Abstract
Atopic dermatitis (AD) is among the most common inflammatory skin diseases in children and adults in industrialized countries. Up to one-third of adults (probably a smaller proportion in childhood) suffer from moderate-to-severe AD, whose recommended treatment is usually based on systemic therapies. The currently available therapeutics are limited, and AD management becomes challenging in most cases. Over the last few years, new advances in the understanding of AD pathogenic mechanisms and inflammatory pathways have led to the identification of specific therapeutic targets and new molecules have been tested. Dupilumab is a fully human monoclonal antibody directed against the IL-4 receptor α subunit that is able to block the signaling of both IL-4 and IL-13 and achieve rapid and significant improvements in adults with moderate-to-severe AD. Dupilumab is ready to inaugurate a long and promising biological target treatment option for Th2 cell-mediated atopic immune response that characterizes AD.
Introduction
Atopic dermatitis (AD) is one of the most common inflammatory skin diseases affecting up to 25% of children in industrialized countries.Citation1 One-third of cases persist into adulthood, comprising a prevalence of 2%–10%.Citation2–Citation4 AD is not just a skin disease as it may represent the first manifestation of the so-called atopic march, a spectrum of interconnected disorders, including rhinitis, conjunctivitis, and asthma, that may follow skin symptoms later in life.Citation2–Citation6
Because the prevalence of AD is lower in rural and nonindustrialized countries,Citation4 the hygiene hypothesis, in which the lack of exposure to antigens in early life would induce immune imbalance, favoring a proinflammatory Th2 response that drives the immune dysregulation in AD, has been proposed.Citation4,Citation7,Citation8
Immunopathogenesis of AD
AD pathogenesis represents a complex mechanism, including a defective epidermal barrier, caused by an altered expression of keratinocyte differentiation genes (eg, cornified cell envelope-related genes) and an abnormal content of extracellular lipids, resulting in increased transepidermal water loss and permeation to allergens, irritants, and microbes.Citation9–Citation11
Beside this intrinsic impairment of the keratinocyte differentiation process, AD lesional skin shows a marked infiltration of T cells, both CD4+ and CD8+ T cells, dendritic cells (DCs), Langerhans cells, and other immune cells, including eosinophils, mast cells, and IgE-producing plasma cells.Citation9 Classically, AD is considered as a Th2-dominant disease, as an enhanced signal of the Th2 pathway is detected in AD lesional skin and, to a lesser strength, in non-lesional skin.Citation5,Citation12,Citation13 The increased expression of Th2-derived cytokines, namely, IL-4, IL-5, IL-13, and IL-31, is correlated with high levels of Th2 chemoattractants and activating factors such as CCL-17, CCL-18, CCL-22, CCL-26, CCL-27, and TSLP.Citation14,Citation15
IL-4 and IL-13
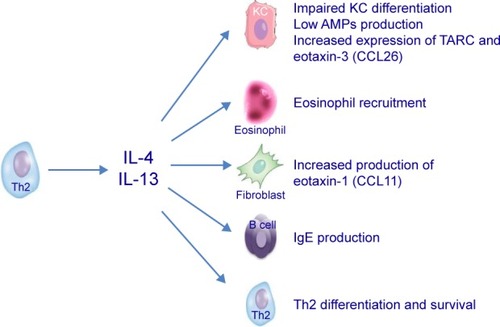
The centrality of the Th2 cytokines is due to their capability of 1) inducing IgE class switching; 2) promoting Th2 survival; 3) recruitment of eosinophils; 4) mediating pruritus; and 5) inhibiting keratinocyte terminal differentiation and AMP production (). Particularly, IL-4 and IL-13 are considered as the Th2-signature cytokines and master mediators in AD pathogenesis as they act on various cells involved in AD (ie, keratinocytes, T cells, DCs, and eosinophils), signaling through the same receptor, the IL-4Rα receptor. Although the immune response is polarized toward a Th2 response, other T-cell subsets participate in AD pathogenesis, including T22 cells and both CD4+ and CD8+ T cells producing IL-22-, IL-17-, and IFNγ-secreting cells.Citation16–Citation19 Based on the dominant pathways driving AD inflammation, intrinsic AD may be distinguished from the extrinsic form. Indeed, the extrinsic form (~80% of AD cases) shows high IgE serum levels associated with a Th2-skewed immune polarization and a less pronounced T22 signal, whereas the intrinsic form (the remaining 20%) is characterized by low IgE titers and a Th2 response, with a marked upregulation of the Th17 and Th22 axes.Citation20 In this scenario, key mediators, such as IL-4, IL-13, and IL-22, have been identified as therapeutic targets for the development of new agents that selectively inhibit their signaling. One of the promising agents that is currently being developed for the treatment of AD is dupilumab, an IL-4Rα antagonist.
Clinical phenotypes and endophenotypes of AD toward personalized treatment
AD is characterized by a wide range of heterogeneity either in the onset (ie, infant–adolescent–adult), course, and presentation (different manifestation of eczema among either the age, the clinical features, or the area involved) or in the comorbidities (eg, the presence of atopy and normal IgE distinguishes an intrinsic AD from an extrinsic or IgE-associated AD).Citation21,Citation22 All these variants are due to the complex interactions between individual genetic and environmental factors involved in AD that lead to epidermal barrier dysfunction, innate and adaptive abnormalities of the immune system (an initial Th2 phase followed by a chronic Th1 phase), and cutaneous microbiome dysbiosis. Despite all these AD variants, the diagnosis is clinical and no diagnostic biomarkers dissecting AD from other inflammatory disorders have been identified yet.Citation23–Citation27 Since AD involves more than one subtype, the discovery, validation, and use of objective markers will achieve a more personalized clinical and treatment approach.Citation28
Nowadays, treatment choice is based on disease severity and symptoms, namely, pruritus and the “trouble sleeping”. The commonly used assessment tools, both in trial setting and in daily practice, include the Eczema Area and Severity Index (EASI), a composite score assessing objective signs, and the SCORing Atopic Dermatitis (SCORAD), evaluating both signs and symptoms. Management and treatment in the short (flares) and long periods may become challenging because of the individual symptomatic variability and the limited array of therapeutics currently available.Citation29–Citation31
Current treatment for AD
AD treatment includes “basic therapy” focused on emollients and moisturizing compounds, on the avoidance of specific or unspecific triggering factors, and on educational programs/psychological counseling.Citation30–Citation32
First-line pharmacological treatment is based on the use of topical medications: topical corticosteroids and topical calcineurin inhibitors, namely, tacrolimus and pimecrolimus. Topical agents represent the mainstay of therapy in patients with mild-to-moderate disease and are used either for the management of exacerbation or, more recently, for proactive therapy.Citation29–Citation31,Citation33 The long-term intermittent (twice weekly) anti-inflammatory therapy aims to prevent relapses and to keep the skin free of eczema. This strategy has been proven successful in improving the clinical management and is, notably, associated with pharmacoeconomic benefits.Citation29–Citation31
Phototherapy (ultraviolet A1 [UVA1] wavelength or ultraviolet B [UVB] 311 nm) can be adjuncted in controlling challenging cases not responsive to topical treatments, such as second-line treatment, with good results.Citation29–Citation31
Up to one-third of adults (probably a smaller proportion in childhood) suffer from moderate-to-severe AD, whose recommended treatment is usually based on systemic therapies. The treatment choice depends on AD severity, patient’s features, physician’s experience, and drug availability.Citation5 Although the majority of evidence exists in adult populations, four systemic immunosuppressive drugs have also demonstrated to be efficacious in adults and children: cyclosporine, mycophenolate mofetil, methotrexate, and azathioprine.Citation33,Citation34 Among them, cyclosporine is the only drug approved for the treatment of AD. According to the European Guidelines, other systemic medications are suggested in case of no response to cyclosporine and/or when contraindications to cyclosporine occur.Citation29–Citation31,Citation33 No biologic drugs targeting AD-signature mediators have been approved yet. Nevertheless, off-label use, sometimes successful, of biologics approved for other indications, such as omalizumab, ustekinumab, and rituximab, has been reported.Citation35
Thus, the current therapeutic paradigm in AD is limited and its management becomes challenging in most cases. Furthermore, some side effects of these medications, such as nephrotoxicity and risk of hypertension during cyclosporine treatment, limit their use.
Dupilumab: developing the new era in the treatment of AD
Over the last few years, new advances in the understanding of AD pathogenic mechanisms and inflammatory pathways have led to the identification of specific therapeutic targets, similar to what occurred in the past decade in psoriasis.Citation28,Citation36 Th2 cells and their key cytokines, such as IL-4, IL-13, IL-5, TSLP, and IgE, constitute emerging targets for new compounds that have been or are currently being tested in clinical trials.Citation28,Citation37 In the last few years, some biologics, such as omalizumab (anti-IgE) and mepolizumab (anti-IL-5), have been previously tested in patients with AD, although no significant benefits were described.Citation33,Citation38–Citation40 On the contrary, the blockade of IL-4/IL-13-mediated signaling through the receptor antagonism was proven to be a successful therapeutic strategy in AD. Dupilumab is a fully human monoclonal antibody directed against the IL-4 receptor α subunit, which is a component of Type I and Type II IL-4 receptors and the IL-13 receptor system. Because of this binding, dupilumab is able to effectively inhibit the IL-4 and IL-13 signaling, with pivotal effect in Th2 inflammatory atopic response ().Citation41 IL-4 and IL-13 increase the expression of important chemokines such as TARC and eotaxin-3 and attract Th2 cells and eosinophils. They are also important in AD because they contribute directly to barrier dysfunction (acting on keratinocyte differentiation and barrier protein, lipids, and production of antimicrobial peptides).Citation42
Mechanistic study
To define dupilumab’s spectrum of action on AD skin, a comprehensive gene expression analysis was performed, comparing pretreatment with posttreatment lesional skin and non-lesional skin. Biopsy specimens were obtained from 18 adult patients with moderate-to-severe chronic AD participating in two Phase I multicenter, randomized, double-blind, placebo-controlled trials testing weekly subcutaneous injections of dupilumab 150 or 300 mg or placebo for 4 weeks.Citation42 Disease severity, assessed by EASI, was significantly improved in adults treated with dupilumab compared to the placebo group as EASI-50, representing at least 50% reduction in the EASI score relative to baseline, was achieved by all but one patient in the substudy treated with 300 mg of dupilumab versus none in the placebo group.Citation42
Clinical improvement was associated with significant dose-dependent changes from baseline in the defined AD transcriptome (meant as the array of differentially expressed genes between lesional and non-lesional AD skin) detected in lesional skin by using microarrays in the dupilumab group compared with the placebo arm of the study.Citation13 The altered expression detected in pretreatment lesional skin improved after dupilumab treatment, with 821 genes whose expression was modulated by 300 mg dupilumab versus 275 placebo-modulated probes.Citation42 In particular, dupilumab downregulated a large set of genes codifying for inflammatory mediators and epidermal proliferation markers, while it upregulated genes involved in the structural, lipid metabolism, and barrier-related functions. Particularly, dupilumab was able to suppress key pathogenic circuits mediated by Th2-derived products, as demonstrated by the reduction in IL-4 and IL-13 levels at skin lesions observed in dupilumab-responder patients.Citation42 As confirmed by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR), dupilumab was able to suppress the expression of proinflammatory genes related not only to a Th2 and eosinophil response (eg, CCL13 and CCL26) but also to the IL-17/IL-22 signaling and Th1 pathway.Citation42 Overall, these mechanistic changes induced by dupilumab, including the suppression of immune cell activation (ie, T cells, DCs, and eosinophils) and the amelioration of keratinocyte proliferation and differentiation, were correlated with clinical improvement of AD, and these effects were dose dependent in dupilumab-treated patients.
Phase I and II studies
The first controlled studies (Phase I and II) testing dupilumab for the treatment of AD were performed in 2014.Citation35,Citation43 In adults with moderate-to-severe AD, dupilumab, administered as 4- to 12-week monotherapy or in association with topical corticosteroids, achieved rapid and significant improvements.
Reduction in disease severity was observed, starting from the first week, obtaining 74% decrease in EASI after 12 weeks of treatment. Similarly, a significant reduction in pruritus levels (55.7% in the treatment group versus 15% in the placebo group) was noted, with positive repercussion on patients’ quality of life (). Clinical improvements were proportional to the drug dose.Citation43,Citation44 During the dupilumab treatment, early and sustained improvement of patient-reported outcomes (PROs) in sleep, mental health, and health-related quality of life were highlighted.Citation43,Citation45
Table 1 Clinical scores and patient-related outcomes of Phase II and III dupilumab studies
Safety data showed no evidence of drug-related serious adverse events or organ toxicity, with nasopharyngitis and headache among the most common side effects.Citation44
The clinical improvement was correlated with Th2-related biomarker changes at serum and tissue levels and normalization of gene expression in lesional skin, appearing similar to the non-lesional gene expression profile.Citation43 The latter data suggest that the epidermal abnormalities associated with AD might be somehow reduced with dupilumab treatment.Citation43,Citation46
Overall, the vigorous effects of dupilumab in improving clinical and laboratory signs confirm the pivotal pathogenic role of IL-4 and IL-13 signaling in adulthood AD and further support the use of Th2 cytokine antagonists in the treatment of this disease.Citation43,Citation47
Phase III trials
In 2016, two randomized, placebo-controlled, worldwide Phase III trials were conducted, enrolling 671 adult patients suffering from moderate-to-severe AD whose disease was inadequately controlled by topical treatment (patients suffering from AD variants were included, eg, intrinsic, extrinsic, with early onset, or with late onset). In these studies, dupilumab significantly improved the signs and symptoms of AD (improvement in EASI, SCORAD, body surface area [BSA], pruritus [visual analog scale {VAS}], Investigator’s Global Assessment [IGA], Global Individual Sign Score [GISS]) as well as scale value of anxiety, depression, and quality of life, as compared with placebo ().Citation48
The data published are promising, and trials of longer duration are needed to assess the long-term effectiveness and safety of dupilumab.Citation48
New treatment era for AD
The approval for the marketization of dupilumab is approaching, and it may offer a significant therapeutic advancement in AD treatment. Unlike the currently available systemic treatments, dupilumab is going to profoundly change the therapeutic paradigm and the long-term management of AD, inaugurating a novel efficacious targeted therapeutic approach for patients with moderate-to-severe AD. Similar to biologic agents approved for psoriasis, dupilumab seems to offer the opportunity of a long-term control of the disease, being effective and overall safe.Citation33,Citation36,Citation37,Citation40,Citation43,Citation48
Dupilumab recently received US Food and Drug Administration breakthrough therapy designation for AD, with ongoing trials in both adult and pediatric populations.Citation33,Citation40,Citation43,Citation48
Although the results of this study are thrilling, it is still not possible to translate the published results to the children, the most commonly affected population, since only patients aged >18 years were enrolled up to now.Citation33,Citation40,Citation43,Citation48,Citation49 The future use of dupilumab in pediatric patients could potentially provide insights about its impact as early intervention in modifying the course of the disease.
Finally, pharmacoeconomically, we do not know how much this medication will cost; compared to the other biologics used in dermatology, it would be thought that dupilumab will likely cost tens to thousands of euro per year, which is significantly higher than the currently available systemic medications.Citation33 As a consequence, dupilumab will likely be reserved for cases of severe AD unresponsive to traditional modalities.Citation33 Currently, there are no studies that compare dupilumab to other medications, and it is difficult to compare the results from Phase I, II, and III studies involving dupilumab to the results achieved with other drugs. The tolerability of dupilumab seems high, maybe higher than that of systemic medications available; even the long-term side effects are not known.
Conclusion
With no doubts, dupilumab is ready to inaugurate a long and promising biological target treatment option for Th2 cell-mediated atopic immune response that characterizes AD and asthma.Citation33,Citation43,Citation48 Interestingly, mepolizumab (anti-IL-5) and omalizumab (anti-IgE), which have been studied in asthma with the same success as that of dupilumab, did not achieve enough results in AD. This fact may underlie a pivotal role of IL-4 and IL-13 among the Th-2 signal cytokines in AD.
Dupilumab initiates a decade that will be probably characterized by a great number of clinical trials in these areas, focusing not only on IL-4 and IL-13 but also on IL-31, IL-22, and TSLP, providing new hopes and insights for future therapeutic (and maybe prevention) approaches in AD.Citation33,Citation35,Citation50
Disclosure
The authors declare no affiliation or significant financial involvement in any organizations or entity with a direct financial interest in the subject matter or materials discussed in the present manuscript. The authors report no conflicts of interest in this work.
References
- ShawTECurrieGPKoudelkaCWSimpsonELEczema prevalence in the United States: data from the 2003 National Survey of Children’s HealthJ Invest Dermatol20111311 67 7320739951
- OdhiamboJAWilliamsHCClaytonTORobertsonCFAsherMIISAAC Phase Three Study GroupGlobal variations in prevalence of eczema symptoms in children from ISAAC Phase ThreeJ Allergy Clin Immunol20091246 1251 1258.e2320004783
- BosJDBrenninkmeijerEESchramMEMiddelkamp-HupMASpulsPISmittJHAtopic eczema or atopiform dermatitisExp Dermatol2010194 325 33120100192
- BieberTAtopic dermatitisAnn Dermatol2010222 125 13720548901
- MalajianDGuttman-YasskyENew pathogenic and therapeutic paradigms in atopic dermatitisCytokine2015732 311 31825542094
- LeungDYGuttman-YasskyEDeciphering the complexities of atopic dermatitis: shifting paradigms in treatment approachesJ Allergy Clin Immunol20141344 769 77925282559
- RomagnaniSThe increased prevalence of allergy and the hygiene hypothesis: missing immune deviation, reduced immune suppression, or both?Immunology20041123 352 36315196202
- GarnHRenzHEpidemiological and immunological evidence for the hygiene hypothesisImmunobiology20072126 441 45217544829
- GittlerJKKruegerJGGuttman-YasskyEAtopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitisJ Allergy Clin Immunol20131312 300 31322939651
- SeidenariSGiustiGObjective assessment of the skin of children affected by atopic dermatitis: a study of pH, capacitance and TEWL in eczematous and clinically uninvolved skinActa Derm Venereol1995756 429 4338651017
- Guttman-YasskyESuárez-FariñasMChiricozziABroad defects in epidermal cornification in atopic dermatitis identified through genomic analysisJ Allergy Clin Immunol20091246 1235 1244.e5820004782
- Guttman-YasskyEKruegerJGPsoriasis: evolution of pathogenic concepts and new therapies through phases of translational researchBr J Dermatol20071576 1103 111517714560
- Suárez-FariñasMTintleSJShemerANonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalitiesJ Allergy Clin Immunol20111274 954 964 e1 e421388663
- BoguniewiczMLeungDYAtopic dermatitis: a disease of altered skin barrier and immune dysregulationImmunol Rev20112421 233 24621682749
- FujitaHThe role of IL-22 and Th22 cells in human skin diseasesJ Dermatol Sci2013721 3 823746568
- NogralesKEZabaLCShemerAIL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cellsJ Allergy Clin Immunol20091236 1244 1252.e219439349
- ZhengYDanilenkoDMValdezPInterleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosisNature20074457128 648 65117187052
- KaminishiKSomaYKawaYMizoguchiMFlow cytometric analysis of IL-4, IL-13 and IFN-gamma expression in peripheral blood mono-nuclear cells and detection of circulating IL-13 in patients with atopic dermatitis provide evidence for the involvement of type 2 cytokines in the diseaseJ Dermatol Sci2002291 19 2512007717
- AkkocTde KoningPJRückertBBarlanIAkdisMAkdisCAIncreased activation-induced cell death of high IFN-gammaproducing T(H)1 cells as a mechanism of T(H)2 predominance in atopic diseasesJ Allergy Clin Immunol20081213 652 658.e118328893
- Suárez-FariñasMDhingraNGittlerJIntrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitisJ Allergy Clin Immunol20131322 361 37023777851
- FlohrCMannJNew insights into the epidemiology of childhood atopic dermatitisAllergy2014691 3 1624417229
- GooderhamMLyndeCWPappKReview of systemic treatment options for adult atopic dermatitisJ Cutan Med Surg2017211 31 3927635033
- BieberTAtopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicineAllergy20126712 1475 148223106343
- D’ErmeAMWilsmann-TheisDWagenpfeilJIL-36γ (IL-1F9) is a biomarker for psoriasis skin lesionsJ Invest Dermatol20151354 1025 103225525775
- HelloMAubertHBernierCNéelABarbarotSAtopic dermatitis of the adultRev Med Interne2016372 91 9926617291
- BieberTD’ErmeAMAkdisCClinical phenotypes and endophenotypes of atopic dermatitis: Where are we and where should we go?J Alllergy Clin Immunol20171394S S58 S64
- BieberTAkdisCLauenerRGlobal Allergy Forum and 3rd Davos Declaration 2015: atopic dermatitis/eczema: challenges and opportunities toward precision medicineAllergy2016715 588 59227023268
- MansouriYGuttman-YasskyEImmune pathways in atopic dermatitis, and definition of biomarkers through broad and targeted therapeuticsJ Clin Med201545 858 87326239452
- WollenbergAOranjeADeleuranMEuropean Task Force on Atopic Dermatitis/EADV Eczema Task ForceETFAD/EADV eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patientsJ Eur Acad Dermatol Venereol2016305 729 74727004560
- RingJAlomarABieberTEuropean Dermatology Forum (EDF)European Academy of Dermatology and Venereology (EADV)European Federation of Allergy (EFA)European Task Force on Atopic Dermatitis (ETFAD)European Society of Pediatric Dermatology (ESPD)Global Allergy and Asthma European Network (GA2LEN)Guidelines for treatment of atopic eczema (atopic dermatitis) part IJ Eur Acad Dermatol Venereol2012268 1045 106022805051
- RingJAlomarABieberTEuropean Dermatology ForumEuropean Academy of Dermatology and VenereologyEuropean Task Force on Atopic DermatitisEuropean Federation of AllergyEuropean Society of Pediatric DermatologyGlobal Allergy and Asthma European NetworkGuidelines for treatment of atopic eczema (atopic dermatitis) Part IIJ Eur Acad Dermatol Venereol2012269 1176 119322813359
- D’ErmeAMHohlDUse of emollient in atopic dermatitis preventionDermatol Ther2016294 286 28726950266
- McGregorSFarhangianMEFeldmanSRDupilumab for the treatment of atopic dermatitis: a clinical trial reviewExpert Opin Biol Ther20151511 1657 1660
- NotaroERSidburyRSystemic agents for severe atopic dermatitis in childrenPaediatr Drugs2015176 449 45726547214
- BiedermannTWerfelTStatus quo and prospects for systemic therapy of atopic dermatitis. Biologics ante portasHautarzt2015662 108 11325645542
- D’ErmeAMThe beginning of biological treatment era in the atopic dermatitis managementDermatol Ther2016293 208 20926331894
- LaufferFRingJTarget-oriented therapy: emerging drugs for atopic dermatitisExpert Opin Emerg Drugs2016211 81 8926808004
- WangHHLiYCHuangYCEfficacy of omalizumab in patients with atopic dermatitis: a systematic review and meta-analysisJ Allergy Clin Immunol20161386 1719 1722.e127543070
- OldhoffJMDarsowUWerfelTAnti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitisAllergy2005605 693 69615813818
- BlakelyKGooderhamMPappKDupilumab, a monoclonal antibody for atopic dermatitis: a review of current literatureSkin Therapy Lett2016212 1 5
- KovalenkoPDiCioccioATDavisJDExploratory population PK analysis of dupilumab, a fully human monoclonal antibody against IL-4Rα, in atopic dermatitis patients and normal volunteersCPT Pharmacometrics Syst Pharmacol2016511 617 62427778477
- HamiltonJDSuárez-FariñasMDhingraNDupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitisJ Allergy Clin Immunol20141346 1293 130025482871
- BeckLAThaçiDHamiltonJDDupilumab treatment in adults with moderate-to-severe atopic dermatitisN Engl J Med20143712 130 13925006719
- ThaçiDSimpsonELBeckLAEfficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trialLancet201638710013 40 5226454361
- SimpsonELGadkariAWormMDupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): a phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD)J Am Acad Dermatol2016753 506 51527268421
- WollenbergABieberTProactive therapy of atopic dermatitis – an emerging conceptAllergy2009642 276 27819076538
- HamiltonJDUngarBGuttman-YasskyEDrug evaluation review: dupilumab in atopic dermatitisImmunotherapy2015710 1043 105826598956
- SimpsonELBieberTGuttman-YasskyESOLO 1 and SOLO 2 InvestigatorsTwo phase 3 trials of dupilumab versus placebo in atopic dermatitisN Engl J Med201637524 2335 234827690741
- SantiniGMoresNMalerbaMDupilumab for the treatment of asthmaExpert Opin Investig Drugs2017263 357 366
- OzdemirCMonoclonal antibodies in allergy; updated applications and promising trialsRecent Pat Inflamm Allergy Drug Discov201591 54 6525706526