Abstract
A drying apparatus combining microwave energy and hot air was developed to dry materials isothermally and its applicability for hygroscopic porous materials has been demonstrated. With on‐line sample mass measurements, the apparatus is suitable for determining drying kinetics and investigating the mechanism(s) that limit the drying process for a variety of food materials. Drying curves have been obtained from bread samples using the isothermal drying apparatus. These drying curves were analyzed to compare effective diffusivities calculated from convective hot air conditions (2.35–4.21 × 10−9 m2/s) to effective diffusivities calculated from isothermal conditions (7.6–18.3 × 10−9 m2/s), and a significant difference was shown. However, Fick's diffusion equation was shown only to predict drying time and not drying rate. The results of this research showed that proper parameter evaluation did not solve the problem of accurately predicting moisture transfer. Therefore a new model needed to be developed to predict moisture transfer. We hypothesized that during isothermal drying of hygroscopic porous material evaporation is the governing moisture loss mechanism, and Fick's second law was unable to predict moisture loss when this mechanism occurs.
Keywords:
Introduction
Drying of food materials has been widely accepted as an internally controlled process,Citation[1],Citation[2] where Fick's second law of diffusion has been used to predict moisture loss during drying:Citation3–5
Achieving isothermal drying condition may have been possible in high moisture non‐porous materials.Citation6–8 For porous materials, the appearance of significant temperature gradients in the samples has been shown.Citation[9],Citation[10] Roberts et al.Citation[11] showed that it is very unlikely that the isothermal assumption would be satisfied in a hygroscopic porous material such as bread during convective drying. Apparently, there is a need to develop an isothermal drying apparatus for porous as well as non‐porous materials. Without such an apparatus, the effective diffusivity may never be determined accurately.
Microwave heating is known for its fast heating rate, which is quite useful in drying studies for bringing the sample temperature from an initial temperature to a target temperature as quickly as possible. The heating rate can be as high as 10–15°C/min for a piece of bread heated in a microwave oven.Citation[12] The microwave penetration depth increases with increasing sample porosity. Due to the large penetration depth relative to the sample size that would be required for conducting drying kinetics experiments, microwave energy may be utilized in a drying apparatus to provide a solution to achieve isothermal conditions necessary to determine effective moisture diffusivity of hygroscopic porous materials.
However, there are some obstacles that need to be overcome. Uneven temperature development due to an uneven electric field distribution is one of the major problems associated with microwave heating. There is no warrenty of homogeneous heating even when a small piece of hygroscopic material is heated in a regular household oven. Without a control mechanism, the temperature of a sample during microwave heating can not be regulated. GoedekenCitation[13] designed a microwave oven with a cavity measuring 0.9 m × 0.9 m × 0.9 m and two mode stirrers. The author further demonstrated that homogeneous and reproducible heating could be achieved in the oven by heating a variety of food materials. Tong et al.Citation[14] developed a microwave oven with a feedback temperature loop using a optical fiber temperature sensing device interfaced to a PC to control the temperature of bread samples. The temperature was regulated within ± 0.5°C of the desired temperature by adjusting the microwave power. FuCitation[15] applied the microwave temperature controller to measured flavor migration kinetics in a pre‐gelatinized porous dough system at different temperatures. Nevertheless, the author's data indicated that the surface temperature was usually lower than the center temperature when controlling the temperature of a hygroscopic porous materials using the microwave temperature controller due to evaporative cooling effects.
The objectives of this research were: (1) to develop a drying apparatus that combines microwave energy as well as convective hot air; (2) to demonstrate the applicability of this apparatus on hygroscopic porous materials by measuring the temperature distributions in bread during drying at different temperatures; (3) to develop a mechanism for inserting the optical fiber temperature probe without interfering on‐line mass measurements; (4) to obtain drying kinetics from the isothermal apparatus; (5) determine the effective moisture diffusivities at different temperatures; and (6) analyze diffusion model prediction to experimental data.
Materials and Methods
Sample Preparation
Bread samples, with a moisture content of 46% (w.b.) and a porosity of 0.6, were prepared using the following recipe: 540 g of flour (54%, w/w) and 40 g of baking powder (4%, w/w) 20 g vegetable shortening (2%, w/w) and 400 g water (40%, w/w). A proofing time of 20 min was used. The bread was steam‐cooked for 1.5 hours to reduce crust formation and to obtain a uniform a loaf with respect to moisture and porosity. These samples were bore‐cut using a cylindrical cutter to produce samples with a diameter of 0.011 m.
Analysis of the bread sample was conducted before drying kinetics were conducted. EquationEquation 2 was then used to calculate the sample porosity, Φ:
Isothermal Drying Apparatus
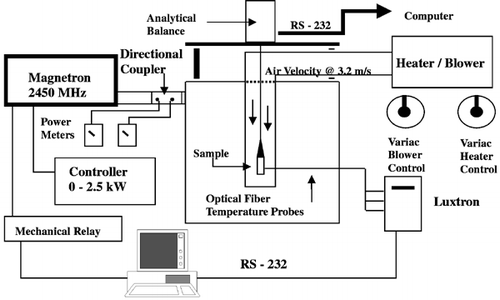
The microwave oven used was the same developed by GoedekenCitation[13] with modifications to integrate constant temperature convective hot air into cavity. The schematic diagram of the isothermal drying apparatus is shown in .
The cavity of the special design large microwave oven system was constructed by the Microwave Research Center (Marlborough, NH). The cavity was made of aluminum and has the dimensions 0.9 m × 0.9 m × 0.9 m. The microwave power generator (Model 4003, Astex/Gerling Laboratories, Modesto, CA) provided 0 to 2.5 kW of power at the frequency of 2450 MHz. A controller (Model number, Astex/Gerling Laboratories, Modesto, CA) regulated the power. Connecting the magnetron to the cavity was WR‐284 waveguide pieces. Two power meters (Model 435B, Hewlett Packard Corp., Santa Clara, CA) were connected to a directional coupler in the waveguide section to monitor the incoming and outgoing powers. Two mode stirrers were mounted on opposite sides of the cavity walls rotating at a speed approximately 30–35 rpm. The purpose of the mode stirrers along with the large sized cavity was to promote uniform power distribution within the microwave cavity, which was verified by Goedeken.Citation[13]
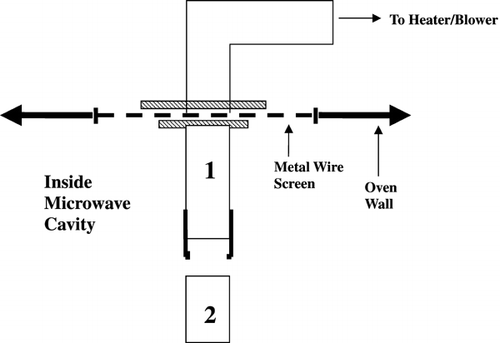
The hot air was brought into the cavity by an air‐flow polycarbonate tubing (0.076 m O.D., 0.07 m I.D.; AIN Plastics, South Brunswick, NJ). shows the air‐flow tubing inside and outside the microwave cavity. An area (0.41 m × 0.51 m) of the top cavity wall was cut out and replaced with metal wire screen with small perforations that would prevent the leakage of microwaves and at the same time allow air to flow through. The inner air‐flow tubing and the outer air‐flow jointed together by plastic nuts and bolts at the metal wire screen. The lower portion of the inner air‐flow tubing was detachable to allow easy insertion of the optical fiber temperature probes into the sample. The detachable piece had a long narrow slit to allow clearance of the probes leading through the tube and into the sample. The other end of outer air‐flow tubing was attached to a blower/heater. The blower/heater (Vapo‐Vent Blower/Heater, Hycel, Inc., Houston, TX) was used to provide constant temperature convective hot air to the sample to be dried. A fiberglass‐insulated jacket was used to insulate the outer air‐flow tubing. The blower/heater unit was connected to two variable transformers (Type 3PN1010, Staco Energy Products, Co., Dayton, OH), one controlled the air flow rate from the blower, and the another controlled the temperature of the heater. The air velocity used throughout this study was 3.2 m/s.
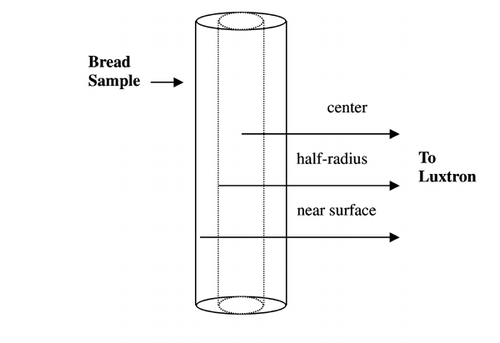
Temperature measurements in the inner air‐flow tubing and bread sample were conducted using optical fiber temperature probes and a multichannel temperature sensing unit (Model 750 Fluoroptic Thermometry System, Luxtron Corporation, Santa Clara, CA), which was connected to a computer via. RS‐232 interface. A custom program was developed to acquire the time–temperature data as well as to control the bread temperature. Temperatures were monitored at the center, half‐radius, and near surface, as shown in , and the on–off control was based on the center temperature. The high and low temperature limits were entered to the computer. The computer communicated with a mechanical relay to turn on and off the magnetron. When the sample temperature reached the high limit, a relay connecting the power was switched off, and when the temperature dropped to the lower limit, the relay was turned on again. The high and low limits were usually within 0.3°C of the target temperature. The tightness of the temperature control using a microwave temperature controller depended upon the microwave power.Citation[14] The optimal microwave power to be applied relied on the sample size as well as the moisture content of the sample. As the sample dried, the power level was manually reduced. Temperature at the center, half‐radius, and near surface as shown in were monitored while the on–off control was based on the center temperature.
An analytical balance interfaced to a PC via RS‐232 connection was used to measure mass loss of the bread sample on‐line during the drying experiments. A custom program was used to acquire the mass change with time.Citation[12] A bread sample was suspended beneath the balance into the microwave oven cavity and within the air‐flow tubing. The drying kinetic experiments were initiated after the isothermal drying conditions in the bread sample had been verified. Only one optical fiber temperature probe was used to monitor the center temperature for feedback temperature control. The rest of the experimental parameters were kept the same as those used in the temperature mapping studies. Since the probe was inserted in the bread sample, it was necessary to assure that the probe did not interfere with the mass loss measurements. A bread sample was suspended from the analytical balance with one fiber optic probe inserted at the center. Aluminum foil squares with known mass were added and removed from the dish on the balance and the change of mass was recorded.
Diffusion Coefficient Determination
Once the proper schedule of when to turn down the air temperature and microwave power during isothermal drying were determined and reproducibility shown, drying experiments were conducted using just one probe at the center of the sample. The drying curves were from 0.6 porosity bread samples with 0.011 m diameter, and the drying conditions were air velocity of 3.2 m/s and temperatures of 40, 50, 60, and 70°C.
The solution of the diffusion equation for one dimensional moisture loss in a infinite cylinder is (5):
Arrhenius plot was made using the effective diffusivities based on isothermal experiments, ln(D eff) vs. 1/T. The activation energy was calculated from the slope of this plot as shown:
The diffusion model prediction was compared to experimental data at each temperature using EquationEq. 5.
Results and Discussion
Isothermal Drying Apparatus
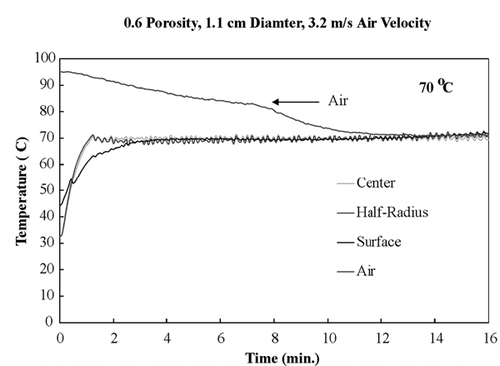
The center temperature of the sample will increase at a greater rate than the surface temperature when only microwave energy alone is used as the heating source due to evaporative cooling effects at the surface. Therefore, to acquire isothermal conditions as quickly as possible, the air temperature had to be greater than the center set‐point temperature of the sample. However, the air temperature had to be reduced incrementally to the center temperature because the evaporative cooling effects were diminishing as drying proceeded. Using a Luxtron probe in the drying chamber to measure temperature of the incoming air, the time when the air temperature had to be reduced was monitored. The starting air temperature for the 70°C isothermal experiments was 95°C, which is 25°C greater than the set‐point temperature. The other isothermal experiments had starting air temperature at 15°C greater than the set‐point temperature.
The objective was to obtain isothermal conditions as quickly as possible. The higher the set‐point temperature the greater the initial microwave power had to be used. Care was to be taken not to have the initial input power too high for the sample temperature, especially the center region, would over‐shoot the desired set‐point temperature significantly. Thus, temperature gradients would occur from the beginning of the experiment making control throughout drying difficult. The initial input power was 700 W for 40°C experiments, 825 W for 50°C experiments, and 1050 W for 60 and 70°C experiments. Reduction of power during the drying experiments were on the order of 100 W increments at times dependent on the temperature experiment.
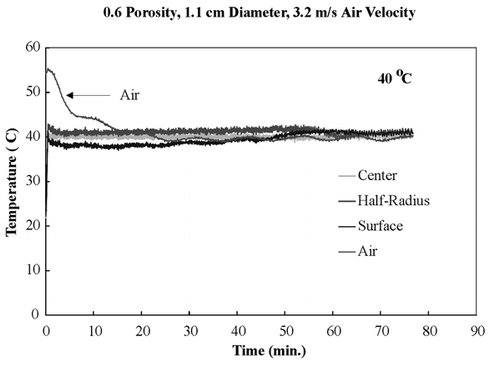
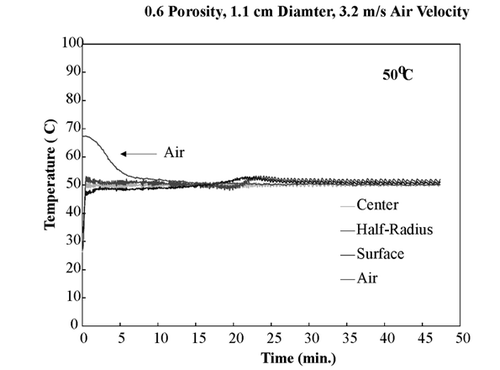
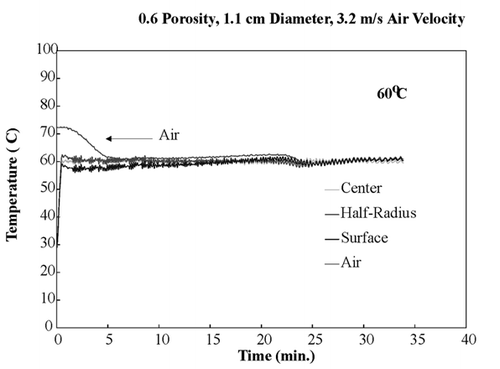
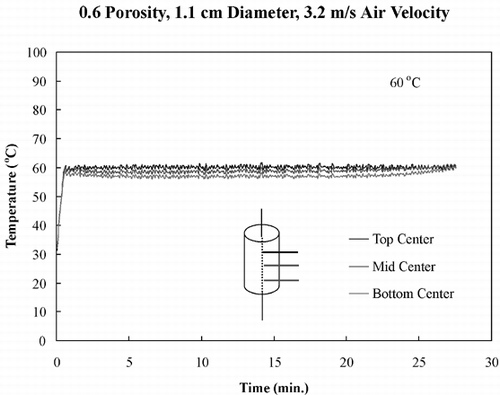
shows temperature profiles of bread samples being heated isothermally at 40, 50, 60, and 70°C. These temperature profiles show that the sample obtained isothermal conditions within the first few minutes and maintained these conditions throughout drying. To ensure that the temperature within the sample was the same in the z‐direction, all three fiber optic probes were positioned in the center of a sample and dried at 60°C. shows the temperature profiles of the top center, mid‐center, and bottom‐center of the bread sample, and similar temperature profiles of all three positions were observed. This experiment ensured that the bread sample was heating isothermally throughout the entire volume.
The probe interference test was conducted on bread samples at initial moisture content, 0.9 kg/kg dry solid, and on bread samples that were at equilibrium moisture content after being dried at 70°C, 0.025 kg/kg dry solid. The bread samples with initial moisture content were suspended from the balance with the blower turned off to reduce fluctuations to the balance readings due to moisture loss. The bread samples at equilibrium moisture content were suspended from the balance with the blower delivering 70°C convective air. With the bread samples at equilibrium with the convective air, the blower remained on to eliminate reading of an increase in mass due to moisture gain from ambient air. The support for the probes was approximately 0.32 m from the air flow cylinder, thus allowing the probe to be as flexible as possible. shows the balance readings from adding and removing known mass from the balance. The balance recorded the proper mass that were added and removed to it while a probe was inside a bread sample. Therefore it is reasonable to assume that mass loss from the sample with a single probe inserted would also be properly read from the balance. (a) shows the moisture content vs. time curve. The smooth decrease in moisture content represents a typical drying curve and further verifies the on‐line drying data recording was not affected by the probe in the sample. (b) shows the natural logarithm of the unaccomplished moisture content vs. time, and a straight line is observed. The significance of this curve is that effective diffusivity can be determined from the slope of this curve, as shown in EquationEq. 7.
Figure 6. Probe interference test: (a) moisture content vs. time; (b) logarithm of unaccomplished moisture content vs. time.
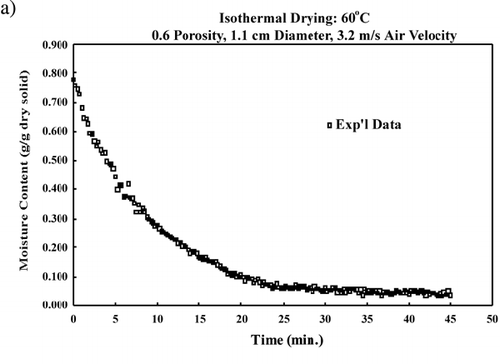
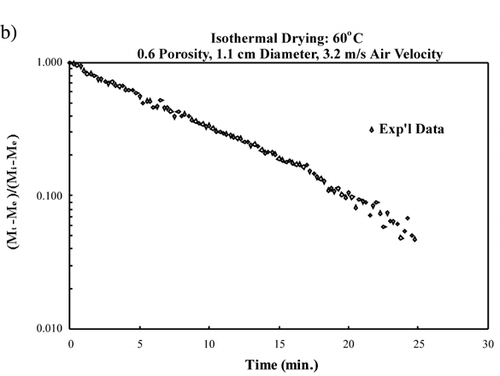
Table 1. Balance readings from the probe interference test
Diffusion Coefficient Determination
gives the average effective diffusivity for each temperature condition along with comparable effective moisture diffusivities calculated from non‐isothermal experiments of the same material in a previous work.Citation[11] Comparing the effective diffusivities calculated from the 50 and 70°C isothermal conditions to the effective diffusivities calculated from 50 and 70°C forced convention experiments, the effective diffusivites determined from true isothermal conditions were three times greater than the effective diffusivites determined from the non‐isothermal conditions. This was expected since the bread samples dried in forced convection conditions revealed pseudo‐wet‐bulb temperature profiles where the center temperatures were much less than the oven temperatures.Citation[11]
Table 2. Comparison of effective diffusivity calculated from isothermal and convective experiments.Footnote a
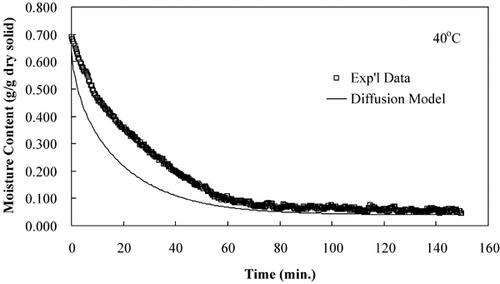
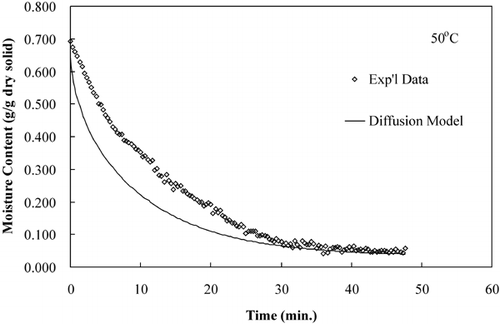
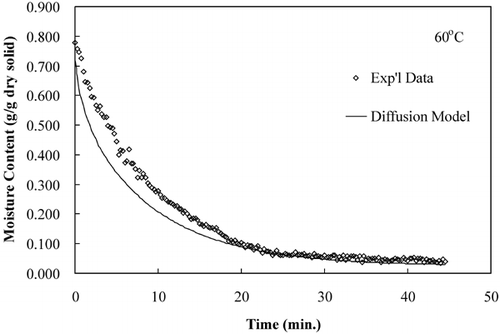
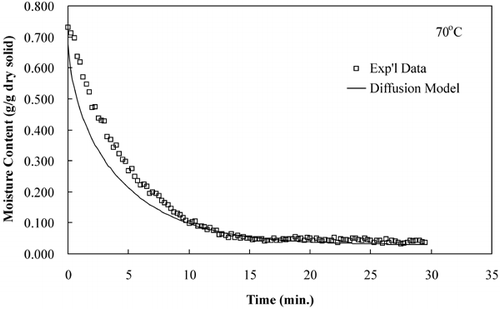
The diffusion model was used to compare predicted moisture loss during drying to that of experimental moisture loss data. show that the diffusion model predicts the drying time but not accurate rate of moisture loss, an observation made by several researchers.Citation15–17
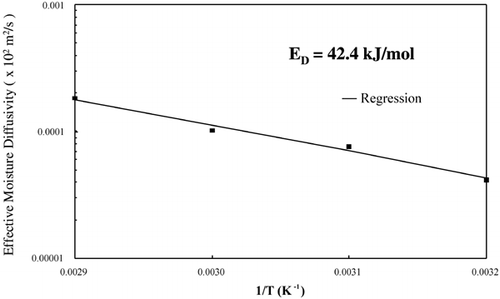
LabuzaCitation[18] reported that a diffusion‐controlled process will have an activation energy less than 34 kJ/mol. The effective diffusivities determined from these isothermal experiments were plotted on an Arrhenius graph, . The activation energy, calculated using EquationEq. 8, was 42.38 kJ/mol. The activation energy determined from isothermal experiments was greater than the activation energy determined from convective hot air experiments, 19.28 kJ/mol.Citation[11] The Arrhenius analysis based on convection drying correlates with a diffusion‐controlled process; however, the Arrhenius analysis based on true isothermal experiments indicates that the drying was not a diffusion‐controlled process. The effective diffusivites and the temperature dependence based on an Arrhenius relationship were more accurately determined in the isothermal drying experiments than the convective drying experiments due to more uniform sample temperatures, which were immediately established.
Arrhenius analysis of effective diffusivity determined during isothermal drying resulted in an activation energy (42.4 kJ/mol) greater than a diffusion‐controlled mechanism (34 kJ/mol) and slightly greater than latent heat of vaporization (40.7 kJ/mol). This significant increase in activation energy suggests that the limiting mechanism is one which requires more energy than is needed for diffusion. Along with the inaccurate prediction by the diffusion equation, this analysis suggests vaporization within the porous material could be the controlling mechanism for moisture loss during isothermal drying. This mechanism was first suggested by Tong et al. (19) when linear moisture loss profile was observed during isothermal drying at 100°C.
Conclusion
An apparatus was successfully designed and tested to obtain isothermal conditions in bread samples using combined microwave and convective hot air and a feedback temperature controller. The temperature throughout the samples were immediately established at the desired temperature and maintained throughout drying. Drying curves were obtained from this isothermal heating apparatus, and effective moisture diffusivities were properly determined. However, the diffusion model did not predict moisture loss in hygroscopic porous material during isothermal drying. This new apparatus will allow for proper analysis of mass transfer during drying by eliminating heat transfer, and thus a mechanistic mass transfer model for hygroscopic porous materials is possible.
| NOMENCLATURE | ||
| D eff | = |
Effective diffusivity, m2/s |
| E D | = |
Activation energy, kJ/mol |
| N Fi | = |
Fick number, (D eff·t)/R 2, dimensionless |
| M | = |
Moisture content, kg H2O/kg dry solid |
| M i | = |
Initial moisture content, kg H2O/kg dry solid |
| M t | = |
Moisture content at time t, kg H2O/kg dry solid |
| M ∞ | = |
Effective moisture content, kg H2O/kg dry solid |
| R | = |
Sample radius, m |
| R c | = |
Universal gas constant, 0.008314 kJ/mol K |
| s A | = |
Slope of the Arrhenius plot of effective moisture diffusivity |
| s D | = |
Slope of unaccomplished moisture content vs. time, min−1 |
| T | = |
Temperature, K |
| t | = |
Time, min |
References
- King , C. J. 1968 . Rates of moisture sorption and desorption in porous dried foodstuffs . Food Technol. , 22 ( 502 ) : 165 – 171 .
- Young , J. H. 1969 . Simultaneous heat and mass transfer in a porous, hygroscopic solid . Trans. ASAE , 12 : 720 – 725 .
- Crank , J. 1975 . The Mathematics of Diffusion London : Oxford University Press . 2nd Ed.
- Karel , M. 1975 . “ Dehydration of foods ” . In Principles of Food Science. Part II: Physical Principles of Food Preservation Edited by: Fennema , O. R. , Lund , D. B. and Karel , M. 309 – 332 . New York : Marcel Dekker, Inc. .
- Rizvi , S. S.H. 1986 . “ Thermodynamic properties of foods in dehydration ” . In Engineering Properties of Foods Edited by: Rao , M. A. and Rizvi , S. S.H. 133 – 214 . New York : Marcel Dekker, Inc. .
- Jason , A. C. 1958 . “ A study of evaporation and diffusion processes in the drying of fish muscle ” . In Fundamental Aspects of Dehydration of Foodstuffs 103 – 135 . London and New York : Soc. Chem. Ind., MaCMillan Co. .
- Vaccarezza , L. M , Lombardi , J. L. and Chirife , J. 1974 . Kinetics of moisture movement during air drying of sugar beet root . J. Food Technol. , 9 : 317 – 327 .
- Alzamora , S. M. , Chirife , J. and Voillaz , P. 1979 . A simplified model for predicting the temperatures of foods during air dehydration . J. Food Technol. , 14 ( 4 ) : 369 – 380 .
- Nissan , A. H. , Kaye , W. G. and Bell , J. R. 1959 . Mechanism of drying thick porous bodies during the falling rate period. I. The pseudo‐wet‐bulb temperature . A.I.Ch.E. Journal , 5 ( 1 ) : 103 – 110 .
- Toei , R. 1983 . “ Drying mechanism of capillary porous bodies ” . In Advances in Drying Edited by: Mujumdar , A. S. Vol. 2 , 269 – 297 . Washington : Hemisphere Publishing Corp. .
- Roberts , J. S. , Tong , C. H. and Lund , D. B. 2002 . Drying kinetics and time‐temperature distribution of pregelatinized bread . J. Food Sci. , 67 ( 3 ) : 1080 – 1087 .
- Tong , C. H. 1988 . Microwave Heating of Baked Dough Products with Simultaneous Heat and Mass Transfer Madison : Dept. Food Sci., University of Wisconsin . Ph.D. Dissertation
- Goedeken , D. L. 1994 . Microwave Baking of Bread Dough With Simultaneous Heat and Mass Transfer Dept. Food Sci., Rutgers, The State University of New Jersey . Ph.D. Dissertation
- Tong , C. H. , Lentz , R. R. and Lund , D. B. 1993 . A microwave oven with variable continuous power and a feedback temperature controller . Biotechnol. Prog. , 9 : 488 – 496 .
- Fu , Y. C. 1996 . Microwave‐Assisted Heat and Mass Transfer in Food Dept. Food Sci., Rutgers, The State University of New Jersey . Ph.D. Dissertation
- Ceaglske , N. H. and Hougen , O. A. 1937 . The drying of granular solids . Trans. Am. Inst. Chem. Eng. , 33 : 283 – 312 .
- Hougen , O. A. , McCauley , H. J. and Marshall , W. R. Jr. 1940 . Limitations of diffusion equations in drying . Trans. Am. Inst. Chem. Eng. , 36 : 183 – 206 .
- Labuza , T. P. 1972 . Nutrient losses during drying and storage of dehydrated foods . CRC Crit. Rev. Food Technol. , 9 : 217 – 240 .
- Tong , C. H. , Parent , A. and Lund , D. B. 1993 . Temperature and moisture distributions in porous food materials during microwave heating . Presented at the Sixth International Congress on Engineering and Food . May 23–27 1993 , chiba , Japan. ICEF6