Abstract
Samples of kingfish of different age groups were collected from the Batinah and Dhofar coasts of Oman. Fatty acids (FA) were extracted from the dorsal muscle samples of the kingfish and analyzed on a Hewlett Packard Gas Chromatograph Mass Spectrophotometer (GCMS). Individual FA was identified by comparing their retention times with reference samples, which were confirmed by the NIST98 computer mass spectral library. Specimens collected from the Batinah coast contained slightly higher amounts of total fatty acids compared to specimens of the Dhofar area. The results showed that kingfish from the Batinah area had high contents of polyunsaturated fatty acids (PUFA) compared to saturated fatty acids (SAFA). However, there was little or no difference between PUFA and SAFA in samples collected from the Dhofar region. All samples had more eicosapentaenoic acid (20: 5ω3, EPA) than docosahexaenoic acid (22: 6ω3, DHA). The ω3 types FA were 5–8 folds higher in all samples compared to the ω6 type FA. Kingfish from the Batinah region showed higher ω3/ω6 FA ratios than samples from the Dhofar region.
Introduction
The kingfish (narrow‐barred or Indo‐Pacific Mackerel) known locally, as “kanad” is a large pelagic fish, which inhabits the epipelagic zone of shallow coastal waters of Oman. It is the most preferred species for consumption by Omani and other Arabs in the Gulf region. It is in high demand both local and in the international markets and hence has significant social and economic importance for the Sultanate of Oman. There are four species of kingfish namely., Scomberomorus commerson, S. guttatus, S. lineolatus, and Acanthocybium salandri with the S. commerson contributing more than 99% of the total kingfish landed in Oman.
The flora and fauna of the tropical regions are characteristically diverse, compared with temperate regions, and fish obey this general ecological rule.Citation[1] General opinion maintains that freshwater fish have a lower nutritional value than marine fish, since they often contain smaller amounts of the beneficial ω3 polyunsaturated fatty acids (PUFA), i.e., eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Many workers have suggested that fatty fish are advantageous for marketing purpose and for human consumption.Citation[2],Citation[3] Marine fish often contain more fat than freshwater fish.Citation[4] Henderson and TocherCitation[5] in an extensive review of lipid composition in freshwater fish have reported that lipids from freshwater fish contain higher proportions of saturated fatty acids (SAFA) and 18 carbon PUFA. However, low levels of 20 and 22 carbon PUFA, and low ω3/ω6 ratios were found in fresh water fish correspond to marine fish. In recent years interest in the lipids and FA composition of fish has increased. Among the FA, particular emphasis has been placed on the ω3 and ω6 PUFA, because of their health benefits.Citation6–9 It is well documented that lipids rich in EPA and DHA have the ability to reduce the blood lipid levels, particularly the serum triglycerides,Citation[8],Citation[10] which retards the atherosclerotic process and prevents thrombus formation.
The FA compositions of the lipids of fish vary considerably, both within and between species.Citation[4],Citation11–13 The main reason for such differences is due to diet.Citation[14] Besides the diet, environmental factors such as temperature, pH, and salinity play an important role in influencing the composition of FA in fish.Citation[5],Citation[15],Citation[17] The present study reports the content and distribution patterns of FA from 40 fish samples collected from various coastal regions of Oman. Individual FA are analyzed by gas chromatograph mass spectrophotometer on mg g−1 fresh weight. In the present investigation we have compared individual fatty acids between the same species of kingfish (S. commerson) collected from different coastal regions of the Sultanate of Oman.
Materials and Methods
Fresh samples of whole individual Kingfish were purchased from fishermen at two sampling locations of the Al‐Batinah and Dhofar regions for FA analysis. The fish were brought to laboratory within a few hours of catching, frozen at −20°C and samples from the dorsal muscles were taken for fatty acid analysis.
The FA were determined according to the procedure described in AOACCitation[16] with some modifications. Dorsal muscle samples (2–3 g) from each kingfish were mixed with 50 mL of aqueous 1 N KOH and 5 mg of heneicosanoic acid (C19) as internal standard and 0.005% BHT as antioxidant. Samples mixed with aqueous KOH solution were heated at 150°C for 30 min, cooled to room temperature and 150 mL of distilled water was added. Few drops of 0.1% methyl orange were added until the solution became yellow. The pH of the solution was adjusted to 2.0 with 5 N HCl resulting in a light pink color. Fatty acids were vigorously extracted from the acidified solution twice with 100 mL diethyl ether. The diethyl ether was pooled and washed four times with 80 mL of distilled water followed by drying over anhydrous sodium sulfate. The diethyl ether extract containing the FA was reduced to 1–2 mL under vacuum at 30°C. The FA in the diethyl ether extract were converted into methyl esters by adding 2 mL of a 14% solution of boron trifluoride in methanol. The mixture was kept in boiling water for 10–15 min. After cooling to room temperature, 5 mL of saturated NaCl was added to the mixture. The fatty acid methyl esters were extracted from the mixture using 3 mL hexane. Three replicates for each sample were used for the extraction of fatty acid. The hexane extract was evaporated to 1 mL under a stream of nitrogen and used for GCMS analysis.
The GCMS analyses of the methyl esters were performed on a Hewlett Packard 5890 GC equipped with an 30 m × 0.32 mm OmegaWax® (Supelco, Inc. USA) fused‐capillary column and a HP5989B mass detector. Helium was used as carrier gas at velocity of 30 cm2/sec with electronic pressure control and split ratio 40:1. The temperature was programmed from 70–250°C rising 5°C/min. The injector temperature was 250°, the detector 275°, ion source 200°, and quadruple mass filter 100°C. One microliter hexane extract containing the methyl esters of FA was injected using split/splitless injector with the HP6890 auto‐injector. Individual FA were identified by comparing their retention time with commercially available mixtures of saturated fatty acids, and polyunsaturated fatty acids from a marine source (Supelco Inc., USA) and confirmed by NIST98 mass spectral computer library. The FA were quantified (mg g−1 fresh weight) using heneicosanoic acid (C19) as internal standard. Multivariate analysis of variance and regression analysis of FA was done using the Statistical Analysis System (SAS Institute Inc., NC, USA).
Results
The kingfish were collected from different areas of the Batinah and Dhofar coasts; the lengths of fish were measured and they were weighed (). Forty fish of varying lengths and weights from both coasts were used for fatty acid analysis. Al‐Batinah coast is situated on Gulf of Oman, whereas the Dhofar coast is on Arabian Sea and both coasts have different environmental conditions. The water temperature in Batinah coast is usually higher than Dhofar coast, whereas there is no difference in salinity levels. The population of sardines, which is the main source of food for kingfish, is found to be higher on the Batinah than the Dhofar coast (M.R.G. Claereboudt, personal communication).
Table 1. Origin, size, and weight of kingfish (S. commerson) analyzed for fatty acid content
The individual FA and the sums of SAFA, PUFA, FA, FA with ω3, ω6, and ratios ω3/ω6 and FA/PUFA ratios are recorded in . The FA identified ranged from 14 carbons to 22 carbons. The sum of total FA contents was found to be higher in kingfish from the Batinah as compared to the Dhofar coast. In kingfish the concentration of hexadecanoic acid (C16) was the highest among the SAFA, while EPA (C20: 5ω3), was predominant followed by C18: 1ω9, and DHA (C22: 6ω3; ). The sum of PUFA was estimated to be higher than the sum of SAFA from the Batinah and Dhofar kingfishes. Fishes from both coasts contained more than 8 fold amount of ω3 PUFA (C20: 5ω3 + C22: 6ω3) than ω6 type PUFA (C18: 2ω6 + C20: 4ω6). Least Square Means of C14, C16, C16: 1ω7, C18: 1ω9, and C18: 2ω6 FA in kingfish from the Batinah coast were found to be significantly higher (P < 0.05) than the kingfish from the Dhofar coast. However, the sums of total FA, SAFA, PUFA, were significantly higher (P < 0.05) in the kingfish from the Batinah compared to the Dhofar region (). Whereas, the ratio of ω3/ω6 was found to be higher in kingfish from the Dhofar compared to the Batinah region. The variation of the individual FA was small in most of the fishes, both within and between regions ().
Table 2. Fatty acid content of kingfish (S. commerson) from different coastal regions of Oman(mg g−1F.W.)
Table 3. Fatty acid profiles of kingfish from two regions of the Sultanate of Oman
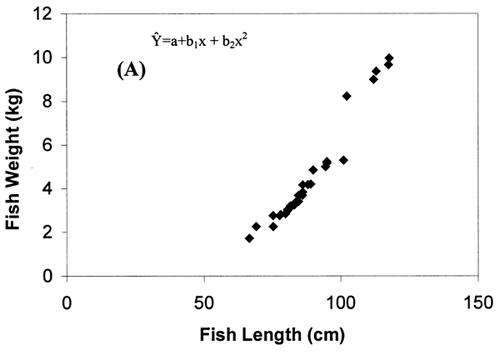
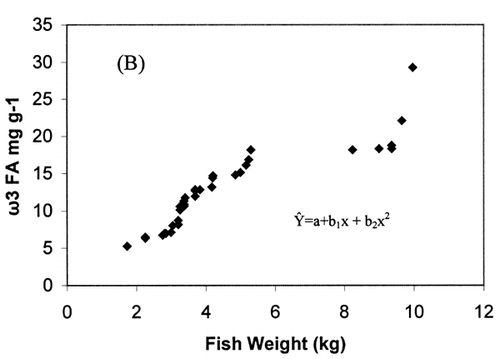
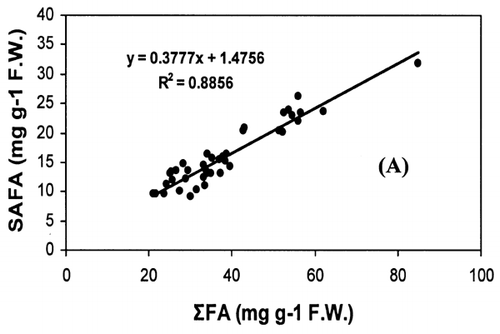
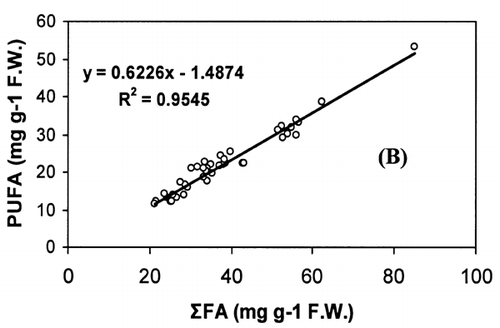
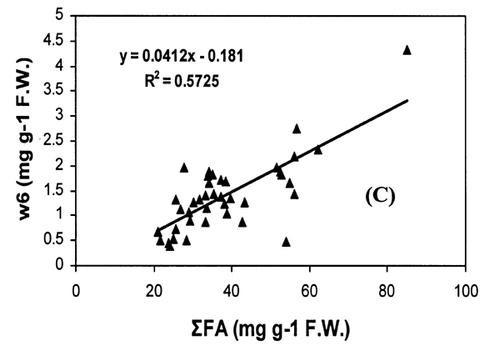
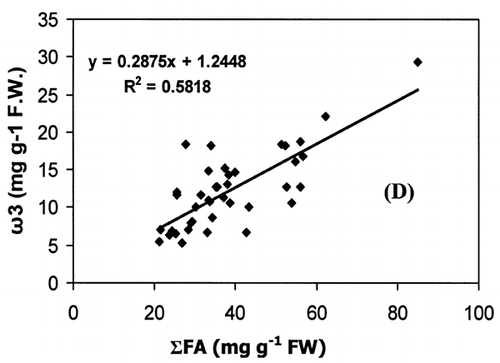
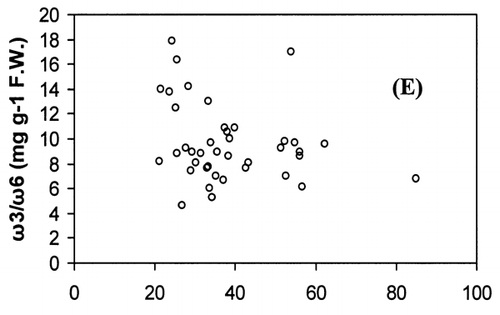
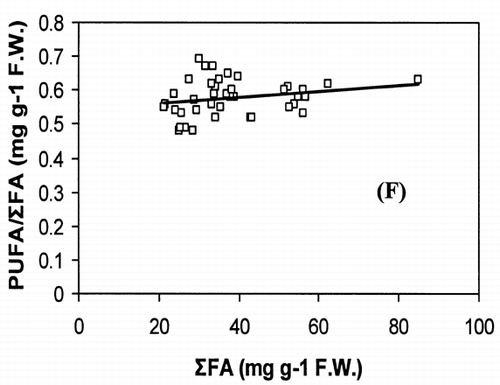
Correlation analysis of all the FA with weight and length revealed a significant correlation (r = 0.94, P < 0.001), []. However, regression analysis showed that the relationship with weight and length was a second order polynomial regression with an R 2 of 92%. The weight decreased by 0.30 kg/cm when the length of fish increased from 66.5 to 77 cm and there after the weight increased by 0.003 kg/cm until the fish reached a length of 117.7 cm. Regression analysis of ω3 ω6 FA with the weight of fish only showed a similar significant second order polynomial regression. []. Fatty acids of ω3 types increased from 5.27 to 18.1 mg g−1 fresh weight by 5.62 mg g−1 when the weight of the fish increased from 1.72 to 5.3 kg and there after the level of ω3 decreased by 0.45 mg g−1 until the fish reached the weight of 9.97 kg. In the case of ω6 type FA, their levels increased from 0.38 to 1.87 mg g−1 fresh weight by 0.74 mg g−1 when the weight of fish increased from 1.72 to 5.3 kg and thereafter the level of ω6 decreased by 0.06 mg g−1 until the fish reached a weight of 9.97 kg. Polyunsaturated fatty acids, SAFA, and ω3, ω6 types of FA gave a linear correlation with sum of FA (Fig. ). The ratio of PUFA and sum of FA decreased with higher fatty acid contents (). In contrast, the ratio of ω3/ω6 exhibited a rather scattered picture showing no clear relationship to sum of FA [].
Discussion
Our results show that the total content of FA regulates the content of SAFA, PUFA, ω3, and ω6 types of FA. This means that the higher the fat content of the fish, the larger the fraction of SAFA, PUFA, ω3, and ω6 FA. However, the average concentration of PUFA was higher than SAFA in fishes from both coasts. The amount of FA of different types was found to be higher in fishes from the Batinah coast compared to the Dhofar coast. This may be due to the different environmental conditions, food sources, and fish stocks found on both coasts, because the Batinah coast is in the Gulf of Oman and the Dhofar coast on the Arabian Sea (First Progress Report on Management of the Sultanate of Oman's Kingfish fishery—personal communication). Among the individual FA, the SAFA (C16 and C18), PUFA (C20: 5ω3 and C22: 6ω3), and monounsaturated fatty acid (C18: 1ω9) were the most abundant FA in all the fish studied. Similar observations were recorded on tropical and temperate fresh water fish.Citation[14],Citation[18] Gruger et al.Citation[19] have reported higher C18: 2ω6 and lower EPA in freshwater fishes than in marine fishes. The concentration of the ω3 type of FA (EPA + DHA) was several folds higher than ω6 types, which can be reckoned a good dietary attribute. The high concentration of EPA and DHA in kingfish (marine) is in agreement with previous findings of several workers.Citation[11],Citation[14],Citation[20]
The concentration of PUFA in total FA (PUFA/sum of FA) and ω3/ω6 ratio is a more important indicator of fat quality than sum of FA.Citation[4] The ratio of PUFA and sum of FA decreases with higher fat contents, whereas ω3/ω6 ratio shows no correlation with total fat. A high ω3/ω6 ratio ranging from 8 to 12 was noticed in fishes from both coasts. A higher ratio of ω3/ω6 (>6) was recorded in many fresh water fish by Ahlgren et al.Citation[4] From the present results, it can be concluded that kingfish in general has a high dietary value with kingfish stock from the Batinah coast being better than Dhofar coast.
Acknowledgments
Authors are grateful to the Fisheries Research Fund, Ministry of Agriculture and Fisheries Wealth, Sultanate of Oman for financial support. Thanks are also extended to the technicians of Marine Science and Fisheries Department for their help in kingfish sampling and date collection.
References
- Lowe‐McConnell , R. H. 1987 . Ecological Studies in Tropical Fish Communities Cambridge , , UK : Cambridge University Press .
- Otwell , W. S. and Richards , W. L. 1981/1982 . Cultured and wild American eels, fat content, and fatty acid composition . Aquaculture , 26 : 67 – 76 .
- Puustinen , T. , Punnonen , K. and Uotila , P. P. 1985 . The fatty acid composition of 12 north European fish species . Acta Med. Scand. , 218 : 59 – 62 .
- Ahlgren , G. , Blomqvist , P. , Boberg , M. and Gustafsson , I. B. 1994 . Fatty acid content of the dorsal muscle–an indicator of fat quality in freshwater fish . J. Fish Biol. , 45 : 131 – 157 .
- Henderson , R. J. and Tocher , D. R. 1987 . The lipid composition and biochemistry of freshwater fish . Progress in Lipid Research , 26 : 281 – 347 .
- Dyerberg , J. , Bang , H. O. , Stoffersen , E. , Moncada , S. and Vane , J. R. 1978 . Eiccosapentaenoic acid and prevention of thrombosis and arthrosclerosis . Lancet , II : 117 – 119 .
- Bang , H. O. , Dyerberg , J. and Sinclair , H. M. 1980 . The composition of Eskimo food in northwestern Greenland . Am. J. Clin. Nutr. , 33 : 2657 – 2661 .
- Leaf , A. C. and Weber , P. C. 1988 . Medical Progress. Cardiovascular effects of n‐3 fatty acids . New Engl. J. Med. , 318 : 549 – 557 .
- Gustafsson , I. B. , Vessby , B. and Nydahl , M. 1992 . Effects of lipid lowering diets enriched with monosaturated and polysaturated fatty acids on serum lipoprotein composition in patients with hyperlipoproteinaemia . Artherosclerosis , 96 : 109 – 118 .
- Boberg , M. 1990 . Clinical effects of fish oil . Naringsfarskning , 34 : 133 – 134 .
- Ackman , R. G. 1989 . Nutritional composition of fats in seafood . Progress in Food & Nutrition Sciences , 13 : 161 – 241 .
- Andrade , A. D. , Rubira , A. F. , Matsushita , M. and Souza , N. E. 1995 . ω3 fatty acids in freshwater fish from South Brazil . J. Am. Oil Chem. Soc. , 72 : 1207 – 1210 .
- Sargent , J. R. , Bell , G. , Bell , M. V. , Henderson , R. J. and Tocher , R. D. 1995 . Requirement criteria for essential fatty acids . J. Appl. Ichthyol. , 11 : 183 – 198 .
- Zenebe , T. , Ahlgren , G. and Boberg , M. 1998 . Fatty acid content of some freshwater fish of commercial importance from tropical lakes in the Ethiopian rift valley . J. Fish Biol. , 53 : 987 – 1005 .
- De Torrengo , M. P. and Brenner , R. 1976 . Influence of environmental temperature on fatty acid de‐saturation and elongation activity of fish liver microsomes . Biochim. Biophys. Acta , 424 : 36 – 44 .
- 1984 . “ A.O.A.C ” . In Official Methods of Analysis Washington DC : Association of Official Analytical Chemists . 14th Ed.
- Farkas , T. 1984 . Adaptation of fatty acid composition to temperature—a study on carp liver slices . Comp. Biochem. Physiol. , 79B : 531 – 535 .
- Clement , S. and Lovelli , R. T. 1994 . Comparison of processing yield and nutrient composition of Nile tilapia and catfish . Aquaculture , 119 : 299 – 310 .
- Gruger , E. H. , Nelson , R. W. and Stansby , M. E. 1964 . Fatty acid composition of oils from 21 species of marine fish, freshwater fish and shellfish . J. Am. Oil Chem. Soc. , 41 : 662 – 667 .
- Exler , J. , Kinsella , J. E. and Watt , B. K. 1965 . Lipids and fatty acids of important finfish: New data for nutrient tables . J. Am. Oil Chem. Soc. , 52 : 154 – 159 .