Abstract
Post rigor horse meat was marinated in 150 mM CaCl2 solution at 4°C and compared against untreated samples in order to evaluate characteristics associated with meat quality. Water holding capacity (WHC) and pH were recorded over 15 days period. Protein degradation was studied by sodium dodecyl sulfate SDS–PAGE electrophoresis, and rheological properties related to texture were evaluated by instrumental compression. Sensory evaluation was carried out with a trained panel. There were no significant differences in pH between treated and untreated samples. Water holding capacity in CaCl2‐treated samples was significantly higher, as a possible consequence of interfilament widening due to Ca2+ ion steric hindrance. Some degradation of high and low molecular weight (HMW and LMW) proteins in Ca2+‐treated samples was observed, although this fact could be due to factors not related to calpain activity. Sensory analysis demonstrated that treated meat had higher scores for overall intensity. There were no significant differences with respect to hardness, chewiness, cohesiveness, and juiciness.
Introduction
Injection and marination of meat with calcium chloride solutions have been extensively studied as a means to increase post‐mortem tenderization.Citation1–4 This has been attributed to activation of calcium‐dependent proteases, generally known as calpains. However, a direct relationship between softening and chemical structure of myofibrillar proteins was demonstrated.Citation[5] There are two types of calpains: calpain I or m, requiring 50 to 70 mM Ca2+ for activation, and calpain II or μ, requiring 1 to 5 µM Ca2+.Citation[6] Softening of bovine meat activated by 1 to 5 µM Ca2+ without notably altering other sensory characteristics was observed.Citation[7],Citation[8] Although calpain activation has been studied in other animal species there is little information regarding horse meat. This species has been used as a substitute for beef in some types of sausages and is consumed in certain geographical areas of the world. However, no specific studies on calcium marination in horse meat have been reported. Therefore, studies on horse meat are important taking into consideration its toughness. The objective of this study was to compare the effects of marination of horse meat in CaCl2 on pH, water holding capacity (WHC), protein degradation, ultrastructural changes by light microscopy, instrumental rheology related to texture and sensory analysis.
Materials and Methods
Meat Samples
Three lots of 200 g of horse meat were taken from the longissiumus dorsi muscle of each one of six animals. Sex, age and pre‐slaughtering handling was not recorded. The animals were stunned with a captive bolt pistol, exanguinated, eviscerated, and sectioned in a local abattoir. Sample collection occurred 24 to 36 hours after slaughtering. Samples were then taken to the laboratory and treatments were applied immediately after arrival, approximately 30 min after removal from the carcass.
Calcium Chloride Treatments
Half of the samples were treated by immersion in 150 mM CaCl2 solution and continuous agitation at 4°C during 48 hours, in a proportion of 1 cm3 of meat to 250 mL of marinating solution. The other half was left untreated as control, vacuum packaged and stored at 4°C. After marination the samples were vacuum‐packaged and stored at 4°C. Values of pH and WHC analysis were carried out at days 3, 6, 9, 12, and 15 of storage.
pH Measurement
Meat was diluted 1:9 with distilled water and pH was measured using a digital potentiometer (Beckman, Fullerton, CA). The analysis was replicated four times.
Water Holding Capacity
Five grams of ground meat were placed in centrifuge tubes and 8 mL 0.6 M NaCl were added. The mixture was stirred with a glass rod for 1 min and centrifuged at 9000 × g for 15 min. The supernatant was measured in a cylinder, and the volume difference from the 8 mL added to the sample was reported as milliliters of 0.6 M NaCl retained/100 g of meat.Citation[9] Analysis were replicated four times, mean values were reported.
Myofibril Protein Extraction
Myofibrillar protein extraction was carried out in two control samples and two samples treated with 150 mM CaCl2 over 48 h taken at random. One gram of meat was added with 10 mL of an extraction solution (0.6 M NaCl+2 mM MgCl+1 mM EDTA + 0.5 mM dithiotheitol + 10 mM sodium phosphate buffer; pH 7.0). It was thoroughly mixed for 1 min and centrifuged at 7800 × g at 4°C for 20 min. The precipitate was resuspended (1:6 proportion) in 50 mM sodium phosphate buffer containing 0.6 M NaCl + 5 mM MgCl + 5 mM sodium pyrophosphate; pH 6.0. The suspension was stirred overnight at 4°C and then centrifuged at 9000 × g for 20 min at 4°C. The precipitate was discarded. The supernatant was then diluted with deionized water at 4°C until ionic strength ca. 0.1 M. It was left standing overnight at 4°C. The precipitate was centrifuged again at 7800 × g for 25 min at 4°C, and the supernatant discarded. The precipitate was resuspended (1:3 proportion) in 50 mM sodium phosphate buffer added with 0.6 M NaCl, stirred overnight at 4°C and finally centrifuged at 14,000 × g for 25 min. The precipitate was also discarded. The solution contained myofibrillar proteins.Citation[10]
Polyacrylamide Gel Electrophoresis (SDS–PAGE)
Polyacrylamide gel electrophoresis was carried out following the procedure described by Laemmli et al.Citation[11] A mixture of 4% sodium dodecyl sulfate (SDS), 10% mercaptoethanol, 0.002% bromophenol solution was added to samples marinated with 150 mM CaCl2 during 48 h as well as to untreated samples. They were incubated for 4 min at 100°C in order to solubilize proteins before application to gels. Ten microliters of sample and 10 µL of low molecular weight (LMW) markers (66 to 14 kDa) were applied in 12% polyacrylamide gels; whereas 10 µL of sample and 10 µL high molecular weight (HMW) markers (500 to 10 kDa) were applied to 3.5% polyacrylamide gels. Markers used were P‐8906 for HMW (584,400 to 97,400 Da), and SDS‐7 for small molecular weight (66,000 to 14,200 Da; Sigma, St. Louis, MO). Staining was carried out using 0.02% Coomasie blue solution. Destaining was done by immersion of the gels in 10% acetic acid and 40% methanol solution for 30 min. Electrophoresis was carried out using a Miniprotean II slab cell (Biorad, Richmond, CA), operated at a constant 200 V supply. This analysis was carried out in duplicate.
Densitometry
The gels obtained by electrophoresis were read in a Pharmacia LKB Ultrascan XL densitometer (Bromma, Sweden). Relative areas were reported (peak area/total area).
Mechanical Properties
Compression tests were carried out using an Instron Universal Texture machine, model D (Canton, MA) fitted with a 10 N load cell. A 5 cm diameter cylindrical probe was used to compress 1 cm3 samples to 50% of its original height. The force was applied perpendicular to the meat fiber direction. Maximum force was recorded as compression.Citation[12] Samples were tested in four replicates, one sample from each animal and two control samples.
Light Microscopy
Meat samples were fixed by immersing in a Bouin solution (75% picric acid saturated solution, 5% concentrated acetic acid, 25% concentrated formol) (5:30 proportion, w/v) and subsequently immersed in xylene for 3 min, then 100% ethanol for 2 min and finally 95% ethanol for 3 min. They were then washed in distilled water for 1 min and stained with hematoxilin and immersed 3 to 4 times for 10 sec in 1 mL concentrated HCl in 99 mL 95% ethanol. Samples were immediately washed with water. The dye turned blue when the samples were placed in a solution containing 1 mL of concentrated ammonia in 80% 99 mL ethanol. They were then washed with water and stained with 1% acid eosine for 15 sec to 2 min, and finally washed with water and dehydrated by immersion in 100% ethanol for 10 to 20 sec and then in xylene for 15 to 20 sec two to three times. Samples were imbeded in paraffin at 65°C. A light Olympus fluorescence microscope Model BH‐2‐RFCA (Tokyo, Japan) fitted with two polarizers was used to make evident the striated structure and its disruption when occurring.
Sensory Texture Evaluation
A Qualitative Descriptive Analysis (QDA) methodCitation[13] was followed. This methodology is used when all or most of the important sensory characteristics need to be evaluated. The sample was presented to 10 panellists in similar conditions, and they quantify each attribute. Subsequently, and during three sessions, the panelists agreed terms which describe the attributes. The means of at least four replicates were plotted in a descriptive profile. In the resulting diagram the intensity of the attributes were marked on lines of the same length. As a standard, the panelists tested untreated samples. The distance from the origin indicated the intensity of the attribute, where 0 = absence of the attribute.Citation[15] Half of the samples for this analysis were marinated in a 150 mM CaCl2 solution, the other half were used as standards. Both type of samples were cooked in a domestic microwave oven at high power during 7 min, to an internal temperature of approximately 70°C, as measured with a thermocouple inserted in one of the samples. Panellists were situated in separted booths, treated and untreated samples were placed in white plastic dishes; the booths were illuminated with day light. A glass of water was also supplied. The terms agreed by the panelists are shown in .
Table 1. Terminology of sensory attributes in QDA
Statistical Analysis
pH and WHC data were analyzed by an analysis of variance procedure and Duncan's multiple range tests (p>0.5). Mean values for each attribute in sensory analysis were subjected to a t‐test between untreated and marinated samples (p>0.5). A SAS package was used.Citation[16]
Results and Discussion
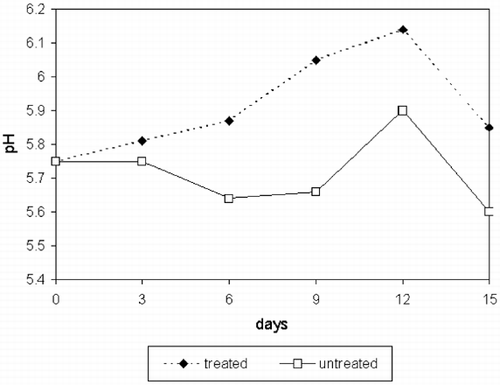
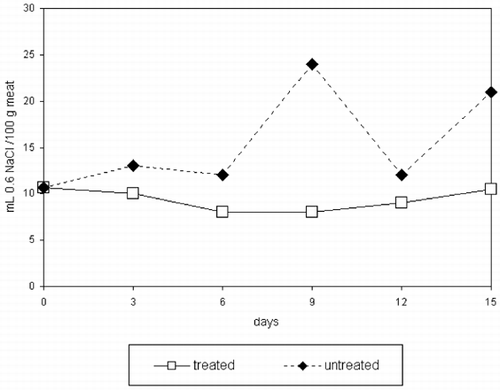
There were no significant differences in pH values for storage time nor treatments (p>0.879 and 0.152, respectively) (). Water holding capacity () was not affected by storage time (p>0.211) but higher values were found for CaCl2‐treated samples compared to controls (p>0.0034). This agrees with other results.Citation[17] It has been suggested that the effect of ions such as Na+ and Cl− on WHC is due to their ability to widening interfilaments due to steric hindrance, hence increasing water retention.Citation[18] This effect has not been detected by sensory panelists as measured by increased juiciness (p>0.2625) ().
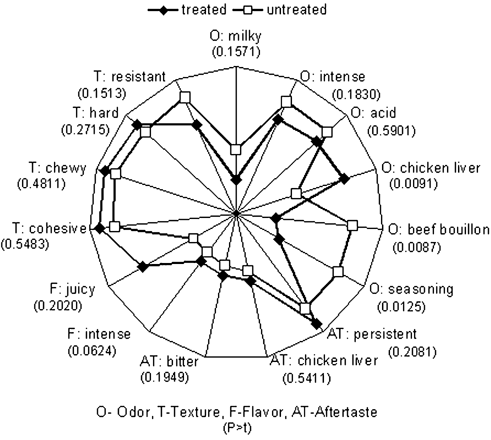
Significant differences were observed in sensory characteristics related to odor (seasoning, beef bouillon, chicken liver) and flavor (intense) (). However other odor characteristics such as acid, intense and milky, as well as texture, flavor (bitter) and aftertaste characteristics had no significant differences between treated and untreated samples. An increase in tenderness and juiciness in CaCl2‐treated beef with no significant differences in flavor intensity or aftertaste was observed.Citation[7] Good acceptability of beef treated with CaCl2 was reported,Citation[2] although tenderness was reduced after 24 h. In a study using high CaCl2 concentrations (300 mM)Citation[19] tenderness and juiciness increased with CaCl2 treatment although acid odor and aftertaste also increased. Our results partially agreed with these authors since chewiness and juiciness were not significantly different between treated and untreated samples, whereas significant differences in seasoning and beef bouillon odor, two desirable attributes, were significantly lower in treated samples while chicken liver odor, an undesirable attribute was measured. None of the aftertaste attributes were significantly different between treated and untreated samples whereas flavor intensity and bitterness were significantly higher in marinated samples (p>0.0311 and 0.0624, respectively). In summary, treatments with CaCl2 did not significantly affect attributes analyzed by QDA, except for an increase in flavor intensity and bitterness and a reduction in seasoning and beef bouillon odors; however treated samples were also higher in chicken liver flavor. As bitter flavor and chicken liver odor increased in treated samples, it was assumed that, although marination did not affect attributes such as texture or after taste, it increased other undesirable organoleptic characteristics.
Hardness was not significantly different in treated as compared to untreated samples as evaluated by a sensory panel (p>0.4102) () or instrumentally (p>0.3941) (). Disruption of myofibril interconnections contribute to meat tenderness, in this study calcium chloride treatments did not promote an extensive disruption resulting in non‐significant differences in hardness between treated and untreated samples (). Fractures caused by the action of Ca2+ ions in beef were previously reported.Citation[20] However, these structural alterations did not promote a significant difference in hardness.
Figure 4. Myofibril micrographs of CaCl2‐treated and untreated horse meat (320 × ). (a) Horse meat treated with 150 mM CaCl2. (b) Untreated horse meat.
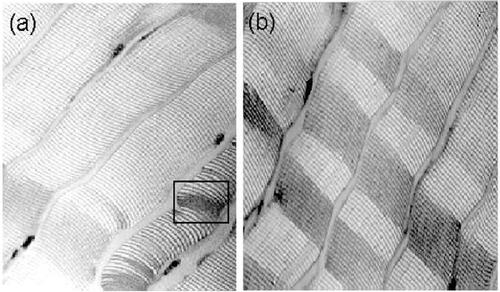
Table 2. Compression test of CaCl2‐treated and untreated horse meat
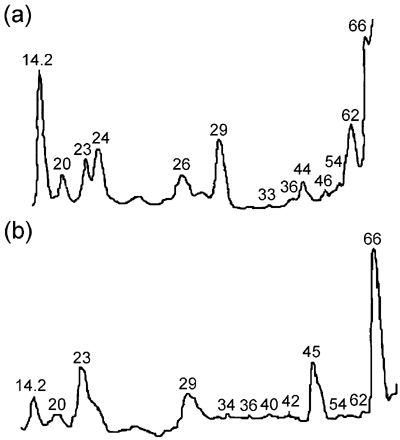
Low molecular weight protein electrophoresis was carried out in order to detect the presence of proteins acting as calpain substrates, whereas HMW protein electrophoresis was carried out to determine possible degradation of HMW skeletal proteins. shows electrophoresis gel densitometries of LMW proteins extracted from CaCl2‐treated and untreated samples. There were 13 peaks of LMW proteins in CaCl2‐treated meat gels [] and 12 peaks of LMW proteins in the untreated meat gels []. A 14 kDa peak could be the result of the presence of light myosin, the concentration of this protein increased in CaCl2‐treated samples as compared to untreated. The presence of this peak was detected in all four replicates of the experiment. Peaks at 26, 29, and 33 kDa could be due to polypeptides from depleted troponin T (29 kDa) as reported by Jaarsveld et al.Citation[21] Desmin (ca. 55 kDa), a calpain substrateCitation[22] is degraded to a 43 kDa polypeptide. Low molecular weight proteins could be produced by degradation of HMW proteins due to myofibril fragmentation as previously reported.Citation[1],Citation[23] These authors found the presence of a 30 kDa peak in marinated beef. Other authors reported the presence of a 32 kDa peak in marinated chicken, beef and pork.Citation[10],Citation[24] The 30 kDa peak was produced by degradation of troponin T.Citation[25] In our study a 29 to 30 kDa peak was observed both in CaCl2‐treated and untreated meat, probably a result of myofibrillar proteolysis but independent from CaCl2 treatment.
Figure 5. Densitometry of LMW protein gel electrophoresis (a) CaCl2‐treated meat, (b) untreated meat.
Desitometers select the most evident peaks in a gel not reading the smallest ones. Therefore, shows the electrophoresis gel densitometries of HMW proteins extracted from CaCl2‐treated [] and untreated samples []. Eight peaks were observed in CaCl2‐treated meat gels, whereas only five were present in untreated samples. Marination could mainly affect HMW proteins. This theory is also supported by oher authorsCitation[23] that reported that beef marination resulted in the presence of more peaks when extracted proteins were analyzed by electrophoresis. Suzuki et al.Citation[26] found the presence of a 98 kDa peak in marinated beef and concluded that this was a degradation product of α‐actinin, the most abundant protein in Z‐lines. Other studies in goose muscle treated with 0.1 M CaCl2 reported severe myofibril fragmentation, resulting in complete disappearance of peaks corresponding to troponin T, α‐actinin, titin and nebulin, and the formation of a 95 kDa component possibly resulting from degradation of proteins of HMW.Citation[23] Takahashi et al.Citation[27] found that titin and nebulin also underwent fragmentation. High molecular weight proteins include intermediate filaments formed by titin of 3000 kDa. After depletion, this protein yields α‐conectin formed by two fragments of 2000 and 1200 kDa. Another protein found in intermediate filaments, nebulin, has a molecular weight of 600 to 900 kDa, depending of the muscle type and age. Electrophoresis of proteins between 250 and 70 kDa, where myosin is the most abundant, was not carried out since the documentation that myosin is not a substrate for calpains.Citation[17]
Figure 6. Densitometry of HMW protein gel electrophoresis (a) CaCl2‐treated meat, (b) untreated meat.
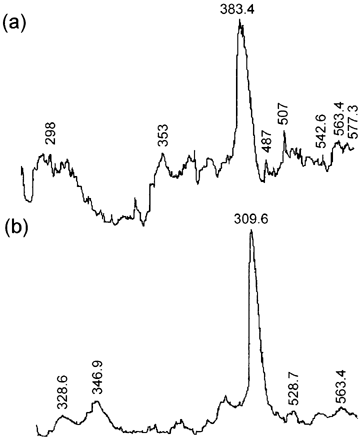
In general, more peaks were observed in CaCl2‐treated meat gels than in control gels, however this could be due not only to calpain activity but also to physicochemical action, as previously demonstrated.Citation[28] The largest relative area of HMW proteins correspond to a peak of approximately 380 kDa, this was probably due to a number of proteins which moved thorough the gel and accumulated. Three peaks were observed in LMW protein gels, around 66 kDa, possibly due to accumulation of other proteins and peptides. A second peak of 29 kDa probably resulted from troponin T degradation.Citation[29] A third peak of 23 kDa could be due to light myosin. During myofibril solubilization at pH = 7.0, enzymes able to degrade these proteins could be inactivated by SDS, pH or absence of Ca2+. In addition, HMW myofibrillar proteins are usually depleted into smaller units, rendering extra bands in electrophoretograms.
Conclusions
These results showed that marination of horse meat did not affect mechanical properties as determined by compression. Water holding capacity, a possible effect of increased protein solubility due to higher pH values in marinated meat, was significantly higher in CaCl2‐treated as compared to untreated samples. This may be the result of interfilament widening due to Ca2+ ion steric hindrance. Protein degradation in marinated meat could be observed as an increase in the number of peaks in the densitometry of electrophoresis gels. Treated meat was scored higher in the intense flavor note. There were no significant differences with respect to hardness, chewiness, cohesiveness, and juiciness. The fractures caused by the action of Ca2+ ions in myofibril ultrastructure did not appear to promote a significant difference in hardness. This was corroborated by sensory tests on hardness and instrumental test on mechanical properties. In summary, marination did not improved horse meat quality although it promoted an increase in WHC by filament widening by Ca2+ and possibly by protein degradation, no significant tenderization occurred. On the other hand a bitter flavor and chicken liver odor were present in marinated samples, both undesirable attributes.
Acknowledgment
Author Pérez‐Chabela thanks CONACYT for an academic scholarship. The results presented here are part of her Ph.D. thesis.
References
- Koohmaraie , M. , Babiker , A. S. , Merkel , R. A. and Dutson , T. R. 1988a . Role of calcium dependent proteases and lysosomal enzymes in postmortem changes in bovine skeletal muscle . J. Food Sci. , 53 : 1253 – 1258 .
- Wheeler , T. L. , Koohmaraie , M. , Lansdell , J. L. , Siragusa , G. S. and Miller , M. F. 1993 . Effect of postmortem injection time, injection level and concentration af calcium chloride and beef quality traits . J. An. Sci. , 71 : 2965 – 2974 .
- Whipple , G. and Koohmaraie , M. 1993 . Calcium chloride marination effects on beef steak tenderness and calpain proteolytic activity . Meat Sci. , 33 : 265 – 269 .
- Yoshizawa , T. , Sorimachi , H. , Tomika , S. , Ishimura , S. and Suzuki , K. 1995 . A catalytic subunit of calpain possesses full proteolytic activity . FEMS‐Letters , 358 : 101 – 103 .
- Wu , J. Y. , Mills , E. W. and Henning , W. R. 1995 . Postmortem delay time and heating rate affect tenderness and ultrastructure of prerigor cooked bovine muscle . J. Food Sci. , 60 : 565 – 570 .
- Koohmaraie , M. , Seideman , S. C. , Schollmeyer , J. E. , Dutson , T. E. and Babiker , A. S. 1988b . Factors associated with the tenderness of three bovine muscles . J. Food Sci. , 53 : 407 – 412 .
- Lansdell , J. L. , Miller , M. F. , Wheeler , T. L. , Koohmaraie , M. and Ramsey , C. B. 1995 . Postmortem injection of calcium chloride effects on beef quality traits . J. An. Sci. , 73 : 1735 – 1740 .
- Shackelford , S. D. , Wheeler , T. A and Koohmaraie , M. 1997 . Tenderness classification of beef. I. Evaluation of beef Longissimus shear force at 1 and 2 days postmortem as a predictor of aged beef tenderness . J. An. Sci. , 75 : 2417 – 2422 .
- Wardlaw , F. B. , Maccaskill , L. H. and Acton , J. C. 1973 . Effect of postmortem muscle changes on poultry meat loaf properties . J. Food Sci. , 38 : 421 – 424 .
- Semejima , K. and Wolfe , F. H. 1976 . Degradation of myofibrillar protein components during postmortem aging of chicken muscle . J. Food Sci. , 41 : 250 – 254 .
- Laemmli , U. K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage t4 . Nature , 227 : 680 – 685 .
- Voisey , P. W. 1976 . “ Instrumental measurement of food texture ” . In Rheology and Texture in Food Quality Edited by: deMan , J. M. , Voisey , P. W. , Rasper , V. F. and Stanley , D. W. 79 – 141 . Westport : The Avi Publishing Co. .
- Siedel , J. and Stone , H. 1985 . Sensory Evaluation Practices New York : Academic Press .
- Stone , H. , Siedel , J. L. and Bloomquist , J. 1980 . Quantitative descriptive analysis . Cereal Foods World , 25 : 642 – 644 .
- Caul , J. F. 1957 . The profile method of flavor analysis . Adv. Food Res. , 7 : 1 – 40 .
- 1996 . SAS User's Guide Cary , North Carolina : SAS Institute . Sas Institute
- Whipple , G. and Koohmaraie , M. 1991 . Degradation of myofibrillar proteins by extractable lysosomal enzymes and m‐calpain, and effects of zinc chloride . J. An. Sci. , 69 : 4449 – 4460 .
- Hamm , R. 1966 . “ Heating of muscle ” . In The Physiology and Biochemistry of Muscle as a Food Edited by: Briskey , E. J. , Cassens , R. G. and Trautman , J. C. 363 – 385 . Madison : The University of Wisconsin Press .
- Morgan , J. B. , Savell , J. W. , Hale , D. S. , Miller , R. K. , Griffin , D. B. , Cross , H. R. and Shackelford , S. D. 1991 . National beef tenderness survey . J. An. Sci. , 69 : 3274 – 3283 .
- Tatsumi , R. and Takahashi , K. 1992 . Calcium‐induced fragmentation of skeletal muscle nebulin filaments . J. Biochem. , 112 : 775 – 779 .
- Jaarsveld , F. P. , Naudé , R. J. and Oelofsen , W. 1997 . The effects of ca ions, edta and storage time on myofibrillar protein degradation, levels of ca2 + ‐dependent proteases and cathepsins B, H, L and D of ostrich skeletal muscle . Meat Sci. , 45 ( 4 ) : 517 – 529 .
- Ho , C. Y. , Stromer , M. H. , Rouse , G. and Robson , R. M. 1997 . Effects of electrical stimulation and postmortem storage an changes an titin, nebulin, desmin, troponin t and muscle ultrastructure an Bos indicus crossbred cattle . J. An. Sci. , 75 : 336 – 376 .
- Rong‐Ghi , R. C. , Kou‐Joang , Lin , Tsai‐Fuh , Tseng and Chaen‐Ping , Wu . 1995 . Postmortem changes in goose (Anser anser) breast muscles as affected by calcium chloride marination . J. Sci. Food Agric. , 68 : 293 – 297 .
- Olson , D. G. , Parrish , F. C. , Dayton , W. R. and Goll , D. E. 1977 . Effect of postmortem storage and calcium activated factor on the myofibrillar proteins of bovine skeletal muscle . J. Food Sci. , 42 : 117 – 124 .
- Koohmaraie , M. 1992 . Effect of pH, temperature and inhibitors on autolysis and catalytic activity of bovine skeletal muscle μ‐calpain . J. An. Sci. , 70 : 3071 – 3080 .
- Suzuki , A. , Matsumoto , Y. , Sato , S and Nonami , Y. 1982 . Ca2 + ‐activated protease in stored muscle . Meat Sci. , 7 : 269 – 278 .
- Takahashi , K. , Kim , O. H. and Yano , K. 1987 . Calcium‐induced weakening of z‐disks in postmortem skeletal muscle . J. Biochem. , 101 : 767 – 773 .
- Pérez‐Chabela , M. L. , Escalona , H. and Guerrero , I. 1998 . Effect of calcium chloride on calpain and quality characteristics of meat from chicken, horse, cattle and rabbit . Meat Sci. , 48 ( 1/2 ) : 125 – 134 .
- Ho , C. Y. , Stromer , M. H. and Robson , R. M. 1994 . Identification of the 30 Kda polypeptide in postmortem skeletal muscle as a degradation product af troponin‐T . Biochemistry , 76 : 369 – 375 .