Abstract
As a progressive chronic disease, age-related macular degeneration (AMD) is the leading cause of irreversible vision impairment worldwide. Experimental and clinical evidence has demonstrated that vascular endothelial growth factor (VEGF) plays a vital role in the formation of choroidal neovascularization. Intravitreal injections of anti-VEGF agents have been recommended as a first-line treatment for neovascular AMD. However, persistent fluid or recurrent exudation still occurs despite standardized anti-VEGF therapy. Patients suffering from refractory or recurrent neovascular AMD may develop mechanisms of resistance to anti-VEGF therapy, which results in a diminished therapeutic effect. Until now, there has been no consensus on the definitions of refractory neovascular AMD and recurrent neovascular AMD. This article aims at clarifying these concepts to evaluate the efficacy of switching drugs, which contributes to making clinical decision more scientifically. Furthermore, insight into the causes of resistance to anti-VEGF therapy would be helpful for developing possible therapeutic approaches, such as combination therapy and multi-target treatment that can overcome this resistance.
Introduction
Age-related macular degeneration (AMD) is a progressive chronic disease. The World Health Organization has indicated that AMD ranks as one of the leading causes of blindness globally due to the aging populations in many countries.Citation1 Neovascular AMD is characterized by pathologic choroidal neovascularization (CNV) that breaks through Bruch’s membrane into the subretinal pigment epithelium space and/or the subretinal space, leading to exudation, hemorrhage, retinal edema, pigment epithelial detachment, and fibrous scarring,Citation2 which may produce serious impairments in visual acuity.
CNV is a process that involves both angiogenesis and inflammation.Citation3 Experimental and clinical evidence has shown that vascular endothelial growth factor (VEGF) is a key component in promoting neovascularization.Citation4–Citation6 Intravitreal anti-VEGF agents have greatly improved visual outcomes.Citation7–Citation13
There are five anti-VEGF agents approved for the treatment of neovascular AMD. Pegaptanib became the first one to be approved by the US Food and Drug Administration (FDA), which selectively binds VEGF165.Citation14–Citation16 The VISION study demonstrated that pegaptanib 0.3 mg given intravitreally every 6 weeks resulted in 70% of patients losing fewer than 15 letters of visual acuity.Citation8 However, pegaptanib has been gradually replaced by the pan-VEGF-A inhibitors. Ranibizumab is a humanized monoclonal Fab fragment, while bevacizumab is a full-length humanized monoclonal antibody. These drugs could neutralize all the active isoforms of VEGF-A.Citation17–Citation19 Ranibizumab was demonstrated to be effective in the MARINA and ANCHOR trials, based on the observation that ∼90% of patients receiving monthly intravitreal treatment with ranibizumab lost fewer than 15 letters after 2 years.Citation7,Citation10 Bevacizumab presented a similar efficacy to ranibizumab in the CATT trials and IVAN study.Citation9,Citation11,Citation20 Aflibercept and conbercept are recombinant fusion proteins that act as soluble decoy receptors for VEGF family members.Citation21–Citation25 In the Phase III VIEW 1 and 2 trials, the administration of an intravitreal aflibercept injection monthly or every 2 months after three initial monthly doses achieved similar visual outcomes comparable to monthly intravitreal ranibizumab.Citation12 Conbercept was tested in the AURORA study, and most patients reported improved functional and morphologic parameters.Citation13 A comparison of current anti-VEGF agents for neovascular AMD is shown in . As the incidence of severe vision loss and blindness has been greatly reduced by 46%–51% in many countries,Citation26–Citation28 anti-VEGF therapy is now considered a first-line treatment for neovascular AMD.
Table 1 Comparison of current anti-VEGF agents for neovascular AMD
Although anti-VEGF agents have shown a dramatic breakthrough in neovascular AMD treatment recently, some patients have poor or nonresponse to anti-VEGF agents with standardized treatment or experience a slow loss of efficacy of anti-VEGF agents after repeated administration over time. Persistent fluid is still common after regular therapy. The CATT revealed that, despite monthly treatment with anti-VEGF agents for 2 years, 51.5% of patients receiving intravitreal ranibizumab and 67.4% of patients treated with bevacizumab had evidence of persistent fluid on time-domain optical coherence tomography (OCT).Citation11 There are still 19.7%–36.6% of patients with active exudation on either angiography or OCT after 1 year of regular 2.0 mg aflibercept treatments (q4wk or q8wk).Citation12
For these phenomena, researchers have offered various descriptions and explanations about the loss of the drug’s effectiveness, such as “incomplete response”,Citation29 “poor response”,Citation30 “nonresponse”,Citation30,Citation31 “unresponsive”,Citation32 “tolerance”,Citation33–Citation35 “tachyphylaxis”,Citation34–Citation40 “treatment resistant”,Citation41–Citation43 “resistance to anti-VEGF”,Citation44 “refractory to anti-VEGF”,Citation45 and “resistance to anti-VEGF treatment”.Citation46 When describing and classifying patients with persistent fluid or recurrent exudation, researchers frequently use the terms “refractory neovascular AMD”,Citation47–Citation50 “recalcitrant neovascular AMD”,Citation51–Citation54 “recurrent neovascular AMD”,Citation44,Citation47,Citation55–Citation57 and “treatment-resistant neovascular AMD”.Citation41–Citation43 However, no present agreement exists on the definition of these terms, and this point has been highlighted and marked as needing further action. Clarifying and consolidating these concepts are of great importance for an effective evaluation of switching to other anti-VEGF drugs, combination therapy, and multi-target treatment. Furthermore, gaining an insight into the causes of resistance to anti-VEGF therapy would be helpful for developing novel strategies to improve the efficacy of anti-angiogenic therapies.
Definition of refractory neovascular AMD and recurrent neovascular
AMD Refractory neovascular AMD
In many clinical trials and scientific papers, researchers frequently use the terms “refractory neovascular AMD” and “recalcitrant neovascular AMD”, but there is still debate regarding what can be defined as “refractory neovascular AMD” or “recalcitrant neovascular AMD”. Some researchers consider patients who show stationary or increased intraretinal or subretinal exudation despite more than three consecutive injections, even if an initial partial response could be observed temporarily, to be suffering from refractory neovascular AMD or recalcitrant neovascular AMD.Citation45,Citation47,Citation48,Citation58,Citation59 Arcinue et alCitation44 concluded that eyes with persistent fluid collection despite at least five monthly consecutive ranibizumab/bevacizumab injections might qualify as refractory neovascular AMD as well.
Previously, many researchers considered that patients with persistent fluid after three initial injections suffer from refractory or recalcitrant AMD, which is mainly based on remarkable vision improvement after three monthly injections. However, as the responses of >30% of patients were delayed after 4 months of treatment in the MARINA and ANCHOR trials,Citation7,Citation10 a response to only three initial injections should not be considered an indicator of visual prognosis. Therefore, some researchers considered to redefine the threshold for refractory or recalcitrant AMD. Broadhead et alCitation41 considered persistent exudation after at least 6-month regular anti-VEGF therapy, which could be defined as “treatment resistance”. Fung et alCitation55 defined “refractory CNV” as persistent fluid on spectral-domain OCT (SD-OCT) at <30 days after the last of six intravitreal injections of an anti-VEGF agent at monthly intervals. Grewal et alCitation52 put forward the concept of “recalcitrant exudative AMD” after 6 months of monthly anti-VEGF treatment.
Since “recalcitrant neovascular AMD” and “refractory neovascular AMD” are synonyms, we recommend the uniform use of “refractory neovascular AMD”. We consider that “refractory neovascular AMD” should be defined in those patients who have a persistence of exudation as evident on clinical examination and also on imaging studies (leakage on fluorescein angiography, or fibrovascular pigment epithelial detachment with intraretinal fluid [IRF] or subretinal fluid [SRF] on SD-OCT), or even increasing hemorrhage compared to the baseline after six consecutive injections at monthly intervals. Nevertheless, structural lesions that can mimic leakage on SD-OCT, such as outer retinal tubulationsCitation60 and chronic intraretinal cysts,Citation61 are considered chronic markers of atrophy and do not require anti-VEGF treatment, which should not be considered as evidence of refractory neovascular AMD.
Our understanding of “refractory neovascular AMD” is consistent with the definition of “recalcitrant exudative AMD” by Grewal et alCitation52 and has some characteristics in common with several experts’ ideas, such as Broadhead et alCitation41 and Fung et al.Citation55 Broadhead et al used the term “treatment-resistant neovascular AMD” and agreed with the idea of receiving standard anti-VEGF therapy for at least 6 months to evaluate the therapeutic response. “Treatment resistance” was another description of “refractory”, but Broadhead et al failed to point out whether the 6-month anti-VEGF therapy was maintained at monthly intervals or at unfixed intervals. Meanwhile, Fung et al offered a definition of “refractory CNV”, which was very similar to our concept of “refractory neovascular AMD”.
These experts consider the persistence of exudation after 6 months of monthly anti-VEGF therapy as an indicator of “refractory neovascular AMD” based on abundant clinical experience in practice and scientific summary from clinical trials. “Refractory AMD” is a really important concept, which contributes to finding the right time of switching treatments and making clinical decision more scientifically. Further multicentric clinical trials are needed to demonstrate that six consecutive, monthly anti-VEGF injections are a turning point of anatomical changes and/or functional changes.
Recurrent neovascular AMD
Apart from persistence of exudation, there are still patients who suffer from the appearance of new retinal hemorrhage or SRF/IRF accumulation after the initial resolution of exudative changes. Kuroda et alCitation56 found that 65.7% of patients experienced a recurrence of retinal exudative change within 12 months and 74.8% reported the same within 24 months.
Yonekawa et alCitation47 considered that “recurrent” means exudation suppressed but requiring frequent injections. In our point of view, eyes have shown complete resolution of retinal exudative change after regular anti-VEGF treatment; once the treatment is withdrawn, multiple recurrences (a minimum of two) of new or increased IRF or SRF with or without vision changes or symptoms are defined as “recurrent neovascular AMD”. Our understanding of “recurrent neovascular AMD” is consistent with Arcinue et al.Citation44 Furthermore, only one recurrence of exudation could be diagnosed as the recurrence of neovascular AMD, instead of “recurrent neovascular AMD”. Recurrent retinal exudation in patients receiving uninterrupted treatment is preferable to experiencing “refractory neovascular AMD” after an initial response.
Despite multiple recurrences of exudation, some patients with recurrent neovascular AMD respond well to frequent retreatment and eventually become dry macular. However, other patients slowly become less responsive over time and maintain persistent exudation. These patients could be qualified as “refractory neovascular AMD”.
Resistance to anti-VEGF therapy resulting in a diminished therapeutic effect
Regardless of whether the diagnosis is refractory neovascular AMD or recurrent neovascular AMD, various clinical manifestations are caused by significant interindividual differences in response to an anti-VEGF agent. There is no authoritative consensus as to how to classify “responder status”. Recently, Amoaku et alCitation30 categorized the response to anti-VEGF therapies in neovascular AMD. It is divided into optimal (good) response, poor response, and nonresponse based on both functional and morphological outcomes. We consider it an appropriate definition/categorization of the response of neovascular AMD to anti-VEGF therapies. Patients who have poor response or nonresponse to anti-VEGF under the standardized treatment may gradually develop mechanisms of resistance to anti-VEGF therapy.
There is currently no consensus on the definition of “resistance to anti-VEGF therapy”. Tranos et alCitation46 considered that half of the patients who did not improve and ∼10% of the patients who had no response at all despite ongoing therapies with the current standard anti-VEGF approach were resistant to anti-VEGF therapy. Bakall et alCitation62 reported that some patients, however, had a good initial response with a resolution of fluid but then developed recurrent exudation and became resistant to further treatment. We consider patients who showed poor response or nonresponse to the initial therapy, or who had a successful initial response to anti-VEGF therapy but experienced a slow loss of response as “resistant to anti-VEGF therapy”.
Some ophthalmologists make no distinction between “resistant”, “refractory”, and “recurrent”. The term “resistant” is aimed at describing the status of a diminished therapeutic effect despite continuous treatment, while “refractory” or “recurrent” focuses on describing the characteristics of AMD itself, as previously explained. Therefore, phrases such as “resistance to anti-VEGF therapy”, “refractory neovascular AMD”, and “recurrent neovascular AMD” may be more useful and effective. In addition, it is also essential to distinguish “resistance to anti-VEGF therapy” and “resistance to anti-VEGF agents”. The former is a broader concept that encompasses “resistance to anti-VEGF agents”.
Causes of resistance to anti-VEGF therapy and possible therapeutic approaches
Resistance can occur at any time during the course of therapy.Citation41 Anti-VEGF therapy may fail from the beginning or following an initial successful treatment period. An incomplete effect of the initial therapy may be caused by several clinical factors, including misdiagnosis and genetic predisposition. Resistance to anti-VEGF agents and sustained activation of other pathogenic pathways result in the development of persistent or recurrent exudation after an initial successful treatment period. We draw on these facets to provide a framework to show why the phenomenon of resistance to anti-VEGF therapy occurs and how to deal with it ().
Figure 1 A framework to show the causes of resistance to anti-VEGF therapy and possible therapeutic approaches.
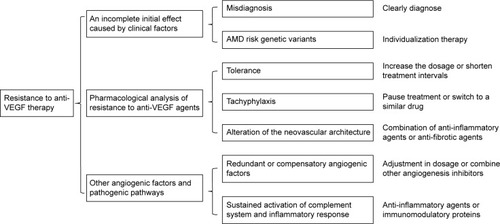
An incomplete initial effect caused by clinical factors
Misdiagnosis
Misdiagnosis appears to be one common clinical factor that results in poor response or nonresponse to anti-VEGF therapy. Previous research has shown that 46.2% of patients with a poor response to treatment require revision of the primary diagnosis. For example, the misdiagnosis of polypoidal choroidal vasculopathy (PCV) as CNV and a lack of distinction between retinal angiomatous proliferation (RAP) and typical CNV have been described at length in several papers.Citation40,Citation45
In contrast to CNV, which is secondary to AMD (CNV–AMD) in Western populations, PCV, an important variant of AMD, appears to be the predominant subtype of neovascular AMD in Asian populations.Citation63 PCV may account for as high as 22.3%–61.6%Citation64–Citation72 of cases in Asians and 8%–13%Citation73 of Caucasian patients who present with presumed neovascular AMD. PCV may mimic CNV on fundus photography and fluorescence fundus angiography, further confusing the diagnosis. Focal hyperfluorescent polyps on early-phase indocyanine green angiography are still the gold standard for diagnosis.Citation74 Considering the lower prevalence of PCV in Caucasian patients, Western ophthalmologists are relatively less experienced in its diagnosis and treatment than Asian experts. Therefore, there might be higher rates of misdiagnosis of patients with PCV in Western countries than in Asia. On the other hand, as indocyanine green angiography is not a routine examination, misdiagnosis is still common worldwide.
Because the role of VEGF in the pathogenesis of PCV is believed to be substantially less important than in CNV, patients with PCV who are misdiagnosed for CNV may be resistant to anti-VEGF agents (ranibizumab and bevacizumab). Therefore, the diagnosis must be reevaluated, and more attention should be paid toward avoiding this misdiagnosis. If a patient has received a diagnosis of PCV, the treatment options should be changed. The optimal treatment for PCV requires further clarification.Citation75 PCV is usually treated with anti-VEGF monotherapy, photodynamic therapy (PDT) monotherapy, or a combination of anti-VEGF/PDT therapy, but ranibizumab and bevacizumab have limited effect on polypoidal lesions. Aflibercept, a new anti-VEGF drug, has been demonstrated to improve both visual acuity and macular morphology in a large number of treatment-naive eyes with PCV.Citation76
RAP, which is also known as a variant of neovascular AMD, represents an estimated 10%–12%Citation77,Citation78 of newly diagnosed neovascular AMD lesions. Freund et alCitation79 considered RAP to be a type 3 neovascularization in order to distinguish it from the type 1 and 2 CNV anatomic classifications. However, RAP may mimic type 1 and 2 CNV on fluorescence fundus angiography. There is a characteristic hyperfluorescent “hot spot” in early RAP lesions on indocyanine green angiography, which has previously been considered the best approach to diagnose RAP.Citation80 OCT angiography is a new noninvasive, motion contrast imaging modality for retinal microvasculature. OCT angiography will play an important role in the early diagnosis of RAP to reduce the rate of misdiagnosis.Citation81
RAP differs from typical neovascular AMD in its natural course and has previously been reported to have poor visual gain in response to anti-VEGF monotherapy.Citation82–Citation86 However, a subanalysis of CATT found that RAP had an optimal response to anti-VEGF therapy.Citation87–Citation89 Applying PDT simultaneously with intravitreal anti-VEGF agents effectively maintained or improved patients’ visual acuity and reduced or eliminated edema in the short term.Citation90
AMD risk genetic variants
AMD is influenced by both environmental and genetic factors. Numerous genetic variants, such as CFH, HTRA1/ARMS2, C3, CFB/C2, and APOE genes, confer significant risk for the development of AMD.Citation91 However, genetic testing is not considered to be included in the standard AMD diagnosis or treatment at present. Some ophthalmologists have speculated that a genetic predisposition may also contribute to resistance to anti-VEGF therapy.
Polymorphism rs1061170 (T1277C, Y402H) has been found to be strongly associated with exudative AMDCitation92 and AMD progression.Citation93 When investigating the association between polymorphism rs1061170 and the treatment response of neovascular AMD, patients harboring homozygous for the variant risk C-allele (CC genotype) are consistent with a decreased response to treatment by ∼1.6-fold when compared to patients carrying homozygous for the ancestral T-allele (TT genotype).Citation94 Lee et alCitation95 found that patients harboring homozygous for the CFH Y402H risk allele had a significantly higher risk (37%) of requiring additional ranibizumab injections. In other words, the response to treatment of AMD with ranibizumab differed according to the patient’s specific CFH genotype.
As for ARMS2 gene, Abedi et alCitation96 found single nucleotide polymorphism rs10490924 (A69S) in the LOC387715/ARMS2 gene with poor outcome of intravitreal anti-VEGF injections in neovascular AMD. A literature-based meta-analysis was performed of studies relevant to A69S polymorphism in the ARMS2 gene and the response to anti-angiogenesis treatment by Hu et al.Citation97 They also found A69S could be considered predictive of the anti-angiogenic effects, especially in Asian populations.Citation97
These patients with AMD risk genetic variants might have higher background levels of inflammation, which may continue to affect the disease progression and probably lead to a more rapid recurrence of neovascularization, which produces a diminished therapeutic effect.Citation95 It is conceivable that future AMD treatments may depend on the patient’s individual genetic risk profile to develop individualized therapy.Citation98 For example, intravitreal exogenous CFH or CFH-related complement inhibitors may be a beneficial therapy for patients with polymorphism rs1061170.
Pharmacological analysis of resistance to anti-VEGF agents
Tolerance
Drug tolerance is a pharmacology concept, where a subject’s reaction to a specific drug and the physiological concentration of the drug are reduced followed by repeated use, subsequently requiring an increased dosage or shorter dosing time intervals to achieve the desired effect.Citation99 However, efficacy is not restored even when the treatment is halted temporarily.Citation100 Drug tolerance could be divided into several different types, including pharmacodynamic tolerance, pharmacokinetic (metabolic) tolerance, and behavioral tolerance (for certain psychoactive drugs).
During anti-VEGF therapies, pharmacodynamic tolerance may be caused by the increased expression of VEGF (especially derived from those macrophages that locate within the choroidal neovascular tissue and respond to VEGF inhibition by upregulating the production of VEGF itself), increased expression of VEGF receptors, changes in signal transduction, or a shift of the stimulus for CNV growth toward other growth factors.Citation34 Pharmacokinetic tolerance occurs because a decreased quantity of the substance reaches the site it affects. A systemic immune response, the development of neutralizing antibodies,Citation34 increased clearance from the eye, or reflux of the drug following injection may all result in pharmacokinetic tolerance. The Biologics License Application states that the baseline incidence of immunoreactivity to ranibizumab is 0%–3%, which rises to ∼1%–6% after monthly dosing with ranibizumab for 12–24 months based on 1-year clinical efficacy and safety data from two pivotal Phase III trials, ANCHOR and MARINA, and the Phase I–II FOCUS trial.Citation36 Theoretically, it is therefore necessary to increase the dosage or shorten treatment intervals if tolerance has developed.
Several studies have investigated the relationship between increasing the dose and further anatomical and visual outcomes. The HARBOR trialCitation101 and Forooghian et al’sCitation36 study demonstrated that high-dose ranibizumab/bevacizumab given monthly did not restore therapeutic responses in eyes that had developed a tolerance, while the evaluation of high-dose ranibizumab (2.0 mg) in the management of AMD in patients with persistent/recurrent macular fluid (LAST) studyCitation55 and Brown et al’sCitation51 trial found that 2.0 mg of ranibizumab could maintain anatomical results and preserve or improve best-corrected visual acuity in patients with persistent or recurrent SRF or IRF despite previous standard anti-VEGF therapy. Compared to Forooghian et al’s study, the LAST study, and Brown et al’s trial, the conclusion of the HARBOR trial may be more persuasive because of that study’s relatively larger sample. The study indicated that intravitreal high-dose anti-VEGF agents may not be readily effective at restoring a complete therapeutic response in all patients. Apart from unclear efficacy, the treatment is also an economic burden for patients when the dosage is increased, which makes it difficult to apply in clinical practice.
Few large trials have evaluated the effect of increasing the frequency of treatment to more than once a month. Stewart et alCitation102 found that dosing a drug (ranibizumab, bevacizumab, and aflibercept) every 2 weeks resulted in markedly improved trough binding activity, so the short-term use of biweekly dosing may be an attractive treatment option for those eyes that respond within 2 weeks of an injection but then rebound with increased macular fluid after a month. Treatment every 2 weeks may present a challenge for patients with poor compliance and also carries a significant cost implication. Moreover, shorter dosing time intervals of every 2 weeks have not yet been approved by the FDA for neovascular AMD.
Tachyphylaxis
Tachyphylaxis is a medical term describing an acute (sudden) decrease in the response to a drug after its administration.Citation103 It can occur after an initial dose or following a series of small doses. Keane et alCitation104 was the first to suggest that possible tachyphylaxis had appeared after treatment with ranibizumab, while other researchers have considered that tachyphylaxis may occur as early as after two injections.Citation37–Citation39,Citation105 Tachyphylaxis cannot be overcome by increasing the dosage. However, efficacy can be restored if the medication is stopped for a short while or if the interval between doses is increased. However, the mechanism of tachyphylaxis during anti-VEGF therapies for exudative AMD is still not clear.
If tachyphylaxis occurs, clinicians should stop the treatment for a while or switch to a similar drug with different properties.Citation34 The majority of these therapies involve switching patients from bevacizumab to ranibizumab,Citation37,Citation106–Citation109 from ranibizumab to bevacizumab,Citation37,Citation105,Citation107–Citation110 and from bevacizumab/ranibizumab to aflibercept.Citation42,Citation43,Citation47,Citation52,Citation62,Citation111–Citation119
The proposed mechanism of switching between two anti-VEGF drugs, bevacizumab and ranibizumab, could be due to the different molecular sizes and associated transport of these molecules through the retina and into the subretinal space. Ranibizumab was found diffusely across the retina after intravitreal injection because of its smaller size. Bevacizumab may also reach the subretinal space with a different distribution in the retina after intravitreal injection.Citation120 Aflibercept is a novel VEGF inhibitor with a higher binding efficacy and a wider spectrum of action than both bevacizumab and ranibizumab.Citation21 Aflibercept may help patients with persistent fluid despite standard treatment with ranibizumab and bevacizumab. Fourteen trials have all demonstrated that patients who are resistant to ranibizumab or bevacizumab have a therapeutic, anatomical structure response when switched to aflibercept, but only five of themCitation43,Citation111–Citation113,Citation118 experienced improved visual outcomes.
Conbercept has a similar molecular structure to that of aflibercept, which is also a recombinant fusion protein of the ligand-binding elements of VEGF receptors.Citation22 Conbercept was approved by the China FDA in December 2013 and has not yet reached the market in other countries. Therefore, there was no evidence to verify the efficacy of switching to conbercept when tachyphylaxis occurs. Given its similar structure to aflibercept, excellent safety and efficacy profile, conbercept is expected to be effective for such patients, but further investigation is needed.
Alteration of the neovascular architecture
Vascular endothelial cells (ECs) play a crucial role in vascular formation. EC mutations may potentially lead to conformational changes in receptors and affect the expression profile and the resultant sensitivity to available antiangiogenic agents.Citation121 In addition, anti-VEGF therapy may promote apoptosis of ECs, leading to empty vascular sleeves formed by the persistence of pericytes and the vascular basement membrane. These empty vascular sleeves serve as channels for EC proliferation when anti-VEGF therapy is halted,Citation122 which might be one of the reasons for the regression of CNV.
Oncologists have demonstrated that tumor vessels have enhanced vessel diameter, mature pericytes, immunoreactivity for desmin, platelet-derived growth factor receptor-β, and late-stage maturity marker α smooth muscle actin to enhance vascular maturity during antiangiogenic blockade. Prolonged antiangiogenesis significantly alters the expression of angiogenic factors implicated in vascular mural cell recruitment, causing extensive morphological changes in the vessels.Citation123 We could speculate that there may be similar changes in CNV architecture during prolonged antiangiogenic blockade, which forms a complicated barrier to current therapy.
Chronic inflammation may cause permanent structural damage to the vascular walls of the CNV complex, which could conceivably result in permanent abnormal vascular permeability and persistent exudation that is no longer amenable to anti-VEGF therapy.Citation101 Inflammatory stimulation could also increase fibrosis of the CNV, which acts as a resorption barrier and decreases patients’ sensitivity to anti-VEGF drugs.
Mutations in ECs, maintenance of vascular sleeves, vascular remodeling, and chronic inflammatory changes of CNV can be influential in their therapeutic effects. There is currently still no effective therapy for EC mutations and apoptosis during antiangiogenic therapy. However, the combination of anti-inflammatory agents or anti-fibrotic agents might play a role in delaying the process of chronic inflammatory changes of CNV.
Various redundant proangiogenic factors and other pathogenic pathways
Redundant or compensatory angiogenic factors
Although VEGF is a key driver of the formation of CNV, many other proangiogenic factors could also promote angiogenesis, such as fibroblast growth factor, transforming growth factor, tumor necrosis factor, interleukins, platelet-derived growth factor (PDGF), and placenta growth factor. VEGF signaling might be closely linked to other pathways, such as PDGFCitation124,Citation125 and fibroblast growth factorCitation126,Citation127 signaling. An increase in the expression of these factors may possibly fuel alternate signaling pathways for angiogenesis, which could trigger VEGF-independent neovascularization and cause resistance to mono anti-VEGF drugs.
Treatment may require dynamic adjustment in the dosage of the therapy or a combination with other angiogenesis inhibitors, such as anti-PDGF agents. PDGF participates in the recruitment of pericytes; thus, anti-PDGF therapy could prevent the pericytes from protecting the vessels, possibly increasing neovascular sensitivity to anti-VEGF therapy.Citation128 Fovista is an anti-PDGF agent. A Phase IIb clinical trial has demonstrated that patients who received ranibizumab combined with Fovista obtained a significantly higher final visual acuity than those administered ranibizumab monotherapy. A Phase III randomized, double-blind, controlled trial is underway.
Sustained activation of the complement system and inflammatory response
In addition to angiogenesis, complement activation and inflammation have also been implicated in the pathogenesis of AMD. Anti-VEGF therapy can only inhibit VEGF-induced neovascularization, but sustained activation of the complement system and inflammatory response may reduce the sensibility to anti-VEGF agents.
Neovascular AMD, with its various pathogens and multiple pathogenic mechanisms, is a complicated disease that requires multi-targeted and comprehensive treatment, such as a combination of anti-inflammatory agents or immunomodulatory proteins. Triamcinolone is a long-acting synthetic corticosteroid that has been used intravitreally to reduce macular edema. Schaal et alCitation39 have found that the combination of triamcinolone acetate and anti-VEGF therapy may lessen the effect of decreased bioefficacy after repeated intravitreal injections. Tandospirone, a serotonin receptor agonist, has a local neuroprotective, anti-inflammatory effect and is being investigated at present. Complement system-modulating substances, such as antibodies (LFG316, FCFD4514S, eculizumab), peptides (POT-4), aptamers (ARC1905), and antibody fragments (lampalizumab), show promising prospects in AMD therapy.Citation98
Conclusion
Five anti-VEGF agents have been introduced in the field of ophthalmology since 2004. These agents have brought dramatic changes in the treatment of neovascular AMD, with fewer patients losing their vision and a reasonable proportion showing vision improvement. Despite the outstanding advances made by anti-VEGF therapy, most patients require repeated injections frequently and long-term follow-up regularly. The SEVEN-UP studyCitation129 showed that the mean visual acuity gradually decreased during long-term follow-up with retreatment using a pro re nata regimen when patients exited from the MARINA or ANCHOR trial. These findings indicated that anti-VEGF therapy is a long and arduous process. Emerging terms such as “refractory neovascular AMD” and “recurrent neovascular AMD” are widely used today. As novel anti-VEGF agents, aflibercept and conbercept have a higher binding efficacy and a wider spectrum of action than both bevacizumab and ranibizumab.Citation21,Citation22 Switching to aflibercept or conbercept may be effective for patients resistant to treatment with bevacizumab or ranibizumab. To consolidate and define these concepts is of great importance in clinical decision making with regard to the switching opportunity and also an evaluation of its effects.
We have to realize that beyond VEGF, there are still abundant angiogenic signaling cascade and other pathways that are related to the pathophysiology of neovascular AMD altogether. Many investigational drugs have the potential to not only reduce patient visits and injections but also improve outcomes by targeting additional pathways, increasing the target’s affinity, and lengthening treatment durability.Citation128 Insight into the mechanisms of resistance to anti-VEGF therapy would be helpful to guiding treatment decisions regarding when to switch to other anti-VEGF drugs or choose a combination therapy or multi-target treatment, which will be a real breakthrough in the treatment of neovascular AMD.
Acknowledgments
This study was supported by grants from the National Science Foundation for Distinguished Young Scholars (81425006).
Disclosure
Dr Sun is a consultant to Novartis International AG (Basel, Switzerland) and Chengdu Kanghong Biotechnology Co. Ltd. (Chengdu, People’s Republic of China). This review received no specific grant from any funding agency in either the commercial or not-for-profit sectors. The authors report no other conflicts of interest in this work.
References
- WHO [webpage on the Internet]Prevention of Blindness and Visual ImpairmentPriority Eye Diseases2012 Available from: http://www.who.int/blindness/causes/priority/en/index7.htmlAccessed May 6, 2016
- LimLSMitchellPSeddonJMHolzFGWongTYAge-related macular degenerationLancet201237998271728173822559899
- CampaCCostagliolaCIncorvaiaCInflammatory mediators and angiogenic factors in choroidal neovascularization: pathogenetic interactions and therapeutic implicationsMediators Inflamm2010201014
- SpilsburyKGarrettKLShenWYConstableIJRakoczyPEOver-expression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularizationAm J Pathol2000157113514410880384
- IshibashiTHataYYoshikawaHNakagawaKSueishiKInomataHExpression of vascular endothelial growth factor in experimental choroidal neovascularizationGraefes Arch Clin Exp Ophthalmol199723531591679085111
- WongTYLiewGMitchellPClinical update: new treatments for age-related macular degenerationLancet2007370958320420617658379
- BrownDMKaiserPKMichelsMRanibizumab versus verteporfin for neovascular age-related macular degenerationN Engl J Med2006355141432144417021319
- GragoudasESAdamisAPCunninghamETJrFeinsodMGuyerDRVEGF Inhibition Study in Ocular Neovascularization Clinical Trial GroupPegaptanib for neovascular age-related macular degenerationN Engl J Med2004351272805281615625332
- GroupCRMartinDFMaguireMGRanibizumab and bevacizumab for neovascular age-related macular degenerationN Engl J Med2011364201897190821526923
- RosenfeldPJBrownDMHeierJSMARINA Study GroupRanibizumab for neovascular age-related macular degenerationN Engl J Med2006355141419143117021318
- Comparison of Age-related Macular Degeneration Treatments Trials Research GroupMartinDFMaguireMGRanibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year resultsOphthalmology201211971388139822555112
- HeierJSBrownDMChongVSchmidt-ErfurthUVIEW 1 and VIEW 2 Study GroupsIntravitreal aflibercept (VEGF trap-eye) in wet age-related macular degenerationOphthalmology2012119122537254823084240
- LiXXuGWangYAURORA Study GroupSafety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA studyOphthalmology201412191740174724793528
- RuckmanJGreenLSBeesonJ2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domainJ Biol Chem19982733220556205679685413
- CriswellMHHuWZSteffensTJLiRMargaronPComparing pegaptanib and triamcinolone efficacy in the rat choroidal neovascularization modelArch Ophthalmol2008126794695218625941
- MorjariaRChongNVPharmacokinetic evaluation of pegaptanib octasodium for the treatment of diabetic edemaExpert Opin Drug Metab Toxicol20141081185119224856361
- BakriSJSnyderMRReidJMPulidoJSEzzatMKSinghRJPharmacokinetics of intravitreal ranibizumab (Lucentis)Ophthalmology2007114122179218218054637
- BakriSJSnyderMRReidJMPulidoJSSinghRJPharmacokinetics of intravitreal bevacizumab (Avastin)Ophthalmology2007114585585917467524
- PapadopoulosNMartinJRuanQBinding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumabAngiogenesis201215217118522302382
- InvestigatorsISChakravarthyUHardingSPRanibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trialOphthalmology201211971399141122578446
- StewartMWRosenfeldPJPredicted biological activity of intravitreal VEGF TrapBr J Ophthalmol200892566766818356264
- LuXSunXProfile of conbercept in the treatment of neovascular age-related macular degenerationDrug Des Devel Ther2015923112320
- HolashJDavisSPapadopoulosNVEGF-Trap: a VEGF blocker with potent antitumor effectsProc Natl Acad Sci U S A20029917113931139812177445
- ZhangMYuDYangCThe pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularizationPharm Res200926120421018854954
- WuZZhouPLiXStructural characterization of a recombinant fusion protein by instrumental analysis and molecular modelingPLoS One201383e5764223469213
- BlochSBLarsenMMunchICIncidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010Am J Ophthalmol20121532209213.e20222264944
- SkaatAChetritABelkinMKinoriMKalter-LeiboviciOTime trends in the incidence and causes of blindness in IsraelAm J Ophthalmol20121532214221.e21122264945
- SloanFAHanrahanBWThe effects of technological advances on outcomes for elderly persons with exudative age-related macular degenerationJAMA Ophthalmol2014132445646324458013
- WykoffCCBrownDMMaldonadoMECroftDEAflibercept treatment for patients with exudative age-related macular degeneration who were incomplete responders to multiple ranibizumab injections (TURF trial)Br J Ophthalmol201498795195524518078
- AmoakuWMChakravarthyUGaleRDefining response to anti-VEGF therapies in neovascular AMDEye (Lond)201529672173125882328
- van AstenFRoversMMLechanteurYTPredicting non-response to ranibizumab in patients with neovascular age-related macular degenerationOphthalmic Epidemiol201421634735525157998
- YazdiMHFaramarziMANikfarSFalavarjaniKGAbdollahiMRanibizumab and aflibercept for the treatment of wet age-related macular degenerationExpert Opin Biol Ther20151591349135826076760
- LiHLeiNZhangMLiYXiaoHHaoXPharmacokinetics of a long-lasting anti-VEGF fusion protein in rabbitExp Eye Res201297115415921933673
- BinderSLoss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance?Br J Ophthalmol20129611222157632
- ArjamaaOMinnHResistance, not tachyphylaxis or toleranceBr J Ophthalmol20129681153115422510583
- ForooghianFCukrasCMeyerleCBChewEYWongWTTachyphylaxis after intravitreal bevacizumab for exudative age-related macular degenerationRetina200929672373119516114
- GasperiniJLFawziAAKhondkaryanABevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisationBr J Ophthalmol2012961142021791509
- AvgikosKNHorganSESivarajRRHuKTachyphylaxis and bevacizumabOphthalmology2009116918311832 author reply 183219729101
- SchaalSKaplanHJTezelTHIs there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration?Ophthalmology2008115122199220518930553
- EghojMSSorensenTLTachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumabBr J Ophthalmol2012961212321733918
- BroadheadGKHongTChangAATreating the untreatable patient: current options for the management of treatment-resistant neovascular age-related macular degenerationActa Ophthalmol201492871372324925048
- GharbiyaMIannettiLParisiFDe VicoUMungoMLMarencoMVisual and anatomical outcomes of intravitreal aflibercept for treatment-resistant neovascular age-related macular degenerationBiomed Res Int2014201427375424895562
- ChangAALiHBroadheadGKIntravitreal aflibercept for treatment-resistant neovascular age-related macular degenerationOphthalmology2014121118819224144450
- ArcinueCAMaFBarteselliGSharpstenLGomezMLFreemanWROne-year outcomes of aflibercept in recurrent or persistent neovascular age-related macular degenerationAm J Ophthalmol20151593426436.e42225461263
- Moon daRCLeeDKKimSHYouYSKwonOWAflibercept treatment for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy refractory to anti-vascular endothelial growth factorKorean J Ophthalmol201529422623226240506
- TranosPVacalisAAsteriadisSResistance to antivascular endothelial growth factor treatment in age-related macular degenerationDrug Des Devel Ther20137485490
- YonekawaYAndreoliCMillerJBConversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degenerationAm J Ophthalmol201315612935.e2223668679
- ShinJYWooSJAhnJParkKHAnti-VEGF-refractory exudative age-related macular degeneration: differential response according to features on optical coherence tomographyKorean J Ophthalmol201327642543224311928
- HiranoYYasukawaTTsukadaAYokoyamaSItoYNakazawaYResolution of exudative changes refractory to ranibizumab after aflibercept injections at the margin of inferior staphyloma in tilted disc syndromeOphthalmic Surg Lasers Imaging Retina201546338438625856827
- ChoMBarbazettoIAFreundKBRefractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathyAm J Ophthalmol200914817078.e7119403115
- BrownDMChenEMarianiAMajorJCJrGroupSSSuper-dose anti-VEGF (SAVE) trial: 2.0 mg intravitreal ranibizumab for recalcitrant neovascular macular degeneration-primary end pointOphthalmology2013120234935423131717
- GrewalDSGillMKSarezkyDLyonATMirzaRGVisual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month resultsEye (Lond)201428789589924833178
- PuliafitoCARecalcitrant neovascular macular degeneration after anti-VEGF therapy: an ongoing challengeOphthalmic Surg Lasers Imaging Retina201344210923510034
- Kanesa-ThasanAGrewalDSGillMKLyonATMirzaRGQuantification of change in pigment epithelial detachment volume and morphology after transition to intravitreal aflibercept in eyes with recalcitrant neovascular AMD: 18-month resultsOphthalmic Surg Lasers Imaging Retina201546663864126114844
- FungATKumarNVanceSKPilot study to evaluate the role of high-dose ranibizumab 2.0 mg in the management of neovascular age-related macular degeneration in patients with persistent/recurrent macular fluid <30 days following treatment with intravitreal anti-VEGF therapy (the LAST Study)Eye (Lond)20122691181118722878451
- KurodaYYamashiroKMiyakeMFactors associated with recurrence of age-related macular degeneration after anti-vascular endothelial growth factor treatment: a retrospective cohort studyOphthalmology2015122112303231026271842
- JoondephBCAnti-vascular endothelial growth factor injection technique for recurrent exudative macular degeneration in a telescope-implanted eyeRetin Cases Brief Rep20148434234425372544
- Pinheiro-CostaJCostaJMBeatoJNSwitch to aflibercept in the treatment of neovascular AMD: one-year results in clinical practiceOphthalmologica20152333–415516125896317
- TozerKRollerABChongLPCombination therapy for neovascular age-related macular degeneration refractory to anti-vascular endothelial growth factor agentsOphthalmology2013120102029203423714319
- ZweifelSAEngelbertMLaudKMargolisRSpaideRFFreundKBOuter retinal tubulation: a novel optical coherence tomography findingArch Ophthalmol2009127121596160220008714
- WolffBMaftouhiMQMateo-MontoyaASahelJAMauget-FaysseMOuter retinal cysts in age-related macular degenerationActa Ophthalmol2011896e496e49921631905
- BakallBFolkJCBoldtHCAflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumabAm J Ophthalmol201315611522.e1123706500
- LimLSCheungCMWongTYAsian age-related macular degeneration: current concepts and gaps in knowledgeAsia Pac J Ophthalmol (Phila)201321324126107866
- ChangYCWuWCPolypoidal choroidal vasculopathy in Taiwanese patientsOphthalmic Surg Lasers Imaging200940657658119928723
- WenFChenCWuDLiHPolypoidal choroidal vasculopathy in elderly Chinese patientsGraefes Arch Clin Exp Ophthalmol2004242862562915257461
- LiuYWenFHuangSSubtype lesions of neovascular age-related macular degeneration in Chinese patientsGraefes Arch Clin Exp Ophthalmol2007245101441144517406882
- ShoKTakahashiKYamadaHPolypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristicsArch Ophthalmol2003121101392139614557174
- CoscasGYamashiroKCoscasFComparison of exudative age-related macular degeneration subtypes in Japanese and French Patients: multicenter diagnosis with multimodal imagingAm J Ophthalmol20141582309318.e30224844973
- MarukoIIidaTSaitoMNagayamaDSaitoKClinical characteristics of exudative age-related macular degeneration in Japanese patientsAm J Ophthalmol20071441152217509509
- ByeonSHLeeSCOhHSKimSSKohHJKwonOWIncidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patientsJpn J Ophthalmol2008521576218369702
- CheungCMLiXMathurRA prospective study of treatment patterns and 1-year outcome of Asian age-related macular degeneration and polypoidal choroidal vasculopathyPLoS One201496e10105724978485
- MoriKHorie-InoueKGehlbachPLPhenotype and genotype characteristics of age-related macular degeneration in a Japanese populationOphthalmology2010117592893820132989
- CiardellaAPDonsoffIMHuangSJCostaDLYannuzziLAPolypoidal choroidal vasculopathySurv Ophthalmol2004491253714711438
- CiardellaAPDonsoffIMYannuzziLAPolypoidal choroidal vasculopathyOphthalmol Clin North Am200215453755412515086
- WongCWWongTYCheungCMPolypoidal choroidal vasculopathy in AsiansJ Clin Med20154578282126239448
- YamamotoAOkadaAAKanoMOne-Year results of intravitreal aflibercept for polypoidal choroidal vasculopathyOphthalmology201512291866187226088619
- KuhnDMeunierISoubraneGCoscasGImaging of chorioretinal anastomoses in vascularized retinal pigment epithelium detachmentsArch Ophthalmol199511311139213987487600
- FreundKBKlaisCMEandiCMSequenced combined intravitreal triamcinolone and indocyanine green angiography-guided photodynamic therapy for retinal angiomatous proliferationArch Ophthalmol2006124448749216606873
- FreundKBHoIVBarbazettoIAType 3 neovascularization: the expanded spectrum of retinal angiomatous proliferationRetina200828220121118301024
- YannuzziLAFreundKBTakahashiBSReview of retinal angiomatous proliferation or type 3 neovascularizationRetina200828337538418327130
- DansinganiKKNaysanJFreundKBEn face OCT angiography demonstrates flow in early type 3 neovascularization (retinal angiomatous proliferation)Eye (Lond)201529570370625744441
- HartnettMEWeiterJJStaurenghiGElsnerAEDeep retinal vascular anomalous complexes in advanced age-related macular degenerationOphthalmology199610312204220539003338
- BottoniFMassacesiACigadaMViolaFMusiccoIStaurenghiGTreatment of retinal angiomatous proliferation in age-related macular degeneration: a series of 104 cases of retinal angiomatous proliferationArch Ophthalmol2005123121644165016344434
- BresslerNMRetinal anastomosis to choroidal neovascularization: a bum rap for a difficult diseaseArch Ophthalmol2005123121741174316344449
- HemeidaTSKeanePADustinLSaddaSRFawziAALong-term visual and anatomical outcomes following anti-VEGF monotherapy for retinal angiomatous proliferationBr J Ophthalmol201094670170519854733
- RouvasAAChatziralliIPTheodossiadisPGMoschosMMKotsolisAILadasIDLong-term results of intravitreal ranibizumab, intravitreal ranibizumab with photodynamic therapy, and intravitreal triamcinolone with photodynamic therapy for the treatment of retinal angiomatous proliferationRetina20123261181118922466469
- ScottAWBresslerSBRetinal angiomatous proliferation or retinal anastomosis to the lesionEye (Lond)201024349149620019765
- YingGSHuangJMaguireMGComparison of Age-related Macular Degeneration Treatments Trials Research GroupBaseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degenerationOphthalmology2013120112212923047002
- GharbiyaMParisiFCrucianiFBozzoni-PantaleoniFPrannoFAbdolrahimzadehSIntravitreal anti-vascular endothelial growth factor for retinal angiomatous proliferation in treatment-naive eyes: long-term functional and anatomical results using a modified PrONTO-style regimenRetina201434229830523807188
- SaitoMShiragamiCShiragaFNagayamaDIidaTCombined intravitreal bevacizumab and photodynamic therapy for retinal angiomatous proliferationAm J Ophthalmol20081466935941.e93118723139
- ChamberlainMBairdPDiraniMGuymerRUnraveling a complex genetic disease: age-related macular degenerationSurv Ophthalmol200651657658617134647
- SouiedEHLevezielNRichardFY402H complement factor H polymorphism associated with exudative age-related macular degeneration in the French populationMol Vis2005111135114016379025
- SeddonJMFrancisPJGeorgeSSchultzDWRosnerBKleinMLAssociation of CFH Y402H and LOC387715 A69S with progression of age-related macular degenerationJAMA2007297161793180017456821
- ChenHYuKDXuGZAssociation between variant Y402H in age-related macular degeneration (AMD) susceptibility gene CFH and treatment response of AMD: a meta-analysisPLoS One201278e4246422905135
- LeeAYRayaAKKymesSMShielsABrantleyMAJrPharmacogenetics of complement factor H (Y402H) and treatment of exudative age-related macular degeneration with ranibizumabBr J Ophthalmol200993561061319091853
- AbediFWickremasingheSRichardsonAJIslamAFGuymerRHBairdPNGenetic influences on the outcome of anti-vascular endothelial growth factor treatment in neovascular age-related macular degenerationOphthalmology201312081641164823582991
- HuZXiePDingYYuanDLiuQAssociation between variants A69S in ARMS2 gene and response to treatment of exudative AMD: a meta-analysisBr J Ophthalmol201599559359825185256
- WeberBHCharbel IssaPPaulyDHerrmannPGrassmannFHolzFGThe role of the complement system in age-related macular degenerationDtsch Arztebl Int2014111813313824622760
- RogersFBMedical subject headingsBull Med Libr Assoc19635111411613982385
- WestfallTCWestfallDPGoodman and Gilman’s. The Pharmacological Basis of Therapeutics12th edNew York, NYMcGraw-Hill2011
- BusbeeBGHoACBrownDMTwelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degenerationOphthalmology201312051046105623352196
- StewartMWRosenfeldPJPenhaFMPharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye)Retina201232343445722374154
- BunnelCAIntensive Review of Internal MedicineBoston, MAHarvard Medical School2009
- KeanePALiakopoulosSOngchinSCQuantitative subanalysis of optical coherence tomography after treatment with ranibizumab for neovascular age-related macular degenerationInvest Ophthalmol Vis Sci20084973115312018408176
- AlmonyAMansouriAShahGKBlinderKJEfficacy of intravitreal bevacizumab after unresponsive treatment with intravitreal ranibizumabCan J Ophthalmol201146218218521708088
- KentJSIordanousYMaoAPowellAMKentSSSheidowTGComparison of outcomes after switching treatment from intravitreal bevacizumab to ranibizumab in neovascular age-related macular degenerationCan J Ophthalmol201247215916422560422
- KaiserRSGuptaOPRegilloCDRanibizumab for eyes previously treated with pegaptanib or bevacizumab without clinical responseOphthalmic Surg Lasers Imaging2012431131921986085
- EhlkenCJungmannSBohringerDAgostiniHTJunkerBPielenASwitch of anti-VEGF agents is an option for nonresponders in the treatment of AMDEye (Lond)201428553854524722504
- AslankurtMAslanLAksoyAErdenBCekicOThe results of switching between 2 anti-VEGF drugs, bevacizumab and ranibizumab, in the treatment of neovascular age-related macular degenerationEur J Ophthalmol201323455355723516253
- Pinheiro-CostaJFreitas-da-CostaPFalcaoMSBrandaoEMFalcao-ReisFCarneiroAMSwitch from intravitreal ranibizumab to bevacizumab for the treatment of neovascular age-related macular degeneration: clinical comparisonOphthalmologica2014232314915525196907
- KumarNMarsigliaMMrejenSVisual and anatomical outcomes of intravitreal aflibercept in eyes with persistent subfoveal fluid despite previous treatments with ranibizumab in patients with neovascular age-related macular degenerationRetina20133381605161223549101
- Fassnacht-RiederleHBeckerMGrafNMichelsSEffect of aflibercept in insufficient responders to prior anti-VEGF therapy in neovascular AMDGraefes Arch Clin Exp Ophthalmol2014252111705170924614949
- SinghRPSrivastavaSEhlersJPBediRSchachatAPKaiserPKA single-arm, investigator-initiated study of the efficacy, safety and tolerability of intravitreal aflibercept injection in subjects with exudative age-related macular degeneration, previously treated with ranibizumab or bevacizumab: 6-month interim analysisBr J Ophthalmol201498suppl 1i22i2724836866
- FerronePJAnwarFNaysanJEarly initial clinical experience with intravitreal aflibercept for wet age-related macular degenerationBr J Ophthalmol201498suppl 1i17i2124795335
- HallLBZebardastNHuangJJAdelmanRAAflibercept in the treatment of neovascular age-related macular degeneration in previously treated patientsJ Ocul Pharmacol Ther201430434635224552305
- MessengerWBCampbellJPFaridiAInjection frequency and anatomic outcomes 1 year following conversion to aflibercept in patients with neovascular age-related macular degenerationBr J Ophthalmol20149891205120724795334
- HoVYYehSOlsenTWShort-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitorsAm J Ophthalmol201315612328.e2223664153
- HeussenFMShaoQOuyangYJoussenAMMullerBClinical outcomes after switching treatment from intravitreal ranibizumab to aflibercept in neovascular age-related macular degenerationGraefes Arch Clin Exp Ophthalmol2014252690991524362854
- ChoHShahCPWeberMHeierJSAflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumabBr J Ophthalmol20139781032103523766432
- GaudreaultJFeiDBeyerJCPharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbitsRetina20072791260126618046235
- AbdullahSEPerez-SolerRMechanisms of resistance to vascular endothelial growth factor blockadeCancer2012118143455346722086782
- InaiTMancusoMHashizumeHInhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghostsAm J Pathol20041651355215215160
- HuangJSofferSZKimESVascular remodeling marks tumors that recur during chronic suppression of angiogenesisMol Cancer Res200421364214757844
- WuEPalmerNTianZComprehensive dissection of PDGF-PDGFR signaling pathways in PDGFR genetically defined cellsPLoS One2008311e379419030102
- AbramssonALindblomPBetsholtzCEndothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumorsJ Clin Invest200311281142115114561699
- RusnatiMPrestaMFibroblast growth factors/fibroblast growth factor receptors as targets for the development of anti-angiogenesis strategiesCurr Pharm Des200713202025204417627537
- PrestaMDell’EraPMitolaSMoroniERoncaRRusnatiMFibroblast growth factor/fibroblast growth factor receptor system in angiogenesisCytokine Growth Factor Rev200516215917815863032
- KaiserPKEmerging therapies for neovascular age-related macular degeneration: drugs in the pipelineOphthalmology20131205 supplS11S1523642781
- RofaghaSBhisitkulRBBoyerDSSaddaSRZhangKSEVEN-UP Study GroupSeven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP)Ophthalmology2013120112292229923642856
