Abstract
Water absorption process during soaking of African breadfruit (ABF) seeds was studied at five typical soaking temperatures, ranging between 30 and 70°C. The progress of water absorption by the seeds followed an exponential increase with increase in temperature. The experimental data were fitted to three empirical equations. All the equations were able to explain over 90% of the experimental data. The predicted water absorption capacity (M e ), which ranged between 68 and 92/100 g dry solid were not significantly affected by temperature changes (p > 0.05) while the time to achieve maximum water absorption capacity ranged between 9 and 140 h. The water absorption rate constant in Singh–Kulshrestha's model was more sensitive to temperature changes than from the others. By applying Arrhenius equation, it was shown that water absorption at 30–70°C was the predominant process responsible for the changes in mass of ABF seeds. The differences in the experimental and predicted data from the three models were compared to evaluate their goodness of fit. The chi-square and the root mean square deviation (RMSD) showed that Singh–Kulshrestha and Pilosof's models described better the moisture variation in soaked ABF seeds with time. In terms of residual moisture plots, Singh–Kulshrestha's model gave a more random distribution at all soaking temperatures, making it a better fitting equation.
Introduction
African breadfruit (Treculia africana) is a tropical tree crop belonging to the family Moraceae that grows numerously in the humid zone of West Africa either as wild or cultivated specie. A mature tree yields about 700–3500 kg fruit per annum.Citation1 Mature and ripe fruit are fermented to extract its edible seeds. The seeds are highly nutritious and have the potential of replacing most leguminous grains in food formulation. The nutritional composition of the seeds has been documented in some reportsCitation2 Citation3 Citation4 Citation5 where it was shown that the crude protein and fat content of the raw seeds ranged between 19–23% and 17–20%, respectively. In Nigeria, the seeds are dehulled, boiled, and eaten as a main dish or as part of a dish. It may also be roasted and eaten as snack.
With increasing awareness of the potential food and industrial value of African breadfruit (ABF) seeds, research efforts towards enhancing its food and industrial utilization have equally increased. Some reportsCitation6 Citation7 Citation8 have shown that the grains are potential raw material for many cottage and commercial food products. It has also been reported that using simple technology, the flour produced from the seeds has functional properties that makes it useful for food product supplementation.Citation9 Citation10
Soaking is central to food processing uses of African breadfruit (ABF) seeds. For example, the seeds are preconditioned in ordinary or warm water prior to malting.Citation6 Citation9 Hot water soaking also enhances its dehulling process.Citation10 Similarly, ABF seeds are soaked in water in preparation for wet milling to produce a paste used as thickening agent in soups while OdoemalamCitation9 also showed that soaking enhanced certain functional properties of flour made from such seeds. During soaking, liquid water is progressively sorbed, the extent of which depends principally on the soaking temperature, time and some physicochemical characteristic of the food material. However, to achieve the optimum moisture condition required for specific processing needs, an accurate means of predicting the moisture contents at any time during soaking is vital.
The water absorption behavior in soaked food grains has been a subject widely studied. More recently, a two-dimensional image analysis was carried out on basmati rice to determine the hot water hydration kinetics.Citation11 Maroulis et al.Citation12 also reported the application of an empirical model not based on diffusion law but which incorporated effect of moisture and temperature on the moisture diffusivity. Empirical equations are relatively simpler to apply by a food engineer compared to those equations based on diffusion laws. Despite this fact, reports on the comparative evaluation of empirical models for predicting kinetics of water uptake in soaked food grains are very few in literatures. An empirical model proposed by PelegCitation13 has been found to accurately predict moisture uptake in soaked cereals and leguminous food grains.Citation14 Citation15 Citation16 Apart from food grains, the empirical equation proposed by PelegCitation13 has been demonstrated to be equally applicable to study moisture uptake of powdered food materials despite the dissimilarity in their physical characteristics. Maia and Cal-VidalCitation17 studied water vapor uptake by citrus juice powder under different relative humidity by applying an empirical equation proposed by Pilosof.Citation18 The equation gave a good fit to the experimental data. However, till date, published reports evaluating its applicability to food grains are scarce. The third equation proposed by Singh and Kulshrestha,Citation19 which was used to model water absorption data for soybean and pigeon pea, has equally received less attention.
The objectives of this study, therefore, were to (1) generate water absorption data for the ABF seeds at various practical soaking temperatures and (2) to evaluate the applicability of three empirical models in the prediction of moisture contents of ABF seeds during soaking.
Description of the Models
In this study, applicability of the two-parameter equations proposed by Peleg,Citation13 Pilosof,Citation18 and Singh and KulshresthaCitation19 were tested. Their original and the linear forms are shown in Table . However, the nonlinear form of Pilosof et al.'s equation was used and parameters A and B in this equation represent the water absorption capacity (dimensionless) and the time (hours) needed to absorb half of the maximum amount of water, respectively. The latter is likened to the half-life of the water absorption process, which is also a measure of the water absorption rate. Also, parameter A in Peleg's model is the water absorption rate constant (hour per % wt. dry basis) while B is the characteristic sorption parameter (per % wt. dry basis). The parameters A and B in Singh–Kulshrestha's equation are the absorption rate constant (per hour) and water absorption capacity (g/g dry solid), respectively. As soaking time is increased i.e., as t → ∞, moisture equilibrium is approached. Thus, Peleg's model is transformed into:
Table 1 Some physical characteristics of ABF seeds
The Eq. (Equation1) is used to calculate the equilibrium moisture content (M e ) in percent weight (dry basis). Pilosof's equation was originally built for dehydrated foods (powder materials) having very low moisture (<5%). Since the seeds studied here have relatively higher initial moisture (9.28% dry basis), the influence of the initial moisture is expected to be significant. Hence, the modified form of Pilosof's equation shown below was also tested:
Materials and Methods
Matured fruits were harvested in a farm site located in Ago Iwoye, the South West of Nigeria. The seeds were extracted following the traditional steps similar to that described elsewhere.Citation20 The seeds were then washed with sharp sand to remove the superficial mucilage and sun dried for about 72 h. The dried seeds were further cleaned to remove any adhering sand particles, fibers, and damaged seeds. The physical characteristics of the seeds (minor and major diameter, thickness, 1000 seed mass, seed coat thickness, and sphericity) were determined following the method of Suthar and Das.Citation21 The results are shown in Table . Separate portions of the seeds were then preconditioned for about an hour in an economy size incubator (Gallenkamp, UK). Soaking of the seeds was carried out at 30, 40, 50, 60, and 70°C. The same temperatures were used in preconditioning the seeds prior to soaking experiment.
About 70 mL of distilled water separately poured into glass tubes were placed in a thermostatically controlled Clifton water bath (Model NE 1-22, Nickel Electro Ltd., England) at set temperatures described above. After the water in the tubes had attained the desired bath temperature, 10 g of preconditioned seeds were charged into the tubes. At 40 min interval, soaked seeds were carefully transferred onto a paper towel on which the seeds were rolled to remove the superficial water as described by Hsu et al.Citation22
The mass of the seeds was then determined to 0.01 g accuracy on a digital balance (Model PB602, Mettler Toledo, Switzerland). The determinations were done in duplicate. The moisture contents were determined in a digital laboratory oven (Gallenkamp, UK) by drying the seeds at 105°C until constant weights were achieved.Citation23
The moisture absorption data derived from the foregoing were subjected to Linear Regression (LR) analysis to determine the values of the parameters in each model equation except for the Pilosof's equation that was solved using iterative nonlinear regression (NLR). The difference between the experimental and the predicted moisture contents, using the empirical equations, was compared by applying a chi-square test as described in Mead and Curnow,Citation24 the root mean square deviation (RMSD) as defined by Engels et al.,Citation25 and the plot of residuals against the soaking time.
Results and Discussion
Water Absorption Characteristics
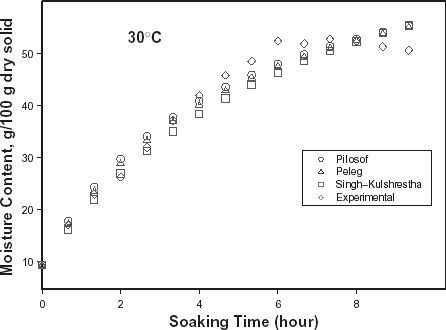
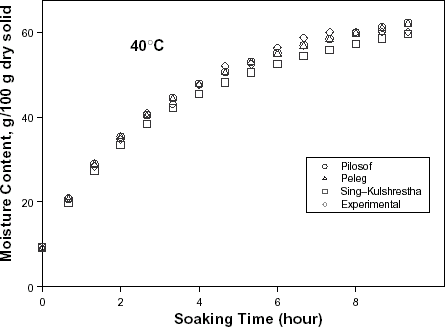
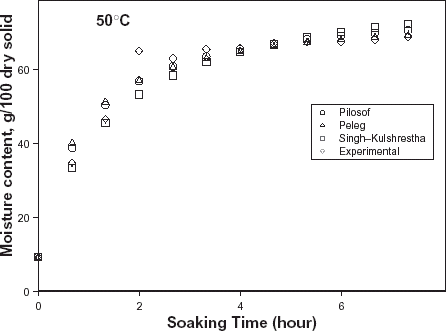
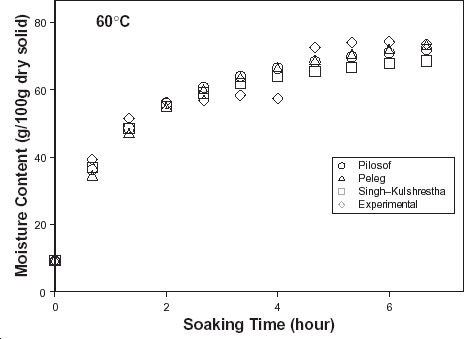
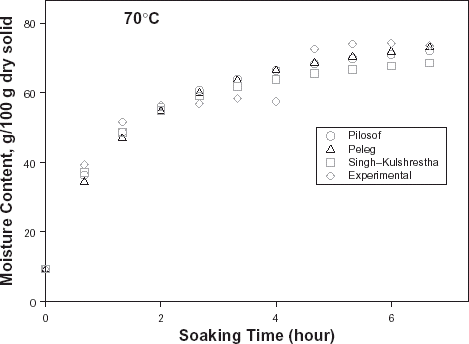
The typical moisture absorption characteristics of the seeds are depicted in Fig. . The water absorption trend followed an exponential growth pattern; there was a very rapid rate of water intake initially which later reduced almost infinitely. The rates also increased with temperature because of increased rate of diffusion of water molecules. The curves are similar to those published for some cereals and leguminous seeds.Citation14 Citation15 Citation16 However, the ABF seeds demonstrated some kind of instability during soaking at the higher temperatures (50, 60, and 70°C) as the seeds’ weight fluctuated while some fissures appeared on the seeds at these temperatures. A similar effect has been reported in rice grains by Lan et al.Citation26 These authors explained this change as an effect of rapid moisture uptake low moisture grain causing a combination of thermal, moisture, and mechanical stress. Some other authorsCitation19 explained that there are usually some structural components of the seeds that resist moisture sorption thereby not contributing to swelling. A reduction in swelling for the same degree of moisture sorbed, could lead to higher internal pressure development which could cause cell wall rupture and could partly explain why ABF seeds were less stable at higher soaking temperatures.
Figure 1. Water absorption characteristics curves for T. africana seeds at various soaking temperatures.
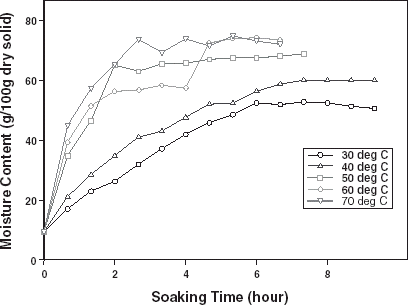
The contribution of chemical composition to water absorption process in biological material is very significant.Citation26 Proteins and carbohydrates are the chief components that absorb water in foods and proteins in particular have the ability to absorb more water than carbohydrates.Citation27 Citation28 Therefore, at the same temperature, grains that have higher protein contents would, therefore, be expected to have higher water absorption capacity and probably faster absorption rate. Looking at the water absorption curve at 50°C for ABF seeds and that presented by Sopade et al.Citation15 for some maize, millet, and sorghum (about 9–11% protein), the former quickly reached the maximum absorption capacity (about 70% dry basis) and the curvilinear section of the curve also ended within 2 h of soaking, compared to about 60 to 100% moisture content (dry basis) which ended the curvilinear section of the curves between 6 to12 h. To further corroborate this observation, the work of Sopade and ObekpaCitation14 showed that Soybean, Cowpea, and Peanut (about 24–42% protein) reached maximum absorption capacity of about 90 to 170% (dry basis), while the curvilinear period lapsed for a maximum of about 4 h of soaking even at a lower temperature (40°C). It should be noted that the curvilinear section of such curves represents a very short period of active water uptake and is usually the rate-determining period.
Water Absorption Rate
In order to have reliable kinetic parameter estimation, data generation was terminated at instances when disintegration of the seeds was conspicuous. Note the termination of the water absorption curves at various times of soaking (Fig. ). The regression coefficients of each model are also indicated in Table .
Table 2 Original and linear forms of the water absorption models
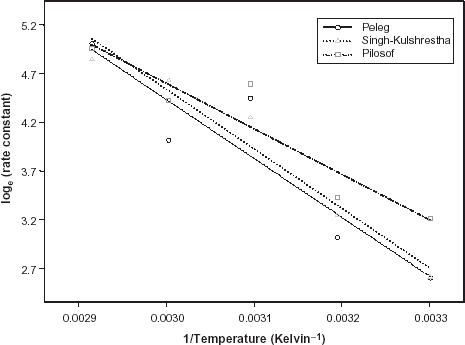
The equivalent of the absorption rate constant in the Peleg's equation is the reciprocal of A while B is the absorption rate constant in Singh–Kulshrestha's equation. Thus, the values of 1/A and B varied between 13.5 and 149.5 g/100 g dry solid per hour and 0.136–1.268 g/g dry solid per hour, respectively. The values of the absorption rate constant calculated using Eq. (Equation6) for Pilosof's model ranged between 0.288 and 1.423 g/g dry solid per hour. As the soaking temperature increased, both values increased because higher temperature caused increased rate of diffusion of water molecules. Using paired sample t-test, there were no significant difference between the rate constants values (p > 0.05). This implies that there is good agreement between the rate constant values derived from the three water absorption models.
Water Absorption Capacity
The knowledge of the water absorption capacity and the corresponding time to achieve it is practically vital for process calculation. For example, it has been shown that allowing grains to approach moisture saturation is advantageous in reducing mechanical stress required for wet milling.Citation29 Table shows the result of predicted water absorption capacity (M e ) and the corresponding soaking duration to achieve the maximum absorption (t e ) at different soaking temperature. The predicted values of M e and t e using Peleg and Singh–Kulshrestha's models were the same at all temperatures. However, the estimated times from Pilosof's model were smaller at each temperature. The usual duration of exposure of biological material to moisture in experiments carried out at ambient conditions is usually in the order of 24–48 hCitation13 due to their instability at longer exposure. For some food materials especially grains that exhibit hard to soak properties due to some physical and compositional factors, longer hours may be required to achieve required moisture content. Similar observations were made with soaked sorghum, millet and maize,Citation29 in which a minimum and maximum of about 54 and 102 h of soaking time were observed for the grains. In the present study, the longest experimental duration for a run was about 11 h after which the experimental run was terminated to avoid collecting data that may not truly represent water absorption process as it was observed that at such instances, the soak water had started turning conspicuously turbid indicating active leaching of soluble. However, the predicted moisture saturation times for ABF seeds are far longer than what could be practically realized. This means that all the models have the capability of predicting long-range moisture absorption from experimental data generated within a relatively shorter duration. The idea of the time required to achieve maximum absorption capacity obtained from this work is at least useful to show that achieving true equilibrium moisture content during soaking may be practically impossible. The water absorption capacity of ABF seeds is similar to those of sorghum and groundnut and lower than those of soybean, pigeon pea, and cowpea as mentioned earlier (Table ).
Table 3 Regression coefficients in the water absorption models
Temperature Dependence
The influence of temperature on the water absorption process in food grains has been viewed from two perspectives. Firstly, it informs how faster or slower the progress of water uptake becomes per degree rise in soak water temperature. Secondly, it has also been mentioned as an additional indicator of the precision required to control temperature during soaking.Citation15 The influence of temperature on the water absorption rate is often examined using the linear form of Arrhenius relation:
Table 4 Temperature dependence of the absorption rate constants
Table 5 Published water absorption parameters for some cereals and legumes
Goodness of Fit
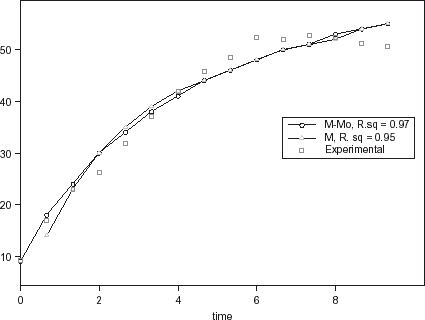
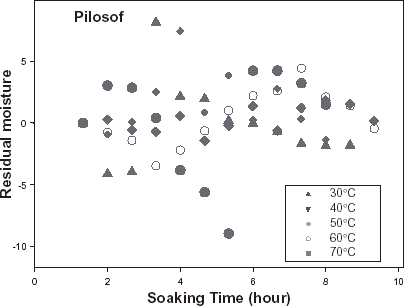
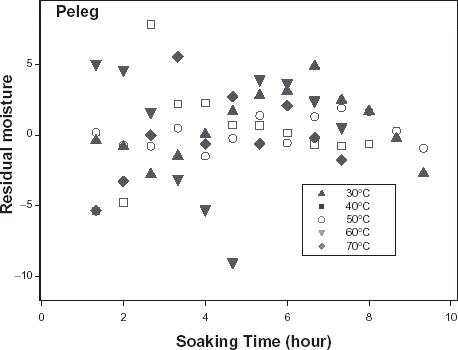
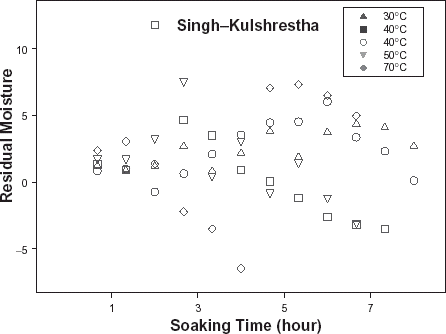
The relative goodness of fit of the modified form of Pilosof's equation (Eq. (5)) was compared to the original form (Eq. (Equation1)). Figure depicts the fitness of the experimental data at 30°C to both forms of the equation. The effect of the initial moisture content is only conspicuous at the early period of the experiment. The modified form of the equation was similarly found to better describe the experimental data at all other temperature (results not shown here). This implies that for any material having moisture content above 5%, the effect of the initial moisture content would be significant at some stage of absorption process when Pilosof's equation is being applied. Thus, the modified Pilosof's equation was considered and further compared with the two other equations to determine their relative predictive power. The agreement between the predicted and the experimental data is summarized in Table and Figs. , , , , . For the entire model, the calculated chi-square values were far less than the critical values. This implies that the predicted moisture contents by the models are not significantly different (p > 0.05) from the experimental values and it further demonstrates the reliability on any of these models to predict moisture absorption at all soaking temperature for ABF seeds. To determine which model was better in describing the experimental data, the values of the RMSD were calculated (Table ) along with the plots of residual moisture contents against soaking time (Figs. , , ). Generally, all the models gave better fit to the data at 40 and 70°C while fitness were poorer at 30 and 60°C. The values of chi-square and RMSD of the Pilosof and Singh–Kulshrestha's model are closer for most of the soaking temperatures. Pilosof's model gave the least maximum difference between the experimental and the predicted moisture data and the least maximum RMSD value. However, Singh–Kulshrestha's model gave a more random residual plot than the two other models. Since lower values of Chi-square and RMSD and increased randomness of the residual plot indicate better fit, Singh–Kulshrestha's and the modified form of Pilosof's model give better fit to the water absorption data in ABF seeds.
Table 6 Estimates of equilibrium moisture (M e ) and time to reach equilibrium (t e )
Table 7 Chi-square and root mean square deviation (RMSD) values
Conclusions
The water absorption profile of ABF seeds at five different soaking temperatures studied followed an exponential growth pattern. ABF seeds were less stable at higher soaking temperatures (50–70°C). ABF seeds have slightly higher water absorption capacity compared to some cereals (maize and millet), chickpea and fieldpea, and lower absorption capacity than legumes (soybean and cowpea) as reported elsewhere. The water absorption rate constant from Singh–Kulshrestha's equation was more sensitive to temperature change. Moreover, the activation energy of hydration, E a , obtained for ABF is within the range obtained for other food grains. Temperature did not have significant influence on the water absorption capacity of ABF seeds. All the models gave good description of the moisture absorption data at all temperature and demonstrated the ability to predict long-range moisture absorption data for experiments that have practically short-term duration. Conclusively, Singh–Kulshrestha's and the modified Pilosof's models gave an overall better description of the moisture absorption data.
References
- Popeno , W. 1985 . Manual on the Tropical and Sub-Tropical Fruits, , 4th Ed. 407 – 415 . Singapore : Longman Publisher Ltd. .
- Edet , E.E. , Eka , O.U. and Ifon , E.T. 1984 . Chemical evaluation of the nutritive value of seeds of African breadfruit (Treculia africana) . Food Chemistry , 17 : 41 – 47 .
- Ekpeyong , I.E. 1985 . Chemical composition and amino acid contents of African breadfruit (Treculia africana) seeds . Food Chemistry , 17 : 59 – 64 . [CROSSREF]
- Osagie , A.U. and Odutuga , A.A. 1986 . Chemical characterization and edibility of the oils extracted from four Nigerian oil seeds . Nigerian Journal of Pure and Applied Sciences , 1 : 35 – 42 .
- Giami , S.Y. , Adindu , M.N. , Hart , A.D. and Denenu , E.O. 2001 . Effect of heat processing on in vitro digestibility and some chemical properties of African breadfruit (Treculia africana) seeds . Plant Foods for Human Nutrition. , 56 : 117 – 126 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Okechukwu , P.E. , Okaka , J.C. and Chukwu Nenye , I.B. 1980 . Preliminary investigation into the malting characteristic of Treculia africana . Nigerian Food Journal , 2 : 149 – 151 . [CSA]
- Anazonwu-Bello , J.N. Proceeding of Symposium on the Development of Indigenous Technology . Aug 21–23 , Enugu. Indigenous Foods and Nutritional Adequacy , pp. 54 – 59 . Enugu, , Nigeria : Ministry of Science and Technology .
- Ejiofor , M.A.N. and Okafor , J.C. 1996 . Prospect for commercial exploitation of Nigerian indigenous trees, vegetables, fruits and seeds through food and industrial products formulation . International Tree Crop Journal , 9 : 119 – 129 .
- Odoemelam , S.A. 2000 . Functional properties of African breadfruit (Treculia africana) flour as influenced by processing . Global Journal of Pure and Applied Sciences , 6 : 389 – 393 .
- Badifu , G.I.O. and Akubor , P.I. 2001 . Influence of pH and sodium chloride on selected functional and physical properties of African breadfruit (Treculia africana) kernel flour . Plant Foods for Human Nutrition , 56 : 105 – 115 . [PUBMED] [INFOTRIEVE] [CSA] [CROSSREF]
- Ramesh , M.N. 2001 . An application of image analysis for the study of kinetics of hydration of milled rice in hot water . International Journal of Food Properties , 4 ( 2 ) : 271 – 284 . [CROSSREF]
- Maroulis , Z.B. , Saravacos , G.D. , Panagioutou , N.M. and Krokida , M.K. 2001 . Moisture diffusivity data compilation for foodstuffs: effect of material moisture content and temperature . International Journal of Food Properties , 4 ( 2 ) : 225 – 237 . [CROSSREF]
- Peleg , M.A. 1988 . An empirical model for the description of moisture sorption curves . Journal of Food Science , 53 : 1216 – 1219 .
- Sopade , P.A. and Obekpa , J.A. 1990 . Modelling water absorption in soybean, cowpea and peanuts at three temperatures using Peleg's equation . Journal of Food Science , 55 : 1084 – 1087 .
- Sopade , P.A. , Ajisegiri , E.S. and Badau , M.H. 1992 . The use of Peleg's equation to Model water absorption in some cereal grains during soaking . Journal of Food Engineering , 15 : 269 – 283 . [CROSSREF]
- Hung , T.V. , Liv , L.H. , Black , R.G. and Trewhella , M.A. 1993 . Water absorption in chickpea (C. arientum) and field pea (P. sativum) cultivars using Peleg's model . Journal of Food Science , 58 : 848 – 852 .
- Maia , M.C.A. and Cal-Vidal , J. 1994 . Kinetics of water uptake by citrus juices in powder form . International Journal of Food Science and Technology , 29 : 137 – 141 . [CSA]
- Pilosof , A.M.R. , Boquet , R. and Bartholomai , G.B. 1985 . Kinetics of water uptake by food powders . Journal of Food Science , 50 : 278 – 282 .
- Singh , B.P.N. and Kulshrestha , S.P. 1987 . Kinetics of water sorption by Soybean and Pigeonpea grains . Journal of Food Science , 52 : 1538 – 1541, 1544 .
- Nwufor , M.I. and Mba , P.C. 1988 . Study on the post-harvest rots of African breadfruit (Treculia africana) seeds in Nigeria . International Biodeterioration , 24 : 17 – 23 . [CROSSREF]
- Suthar , S.H. and Das , S.K. 1996 . Some physical properties of Karingda [Citrullus lanatus (Thumb) Mansf] seed . Journal of Agricultural Engineering Research , 65 : 15 – 22 . [CROSSREF]
- Hsu , K.H. , Kun , C.J. and Wilson , L.A. 1983 . Factors affecting water uptake of soybeans during soaking . Cereal Chemistry , 60 : 208 – 211 .
- AOAC. 1995 . Official Methods of Analysis Washington, DC : Association of Official Analytical Chemists .
- Mead , R. and Curnow , R.N. 1983 . Statistical Method in Agriculture and Experimental Biology London : Chapman and Hall .
- Engels , C. , Hendrickx , M. and Tobback , P. 1987 . Limited multilayer desorption of brown, parboiled rice . International Journal of Food Science and Technology , 22 : 219 – 223 . [CSA]
- Mayer , A. and Polja , R.M. 1975 . The Germination of Seeds, , 2nd Ed. Oxford : Pergamon Press .
- Moss , R. 1977 . The influence of endosperm structure, protein content and grain moisture on the rate of water penetration into wheat during conditioning . Journal of Food Technology , 12 : 275 – 283 .
- Busk , G.C. 1984 . Polymer-water interactions in gelation . Food Technology , 58 : 59 – 64 .
- Ituen , E.U.U. , Mittal , J.P. and Adeoti , J.S. 1986 . Water absorption in cereal grains and its effect on their rupture stress . Journal of Food Process Engineering , 8 : 147 – 158 .
- Cox , B.G. 1994 . Modern Liquid Phase Kinetics New York : Oxford University Press .
- Lin , S.H. 1994 . Water uptake and gelatinization of white rice . Food Science and Technology , 26 : 276 – 278 .