Abstract
Sorption isotherms find numerous applications in food science and technology. They can be measured by different methods, but static desiccator method is one of the most frequently used. It is simple and many samples can be placed in one desiccator. Hence, isotherms of many products can be obtained in one experiment. In this work 20 products, mainly herbs and spices, were placed in one desiccator and 13 desiccators were used to obtain water sorption isotherms. Water activity of each sample was measured in Hygroscope DT2. The effect of the individual operations such as opening the desiccator, transferring samples to the balance and measuring water activity on the results was assayed. It was shown that the most important is the disturbance of equilibrium caused by opening the desiccator, taking the sample and closing it again. The disturbances cause adsorption of water from the surrounding air by samples with low water activities, and desorption of water from the samples having high water activity. Analysis showed that the process of desorption is slow and affects results insignificantly. On the other hand adsorption is fast and leads to the increase of water activity of samples. The increase depends on water activity of the sample and number of openings of the desiccator. With 20 openings the increase in water activity can be two to threefold in the range 0 ≤ a w ≤ 0.1. Some recommendations for standardization of methods using static desiccator method are suggested.
Introduction
Sorption isotherms find numerous applications in food science and technology. They are used to determine sorption—desorption enthalpies and to deduce the state of water in food. Moreover, some information about porosity, pore size distribution and adsorption surface area can be drawn from sorption isotherm.Citation[1] The state of food components, especially polymers, affects the course of sorption isotherms. Hence, information about crystallinity or the presence of amorphous domains can be obtained investigating food sorption isotherms.
Food sorption isotherms are used in food processing, especially in drying, mixing powders, and dry products, packaging and in storage. Sorption isotherms, theoretically representing the state of equilibrium, make it possible to establish end‐point of drying, to predict final water activity of dry mixes, to choose proper packaging and to establish optimal water content for products intended for long storage.
Sorption isotherms can be obtained by different methods. Generally it can be done either by measurement of water activity of food at different water contents, or by adjustment of water activity.Citation[2] Direct measurement of vapor pressure over food has been extensively used to measure water activity and is recommended as a reference method.Citation[3] In this method to obtain sorption isotherm measurements of vapor pressure over food containing different amounts of water are necessary. The most popular method to obtain sorption isotherm of food is the gravimetric method in which food samples are brought to equilibrium with the surrounding environment of known water activity. Sulfuric acid solutions and saturated salt solutions are used to adjust humidity of air to required values. The experiment is usually done in desiccators in which food samples are placed over the solution on a support and kept for several days or few months. The process of equilibration can be done in desiccators with or without vacuum.Citation[4] Citation[5] It was shown that equilibration of samples under vacuum or without vacuum yields different water contents.Citation[6] Hence, it is an important parameter in methods of obtaining food sorption isotherms.
Gravimetric method has been thoroughly investigated in the COST90 projectCitation[7] and a reference material as well as construction of sorbostats was recommended. Moreover, the standardization of the individual operations such as opening the desiccator, transferring samples to the balance, etc., was stressed since they might influence the final results.
The aim of this work was to investigate the effect of individual operations carried on during gravimetric method on the course of water sorption isotherms.
Material and Methods
As a material of investigation 20 different products, mostly herbs and spices, were used in this experiment. Products were used in the form of small particles. About 1 g of each product was placed in small aluminum containers and equilibrated in desiccators at different relative humidities. In the water activity range 0 < a w ≤ 0.75 equilibration was done for 90 days and it was assumed that materials reached the state of equilibrium (12 desiccators). At higher water activities successive weightings were done every week until the samples were discarded (these samples did not reach the state of equilibrium—three desiccators). In each desiccator 20 products were placed for equilibration. The experiment was done in duplicates.
Equilibrated sample was taken individually from desiccators and weighted on the electronic balance. Then it was transferred to the Hygroscope DT2 (Rotronic AG) and its water activity was measured. Next sample was taken from the desiccator when measurement of water activity of the previous sample was completed. Equilibration and all operations were done in a constant temperature room at 25 ± 1°C. Relative humidity of air in the room was in the range 40–50%.
Results
Opening and closing the desiccator, transferring the sample to balance and weighing took on the average 2 min. At that time the sample was in contact with the surrounding air since aluminum containers had no cups. Measurement of water activity in the Hygroscope DT2 depended on the wetness of the sample. In preliminary experiments it was found that 30 min is a sufficient time, and all samples were kept for that time in the instrument. Discarding the sample and cleaning the instrument took another few minutes.
Since water activity of two samples could be measured at a time in the Hygroscope DT2, the desiccator was open every 20 min. The experiment was done in such a way that samples from one desiccator were weighted in sequence, and then the next desiccator was open.
Measurement of water activity of samples equilibrated in desiccators showed that during 90 days of storage a state of equilibrium was reached in most of sorbostats (). At low water activities, where desorption of water occurred, especially over anhydrous CaCl2 the equilibrium was not reached during 90 days of storage. At a w > 0.75, due to danger of microbial spoilage, the storage time was less than three weeks and was too short to reach the state of equilibrium. In the range of water activities 0.03–0.75 the difference between expected and measured values was statistically not significant. Variance analysis yielded F = 0.11 at 71 degrees of freedom, while statistical significance occurred at F ≥ 3.98.
Table 1. Water activity of samples stored in desiccators
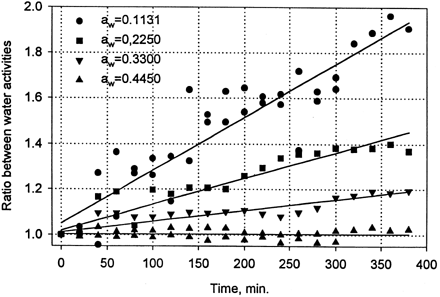
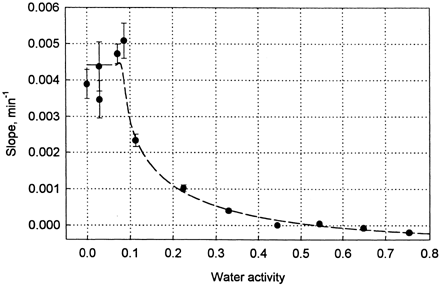
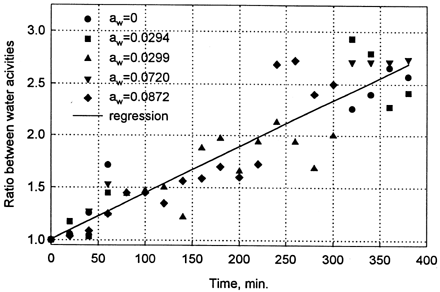
All procedures and operations done in this experiment disturbed the state of equilibrium in desiccators, and the degree of alternation was dependent on the relative humidity of atmosphere in sorbostats. At low water activities the disturbance was very pronounced and water activity of samples weighed after 20 openings of desiccator was 2.65 times higher than that measured at the first opening of the desiccator (). The relationship between the time of experiment (number of openings) and increase in water activity of samples was linear and practically independent on water activity within the range 0 < a w < 0.1. In that range the slope of the straight line varied between 0.0035 and 0.0051 min−1. The slope decreased substantially at a w > 0.1 and at water activity larger than 0.4 reached values close to zero (). It means that at a w > 0.4 number of openings of desiccator had no influence on water activity of weighted samples. At water activities larger than 0.6 the slope attained negative values () but they were statistically not different from zero. It is worth to notice that standard error of estimation of the slope was large at low water activities, and decreased with increasing water activity of the sample.
Figure 1. Relationship between time (number of openings of desiccator) and the increase of water activity of samples in respect to their initial value. Desiccators with water activity below 0.1.
Discussion
Data presented in this work showed that the individual operations, performed during sorption isotherm estimation by the static desiccator method, influence the final result. The influence is especially strong at low water activities and multiple openings of desiccator can cause substantial errors in water activity predictions of equilibrated samples. At water activities below 0.1 multiple openings of desiccator can result in a w of last samples two to three times higher than that which would be expected.
Analysis of results of this experiment leads to a conclusion that the main cause of the increased water activity of samples is the disturbance of the atmosphere in desiccator by its opening, taking the sample and closing. Other operations such as transferring the sample to balance and than to a w measuring equipment had a little effect on the final result.
Results of this work showed that adsorption processes are much faster than the desorption ones. In adsorption the mass transfer resistance is on the gas side while desorption requires transport of water from the inside of the sample to its surface. The resistances are different because water diffusion coefficients in air and in food differ by as much as five orders of magnitude.
Opening the desiccator and taking the sample causes turbulence and exchange of air that contained in desiccator with the surrounding one. When desiccator is closed a process of equilibration begins. On the adsorption side (water activities lower than in the surrounding air) the samples and the solution in the desiccator adsorb the surplus water. Since samples are on the support and over the surface of the solution it can be expected that most of water is adsorbed by the samples before it reaches the surface of the solution. Air with low humidity has a density larger than that with high humidity. Hence, natural convection in desiccators with samples of low water activity will not occur. On the desorption side low humidity air (surrounding air) is heavier than the high humidity air present in the desiccator. Hence, it descends toward the surface of the solution and desorption of water from the samples is limited.
Low mass transfer resistance and lack of natural convection currents in desiccators with low water activities cause fast adsorption of water and increased water activity of samples after each opening of the sorbostat. On the other hand, large mass transfer resistances and natural convection in desiccators with high water activities limit process of desorption and each opening of the sorbostat causes little change in water activity of the samples.
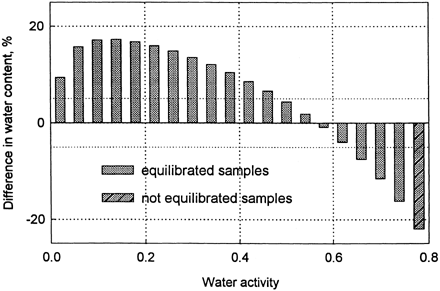
Most foods have a sigmoid shaped sorption isotherms. It means that at low water activities small change in water content results in small change in water activity, while at high water activities small change in water activity correspond to large change in water content. Taking this into account sorption isotherm of marjoram was analyzed. Water sorption isotherm based on desiccators water activity was calculated and compared with the isotherm based on measured water activities of samples being in the state of equilibrium (0.03 < a w < 0.75). The difference expressed in per cent () shows that at low water activities water content of marjoram was overestimated by more than 15%, and in high water activities the underestimation of water content reached also some 15%. The range of water activities close to the relative humidity of the surrounding air yielded differences not exceeding ±5%.
Recommendations
| 1. | Doing experiment with multiple samples placed in one desiccator use weighting bottles with covers. At the first opening of the desiccator close all weighting bottles and then begin weighing the samples. | ||||
| 2. | If, for any reason, weighting bottles are not used as sample holders measurement of water activity of each sample is necessary after it is weighed. This must be done for all samples with water activity lower than the relative humidity of the surrounding air. At higher water activities of samples stored long enough it can be assumed that the state of equilibrium was reached and the samples attained expected water activity. | ||||
| 3. | It should be always specified what kind of sample holders was used and how water activity of samples was estimated. Without these information the results are meaningless because they can be loaded with large systematic (methodical) errors. | ||||
References
- Gal , S. 1983 . “ The need for, and practical applications of, sorption data ” . In Physical Properties of Foods Edited by: Jowitt , R. , Escher , F. , Hallström , B. , Meffert , H. F.T. , Spiess , W. E.L. and Vos , G. 13 – 24 . London : Applied Science Publ. .
- Rizvi , S. S.H. 1986 . “ Thermodynamic properties of foods in dehydration ” . In Engineering Properties of Foods Edited by: Rao , M. A. and Rizvi , S. S.H. 133 – 214 . New York : Marcel Dekker .
- Rahman , M. S. , Sablani , S. S. , Guizani , N. , Labuza , T. P. and Lewicki , P. P. 2002 . “ Direct manometric determination of vapour pressure ” . In Current Protocols in Food Analytical Chemistry, Supplement 1 A2.4.1 – A2.4.6 . New York : John Wiley & Sons .
- Labuza , T. P. , Kaanane , A. and Chen , J. Y. 1985 . Effect of temperature on the moisture isotherms and water activity shift of two dehydrated foods . J. Food Sci. , 50 : 385 – 391 .
- Lomauro , C. J. , Bakshi , A. S. and Labuza , T. P. 1985 . Moisture transfer properties of dry and semi moist foods . J. Food Sci. , 50 : 397 – 400 .
- Laaksonen , T. J. , Roos , V. H. and Labuza , T. P. 2002 . Comparisons of the use of desiccators with or without vacuum for water sorption and glass transition studies . Int. J. Food Prop. , 4 : 545 – 563 .
- Spiess , W. E.L. and Wolf , W. R. 1983 . “ The results of the COST90 project on water activity ” . In Physical Properties of Foods Edited by: Jowitt , R. , Escher , F. , Hallström , B. , Meffert , H. F.T. , Spiess , W. E.L. and Vos , G. 65 – 87 . London : Applied Science Publ. .