Abstract
Modulated Differential Scanning Calorimetry (MDSC) was used to investigate thermal transitions of pomegranate extracts as a function of concentration. The transitions were the glass transition temperatures (T
g
) and maximum freeze-concentration condition (), melting points (T
f
), the onset temperatures of ice melting (
), and latent heat of fusion (H
L
). In addition, the specific heat (C
p
) below and above freezing was also determined. The soluble solid contents for the pomegranate extracts ranged from 20 to 53°Brix. The T
g
and
values ranged from −43.3 to −49.3 and from −44.9 to −52.0°C, respectively. On the other hand, T
f
and
ranged from −2.6 to −16.7 and from −39.6 to −45.3°C, respectively. The latent heat of fusion was between 61.6 and 204.7 kJ/kg. Below freezing, a linear relationship was established between specific heat and temperature, while above freezing range the change of specific heat was relatively low.
Introduction
The pomegranate is a popular exotic fruit whose origins are from the Middle East and Asia. Pomegranates typically grow well in the regions where the temperature is mild and low humidity similar to that in the Western part of Saudi Arabia particularly the Taif city and its neighboring villages. The edible part of the pomegranate is mainly consumed fresh, but is also used in the preparation of fresh juice, which is a very popular beverage. The separated edible part of the fruit contains significant quantities of micronutrients essential for health.Citation1 These include considerable amounts of sugars, vitamins, polysaccharides, polyphenols, and minerals.Citation2 In Saudi Arabia, the juice sacs may be frozen intact or the extracted juice may be concentrated and frozen, for further use. Pomegranate juice is widely made into grenadine for use in mixed drinks. The juice is regarded as a delicacy and is made into excellent sherbet and it is consumed with the addition of water and sugar. It is also used in preparing syrups, jellies, marmalades, and pomegranate molasses. Pomegranate molasses (called dibs rumman in Arabic) is heavy pomegranate syrup used in some parts of the world in cooking and also to marinate meat; due to its content of proteolytic enzymes, which acts as a meat tenderizer.
Carbohydrate solutions such as fruit juices and extracts such as pomegranate extracts contain dissolved solutes of a nature such that they are kinetically inhibited from solidifying at the eutectic point. Previous studies had shown that solutions of these types have shown problems such as voids, collapse, puffing, foaming, high hygroscopicity, and early caking during storage.Citation3 Citation4 Citation5 Citation6 Citation7 Citation8 During the freezing process of liquid foods, water usually crystallizes as almost pure ice while the solution becomes more concentrated. In a previous study, it was reported that when pomegranate extracts were frozen, the concentration of the extracts result in the formation of concentrated amorphous solutions, which may result from the rapid removal of water from the extracts occurring during the freezing process.Citation5 In that study, the high sugar content of extracts above 17°Brix was primarily responsible for the difficulties of freeze-drying.
Differential Scanning Calorimeter (DSC) and Modulated Differential Scanning Calorimetric (MDSC) analyses were previously performed on sugar solutions and other materials,Citation9 Citation10 Citation11 Citation12 Citation13 Citation14 Citation15 Citation16 and no references has been found for pomegranate juice and extracts. Knowledge of the thermal behavior of pomegranate extracts at low temperatures is essential for process design evaluation, quality control, and for identifying consumers' acceptability. The purpose of the present study was to investigate the thermal transitions of pomegranate extracts as a function of concentration with the aid of a modulated differential scanning calorimeter.
Materials and Methods
Extract Preparation and Water Activity Measurement
Fully ripe, reddish yellow pomegranate fruits (Punica granatum L.) were cut into halves with a sharp knife and manually pressed to extract juice using a stainless steel hand operated juice extractor. The extraction process was followed by a two stage filtration where one layer cheese cloth was used for prefiltration and a three layers set up was used for final filtration of juice. The extracted juice was vacuum concentrated using a pilot-scale falling film evaporator at temperature of 60–65°C and vacuum of 560 mm Hg.Citation17 Intermediate juice concentrates of pomegranate extracts were prepared by diluting the resulting concentrates with predetermined amounts of distilled water. These concentrations were 20, 30, 40, and 53°Brix. All samples were kept refrigerated at 4°C prior to test experiments. Water activity values of extracts, a w , were measured using an AquaLab water activity meter of accuracy ±0.003 (Model CX-2, Decagon Devices Inc., Pullman, WA) with chilled-mirror dew point sensor. Water activity measuring equipment was calibrated with distilled waterCitation18 and the standard solution 0.5 molal KCl (Decagon Devices Inc., Pullman, WA).
Modulated Differential Scanning Calorimetry
The modulated DSC analyses were conducted (DSC Q100, TA Instruments, Ltd., Leatherhead, England) over a temperature range from −60 to 65°C. The linear heating rate was 5°C/min, and the heating-only modulation condition used was a period of 100 s with an amplitude of 1°C. This set of conditions was designed to increase sensitivity for detecting weak transitions. Baseline, temperature, heat flow, and heat capacity calibrations were carried out on the instrument using indium and sapphire.Citation19 Triplicate pomegranate extract samples of 10–20 mg were hermetically sealed (Sample Encapsulating Press, TA Instruments) in preweighed 40 µL aluminum pans. The annealing of different pomegranate samples was performed according to the method reported by Roos.Citation20 The only deviation of this study was that the low end of the scanning scale was −70°C and the rate of cooling and heating was 5°C/min. Annealing was considered essential in order to attain maximum freeze-concentration and to increase the chances of detecting accurate values. All analyses were achieved with the use of dry nitrogen gas (AGA, 99.9% N2) at 50–60 mL/min to eliminate water condensation in the measuring cell.
Results and Discussion
Modulated Differential Scanning Calorimetry
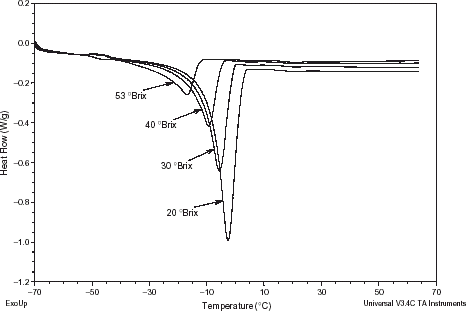
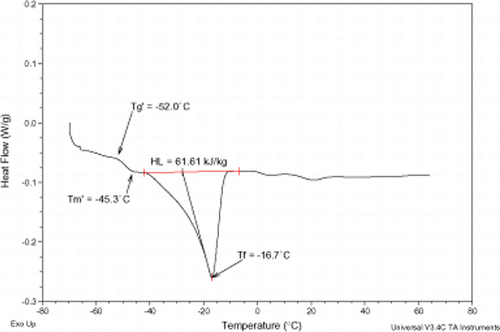
The thermograms of pomegranate extracts are shown in Fig. . Different pomegranate extracts exhibited different broad endothermic peaks of first-order transition between −14 to 4, −20 to 1, −24 to −4, and −37 to −11°C for the 20, 30, 40, and 53°Brix, respectively. Peak temperatures, referred to as melting points (fusion temperatures) (T f ) in Table , shifted toward higher temperatures as extract concentration decreased. The MDSC thermograms of pomegranate extracts were within the range of food materials, and were found to be comparable with those in published literature.Citation20 Citation21
Table 1 Glass transition (T g ) and melting point (T f ) temperatures of various pomegranate extracts at different water activities
The thermograms were analyzed for the glass transition temperatures (T g ) using the TA Instruments Universal Analysis 2000 software. The T g midpoint values for the different pomegranate extracts studied and their corresponding water activities (a w ) are given in Table . The values of T g are the midpoint of the glass transition temperature range. Three scans were made to quantify all measured properties at any given concentration and temperature. The T g values generally decreased with increasing extract concentration. The T g values obtained in the present study were in good agreement with those in the literature.Citation22 Citation23 Citation24 The glass transition temperature of the different extracts was found to be a function of water activity at a constant temperature. It should also be noted that for the extracts with water activities equal/higher than 0.946 the glass transition phenomena is not clear compared with extracts of water activity less than the mentioned values.
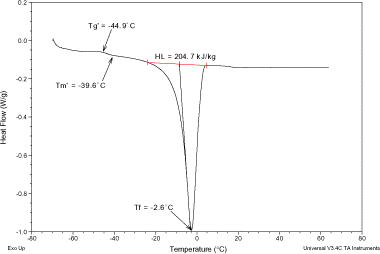
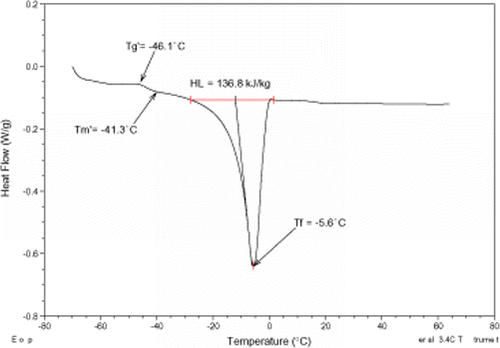
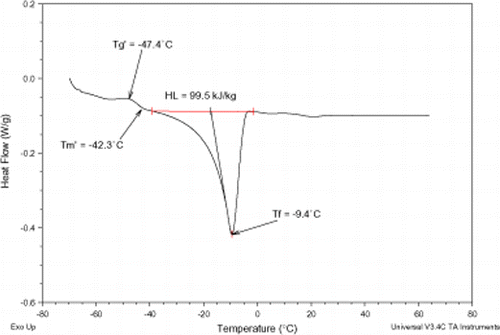
Figures , , , present MDSC plots for the glass transition and the onset of ice melting temperatures in the maximally freeze-concentrated state of different pomegranate extracts, and
, respectively. The maximum freeze-concentration of carbohydrate solutions is often achieved at a temperature above
but below
. The values of
and
of various pomegranate samples were determined (Table ) and the results demonstrated the importance of annealing for these extracts. The results reported here revealed that the
and
values were influenced by water. This effect is of particular importance when studying the physical state of amorphous carbohydrate solutions in relation to the stability of frozen materials.Citation25
Citation26 On the whole, the
and
values seemed similar to the glass transition melting temperatures reported for low molecular weight carbohydrates.Citation20 The latent heat of fusion (H
L
) values cover a temperature range from −60 to 65°C (Figs. , , , ) and ranged from 61.6 to 204.7 kJ/kg, Table . The H
L
values were generally in agreement with those reported by RoosCitation10 for strawberries concentrates.
Table 2
Glass transition temperatures ( ) and the onset temperatures of ice melting (
) and the onset temperatures of ice melting ( ) in the maximally freeze-concentrated state of different pomegranate extracts, and latent heat of fusion (H
L
)
) in the maximally freeze-concentrated state of different pomegranate extracts, and latent heat of fusion (H
L
)
Specific Heat Below and Above Freezing
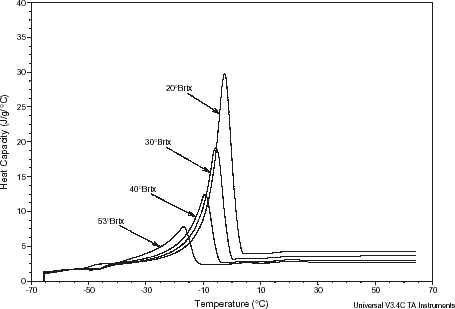
Figure shows MDSC specific heat curves for various pomegranate extracts. The peaks in the curves were due to the phase change during the melting process. MDSC scans illustrate typical changes in specific heat below and above melting due to changes in water status. Linear regression analyses were performed on the results in order to correlate the specific heat values with temperature below freezing. A summary of specific heat data and the correlation coefficients is given in Table . The results indicated that the specific heat increased with increasing temperature, but more prominently at higher temperatures. In other words, the obtained results suggest the occurrence of straight-lined dependencies of the specific heat on temperature for all extracts below freezing. Also, for any particular temperature interval studied, the specific heat values increased with concentration. Keppeler and ArboledaCitation27 reported similar trend for sucrose solutions. The high rate of increase could be attributed to the increased melting of the solutions, which required more heat (heat of fusion) for small increase of temperature.
Table 3 Linear regression correlation coefficients of specific heat values vs. temperature below freezing of different pomegranate extracts
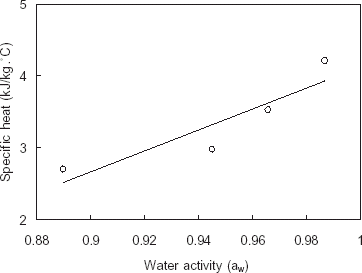
The specific heat (C p ) of pomegranate extracts as a function of water activity (a w ) is shown in Fig. . The correlation coefficient (R 2) was 0.84, and the regression equation was as follows:
Table 4 Specific heat values (C p ) above freezing of pomegranate extracts and their corresponding standard deviations (%)
Conclusions
In the present study, it was shown that thermal transitions of different concentrates of pomegranate, a natural carbohydrate material, was strongly temperature and moisture content dependent. Different concentrations had different glass transitions, which indicate the amorphous state of the pomegranate solution at lower freezing temperatures. The presented values may be used to evaluate the behavior of the material at different thermal processing stages. The MDSC method is simple and rapid technique for the determination of the thermal transitions of pomegranate extracts.
References
- Saxena , A.K. , Mana , J.K. and Berry , S.K. 1987 . Pomegranates: postharvest technology, chemistry and processing . Indian Food Packer , 41 ( 4 ) : 43 – 60 .
- Melgarejo , P. , Salazar , D.M. and Artés , F. 2000 . Organic acids and sugars composition of harvested pomegranate fruits . European Food Research and Technology , 211 ( 3 ) : 185 – 190 . [CROSSREF]
- Bellows , R.J. and King , C.J. 1973 . Product collapse during freeze drying of liquid foods . AIChE Symposium Series , 69 ( 132 ) : 33 – 41 .
- Flink , J.M. 1975 . “ Application of freeze-drying for preparation of dehydrated powders from liquid food extracts ” . In Freeze-drying and Advanced Food Technology Edited by: Goldblith , S.A. , Ray , L. and Rothmayer , W.W. London : Academic Press .
- Hobani , A.I. , Hassan , B.H. and Al-Khalifah , A.S. 1996 . Pilot scale freeze-drying of pomegranate juices and roselle extracts . Misr. Journal of Agricultural Engineering , 13 ( 2 ) : 363 – 375 .
- Maltini , E. 1975 . “ Thermal phenomena and structural behavior of fruit juices in the pre-freezing stage of the freeze-drying process ” . In Freeze-drying and Advanced Food Technology Edited by: Goldblith , S.A. , Ray , L. and Rothmayer , W.W. London : Academic Press .
- Mackenzie , A.P. 1975 . “ Collapse during freeze-drying: quantitative and qualitative aspects ” . In Freeze-drying and Advanced Food Technology Edited by: Goldblith , S.A. , Ray , L. and Rothmayer , W.W. London : Academic Press .
- Tsourouflis , S. , Flink , J.M. and Karel , M. 1976 . Loss of structure in freeze-dried carbohydrates solutions: effect of temperature, moisture content and composition . J. Sci. Fd. Agric. , 27 : 509 – 519 .
- Ferrero , C. and Zaritzky , N.E. 2000 . Effect of freezing rate and frozen storage on starch–sucrose–hydrocolloid systems . J. Sci. Fd. Agric. , 80 ( 14 ) : 2149 – 2158 . [CROSSREF]
- Roos , Y. 1987 . Effect of moisture on the thermal behavior of strawberries studied using differential scanning calorimetry . J. Food Sci. , 52 ( 1 ) : 146 – 149 .
- Roos , Y. and Karel , M.K. 1991 . Amorphous state and delayed ice formation in sucrose solutions . Int. J. Food Sci. Technol. , 26 ( 6 ) : 553 – 566 . [CSA]
- Ross , K.A. , Campanella , O.H. and Okos , M.R. 2002 . The effect of porosity on glass transition measurement . Int. J. Food Prop. , 5 ( 3 ) : 611 – 628 . [CROSSREF]
- Laaksonen , T.J. and Ross Yrjö , H. 2001 . Thermal and dynamic-mechanical properties of frozen wheat doughs with added sucrose, NaCl, ascorbic acid, and their mixtures . Int. J. Food Prop , 4 ( 2 ) : 201 – 213 . [CROSSREF]
- Lai , V.M.F. and Lii , C.Y. 1999 . Effects of modulated differential scanning calorimetry (MDSC) variables on thermodynamic and kinetic characteristics during gelatinization of waxy rice starch . Cereal Chem. , 76 ( 4 ) : 519 – 525 .
- Cuq , B. and Icard-Vernière , C. 2001 . Characterisation of glass transition of durum wheat semolina using modulated differential scanning calorimetry . J. Cereal Sci. , 33 ( 2 ) : 213 – 221 . [CROSSREF]
- Baik , O.D. and Mittal , G.S. 2003 . Determination and modeling of thermal properties of tofu . Int. J. Food Prop. , 6 ( 1 ) : 9 – 24 . [CROSSREF]
- Hobani , A.I. 1999 . Rheology of pomegranate juice extracts and concentrates . Alex. J. Agric. Res. , 44 ( 3 ) : 87 – 99 .
- Mohsenin , N.N. 1986 . Physical Properties of Plant and Animal Materials: Structure, Physical Characteristics, and Mechanical Properties, , 2nd Updated and Revised Ed. New York, USA : Gordon and Breach Science Publishers .
- TA Instruments. Modulated DSC™ Compendium, Basic Theory and Experimental Considerations New Castle, DE, , USA : TA-210 TA Instruments, Inc. .
- Roos , Y. 1993 . Melting and glass transitions of low molecular weight carbohydrates . Carbohydr. Res. , 238 : 39 – 48 . [CROSSREF]
- Roos , Y. 1986 . Phase transitions and unfreezable water content of carrots, reindeer meat and white bread studied using differential scanning calorimetry . J. Food Sci. , 51 ( 3 ) : 684 – 686 .
- Krszard , R. , Eugeniusz , K. and Serowik , M. 2001 . Characterization of garlic freezing drying with the use of differential scanning calorimetry . Elect. J. Pol. Agric. Univ. , 4 ( 2 ) : 1 – 6 .
- Maltini , E. 1977 . Studies on the physical changes in frozen acqueous solutions by DSC [differential scanning calorimetry] and microscopic observations . Bull. Inst. Int. Froid. , 1 : 43 – 52 .
- Simatos , D. , Faure , M. , Bonjour , E. and Couach , M. 1975 . The physical state of water at low temperatures in plasma with different water contents as studied by the differential thermal analysis and differential scanning calorimetery . Cryobiology , 12 : 202 – 211 . [PUBMED] [INFOTRIEVE] [CROSSREF]
- Slade , L. and Levine , H. 1991 . Beyond water activity: recent advances on an alternative approach to the assessment of food quality and safety . Crit. Rev. Food Sci. Nutr. , 30 ( 2–3 ) : 115 – 360 . [PUBMED] [INFOTRIEVE]
- Roos , Y. and Karel , M. 1991 . Applying state diagrams to food processing and development . Food Technol. , 45 ( 12 ) : 66, 68 – 71, 107 .
- Keppeler , R.A. and Arboleda , J.R. 1982 . The thermal properties of frozen invert sugar solutions used in food products . J. Food Proc. Eng. , 5 ( 2 ) : 89 – 111 .
- Kapseu , C. and Kayem , G.J. 1994 . Analysis of cottonseed oil and its fractions by differential scanning calorimetery . J. Food Eng. , 21 ( 2 ) : 225 – 234 . [CROSSREF]
- Swern , D. 1979 . Bailey's Industrial Oil and Fat Products, , 4th Ed. Vol. 1 , New York, , USA : Wiley .