Abstract
The rheological behavior of banana puree was determined using a dynamic stress rheometer with a pressure couette fixture, which allowed experiments to be conducted at high temperature. The pressure couette was pressurized with compressed air to 206.8 kPa (gage pressure) and experiments were carried out at temperatures ranging from 30 to 120°C. The shear stress values ranged from 10 to 170 Pa and the shear rate values from 10−5 to 103 s−1. The model that best fitted the experimental data at all temperatures was the Herschel-Bulkley model. There was a usual tendency for the apparent viscosity to decrease with increasing temperature but an increase in apparent viscosity with increasing from 50 to 60°C and from 110 to 120°C was found. This could be due to interaction of polysaccharides present in banana puree. There was a slight difference between the apparent viscosity values for increasing shear stress sweeps and those for the decreasing shear stress sweeps suggesting time dependency of the rheological behavior. Models were proposed to correlate the parameters of the Herschel-Bulkley model and viscosity with temperature.
1. Introduction
Banana was one of the first fruits cultivated by man. Nowadays, it is planted in all humid tropical regions and is the fourth largest fruit crop in the world. Bananas have high energy content (100 cal/100 g of pulp) and are nutritious with readily available carbohydrates, vitamins C, A, B1, B2, and the minerals potassium, magnesium, phosphorus, calcium, and iron.Citation1
The losses in banana production are extremely high (about 40% of the total global production) because of its perishability and the precarious technology employed by farmers. Processing rejected fruits can diminish this loss and one of the best alternatives is banana puree. Banana puree has a longer shelf life than the fruit and can be used as an ingredient for many foods such as baby food, gelatin, cake, bread, pie, yogurt, juice, ice cream, pudding, among others.Citation1 In this case banana puree is reprocessed, sometimes at a high temperature, for example in baby food production.
The viscosity of fluid foods is an important transport property, which is useful in many applications, such as design of food processes and processing equipment, quality evaluation and control of food products, and understanding of the structure of food materials. Theoretical and semi-empirical models do not adequately predict the viscosity of foods because of the complex chemical and physical structure. Therefore, to characterize fluid foods experimental measurements and empirical models are necessary.Citation2
Banana puree undergoes thermal processing at high temperatures during its manufacture. There is a significant change in apparent viscosity when the banana puree is heated. This influences velocity and temperature profiles, as well as the pressure drop inside the processing equipment. Therefore it is necessary to have knowledge of the influence of shear rate and temperature, and their combined effect on the rheological behavior of banana puree.
There are only a few studies on flow properties of banana puree and none has considered temperature above 55°C. CharmCitation3 presented data on the rheological behavior of two commercial brands of banana puree. The experiments were carried out in a Merril co-axial viscometer and a tube viscometer at 25°C. The author suggested that the power law model best represented the data.
Rao and PalominoCitation4 studied flow behavior of banana puree with a tube viscometer at ambient temperature. The researchers concluded that banana puree is a pseudoplastic fluid and that the power law model was the best model to describe its behavior. The power law model was also used for describing the rheological behavior of banana puree at 26.5°C by Holdsworth.Citation5 Barbosa-Cánovas and PelegCitation6 tested commercial banana puree from two different brands in a Haake coaxial viscometer at 25°C. The Herschel-Bulkley model was the best mathematical representation of most experimental curves and for some, the modified Casson model was better, though both models had good fit. Kaletunc-Gencer et al.Citation7 studied banana puree with a Haake coaxial viscometer at 25°C. The authors concluded that both the Herschel-Bulkley model and the modified Casson model could be used to represent the flow curves for banana puree. However, the Casson model did not provide an adequate fit.
The rheological behavior of banana puree with addition of glucose, sodium bisulfite, potassium sorbate, ascorbic acid, and orthophosphoric acid was studied with a Haake concentric cylinder viscometer at 5°C and at 25°CCitation8 and the results were best fitted by the Herschel-Bulkley model. Since the banana puree is also a thixotropic fluid, the Weltmann model also fits the results. Another investigation was carried out to study the influence of pH, temperature, soluble solids concentration, and water activity change by glucose addition on the flow behavior of banana puree. Experiments were conducted with banana puree at pH 3.0 and 5.1, at temperatures ranging from 10–55°C, concentrations from 21.4–50.9°Brix and enough glucose added to achieve the water activity values of 0.89, 0.93, and 0.97. The puree showed a shear thinning behavior with an appreciable yield stress value, following the trend of Herschel-Bulkley model. The addition of glucose decreased the apparent viscosity and increased the temperature dependency of the flow properties. The pH presented no pattern with respect to its effect on flow characteristics, while temperature and concentration greatly influenced the flow properties. An equation was proposed to calculate the consistency coefficient at different temperatures and concentrations.Citation9
This study was aimed to obtain the data on flow properties of banana puree from 30 to 120°C and fit appropriate predictive models. This temperature interval was chosen because the reprocessing of banana puree is done at temperatures within this range.
2. Materials and Methods
2.1. Banana Puree
Banana puree was obtained from a commercial source as acidified single strength aseptic banana puree in 225 kg drums. The puree had been acidified with a combination of citric and ascorbic acid to an average pH of 4.49 ± 0.03, heat treated for enzyme inactivation and aseptically packaged in plastic bags within the drums. The average soluble solids concentration of the puree was of 22.1 ± 0.9°Brix. The composition of the puree can be seen in Table .
Table 1 Composition data of banana puree
Also, raw bananas were bought at the local market and pureed. The banana puree was pushed through a 1 mm screen and acidified with 0.2%(w/w) ascorbic acid and sufficient citric acid to lower the pH to 4.5. The corrected soluble solids content of the puree was 22.2°Brix and the composition of the puree was similar to that shown in Table .
Controlled Stress Rheometer
A dynamic stress controlled rheometer (model SR 5000, Rheometric Scientific, Piscataway, NJ) was used to obtain the flow behavior of banana puree. A pressure couette fixture, which allowed high temperature experiments, was used. The pressurized chamber was connected to the rheometer head by a magnetic coupling (indirect drive) system. A test was carried out to determine the resistance of the bearings so that this could be accounted for in the measurements as recommended by the manufacturer. A water bath (model F34-MD, Julabo USA Inc., Allentown, PA) with a Thermal H10S fluid (Julabo USA Inc., Allentown, PA) was used to heat the couette. The rheometer chamber has a jacket through which the heating fluid recirculates for heating the couette.
Brookfield Rheometer
A Brookfield rheometer model RVT-III was used to perform tests on the banana puree made from raw bananas. The small sample adapter was used with a SC4-28 spindle and a SC4-13R chamber. The temperature was controlled by a UH water bath (MLW, Germany).
Experimental Procedure
The water bath temperature was set to the experimental temperature, from 30 to 120°C. For the higher temperatures (above 80°C), the water bath was closed off from the rheometer jacket, so the heating fluid would remain in the water bath and heat up faster, and the connections to the jacket were only reopened when the temperature was reached. A 10 mL sample of banana puree was loaded into the cup of the pressure cell. The cell was closed and pressurized with compressed air to 206.8 kPa. The cell was loaded onto the rheometer and the stage containing the rheometer head was lowered to make sure that the magnet on the head was aligned to the bearing.
When the temperature of the pressure cell reached the desired experimental temperature, additional ten minutes were allowed to make sure that the temperature of the puree inside was the same as that of the couette. The magnetic upper fixture of the rheometer was turned 10 times in each direction to ensure even distribution of the puree inside the couette and also guarantee a more even temperature distribution.
A steady stress sweep was performed to determine the dependence of apparent viscosity and shear stress with shear rate. The sweep was performed first by increasing shear stress up to a certain value, which ensured that the shear rate remained within an acceptable range, and then the shear stress was decreased back to the initial value using the same sample. The range for the shear stress variation was determined by tests conducted at each temperature. The minimum shear stress was determined by back-calculation from apparent viscosity of the banana puree set at 10 Pa s, which is the maximum viscosity recommended by the manufacturer for use with the pressure couette fixture, so that the bob shaft is not damaged. The maximum shear stress was set to cover a wide range while ensuring that the shear rate would not exceed the instrument's capability, which is 2000 s−1. A logarithmic stress sweep mode was chosen with 200 points per decade and a maximum time per data point of 10 s.
For temperatures up to 70°C experiments were repeated three times at each condition with a fresh sample each time. For the temperature of 80°C and above experiments were repeated five times at each condition with a fresh sample each time. A total of 45 experiments were conducted with an average of 80 points each. The maximum variability allowed between replicates was 5%. Additionally at least two experiments were run at each temperature to determine minimum and maximum shear stress values.
The experiments carried out in the Brookfield rheometer with raw banana puree were at 50, 55, and 60°C. A sample was placed in the chamber and the chamber was fitted into the water jacket. The rheometer was zeroed and the spindle was placed in the sample. The amount of sample used was such that the surface of the puree was level with the top of the chamber. The sample was heated to the desired temperature (approximately 5 min were sufficient to ensure that the temperature was reached) and an up and down test was performed. The speed was increased from 10 rpm (shear rate of 2.8 s−1) to 250 rpm (shear rate of 70.0 s−1) and then decreased back to 10 rpm. The shear stress was measured every 10 s. A new sample was used for each temperature and furthermore, at each temperature three replicates were carried out with a new sample each time with a deviation of less than 5% between replicates.
Analysis of Results
A statistical program (STATGRAPHICS version 4.0, Manugistics, Inc., Rockville, MD) was used to perform regressions on the data for the upward and downward shear rate curves separately at each temperature. Yield stress values were determined at low shear rate and the values compared with those obtained by regression analysis. The models tested were the Bingham and the Power Law as two-parameter models, and the Herschel-Bulkley as a three-parameter model.
The Bingham model is described by the equationCitation5:
The Power Law model has the following equationCitation5:
The Herschel-Bulkley model is an extension of the Power Law by including a yield stress term:
These models have been widely used in the literature and it is well known that K and n are affected by temperature, though K tends to be more sensitive to temperature changes than n.Citation5
3. Results and Discussion
The rheological curves obtained by the upward and downward shear rate change for banana puree within the 30–120°C show that for some temperatures (30 and 70°C), there is practically no change in the values of shear stress at corresponding shear rate for the upward and downward curves indicating that the rheological behavior is not time dependent (as seen in Fig. ). At other temperatures, there seems to be indication of time dependency of the rheological behavior, and thus there is indication of thixotropy for all conditions, except that for 90°C. At 90°C, there is an indication that a structural change happened while the rheological measurement was taking place. At this point, starch gelatinization is normally complete and the peak apparent viscosity of banana starch has been reached.Citation11
Figure 1. Rheological curves for upward and downward shear rate change at temperatures ranging from 30 to 120°C obtained for banana puree.
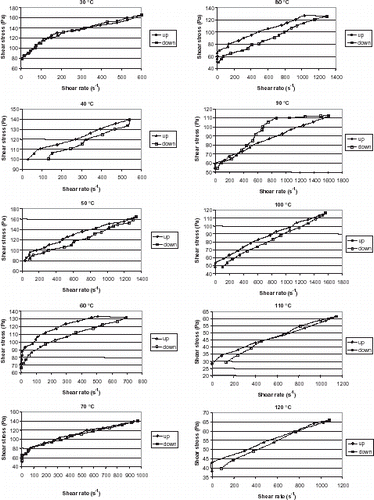
The upward and downward curves at temperatures from 30 to 120°C were fitted with the three models, Bingham, Power Law, and Herschel-Bulkley, by regression analysis. As reported by other authorsCitation8 Citation9 the best model to describe the behavior at all temperatures was the Herschel-Bulkley model. Table presents the parameters obtained. The values of yield stress obtained in this article are considerably larger than those reported by other authors.Citation3 Citation4 Citation5 Citation6 Citation7 Citation9 This could be due to the fact that the puree studied in this article had already undergone heat treatment. However, values of the consistency index, and flow behavior index are similar to those obtained by these authors. There is a large variation between the literature values for yield stress, consistency index and flow behavior index, which can be attributed to different techniques employed by the authors, different temperatures and also soluble solids content of the purees studied.
Table 2 Parameters obtained from experimental data fitting for banana puree flow curves to the Bingham, Power Law, and Herschel-Bulkley models for the upward and downward curves at temperatures from 30 to 120°C
Banana puree has a complex rheological behavior that changes drastically with temperature. In general the puree will flow easier at higher temperatures but there are changes that can be seen particularly from 50 to 60°C and from 110 to 120°C, where the shear stress vs. shear rate graphs are actually above those obtained at other temperatures. A marked change in the rheological behavior of banana puree with increasing temperature was observed. From 50 to 60°C and from 110 to 120°C the behavior was opposite of the expected that would be for the fluidity to increase with increasing temperature. From 90 to 100°C there was small change as seen by the values of shear stress at corresponding shear rate.
The bananas used for making the puree were ripe bananas, which contained 0.9% starch.Citation10 The pasting temperature for banana starch varies from 49.8 to 51.8°C,Citation11 which is the temperature in which the viscosity starts to increase significantly, so the change at this temperature is probably due to the presence of starch in banana puree. The change from 110 to 120°C might be caused by interaction of the polysaccharides that are present in banana puree.
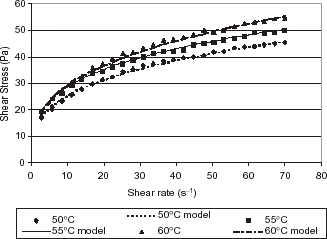
The change in behavior from 50 to 60°C has also been observed in experiments carried out with raw banana puree using a Brookfield rheometer. Figure shows that the fluidity has decreased with increasing temperature.
Figure 2. Shear stress dependency on shear rate for upward experiments at temperatures from 50 to 60°C, in comparison to the Power Law model for raw banana puree.
Models were proposed to correlate the dependency of the Herschel-Bulkley model parameters with temperature. Table shows the models for the yield stress (σ 0), consistency index (K), and the flow behavior index (n) for the upward curves of banana puree. For K and n, two different models were used from 30 to 50°C and from 60 to 120°C, because a single model was not able to explain the data fully since there is a marked change in the rheological behavior from 50 to 60°C.
Table 3 Models for correlating Herschel-Bulkley parameters (yield stress (σ 0), consistency index (K), and the flow behavior index (n)) with temperature for the upward curves obtained for banana puree
The graphs of the parameters σ 0, K, and n (Fig. ) show that these models can explain the data well. The parameters σ 0, K, and n were influenced by the temperature of the banana puree. The yield stress (σ 0) decreased with temperature as expected. For K and n there was a discontinuity when the temperature of the banana puree changed from 50 to 60°C. K tends to decrease with temperature, which is expected since there is a general tendency for the viscosity to decrease with temperature. From 50 to 60°C however, the viscosity increases and so K also increases and then starts to decrease again but with a different model.
Figure 3. Influence of temperature on the Herschel-Bulkley parameters (yield stress (σ 0), consistency index (K ), and the flow behavior index (n)) for the upward curves obtained for banana puree.
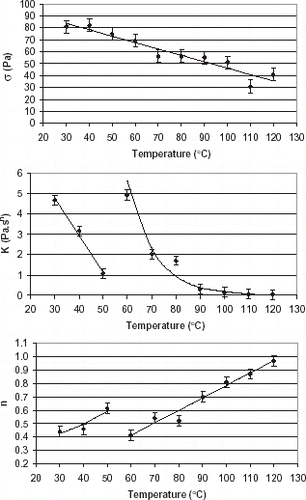
The flow behavior index (n) tends to increase with increasing temperature, showing that the behavior of the puree tends to become closer to that of a Newtonian fluid. From 50 to 60°C there is clearly a structural change in the puree that causes a marked change in rheological behavior (probably due to starch gelation which occurs within this temperature intervalCitation11) therefore n decreases again, since the rheological behavior becomes more pseudoplastic. Then with increasing temperature it increases again and at 120°C its value is close to 1 and the rheological behavior is practically that of a Bingham fluid, once the yield stress is overcome the behavior is Newtonian. This is very important for the thermal processing design of banana puree. The Arrhenius model did not explain the temperature dependency of any of the parameters of the Herschel-Bulkley model.
For the raw bananas the model that best correlated the data was the Power Law model, there is no indication of an appreciable yield stress, which is expected since this puree is unprocessed. The values of K obtained were 12.70 Pa s n at 50°C, 14.91 Pa s n at 55°C, and 13.79 Pa s n at 60°C, that is K increases reaching a maximum at 55°C and then decreases again but to a value still above that at 50°C. This confirms that there is a change in behavior, with an increase in K with temperature between 50 and 60°C. The values of n were 0.30 at 50°C, 0.28 at 55°C, and 0.32 at 60°C. There is a decrease in the n value from 50 to 55°C, so the banana puree does become more pseudoplastic, but at 60°C the value has increased again.
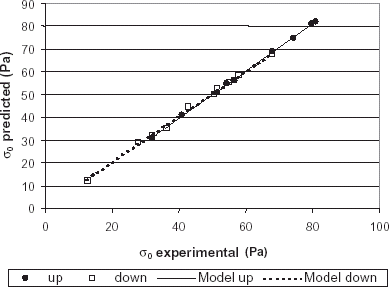
The yield stress measurements were compared to those obtained by regression analysis for the upward and downward curves and a good agreement was found. Figure shows the comparison of the experimental and predicted values.
Figure 4. Comparison of predicted and experimental values of yield stress for upward and downward experiments and the models for their correlation.
The downward curves for the temperatures from 30 to 120°C indicate that the trend is the same as that observed for the upward curves except that the change from 90 to 100°C is marked, whereas the change from 80 to 90°C is small.
Apparent Viscosity Dependency on Temperature at Different Shear Rates
There is an usual tendency for the apparent viscosity to decrease with increasing temperature but an increase in apparent viscosity with increasing from 50 to 60°C and from 110 to 120°C was found (Fig. ). The increase in viscosity from 50 to 60°C and from 110 to 120°C was statistically significant, but a single model could still be fitted to the data, considering that viscosity decreases with temperature for all shear rates. This could be due to changes in the starch structure in banana puree. For a shear rate of 100 s−1 the change is more pronounced from 50 to 60°C. As the shear rate increases the change in the apparent viscosity decreases, which is expected since the banana puree has a pseudoplastic rheological behavior. In industrial operations a product is submitted to a range of shear rates and it is important to know how the viscosity will change with temperature at these shear rates to adequately design the equipments for these operations.
Figure 5. Temperature dependency of apparent viscosity of banana puree at shear rates from 10 to 600 s−1.
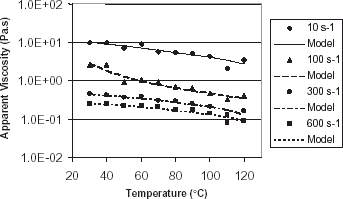
The equations for the models that correlate apparent viscosity with temperature at the different shear rates are shown in Table . The Arrhenius model did not explain the temperature dependency of apparent viscosity of banana puree well.
Table 4 Models for correlation of apparent viscosity with temperature at different shear rates
Conclusions
Banana puree presents a complex rheological behavior that changes drastically with temperature. The model that best represents the rheological behavior for upward and downward curves at all temperatures was the Herschel-Bulkley model. Correlations were found to show the temperature dependency for the parameters and the apparent viscosity of banana puree to temperature. At some temperatures there seems to be indication of time dependency of the rheological behavior (40, 50, 60, 80, 90, 100, 110, 120°C), there is indication of thixotropy, except for 90°C. At 90°C there is an implication that a structural change happens while the rheological measurement is taking place. There is a marked change in the rheological behavior from 50 to 60°C. It is very important to consider this for thermal process design of banana puree. Further study should be undertaken to analyze variation of shear stress at a constant shear rate with time and also within the range 50 to 60°C to determine the exact flow behavior of banana puree.
Nomenclature
| K | = |
Consistency coefficient (Pa s n ) |
| n | = |
Flow behavior index (dimensionless) |
| T | = |
Temperature (°C) |
|
| = |
Shear rate (1/s) |
| η | = |
Apparent viscosity (Pa s) |
| σ | = |
Shear stress (Pa) |
| σ | = |
Yield stress (Pa) |
Acknowledgments
The authors acknowledge the State of São Paulo Research Foundation (FAPESP) and the Brazilian Committee for Postgraduate Courses in Higher Education (CAPES) for the scholarships granted to author Ditchfield. Special thanks to Mr. Carl Ruiz, David Peck, and Dr. Nepal Singh for providing laboratory assistance during the experimental phase of this project.
References
- Carvalho Filho , C.D. and Massaguer , P.R. 1997 . Processamento térmico de purê de banana (Musa cavendishii, Lamb.) em embalagens flexíveis esterilizáveis . Ci. Tecnol. Aliment. , 17 ( 3 ) : 213 – 218 .
- Krokida , M.K. , Maroulis , Z.B. and Saravacos , G.D. 2001 . Rheological properties of fluid fruit and vegetable puree products: compilation of literature data . Int. J. Food Prop. , 4 ( 2 ) : 179 – 200 . [CROSSREF]
- Charm , S.E. 1960 . Viscometry of non-Newtonian food materials . Food Res. , 25 : 351 – 362 .
- Rao , M.A. and Palomino , L.N.O. 1974 . Flow properties of tropical fruit purees . J. Food Sci. , 39 : 160 – 161 .
- Holdsworth , S.D. 1993 . Rheological models used for the prediction of flow properties of food products: a literature review . Trans. Inst. Chem. Eng. Part C , 71 : 139 – 179 .
- Barbosa-Cánovas , G.V. and Peleg , M. 1983 . Flow parameters of selected commercial semi-liquid food products . J. Texture Studies , 14 : 213 – 234 .
- Kaletunc-Gencer , G. , Barbosa-Canovas , G.V. , Normand , M.D. , Rosenau , J.R. and Peleg , M. 1986 . “ Determination of the flow parameters of fluid foods using a digitizer and a personal computer ” . In Food Engineering and Process Applications, , 1st Ed. Edited by: Le Maguer , M. and Jelen , P. Vol. 1 , 15 – 21 . London : Elsevier Applied Science Publishers Ltd. .
- Guerrero , S. , Alzamora , S.M. and Gerschenson , L.N. 1997 . Flow behavior of processed banana puree . Food Sci. Technol. Int. , 3 : 103 – 111 .
- Guerrero , S. and Alzamora , S.M. 1997 . Effect of pH, temperature and glucose addition on flow behaviour of fruit purées I . Banana purée. J. Food Eng. , 33 : 239 – 256 . [CROSSREF]
- Mota , R.V. da , Lajolo , F.M. and Cordenunsi , B.R. 1997 . Composição em carboidratos de alguns cultivares de banana (Musa spp.) durante o amadureciemnto . Ci. Tecnol. Aliment , 17 ( 2 ) : 94 – 97 .
- Mota , R.V. da , Lajolo , F.M. , Ciacco , C. and Cordenunsi , B.R. 2000 . Composition and functional properties of banana flour from different varieties . Starch , 52 ( 2–3 ) : 63 – 68 . [CROSSREF]