Abstract
Rheological properties of four unprocessed unifloral Australian honeys (heath, tea tree, yapunya, and yellow box) and an artificial honey were analysed at 20°C. A model previously used to describe viscosity data of various sugar and sugar mixtures was used to describe the concentration dependence of the viscosity of honey samples with varying moisture contents. The model successfully described the sugar concentration dependence of the unadulterated and medium moisture (70–85% solids) range honey samples.
Introduction
Knowledge of the rheological behavior of honey is of practical interest and is critical during storage, handling and processing.Citation[1],Citation[2] Viscosity, one of the important rheological properties of honey, is influenced by several factors. The viscosity of honey is highly temperature sensitive.Citation[3] As honey is heated, it initially undergoes a very rapid decrease in viscosity per degree rise in temperature. At temperatures in excess of 30°C the change in viscosity is much slower, while between 45°C and 60°C the decrease in viscosity is almost negligible.Citation[4] Generally, as temperature rises, viscosity falls because there is less molecular friction and hydrodynamic forces decrease.Citation[5]
Moisture content is another factor influencing viscosity. The moisture content of honey shows varietal differences and may range from 13%Citation[1] to 29%Citation[6] but is generally considered to have an average of 17.2%.Citation[3],Citation7–9 Generally, higher moisture content results in lower viscosity.
Honeys of similar moisture content but different floral origin vary to some extent in viscosity.Citation[3],Citation[4] These variations are attributed to the composition of individual sugars and to non‐sugar, and colloidal material.Citation[3],Citation[4] The major sugars in honey are the monosaccharides fructose and glucose. Additionally, small amounts of disaccharides (sucrose, maltose, and turanose) are present. The gross physical attributes of honey are largely determined by the types and concentrations of carbohydrates.Citation[10] The higher the number of monosaccharide units the higher the viscosity.Citation[5] While, there is no research published on the sugar concentration dependence of honey viscosity it is proposed that viscosity can be related to the molality of solutes.Citation[11]
Goldsack and FranchettoCitation[12] developed a model to predict the relative viscosity (the ratio of the liquids viscosity to that of water, μ r ) of a single electrolyte solution:
Chirife et al.Citation[13] proposed that EquationEq. 1 could be used to fit viscosity data of some non‐electrolyte solutions. By assuming in the case of non‐electrolytes that the parameter V was very small (∼0), EquationEq. 1 was reduced to:
As honey is basically sugar syrup with 95–99% of the solids being sugar, the object of the current study was to assess whether EquationEq. 3 can adequately describe the concentration dependence of honey viscosity (basically a sugar syrup) and whether undetermined solids in honey influence concentration dependent viscosity.
Materials and Methods
Materials
Honeys, collected from commercial apiarists in Queensland and New South Wales, were supplied by Capliano (Richlands, Queensland, Australia). The floral source of each honey was identified by the apiarist supplying it. The majority of honeys were considered by the apiarists to be species‐specific unifloral specimens. Identification was based on color, aroma, and flavor as well as hive location and season of production.
Four common Australian honey species‐specific varieties, two eucalypt and two non‐eucalypt varieties [heath (Banksia ericifolia), tea tree (Melaleuca quinquenervia), yapunyah (Eucalyptus ochrophloia), and yellow box (Eucalyptus melliodora)] and an artificial honey were investigated in this study. The honey samples assayed here were previously determined to be Newtonian fluids.Citation[14]
Methods
Preparation of Honey Samples
Incorporation of air bubbles and the presence of crystals can interfere with honey viscosity determination. Thus, all honey samples were heated to 55°C in a water bath to dissolve any glucose crystals (or crystal nuclei) which may have been present in the sample. To ensure complete removal of air bubbles, the preheated honey samples were then stored in a temperature‐controlled room at 30°C for 48 hours. To minimise re‐crystallisation, the honey samples were then stored frozen at −18°C until analysis. All the samples were stored in 500 cm3 glass jars and caution exercised to avoid incorporating air bubbles during sample transfer and handling.
Preparation of the Artificial Honey Mixture
Unifloral Australian honey samples (n = 138) from 16 floral sources were analysed for moisture, fructose, glucose, sucrose, and maltose to determine their average moisture and carbohydrate content (unpublished). Based on this analysis an artificial honey mixture was prepared to resemble an “average” Australian honey. While fructose, glucose, sucrose, and maltose account for most of the soluble solids in honey, other constituents (carbohydrates, ash, total acids, and nitrogen) are also present. As gluconic acid is the principle acid in honey, gluconic acid lactone (C6H10O6, molecular weight 178) was added to account for all unknown honey solids in the artificial honey.
D‐glucono‐δ lactone (10.2 g) was added to distilled water (17.5 mL). To this gluconic acid solution, 38 g fructose, 30.2 g glucose, 1.8 g of sucrose, and 2.31 g of maltose were added and heated to 50°C to dissolve the sugars. This produced an artificial honey with moisture content 17.5% w/v.
Preparation of a Dilution Series
Honey samples were removed from frozen storage, heated and mixed to ensure homogeneity. Sub‐samples (50 g) of each honey variety were diluted with distilled water to obtain a series of five solutions with solid contents ranging from 70 to 85% w/w.
Moisture Content
Honey moisture content was determined by measuring refractive index at 20°C. Average refractive index values were converted to honey moisture content using the table given by the AOAC in Method 969.38.Citation[15]
Sugar Content
Honey sugars (glucose, fructose, sucrose, and maltose) were determined by HPLC using AOAC method 977.20.Citation[15]
Viscosity Measurement
A Brookfield DVIII programmable rheometer, fitted with an SCR‐27/13R small sample adapter and Rheocalc version 1.1 software (Brookfield Engineering Labs, MA) was used to determine viscosity at 20°C and shear rate 8.5 sec−1. A temperature controlled water bath and water jacketed sample adaptor were used to regulate the temperatures of the samples at 20 ± 0.5°C. The samples were analysed in triplicate.
Statistical Analysis
Linear regression analysis, mean absolute percentage error (%E) and modeling efficiency (EF) were calculated to validate EquationEq. 3 as a tool for predicting the concentration dependence of honey viscosity. Mean average percentage error and modeling efficiency were calculated as follows;Citation[16]
Results
In log of relative viscosity (μ r ) is plotted against mole fraction (X) of solute. Linear curves were observed for all experimental honeys and the artificial honey when undetermined solids (4.98–16.65%) () are considered as sucrose and glucose respectively. In all cases the correlation coefficient (R 2) was above 0.97.
Figure 1. Plot of viscosity vs. concentration data for (A) artificial, (B) health, (C) tea tree, (D) yapunyah, and (E) yellow box honeys at 20°C, shear rate 8.5 sec−1.
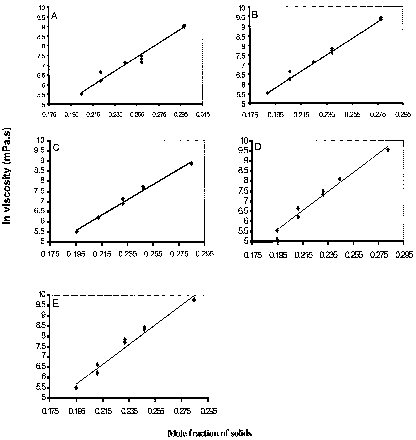
Table 1. Moisture, viscosity, and carbohydrate composition of the honey varieties studied
Values of a and E for individual sugars (glucose, fructose, sucrose, and maltose), presented in , were calculated by plotting viscosity against concentration data (concentration ranging from 0.5 mass % to 70 mass % and viscosity ranging from 1–60 mPa.s) obtained from the literature.Citation[18] In this study, parameter a for individual sugars is close to unity (). Chirife and Buera,Citation[11] also found that values of parameter a for pure and mixed sugar solutions were close to unity. As parameter a is equivalent to the viscosity of a solution with zero solids a limiting value of one at zero solids is expected.Citation[19] Thus, it is reasonable to assume that an average value of a (calculated from the individual a of a the component sugars in a solution) can be applied to a multi‐component sugar solution when applying EquationEq. 3. In this study an average value of a for glucose, fructose, sucrose, and maltose was applied experimentally when predicting viscosity of honey solutions using EquationEq. 3.
Table 2. Calculated parameters of a and E for various sugar solutions and honeys at 20°C
Soesanto and WilliamsCitation[22] found linear additivity of component volumes when assessing viscosity of aqueous sugar solutions and suggest that the concentration dependence of viscosity can be accommodated in terms of average molar volume. Similarly, Chirife and BueraCitation[11] found for an oligosaccharide mixture there was linear additivity for parameter E. Thus, for honeys, which are mixtures of sugars, parameter E was calculated using the following equation,
compares experimental and calculated viscosity for the honey varieties studied. Linear regression analysis of the experimental values for the viscosity against the values calculated using EquationEq. 3 show close correlation, with correlation coefficients (R 2) of 0.97 or greater. For all the honeys investigated, the mean absolute percentage error was below 5% and the modeling efficiency value greater than 0.90.
Table 3. Correlation co‐efficients and mean absolute percentage error for analysis for experimental viscosity vs. viscosity calculated using Eq. (4)
shows composition and viscosity data for the honey varieties studied. Honeys of similar moisture content but different absolute viscosity all obeyed EquationEq. 3 ().
Discussion
The viscosity of honey decreases with increasing moisture content or decreasing percent solids (). This finding is in agreement with previous studies that viscosity decreases with increasing water content.Citation[6],Citation[20],Citation[21] Zaitoun et al.Citation[21] explain this increase in viscosity with decreasing moisture content in terms of the anti‐plasticising effect of sugars compared with water.
The molal concentration dependence of viscosity in honey was studied using the model proposed by Chirife and Buera.Citation[11] If EquationEq. 3 is obeyed, a straight line is obtained when log of relative viscosity (μ r ) is plotted against mole fraction (X) of solute. As noted in , excellent straight lines were observed for all honey samples (R 2 > 0.98) when undetermined solids (4.98–16.65%) () are considered as sucrose and glucose respectively. These findings agree with Soesanto and WilliamsCitation[22] that for individual sugars and sugar mixtures, concentration dependent viscosity is linear at 20°C. Oppen and SchuetteCitation[20] also found that the relationship between moisture content and honey viscosity was linear over the temperature range 30–50°C. Zaitoun et al.Citation[21] however, found a polynomial fit best described the water content dependency of viscosity in white Jordanian honey.
A model's efficiency is the best overall measure of agreement between observed and simulated values.Citation[16]. While there are no absolute criteria for model validation, KlienjenCitation[23] recommends 10% as an upper limit on acceptability for mass percentage error. Modeling efficiency (EF) is a dimensionless statistic that directly relates model predictions to observed data.Citation[16] The calculated modeling efficiency (EF) is an overall indication of goodness of fit where values close to one indicate a “near perfect” fit while any model giving a negative value cannot be recommended.Citation[16] Thus, on the basis of the validation steps applied, the observed values have a good fit to the suggested model [EquationEq. 3]. Mass percentage error has previously been used to assess the “goodness of fit” of temperature dependent viscosity data of Australian honeys with the Arrhenius relationship.Citation[24] Like the current study, the mean absolute error was below 5% indicating that the temperature effect on viscosity can also be described using a previously determined model. It should be noted that viscosity measurement of samples with high moisture contents (30–40%) approached the level of detection of the instrument and thus, were not reliable. This may account for some of variation between calculated and observed viscosity values.
Variation in viscosity measures of honeys of similar moisture contents is usually attributed to non‐sugar, colloidal material. Honeys of similar moisture content but different absolute viscosity () all obeyed EquationEq. 3. Thus, any contribution of non‐carbohydrate, colloidal material to concentration dependent viscosity was minimal in the honey samples assayed. These findings agree with White,Citation[10] that the types and concentrations of carbohydrates largely determine the gross physical attributes of honey.
The model [EquationEq. 3], validated in this study, was developed to describe the concentration dependence of viscosity of pure and mixed oligosaccharide solutions. Honey is a complex multi‐component mixture. While fructose, glucose, sucrose, and maltose account for 85–95%Citation25–27 of the soluble solids in honey, other constituents, including higher carbohydrates, ash, total acids, and nitrogen are also present.Citation[28] As higher carbohydrates account for some of the undetermined solids in honey and larger solute molecules tend to cause higher viscosity,Citation[22] for the purpose of this study, all unknown solids were treated as sucrose except for the artificial honey. In the artificial honey gluconic acid lactone (C6H10O6, molecular weight 178) was added to account for all unknown solids. Because of its similar molecular weight, gluconic acid lactone was treated experimentally as glucose (molecular weight 180). For the experimental honeys unknown solids ranged from 4.98–16.65% (). However, large amounts of unknown solids did not invalidate the model [EquationEq. 3], when these solids were considered as sucrose ().
The viscosity of honey is highly temperature sensitive.Citation[3] Generally, as temperature rises, viscosity falls because there is less molecular friction and hydrodynamic forces decrease.Citation[5] The temperature effect on the viscosity of the honey samples assayed in this study followed an Arrhenius‐type relationship.Citation[14] Therefore the Arrhenius model should also describe the temperature effect of parameter E in the model [EquationEq. 3]. The effect of temperature on parameter E is currently being pursued.
Conclusions
The viscosity of the honeys considered in this investigation shows that viscosity can be predicted using a model based on solute concentration. Future identification of unknown solid components of honey will encourage more precision when attempting to fit experimental data to models based on solute concentration. From the results of this investigation it can be concluded that carbohydrate concentration is the major factor contributing to honey viscosity.
Acknowledgments
The authors gratefully acknowledge the Rural Industries Research and Development Corporation and Capilano Honey, Australia, for providing funds and samples that enabled this work to be conducted.
References
- White , J. W. 1978 . Honey . Adv. Food Res. , 24 : 288 – 354 .
- Assil , H. I. , Sterling , R. and Sporns , P. 1991 . Crystal control in processed liquid honey . J. Fd. Sci. , 56 : 1034 – 1041 .
- White , J. W. 1975 . “ Physical characteristics of honey ” . In Honey: A Comprehensive Survey Edited by: Crane , E. 207 – 239 . London : Morrison and Gibbs Ltd .
- Munroe , J. A. 1943 . The viscosity and thixotropy of honey . J. Ent. , 36 : 769 – 777 .
- Davis , E. A. 1995 . Functionality of sugars: Physiochemical interactions in foods . Am. J. Clin. Nutr. , 62 ( supplement ) : 170S – 177S .
- Junzheng , P. and Changying , J. 1998 . General rheological model for natural honeys in China . J. Food Eng. , 36 : 165 – 168 .
- Doner , L. W. 1977 . The sugars of honey: A review . J. Sci. Fd. Agric. , 28 : 443 – 456 .
- Chandler , B. V. 1977 . Quality of Australian honey . Food Res. Quart. , 37 : 1 – 9 .
- Chandler , B. V. , Fenwick , D. , Orlova , T. and Reynolds , T. 1974 . Composition of Australian honeys . Food Res. Dep. Div. Food Res. CSIRO , 38 : 1 – 39 .
- White , J. W. 1969 . Moisture in honey: Review of chemical and physical methods . J. Assoc. Off Anal. Chem. , 52 ( 4 ) : 729 – 737 .
- Chirife , J. and Buera , M. P. 1997 . A simple model for predicting the viscosity of sugar and oligosaccharide solutions . J. Food Eng. , 33 : 221 – 226 .
- Goldshank , D. E. and Franchetto , R. 1977 . The viscosity of concentrated electrolyte solutions I. Concentration dependence at fixed temperature . Can. J. Chem. , 55 : 1062 – 1072 .
- Chirife , J. E.A. 1984 . Microbial growth at reduced water activities: Some physiochemical properties of compatible solutes . J. Appl. Bacteriol. , 56 : 259 – 268 .
- Mossel , B. L. , Bhandari , B. , D'arcy , B. and Caffin , N. 2000 . Use of an Arrhenius model to predict rheological behaviour in some Australian honeys . Lebensm‐Wiss Technol. , 33 : 545 – 552 .
- 1995 . “ AOAC ” . In Official Methods of Analysis Washington, DC : Association of Official Analytical Chemists . 14th Ed.
- Mayer , D. G. and Butler , D. G. 1993 . Statistical validation . Ecol. Model. , 68 : 21 – 32 .
- Steel , R. G.D. and Torrie , J. H. 1980 . Principles and Procedures of Statistics 633 New York : McGraw‐Hill Book Company . 2nd Ed.
- Lide , D. R. 1999 . CRC Handbook of Chemistry and Physics, A Ready‐Reference Book of Chemical and Physical Data Edited by: Lide , D. R. London : CRC Press . 80th Ed.
- Rao , M. A. 1999 . “ Flow and functional models for rheological properties of fluid foods ” . In Rheology of Foods and Semi‐Solid Foods Edited by: Rao , M. A. 64 – 92 . Gaithersburg : Aspen Publications .
- Oppen , F. C. and Schuette , H. A. 1939 . Viscometric determination of moisture in honey . Industrial Engineering and Chemistry , 11 : 130 – 135 .
- Zaitoun , S. , Ghzawi , A. A. , Al‐Malah , K. I.M. and Abu‐Jdayil , B. 2001 . Rheological properties of selected light coloured Jordainian Honey . Int. J. Food Prop. , 4 ( 1 ) : 139 – 148 .
- Soesanto , T. and Williams , M.C. 1981 . Volumetric interpretation of viscosity for concentrated and dilute sugar solutions . J. Phys. Chem. , 85 : 3338 – 3341 .
- Kleijnen , J. P.C. 1987 . Statistical Tools for Simulation Practitioners New York : Marcel Dekker .
- Bhandari , B. , D'Arcy , B. and Chow , S. 1999 . Rheology of selected Australian honeys . J. Food Eng. , 4 : 65 – 68 .
- Sanz , S. , Perez , C. , Herrera , A. , Sanz , M. and Juan , T. 1994 . La Roja honey composition . Rev. Esp. Cienc. Tecnol. Aliment. , 34 ( 5 ) : 540 – 552 .
- Serra Bonvehí , J. and Granados Tárres , E. 1993 . Physicochemica propertis, composition and pollen spectrum of ling heather (Calluna vulgaris (L) Hull) honey produced in Spain . Apidologie. , 24 : 586 – 596 .
- White , J. W. , Reithof , M. L. , Subers , M. H. and Kushir , I. 1962 . Composition of American honeys . Technical Bulletin U.S. Department of Agriculture , 1261 : 1 – 124 .
- Crane , E. 1980 . A Handbook of Honey 180 Oxford : Oxford University Press .