imaging Sensor. J. Food Eng. 2019, 240, 207–213. DOI: 10.1016/J.JFOODENG.2018.07.032.
- Al-Sarayreh, M.; Reis, M. M.; Yan, W. Q.; Klette, R. Potential of Deep Learning and Snapshot Hyperspectral Imaging for Classification of Species in Meat. Food Control. 2020, 117, 107332. DOI: 10.1016/J.FOODCONT.2020.107332.
- Hassoun, A.; Abdullah, N. A.; Aït-Kaddour, A.; Beşir, A.; Zannou, O.; Önal, B.; Aadil, R. M.; Lorenzo, J. M.; Khaneghah, A. M.; Regenstein, J. M., et al. Food Traceability 4.0 as Part of the Fourth Industrial Revolution: Key Enabling Technologies. Crit. Rev. Food Sci. Nutr. 2022, 1–17. DOI:10.1080/10408398.2022.2110033.
- Fuentes, S.; Viejo, C. G.; Tongson, E.; Dunshea, F. R. The Livestock Farming Digital Transformation: Implementation of New and Emerging Technologies Using Artificial Intelligence. Anim. Heal. Res. Rev. 2022, 23, 59–71. DOI: 10.1017/S1466252321000177.
- Feider, C. L.; Krieger, A.; DeHoog, R. J.; Eberlin, L. S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem. 2019, 91, 4266–4290. DOI: 10.1021/acs.analchem.9b00807.
- Committee on the Environment Public Health and Food Safety. On the food crisis, fraud in the food chain and the control thereof; 2013, https://www.europarl.europa.eu/doceo/document/A-7-2013-0434_EN.html
- Moore, J.; United States Pharmacopeia appendix XVIII: Guidance on Developing and Validating Non-Targeted Methods for Adulteration Detection; USP: Rockville, MA, USA, 2017; pp. 2053–2066.
- Takáts, Z.; Wiseman, J. M.; Gologan, B.; Cooks, R. G. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science. 2004, 306, 471–473. DOI: 10.1126/science.1104404.
- Garrett, R.; Schwab, N. V.; Cabral, E. C.; Henrique, B. V. M.; Ifa, D. R.; Eberlin, M. N.; Rezende, C. M. Ambient Mass Spectrometry Employed for Direct Analysis of Intact Arabica Coffee Beans. J. Braz. Chem. Soc. 2014, 25, 1172–1177. DOI: 10.5935/0103-5053.20140094.
- Correa, D. N.; Santos, J. M.; Eberlin, L. S.; Eberlin, M. N.; Teunissen, S. F. Forensic Chemistry and Ambient Mass Spectrometry: A Perfect Couple Destined for a Happy Marriage? Anal. Chem. 2016, 88, 2515–2526. DOI: 10.1021/acs.analchem.5b02397.
- Swiner, D. J.; Jackson, S.; Burris, B. J.; Badu-Tawiah, A. K. Applications of Mass Spectrometry for Clinical Diagnostics: The Influence of Turnaround Time. Anal. Chem. 2020, 92, 183–202. DOI: 10.1021/acs.analchem.9b04901.
- Walworth, M. J.; Elnaggar, M. S.; Stankovich, J. J.; Witkowski, C.; Norris, J. L.; Van Berkel, G. J. Direct Sampling and Analysis from Solid-Phase Extraction Cards Using an Automated Liquid Extraction Surface Analysis Nanoelectrospray Mass Spectrometry System. Rapid Commun. Mass Spectrom. 2011, 25, 2389–2396. DOI: 10.1002/rcm.5132.
- Rankin-Turner, S.; Ninomiya, S.; Reynolds, J. C.; Hiraoka, K. Sheath-flow probe electrospray ionization (sfPESI) mass spectrometry for the rapid forensic analysis of human body fluids. Anal. Methods. 2019, 11, 3633–3640. DOI: 10.1039/C9AY00698B.
- Mattarozzi, M.; Bianchi, F.; Milioli, M.; Cavazza, A.; Careri, M. An Innovative Method Based on Quick, Easy, Cheap, Effective, Rugged, and Safe Extraction Coupled to Desorption Electrospray Ionization-High Resolution Mass Spectrometry for Screening the Presence of Paralytic Shellfish Toxins in Clams. Talanta. 2016, 147, 416–421. DOI: 10.1016/j.talanta.2015.10.016.
- Haddad, R.; Sparrapan, R.; Eberlin, M. N. Desorption Sonic Spray Ionization for (High) Voltage-Free Ambient Mass Spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2901–2905. DOI: 10.1002/rcm.2680.
- Porcari, A. M.; Fernandes, G. D.; Barrera-Arellano, D.; Eberlin, M. N.; Alberici, R. M. Food Quality and Authenticity Screening via Easy Ambient Sonic-Spray Ionization Mass Spectrometry. Analyst. 2016, 141, 1172–1184. DOI: 10.1039/C5AN01415H.
- Maluly, H. D. B.; de Melo Porcari, A.; da Silva Cunha, I. B.; Pacheco, M. T. B.; Eberlin, M. N.; Alberici, R. M. The Impacts of the Raising Regime of Salmon Species on Their Triacylglycerol Composition Revealed by Easy Ambient Sonic-Spray Ionization Mass Spectrometry. Food. Res. Int. 2019, 120, 19–25. DOI: 10.1016/j.foodres.2019.01.066.
- Massaro, A.; Stella, R.; Negro, A.; Bragolusi, M.; Miano, B.; Arcangeli, G.; Biancotto, G.; Piro, R.; Tata, A. New Strategies for the Differentiation of Fresh and Frozen/Thawed Fish: A Rapid and Accurate Non-Targeted Method by Ambient Mass Spectrometry and Data Fusion (Part A). Food Control. 2021, 130, 108364. DOI: 10.1016/j.foodcont.2021.108364.
- Fiorino, G. M.; Losito, I.; De Angelis, E.; Logrieco, A. F.; Monaci, L. Direct Analysis in Real Time Coupled to High Resolution Mass Spectrometry as a Rapid Tool to Assess Salmon (Salmo Salar) Freshness. J. Mass Spectrom. 2018, 53, 781–791. DOI: 10.1002/jms.4260.
- Balog, J.; Perenyi, D.; Guallar-Hoyas, C.; Egri, A.; Pringle, S. D.; Stead, S.; Chevallier, O. P.; Elliott, C. T.; Takats, Z. Identification of the Species of Origin for Meat Products by Rapid Evaporative Ionization Mass Spectrometry. J. Agric. Food. Chem. 2016, 64, 4793–4800. DOI: 10.1021/acs.jafc.6b01041.
- Rigano, F.; Mangraviti, D.; Stead, S.; Martin, N.; Petit, D.; Dugo, P.; Mondello, L. Rapid Evaporative Ionization Mass Spectrometry Coupled with an Electrosurgical Knife for the Rapid Identification of Mediterranean Sea Species. Anal. Bioanal. Chem. 2019, 411, 6603–6614. DOI: 10.1007/s00216-019-02000-z.
- Song, G.; Zhang, M.; Zhang, Y.; Wang, H.; Li, S.; Dai, Z.; Shen, Q. In Situ Method for Real-Time Discriminating Salmon and Rainbow Trout Without Sample Preparation Using iKnife and Rapid Evaporative Ionization Mass Spectrometry-Based Lipidomics. J. Agric. Food. Chem. 2019, 67, 4679–4688. DOI: 10.1021/acs.jafc.9b00751.
- Black, C.; Chevallier, O. P.; Haughey, S. A.; Balog, J.; Stead, S.; Pringle, S. D.; Riina, M. V.; Martucci, F.; Acutis, P. L.; Morris, M., et al. A Real Time Metabolomic Profiling Approach to Detecting Fish Fraud Using Rapid Evaporative Ionisation Mass Spectrometry. Metabolomics. 2017, 13, 153. DOI: 10.1007/s11306-017-1291-y.
- Kosek, V.; Uttl, L.; Jírů, M.; Black, C.; Chevallier, O.; Tomaniová, M.; Elliott, C. T.; Hajšlová, J. Ambient Mass Spectrometry Based on REIMS for the Rapid Detection of Adulteration of Minced Meats by the Use of a Range of Additives. Food Control. 2019, 104, 50–56. DOI: 10.1016/j.foodcont.2018.10.029.
- Black, C.; Chevallier, O. P.; Cooper, K. M.; Haughey, S. A.; Balog, J.; Takats, Z.; Elliott, C. T.; Cavin, C. Rapid Detection and Specific Identification of Offals Within Minced Beef Samples Utilising Ambient Mass Spectrometry. Sci. Rep. 2019, 9, 6295. DOI: 10.1038/s41598-019-42796-5.
- Gredell, D. A.; Schroeder, A. R.; Belk, K. E.; Broeckling, C. D.; Heuberger, A. L.; Kim, S. -Y.; King, D. A.; Shackelford, S. D.; Sharp, J. L.; Wheeler, T. L., et al. Comparison of Machine Learning Algorithms for Predictive Modeling of Beef Attributes Using Rapid Evaporative Ionization Mass Spectrometry (REIMS) Data. Sci. Rep. 2019, 9, 5721. DOI: 10.1038/s41598-019-40927-6.
- Ross, A.; Brunius, C.; Chevallier, O.; Dervilly, G.; Elliott, C.; Guitton, Y.; Prenni, J. E.; Savolainen, O.; Hemeryck, L.; Vidkjær, N. H., et al. Making Complex Measurements of Meat Composition Fast: Application of Rapid Evaporative Ionisation Mass Spectrometry to Measuring Meat Quality and Fraud. Meat Sci. 2021, 181, 108333. DOI: 10.1016/j.meatsci.2020.108333.
- Birse, N.; Chevallier, O.; Hrbek, V.; Kosek, V.; Hajŝlová, J.; Elliott, C. Ambient Mass Spectrometry as a Tool to Determine Poultry Production System History: A Comparison of Rapid Evaporative Ionisation Mass Spectrometry (REIMS) and Direct Analysis in Real Time (DART) Ambient Mass Spectrometry Platforms. Food Control. 2021, 123, 107740. DOI: 10.1016/j.foodcont.2020.107740.
- Gatmaitan, A. N.; Lin, J. Q.; Zhang, J.; Eberlin, L. S. Rapid Analysis and Authentication of Meat Using the MasSpec Pen Technology. J. Agric. Food. Chem. 2021, 69(11), 3527–3536. DOI: 10.1021/acs.jafc.0c07830.
- Kertesz, V.; Van Berkel, G. J. Fully Automated Liquid Extraction-Based Surface Sampling and Ionization Using a Chip-Based Robotic Nanoelectrospray Platform. J. Mass Spectrom. 2010, 45, 252–260. DOI: 10.1002/jms.1709.
- Montowska, M.; Alexander, M. R.; Tucker, G. A.; Barrett, D. A. Authentication of Processed Meat Products by Peptidomic Analysis Using Rapid Ambient Mass Spectrometry. Food Chem. 2015, 187, 297–304. DOI: 10.1016/j.foodchem.2015.04.078.
- Hiraoka, K.; Rankin-Turner, S.; Ninomiya, S.; Sekine, R.; Wada, H.; Matsumura, M.; Sanada-Morimura, S.; Tanaka, F.; Nonami, H.; Ariyada, O. Point Analysis of Foods by Sheath-Flow Probe Electrospray Ionization/Mass Spectrometry (sfPESI/MS) Coupled with a Touch Sensor. J. Agric. Food. Chem. 2020, 68, 418–425. DOI: 10.1021/acs.jafc.9b06489.
- Law, J. W. F.; Mutalib, N. S. A.; Chan, K. G.; Lee, L. H. Rapid Metho Ds for the Detection of Foodborne Bacterial Pathogens: Principles, Applications, Advantages and Limitations. Front. Microbiol. 2014, 5, 770. DOI: 10.3389/fmicb.2014.00770.
- Qiao, L.; Tang, X.; Dong, J. A Feasibility Quantification Study of Total Volatile Basic Nitrogen (TVB-N) Content in Duck Meat for Freshness Evaluation. Food Chem. 2017, 237, 1179–1185. DOI: 10.1016/J.FOODCHEM.2017.06.031.
- Kuswandi, B.; Nurfawaidi, A. On-Package Dual Sensors Label Based on pH Indicators for Real-Time Monitoring of Beef Freshness. Food Control. 2017, 82, 91–100. DOI: 10.1016/J.FOODCONT.2017.06.028.
- Bi, J.; Tian, C.; Zhang, G. L.; Hao, H.; Hou, H. M. Detection of Histamine Based on Gold Nanoparticles with Dual Sensor System of Colorimetric and Fluorescence. Foods. 2020, 9, 316. DOI: 10.3390/FOODS9030316.
- Pereira, J. A. M.; Porto-Figueira, P.; Andrade, B.; Gonçalves, P.; Pataca, J.; Câmara, J. S. Biogenic Amines in Food: Occurrence and Analytical Challenges for Their Analysis. In Biogenic Amines (BA): Origins, Biological Importance and Human Health Implications; Stadnik, J., Ed.; Hauppauge, NY, USA: Nova Science Publishers, Inc, 2017; pp. 1–23.
- Bülbül, G.; Hayat, A.; Andreescu, S. Portable Nanoparticle-Based Sensors for Food Safety Assessment. Sensors. 2015, 15, 30736–30758. DOI: 10.3390/S151229826.
- Chen, Q.; Hui, Z.; Zhao, J.; Ouyang, Q. Evaluation of Chicken Freshness Using a Low-Cost Colorimetric Sensor Array with AdaBoost–OLDA Classification Algorithm. LWT - Food Sci. Technol. 2014, 57, 502–507. DOI: 10.1016/J.LWT.2014.02.031.
- Sionek, B.; Przybylski, W.; Bańska, A.; Florowski, T. Applications of Biosensors for Meat Quality Evaluations. Sensors. 2021, 21, 7430. DOI: 10.3390/S21227430.
- Choi, J. R.; Yong, K. W.; Tang, R.; Gong, Y.; Wen, T.; Li, F.; Pingguan-Murphy, B.; Bai, D.; Xu, F. Advances and Challenges of Fully Integrated Paper-Based Point-Of-Care Nucleic Acid Testing. TrAc Trends Anal. Chem. 2017, 93, 37–50. DOI: 10.1016/J.TRAC.2017.05.007.
- Apetrei, I. M.; Apetrei, C. Application of Voltammetric E-Tongue for the Detection of Ammonia and Putrescine in Beef Products. Sens. Actuators B Chem. 2016, 234, 371–379. DOI: 10.1016/J.SNB.2016.05.005.
- Mohareb, F.; Papadopoulou, O.; Panagou, E.; Nychas, G. J.; Bessant, C. Ensemble-Based Support Vector Machine Classifiers as an Efficient Tool for Quality Assessment of Beef Fillets from Electronic Nose Data. Anal. Methods. 2016, 8, 3711–3721. DOI: 10.1039/C6AY00147E.
- Dowlati, M.; de la Guardia, M.; Dowlati, M.; Mohtasebi, S. S. Application of Machine-Vision Techniques to Fish-Quality Assessment. TrAc Trends Anal. Chem. 2012, 40, 168–179. DOI: 10.1016/J.TRAC.2012.07.011.
- Grau, R.; Sánchez, A. J.; Girón, J.; Iborra, E.; Fuentes, A.; Barat, J. M. Nondestructive Assessment of Freshness in Packaged Sliced Chicken Breasts Using SW-NIR Spectroscopy. Food. Res. Int. 2011, 44, 331–337. DOI: 10.1016/J.FOODRES.2010.10.011.
- Weng, X.; Luan, X.; Kong, C.; Chang, Z.; Li, Y.; Zhang, S.; Al-Majeed, S.; Xiao, Y. A Comprehensive Method for Assessing Meat Freshness Using Fusing Electronic Nose, Computer Vision, and Artificial Tactile Technologies. J. Sens. 2020, 2020, 1–14. DOI: 10.1155/2020/8838535.
- Hameed, S.; Xie, L.; Ying, Y. Conventional and Emerging Detection Techniques for Pathogenic Bacteria in Food Science: A Review. Trends Food Sci. Technol. 2018, 81, 61–73. DOI: 10.1016/J.TIFS.2018.05.020.
- Pang, B.; Fu, K.; Liu, Y.; Ding, X.; Hu, J.; Wu, W.; Xu, K.; Song, X.; Wang, J.; Mu, Y., et al. Development of a Self-Priming PDMS/Paper Hybrid Microfluidic Chip Using Mixed-Dye-Loaded Loop-Mediated Isothermal Amplification Assay for Multiplex Foodborne Pathogens Detection. Anal. Chim. Acta. 2018, 1040, 81–89. DOI: 10.1016/J.ACA.2018.07.024.
- Shih, C. M.; Chang, C. L.; Hsu, M. Y.; Lin, J. Y.; Kuan, C. M.; Wang, H. K.; Huang, C. T.; Chung, M. C.; Huang, K. C.; Hsu, C. E., et al. Paper-Based ELISA to Rapidly Detect Escherichia Coli. Talanta. 2015, 145, 2–5. DOI: 10.1016/J.TALANTA.2015.07.051.
- Li, X.; Yang, F.; Wong, J. X. H.; Yu, H. Z. Integrated Smartphone-App-Chip System for On-Site Parts-Per-Billion-Level Colorimetric Quantitation of Aflatoxins. Anal. Chem. 2017, 89, 8908–8916. DOI: 10.1021/acs.analchem.7b01379.
- Coskun, A. F.; Wong, J.; Khodadadi, D.; Nagi, R.; Tey, A.; Ozcan, A. A Personalized Food Allergen Testing Platform on a Cellphone. Lab. Chip. 2013, 13, 636. DOI: 10.1039/C2LC41152K.
- Liu, Z.; Zhang, Y.; Xu, S.; Zhang, H.; Tan, Y.; Ma, C.; Song, R.; Jiang, L.; Yi, C. A 3D Printed Smartphone Optosensing Platform for Point-Of-Need Food Safety Inspection. Anal. Chim. Acta. 2017, 966, 81–89. DOI: 10.1016/J.ACA.2017.02.022.
- Valderrama, W. B.; Dudley, E. G.; Doores, S.; Cutter, C. N. Commercially Available Rapid Methods for Detection of Selected Food-Borne Pathogens. Crit. Rev. Food Sci. Nutr. 2016, 56, 1519–1531. DOI: 10.1080/10408398.2013.775567.
- Nabi, B. G.; Mukhtar, K.; Arshad, R. N.; Radicetti, E.; Tedeschi, P.; Shahbaz, M. U.; Walayat, N.; Nawaz, A.; Inam-Ur-Raheem, M.; Aadil, R. M. High-Pressure Processing for Sustainable Food Supply. Sustainability. 2021, 13, 13908. DOI: 10.3390/SU132413908.
- Power, A.; Cozzolino, D. How Fishy is Your Fish? Authentication, Provenance and Traceability in Fish and Seafood by Means of Vibrational Spectroscopy. Appl. Sci. 2020, 10, 4150. DOI: 10.3390/app10124150.
- Hassoun, A.; Gudjónsdóttir, M.; Prieto, M. A.; Garcia-Oliveira, P.; Simal-Gandara, J.; Marini, F.; Di Donato, F.; D’Archivio, A. A.; Biancolillo, A. Application of Novel Techniques for Monitoring Quality Changes in Meat and Fish Products During Traditional Processing Processes: Reconciling Novelty and Tradition. Processes. 2020, 8, 988. DOI: 10.3390/PR8080988.