Abstract
Purpose
We explored the role of tumor size and number in the prognosis of HCC patients who underwent ablation and created a nomogram based on machine learning to predict the recurrence.
Patients and Methods
A total of 990 HCC patients who underwent transcatheter arterial chemoembolization (TACE) combined ablation at Beijing Youan Hospital from January 2014 to December 2021 were prospectively enrolled, including 478 patients with single small HCC (S-S), 209 patients with single large (≥30mm) HCC (S-L), 182 patients with multiple small HCC (M-S), and 121 patients with multiple large HCC (M-L). S-S patients were randomized in a 7:3 ratio into the training cohort (N=334) and the validation cohort (N=144). Lasso-Cox regression analysis was carried out to identify independent risk factors, which were used to construct a nomogram. The performance of the nomogram was evaluated by C-index, receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA) curves. Patients in the training and validation cohorts were divided into low-risk, intermediate-risk, and high-risk groups based on the risk scores of the nomogram.
Results
The median recurrence-free survival (mRFS) in S-S patients was significantly longer than the S-L, M-S, and S-L patients (P<0.0001). The content of the nomogram includes age, monocyte-to-lymphocyte (MLR), gamma-glutamyl transferase-to-lymphocyte (GLR), International normalized ratio (INR), and Erythrocyte (RBC). The C-index (0.704 and 0.71) and 1-, 3-, and 5-year AUCs (0.726, 0.800, 0.780, and 0.752, 0.761, 0.760) of the training and validation cohorts proved the excellent predictive performance of the nomogram. Calibration curves the DCA curves showed that the nomogram had good consistency and clinical utility. There were apparent variances in RFS between the low-risk, intermediate-risk, and high-risk groups (P<0.0001).
Conclusion
S-S patients who underwent ablation had the best prognosis. The nomogram developed and validated in the study had good predictive ability for S-S patients.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third leading cause of cancer-related mortality. It is one of the few cancers with an increasing mortality rate.Citation1,Citation2 Half of the newly diagnosed cases of HCC every year occur in China, posing a serious threat to the lives and health of the Chinese people.Citation3 With the advancement of diagnostic techniques, early diagnosis and monitoring of the HCC have extensively improved. Surgical resection, ablation, and liver transplantation are recommended as radical treatments for patients with early-stage liver cancer.Citation4 Because of decompensated liver function, portal hypertension, and organ shortage, surgical resection, and liver transplantation are limited in many cases.Citation5,Citation6 As a minimally invasive treatment, ablation has a similar prognosis to surgical resection and exhibits the advantages of fewer complications.Citation7,Citation8 However, the prognosis of ablation remains unsatisfactory, with a 5-year recurrence rate of 50–70%, which leads to shorter survival of HCC patients.Citation9 Hence, the recurrence of HCC has become a public health problem that needs urgent attention.
Tumor characteristics such as tumor size and tumor number affect treatment options and prognosis.Citation10,Citation11 They are also major components of the TNM system and BCLC system. Studies at home and abroad have found that for surgical resection, patients with solitary and small (<30mm) HCC (S-S) have the best prognosis compared to other patients.Citation12,Citation13 Yet, in patients who underwent ablation, the therapeutic forecast requires further statistics and confirmation.
Precisely due to S-S patients having a better prognosis, the assessment of recurrence in S-S patients is easily overlooked. According to the guidelines, early-stage patients do not need to receive other treatments after radical therapy, which can lead to a further decline in patient follow-up and monitoring efforts.Citation14 Currently, tumor classification systems such as TNM, Hong Kong Liver Cancer, and Italian liver cancer program have been widely used for treatment selection, but they are not sufficient for predicting relapse. Although predictive markers like AFP, ALBI, NLR, and a number of nomograms are available to predict recurrence in early-stage patients, the nomogram for S-S patients after undergoing ablation is still lacking.Citation15–17 Therefore, the aim of our study was to confirm the prognosis of S-S patients who underwent ablation and to create a nomogram based on the machine learning approach to more accurately predict the recurrence in order to better guide clinical decisions.
Materials and Methods
Patient Selection
Our study retrospectively evaluated 990 HCC patients who underwent TACE combined ablation at Beijing Youan Hospital from January 2014 to December 2021. The diagnosis of HCC was based on the guideline of America Association for the Study of Liver Diseases (ASSLD).Citation18,Citation19 HCC patients included 478 patients with single small HCC (S-S), 209 patients with single large (≥30mm) HCC (S-L), 182 patients with multiple small HCC (M-S), and 121 patients with multiple large HCC (M-L). S-S patients were randomized in a 7:3 ratio into the training cohort (N=334) and the validation cohort (N=144). The inclusion criteria were as follows: a) received ablation and achieved complete ablation. b) Aged 18–75 years. c) Child-Pugh classification was class A or B. d) All patients did not receive other antitumor therapy. The Exclusion criteria were as follows: a) advanced HCC. b) with second primary malignant tumors. c) clinical follow-up data incomplete.
Our research was approved by the Medical Ethics Committee of Beijing Youan Hospital and conducted in accordance with the Helsinki Declaration. The requirement for informed consent was waived by the Ethics Commission because the study was based on deidentified data.
Clinicopathologic Characteristics
The demographic, clinical, and pathological information was collected on HCC patients. Demographics included age, gender, cirrhosis, drinking history, smoking history, hypertension, and diabetes. Clinical and pathological data was composed of alpha-fetoprotein (AFP), alanine transaminase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), albumin (ALB), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), monocyte-to-lymphocyte ratio (MLR), and gamma-glutamyl transferase to lymphocyte ratio (GLR).
Therapeutic Procedure
All of the patients received TACE combined with ablation, which was conducted by experienced interventional radiologists. Under local anesthesia, percutaneous right femoral artery puncture with a modified Seldinger technique was performed. Through the microcatheter, a mixture of doxorubicin (Pfizer Inc., New York, NY, USA) and lipiodol (Guerbet, Villepinte, France) was injected into the blood-supply artery of the cancer. The gelfoam or polyvinyl alcohol particles was used to embolize the feeding artery, and the disappearance of the vessel stain was considered the complete embolization. Ablation was performed 1 to 2 weeks after TACE by radiofrequency, microwave, and argon-helium. The ablation area was expanded by 0.5–1cm to determine complete coverage. Otherwise, the procedure was defined as incomplete ablation. The detailed protocol of ablation was described in our previous study. Immediately following ablation, all patients underwent a contrast-enhanced CT to evaluate the success of the ablation and possible complications. Complete ablation was defined as non-enhancement on contrast-enhanced CT with the ablation zone.
Follow-Up
All patients were regularly followed up at the outpatient clinic. Tumor response was assessed by contrast CT or MRI at 4–6 weeks after ablation. Patients were followed up every three months during the first year and every six months thereafter. Follow-up examinations consisted of imaging examinations, liver function, and blood tests to monitor recurrence. Recurrence was confirmed when the imaging results showed an enhanced signal within. Recurrence-free survival (RFS) was defined as the time from initial treatment to the diagnosis to relapse.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviations, and categorical variables were presented as numbers (percentage). Differences between groups were compared by t-test, Mann–Whitney, ANOVA, and chi-square tests. Survival rates were calculated by the Kaplan-Meier method and compared with the Log rank test. Multivariate Cox regression analysis followed by lasso regression analysis was carried out to identify independent risk factors, which were used to construct a nomogram to predict recurrence. Prediction ability was measured by receiver-operating characteristic (ROC) curves and areas under the curves (AUC). The calibration of the nomogram was evaluated by the Hosmer-Lemeshow test and displayed by the calibration curves. Decision curve analysis (DCA) was employed to determine the clinical effectiveness by calculating the net benefits for a range of threshold probabilities. According to the nomogram score, patients were divided into low-risk, medium-risk, and high-risk groups, and the Kaplan-Meier curves were generated.
All data were analyzed with SPSS (version 26.0, IBM, Armonk, NY, USA) and R software (version 4.1.3) in this study, and the P-value less than 0.05 was considered statistically significant (two-tailed tests).
Result
Baseline Characteristics
A total of 990 HCC patients who underwent TACE combined ablation in Beijing Youan Hospital were enrolled in the study, including 778 (79.6%) males and 202 (20.1%) females. At the time of diagnosis, 212 (21.4%) patients had diabetes. There were 745 (75.3%) patients with Child-Pugh A and 245 (24.7%) patients with Child-Pugh B. The 990 HCC patients included 478 (48.3%) patients with S-S, 209 (21.1%) patients with S-L, 182 (18.4%) patients with M-S, and 121 (12.2%) patients with M-L ().
Table 1 Demographics and Clinical Characteristics for HCC Patients
Efficacy
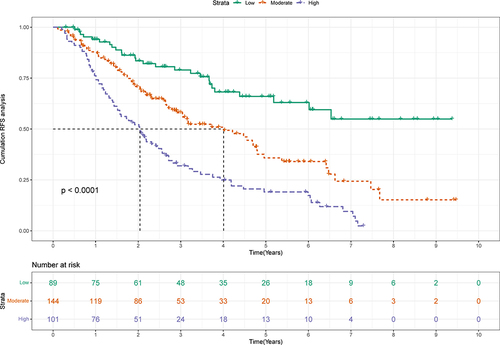
The median follow-up was 4.03 years in this study. The K-M curves indicated a better prognosis in patients with S-S (). The mRFS in S-S patients was significantly longer than the S-L, M-S, and M-L patients (3.8 years VS 1.47 years, 1.47 years, and 1.13 years, P<0.0001). However, the 1-, 3-, and 5-year RFS rates for S-S patients were 86.2%, 56.7%, and 40.1%, and the recurrence rate remained high. Consequently, it is necessary to create a nomogram for patients with S-S to identify signs of relapse in order to provide timely treatment.
Figure 1 Kaplan-Meier plot of RFS for HCC patients.
Next, we randomly divided the patients with S-S into a training cohort (N=334) and a validation cohort (N=161) in a 7:3 ratio. The validation cohort had a similar characteristic as the training cohort (P>0.05). In the two cohorts, the majority of the patients were male (74.9% VS 77.8%, P=0.570), and the average was over 50 years (56.4±9.28 VS 56.1±9.04, P=0.697). Most of the patients had cirrhosis (85.9% VS 85.4%, P=0.997), but the majority of Child-Pugh classifications were A (76.4% VS 79.9%, P=0.257), suggesting that patients had good liver function ().
Table 2 Demographics and Clinical Characteristics for Training and Validation Sets
The Prediction Model Was Built Based on the Lasso-Cox Regression
Independent Prognostic Factors of RFS
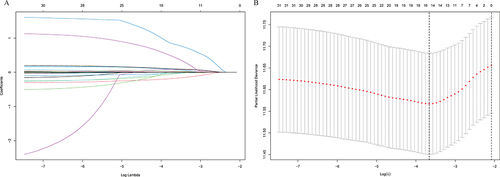
Lasso regression was used to screen the parameters, and the variation characteristics of the coefficient of these variables were shown in . The model exhibited outstanding performance and the least number of independent variables when λ was 0.026 (). The screened variables included age, gender, hypertension, diabetes, antiviral, smoking, drinking, family, cirrhosis, AFP, RBC, MLR, GLR, Alb, and INR. These variables were incorporated into multivariate Cox regression analysis to identify independent prognostic factors associated with recurrence. The final Results obtained were age (HR: 1.02, 95% CI: 1.01–1.04), RBC (HR: 0.93, 95% CI: 0.87–0.99), MLR (HR: 2.57, 95% CI: 1.17–5.65), INR (HR: 3.01, 95% CI: 1.19–7.62) and GLR (HR: 1.00, 95% CI: 1.00–1.01) ().
Table 3 Cox Proportional Hazards Regression to Predict Recurrence Based on Lasso Regression
Develop the Nomogram
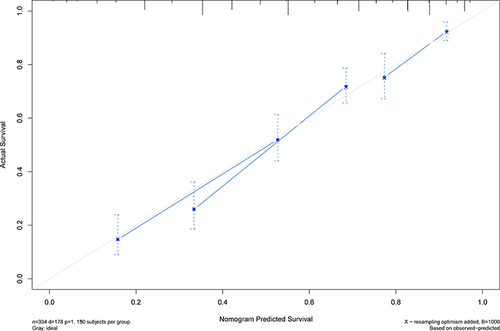
The nomogram was established based on the independent predictors found by the Lasso-Cox regression analysis (). In the training cohort, the C-index was 0.704 (95% CI: 0.67–0.74), with AUCs of 0.726, 0.800, and 0.780 at 1-, 3-, and 5-year, and these indicated the validity of the nomogram in predicting RFS (). The calibration curve showed optimal agreement between the predicted and observed outcomes (). Moreover, the 1-, 3-, and 5-year DCA curves testified that the nomogram had a high net benefit ().
Figure 3 Nomogram, including Age, MLR, GLR, RBC, and INR for 1-, 3-, and 5- years recurrence free survival (RFS) in HCC patients with high HBsAg levels in AFP. The nomogram is valued to obtain the probability of 1-, 3-, and 5- years recurrence by adding up the points identified on the points scale for each variable.
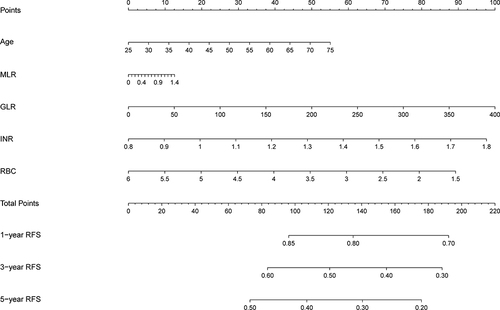
Figure 4 1-, 3-, and 5-year ROC curves of the nomogram in the training cohort.
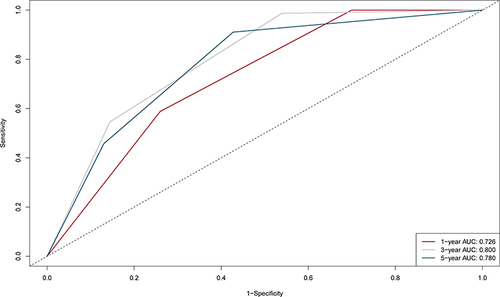
Figure 6 1-, 3-, and 5-year DCA curves of the nomogram in the training cohort. (A) One-year decision curve analysis in the training cohort. (B) Three-year decision curve analysis in the training cohort. (C) Five-year decision curve analysis in the training cohort.
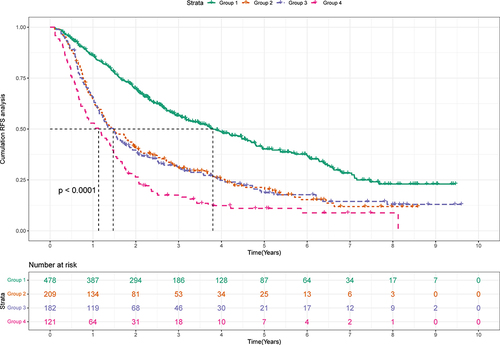
Patients were divided into three groups according to the score of the nomogram: low-risk group, medium-risk group, and high-risk group. And there were apparent variances in RFS (NA, 4.01 years, and 2.04 years) between the low-risk (N=89), medium-risk (N=144), and high-risk (N=101) groups (P<0.0001) (). The 1-, 3-, and 5-year RFS rates were 94.0%, 78.9%, and 65.9% in the low-risk group and 76.2%, 31.9%, and 19.1% in the high-risk group.
Validate the Nomogram
Internal validation was conducted to verify the reliability and stability of the nomogram. In the validation cohort, the C-index was 0.71 (95% CI: 0.66–0.76), and 1-,3-, and 5-year AUCs were 0.752, 0.761, and 0.760, both indicating good discrimination (Figure S1). Besides, all calibration curves demonstrated good consistency, and the DCA curves also showed that the nomogram had good clinical utility (Figures S2 and S3). Patients in the validation cohort were similarly categorized into low-risk, medium-risk, and high-risk groups, and the low-risk group (NA) also had a significantly higher RFS than the high-risk and medium-risk groups (2.3 and 4.8 years) (P<0.0001) (Figure S4). The 1-, 3-, and 5-year RFS rates were 95.8%, 77.8%, and 64.4% in the low-risk group and 76.3%, 35.4%, and 23.2% in the high-risk group. These proved that the nomogram could distinguish patients at high risk of recurrence.
Discussion
HCC is one of the most frequent and fatal malignancies, which seriously threatens human health.Citation20,Citation21 The size and number of tumors are important for staging and play a key role in prognosis.Citation22–24 Our study further explored the role of tumor size and number in tumor progression and prognosis of the HCC patients who underwent ablation and found that patients with single small HCC had the best prognosis. In addition, we created a nomogram by Lasso-Cox regression to predict the prognosis of S-S patients accurately.
Tumor burden, measured as maximum tumor size and number of tumors, has been incorporated as a common element in many prognostic models of HCC.Citation25 Previous studies have revealed that tumor burden is closely associated with patient survival and recurrence.Citation26–29 However, tumor recurrence is still common, even in HCC patients without aggressive features. Furthermore, it might have been insufficient to predict the recurrence of HCC using only tumor burden in all patients with HCC. The results of the study in our center indicated that S-S patients had a better prognosis, with a mRFS of 3.85 years. Because of the good prognosis, monitoring of this group of patients after ablation can be untimely. Furthermore, S-S patients do not require further treatment after radical therapy, which makes follow-up more difficult. However, the rates of 1-, 3-, and 5-year RFS were 86.3%, 56.7%, and 40.2%, respectively. Due to the high recurrence, we need to create a nomogram to predict the population at high risk of recurrence in patients with S-S to help clinicians guide clinical decisions.
Several studies have suggested that liver weight and portal blood flow velocity are reduced in older patients, which can lead to less reparability of the liver in younger patients.Citation30 Tumor progression after treatment is faster in the elderly than in younger patients because of low immunity, resulting in a high recurrence rate and poor prognosis.Citation15 As a classic inflammation-associated cancer, approximately 90% of the liver cancer burden is caused by viral hepatitis, excessive drinking, and non-alcoholic fatty liver disease (NAFLD).Citation31,Citation32 Both the immune microenvironment and inflammatory marker are part of the systemic inflammatory response and exert a fundamental role in tumor initiation, progression, and metastasis.Citation33,Citation34 MLR is the ratio of monocytes to lymphocytes. Monocytes accumulate at sites of inflammation and differentiate into M1 and M2 macrophages as inflammation occurs. Activated circulating monocytes can secrete a variety of pro-inflammatory factors, which are involved in tumor development.Citation35–37 Lymphocyte, which possesses potent anticancer activities, plays critical roles in host immune responses and anti-tumor immunity.Citation38 Tumor invasion and the release of inflammatory factors are responsible for the destruction of hepatocytes, which is characterized by an increase of GGT in peripheral blood.Citation39 GLR could be used as a potential prognostic marker of early recurrence and prognosis, and higher GLR is associated with poor outcomes.Citation40–42 INR is a good measure of hepatic synthetic function. A large cohort study including 2509 HCC patients showed that low INR levels had shorter RFS and OS compared to high INR levels.Citation43 The incidence of MVI was also found to be significantly higher in HCC patients with low INR levels than normal or high INR levels.Citation44 RBC could inhibit tumor growth and metastasis by removing circulating immune complexes.Citation45 Normal RBCs improve the killing effect on tumor cells by increasing the activity of the lymphocyte-activated killer cells and the NK cells through the release of natural killer cell-activating factors.Citation46
The nomogram contains five variables screened by Lasso-Cox regression, including age, MLR, GLR, INR, and RBC. The scores on the nomogram are obtained by drawing a vertical line through the position of the corresponding total score so that it intersects the three lines predicting recurrence, and the values shown at the intersection were predicted RFS at 1, 3, and 5 years. The C-index of the nomogram for training and validation cohorts was 0.704 and 0.71, respectively. The correction and ROC curves verified the predictive performance of the nomogram. The DCA curves revealed that the nomogram had a high positive net benefit, which implied good clinical application potential. Patients in the training and validation cohorts were categorized into low-risk, intermediate-risk, and high-risk groups based on the scores of the nomogram, and there was a statistically significant difference in RFS among the three groups (P<0.0001), suggesting that our nomogram had good ability to differentiate S-S patients.
TACE could mark tumors that are not clearly visualized on imaging and reduce tumor size by embolizing tumor vessels, reducing ablation time, and increasing the success rate of ablation.Citation16 The previous investigation of our team and foreign reports confirmed that the combination therapy with TACE and ablation is superior to TACE alone or ablation alone in RFS and OS.Citation47,Citation48 Simultaneous examination of comprehensive patient features covering demographics, liver function, and inflammatory markers was a major strength of our study. Compared with single-factor analysis, Lasso regression can minimize the multicollinearity in variables.
Our study still had some limitations. First, this was a retrospective cohort study. Thus, further investigation in a prospective clinical trial is warranted. Secondly, as a single-center study, there was a potential selection bias of the patients in the cohort. Because of the long span in our study, there are differences in ablation between periods and medical teams. As such, the results of the present study need external validation to be verified further. Nevertheless, we used up to eight years of follow-up to create an accurate and reliable nomogram to better guide clinical practice for this group of HCC patients with single and small HCC.
Conclusion
In summary, patients with single small HCC had the best prognosis. However, the rates of 1-, 3-, and 5-year RFS were 86.3%, 56.7%, and 40.2% in patients with S-S. For S-S patients, we created an accurate and reliable nomogram to predict recurrence based on the Lasso-Cox regression analysis. The nomogram, including age, MLR, GLR, RBC, and INR, demonstrated adequate discrimination ability, which could better guide clinical decisions.
Ethics Statement
The study protocol was approved by the Ethics Committee of Beijing Youan Hospital and conducted following the ethical principles outlined in the Helsinki Declaration of 1964 and its subsequent amendments, or other ethical standards with equivalent requirements. As a retrospective study as well as to ensure patient confidentiality, the identities of the individuals included in this study were anonymized using computer-generated ID numbers, and thus, patient consent was waived.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no competing interests in this work.
Acknowledgments
The authors highly appreciate all patients who participated in the study.
Data Sharing Statement
All relevant data are available within the manuscript and its Supplementary Material Files. Further enquiries can be directed to the corresponding author (Yonghong Zhang, [email protected]).
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. 2020;18(12):2650–2666. doi:10.1016/j.cgh.2019.07.060
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi:10.1007/s12072-017-9799-9
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
- Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14(4):203–217. doi:10.1038/nrgastro.2016.193
- Wu S, Li Z, Yao C, et al. Progression of hepatocellular carcinoma after radiofrequency ablation: current status of research. Front Oncol. 2022;12:1032746. doi:10.3389/fonc.2022.1032746
- Bai XM, Cui M, Yang W, et al. The 10-year survival analysis of radiofrequency ablation for solitary hepatocellular carcinoma 5 cm or smaller: primary versus recurrent HCC. Radiology. 2021;300(2):458–469. doi:10.1148/radiol.2021200153
- Erstad DJ, Tanabe KK. Hepatocellular carcinoma: early-stage management challenges. J Hepatocell Carcinoma. 2017;4:81–92. doi:10.2147/jhc.S107370
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
- Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
- Tsilimigras DI, Bagante F, Sahara K, et al. Prognosis after resection of Barcelona clinic liver cancer (BCLC) Stage 0, A, and B hepatocellular carcinoma: a comprehensive assessment of the current BCLC classification. Ann Surg Oncol. 2019;26(11):3693–3700. doi:10.1245/s10434-019-07580-9
- Feng LH, Sun HC, Zhu XD, et al. Prognostic nomograms and risk classifications of outcomes in very early-stage hepatocellular carcinoma patients after hepatectomy. Eur J Surg Oncol. 2021;47(3):681–689. doi:10.1016/j.ejso.2020.10.039
- Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
- Wang Q, Qiao W, Zhang H, et al. Nomogram established on account of Lasso-Cox regression for predicting recurrence in patients with early-stage hepatocellular carcinoma. Front Immunol. 2022;13:1019638. doi:10.3389/fimmu.2022.1019638
- Sun Y, Xiong Y, Wang Q, Qiao W, Zhang H, Zhang Y. Development and validation of a nomogram to predict the recurrence of hepatocellular carcinoma patients with dynamic changes in AFP undergoing locoregional treatments. Front Oncol. 2023;13:1206345. doi:10.3389/fonc.2023.1206345
- Johnson P, Zhou Q, Dao DY, Lo YMD. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19(10):670–681. doi:10.1038/s41575-022-00620-y
- Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi:10.1016/s0140-6736(22)01200-4
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
- Toh MR, Wong EYT, Wong SH, et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology. 2023;164(5):766–782. doi:10.1053/j.gastro.2023.01.033
- Yamashita YI, Imai K, Yusa T, et al. Microvascular invasion of single small hepatocellular carcinoma ≤3 cm: predictors and optimal treatments. Ann Gastroenterol Surg. 2018;2(3):197–203. doi:10.1002/ags3.12057
- Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: a multicentre observational study. J Hepatol. 2019;70(5):893–903. doi:10.1016/j.jhep.2019.01.013
- Kadalayil L, Benini R, Pallan L, et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24(10):2565–2570. doi:10.1093/annonc/mdt247
- Müller L, Hahn F, Auer TA, et al. Tumor burden in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: head-to-head comparison of current scoring systems. Front Oncol. 2022;12:850454. doi:10.3389/fonc.2022.850454
- Hung YW, Lee IC, Chi CT, et al. Redefining tumor burden in patients with intermediate-stage hepatocellular carcinoma: the seven-eleven criteria. Liver Cancer. 2021;10(6):629–640. doi:10.1159/000517393
- Ho SY, Liu PH, Hsu CY, et al. Tumor burden score as a new prognostic marker for patients with hepatocellular carcinoma undergoing transarterial chemoembolization. J Gastroenterol Hepatol. 2021;36(11):3196–3203. doi:10.1111/jgh.15593
- Kaewdech A, Sripongpun P, Assawasuwannakit S, et al. FAIL-T (AFP, AST, tumor sIze, ALT, and Tumor number): a model to predict intermediate-stage HCC patients who are not good candidates for TACE. Front Med Lausanne. 2023;10:1077842. doi:10.3389/fmed.2023.1077842
- Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232(1):10–24. doi:10.1097/00000658-200007000-00003
- Schmucker DL. Aging and the liver: an update. J Gerontol a Biol Sci Med Sci. 1998;53(5):B315–20. doi:10.1093/gerona/53a.5.b315
- Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi:10.1038/nrdp.2016.18
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:6917):860–7. doi:10.1038/nature01322
- Chen Q, Li F, Zhong C, et al. Inflammation score system using preoperative inflammatory markers to predict prognosis for hepatocellular carcinoma after hepatectomy: a cohort study. J Cancer. 2020;11(17):4947–4956. doi:10.7150/jca.45274
- Mao S, Yu X, Shan Y, Fan R, Wu S, Lu C. Albumin-Bilirubin (ALBI) and monocyte to lymphocyte ratio (MLR)-based nomogram model to predict tumor recurrence of AFP-negative hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:1355–1365. doi:10.2147/jhc.S339707
- Jiang S, Yang Y, Fang M, Li X, Yuan X, Yuan J. Co-evolution of tumor-associated macrophages and tumor neo-vessels during cervical cancer invasion. Oncol Lett. 2016;12(4):2625–2631. doi:10.3892/ol.2016.5014
- Lippitz BE, Harris RA. Cytokine patterns in cancer patients: a review of the correlation between interleukin 6 and prognosis. Oncoimmunology. 2016;5(5):e1093722. doi:10.1080/2162402x.2015.1093722
- Chen Y, Zhang S, Wang Q, Zhang X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncol. 2017;10(1):36. doi:10.1186/s13045-017-0408-0
- Wang Q, Qiao W, Liu B, et al. The monocyte to lymphocyte ratio not only at baseline but also at relapse predicts poor outcomes in patients with hepatocellular carcinoma receiving locoregional therapy. BMC Gastroenterol. 2022;22(1):98. doi:10.1186/s12876-022-02180-6
- Zhao Z, Zhu Y, Ni X, et al. Serum GGT/ALT ratio predicts vascular invasion in HBV-related HCC. Cancer Cell Int. 2021;21(1):517. doi:10.1186/s12935-021-02214-1
- Li S, Xu W, Liao M, et al. The significance of gamma-glutamyl transpeptidase to lymphocyte count ratio in the early postoperative recurrence monitoring and prognosis prediction of AFP-negative hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:23–33. doi:10.2147/jhc.S286213
- Zhao H, Liu X, Xu R, Guo X, Shen A. Predictive value of and relationship between the gamma-glutamyl transpeptidase to lymphocyte ratio and CT features in hepatocellular carcinoma patients with postoperative adjuvant TACE. J buon. 2021;26(4):1346–1354.
- Zhang W, Bi Y, Yang K, et al. A new model based on gamma-glutamyl transpeptidase to lymphocyte ratio and systemic immune-inflammation index can effectively predict the recurrence of hepatocellular carcinoma after liver transplantation. Front Oncol. 2023;13:1178123. doi:10.3389/fonc.2023.1178123
- Zhang XP, Zhou TF, Wang ZH, et al. Association of preoperative hypercoagulability with poor prognosis in hepatocellular carcinoma patients with microvascular invasion after liver resection: a multicenter study. Ann Surg Oncol. 2019;26(12):4117–4125. doi:10.1245/s10434-019-07504-7
- Liu S, Xu Z, Fang Z, et al. The combination of age, international standardized ratio, albumin and γ-glutamyl transpeptidase (AIAG), tumor size and alpha fetoprotein (AFP) stage as the prognostic model for hepatitis B-related hepatocellular carcinoma. Int J Gen Med. 2021;14:4291–4301. doi:10.2147/ijgm.S323293
- Emlen W, Burdick G, Carl V, Lachmann PJ. Binding of model immune complexes to erythrocyte CR1 facilitates immune complex uptake by U937 cells. J Immunol. 1989;142(12):4366–4371. doi:10.4049/jimmunol.142.12.4366
- Wei J, Wang Z. Establishment of a predictive model for short-term efficacy of transcatheter arterial chemoembolization treatment in hepatocellular carcinoma and its clinical application. J Cancer Res Ther. 2019;15(4):941–946. doi:10.4103/jcrt.JCRT_52_19
- Chen S, Zeng X, Su T, et al. Combinatory local ablation and immunotherapies for hepatocellular carcinoma: rationale, efficacy, and perspective. Front Immunol. 2022;13:1033000. doi:10.3389/fimmu.2022.1033000
- Kim AR, Park E, Kwon SY, et al. Efficacy and safety of combined radiofrequency ablation with transarterial chemoembolization in patients with Barcelona clinic liver cancer stage a hepatocellular carcinoma ineligible for curative treatment. Korean J Gastroenterol. 2019;73(3):167–176. doi:10.4166/kjg.2019.73.3.167