Abstract
The signal transducer and activator of transcription (STAT) family of proteins was originally discovered in the context of normal cell biology where they function to transduce intracellular and extracellular signals to the nucleus, ultimately leading to transcription of specific target genes and downstream phenotypic effects. It was quickly appreciated that the STATs, especially STAT3, play a fundamental role in human malignancy. In contrast to normal biology in which transient STAT3 signaling is strictly regulated by a tightly coordinated network of activators and deactivators, STAT3 is constitutively activated in human malignancies. Constitutive STAT3 signaling has been associated with many cancerous phenotypes across nearly all human cancers, including the upregulation of cell growth, proliferation, survival, and motility, among others. Studies involving candidate preclinical STAT3 inhibitors have further demonstrated that the reversal of these phenotypes results from pharmacologic or genetic inhibition of STAT3, suggesting that STAT3 may be a promising target for clinical interventions. Indeed, a Phase 0 clinical trial involving a STAT3 decoy oligonucleotide demonstrated that STAT3 is a drug-gable target in human tumors. Because of the ubiquity of overactive STAT3 in cancer, its role in promoting a wide variety of cancerous phenotypes, and the strong clinical and preclinical studies performed to date, STAT3 represents a promising target for the development of inhibitors for the treatment of human cancers.
Keywords:
Introduction
The signal transducer and activator of transcription (STAT) family is a group of ubiquitously expressed proteins involved in a wide variety of cellular processes. Canonical STAT signaling involves STAT monomers localized in the cytoplasm where they receive a wide variety of specific upstream signals. Upon activation, STATs dimerize and translocate to the nucleus where they activate transcription of specific target genes, ultimately leading to altered protein expression and cellular phenotype. To date, seven STAT family members have been identified – STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6 – and multiple isoforms of each have also been found. Each STAT protein includes several conserved domains that contribute to protein function (). The N-terminal protein-protein interaction domain (PPID) mediates interaction between neighboring STAT proteins (or other coregulatory proteins) and contributes to cooperative binding of STAT dimers on deoxyribonucleic acid (DNA), leading to the formation of stabilized tetramers.Citation1 This function, while nonessential for transcriptional activation, may contribute to enhanced STAT3 signaling by prolonging DNA binding. The DNA-binding domain is involved in sequence-specific DNA binding, recruitment of coactivators, and the activation of transcription of STAT3 target genes. The Src-homology 2 (SH2) domain is the mediator of STAT dimerization via reciprocal phosphotyrosine binding, a critical step for STAT activation. It is also involved in the recruitment of STAT to phosphotyrosine residues on other proteins, including tyrosine kinases, which then phosphorylate and activate STAT. The carboxy-terminal domains present in STAT1 and STAT2 are involved in further protein–protein interactions that impact STAT function, including those with comodulators of transcription such as the CREB binding protein.Citation2 These domains coordinately determine the varied functions of each STAT protein.
Figure 1 Domain architecture of STATs.
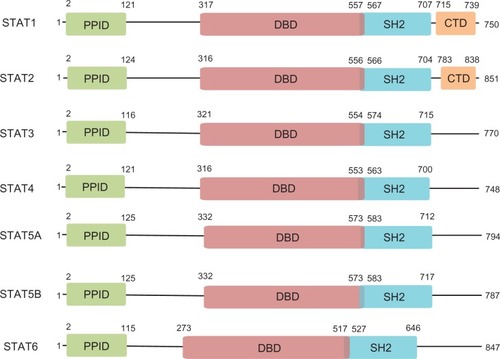
STAT proteins were originally identified in the context of their physiological roles as major effectors of cytokine and chemokine receptor signaling. In recent years, the discovery that dysregulated STAT proteins are key modulators of human malignancy has driven research into the functions of these proteins. It now appears that the contribution of STAT proteins to cancer, especially overexpression and overactivation of STAT3, is crucial for the development and progression of many cancers. As such, STAT3 is a promising target for the development of inhibitors and is the focus of much ongoing research and drug development.
Physiological roles of STAT3
Phosphorylation of STAT3 on tyrosine 705 (Y705) by various upstream kinases is critical for STAT3 activation.Citation3 A large number of protein tyrosine kinases directly phosphorylate STAT3 on Y705, including cytokine and chemokine receptors and their coactivators. Well-studied examples of such kinases include membrane integral receptor tyrosine kinases (RTKs) such as glycoprotein 130, the signaling subunit of the interleukin (IL)-6 receptor and other receptors, the epidermal growth factor receptor (EGFR), the fibroblast growth factor receptor, and non-RTKs that may or may not be associated with receptors such as Janus kinase (JAK), Src kinase, and Abl kinase.Citation3–Citation6 Upon STAT3 activation, dimers directly bind DNA at TT(N)4–6AA consensus sites and regulate transcription of specific target genes.Citation7 The binding affinity of STAT3 for this region is determined by both the nucleotide sequence and cooperative dimer-dimer interactions mediated by the amino-terminal PPID of STAT3.Citation7,Citation8 STAT3 activity can be further modulated by the phosphorylation of serine 727, though the context-specific consequences of serine 727 phosphorylation remain incompletely understood.Citation9,Citation10 In addition, STAT3 activity can be modified by reversible acetylation of lysine 685 (K685), an event that is critical for the formation of stable STAT3 dimers and is required for cytokine-induced STAT3-mediated transcription.Citation11,Citation12
Downregulation of STAT3 occurs by several mechanisms in normal biology. The suppressor of cytokine signaling (SOCS) family of proteins, especially SOCS1 and SOCS3, inhibit STAT3 in a cytokine-inducible manner by binding to and inhibiting upstream JAKs.Citation13,Citation14 Members of the protein inhibitor of activated STAT (PIAS) family, especially PIAS3, are small ubiquitin-like modifier-E3 ligases that bind specifically to STAT3 and abrogate its activity.Citation15 The SOCS and PIAS families together constitute major mechanisms by which STAT3 activity is downregulated quickly following stimulation with specific cytokines under normal conditions. Other proteins, including GRIM-19, can also abrogate STAT3 activity via direct interactions.Citation16 Importantly, enzymatic removal of the phosphate group from Y705 of STAT3 by protein tyrosine phosphatases (PTPs) can also occur. Like the upstream kinases, PTPs that inactivate STAT3 can be membrane integral (PTPR family, including PTPRT and PTPRD) or cytosolic (PTPN family, including PTPN2 and PTPN11).Citation17–Citation20 Additionally, removal of the acetyl group from K685 of STAT3 by deacetylases, including SirT1, can lead to STAT3 downregulation.Citation21,Citation22 The intricacy and redundancy of the many mechanisms of STAT3 activation and deactivation illustrate the importance of maintaining tight control over the STAT3 pathway in normal biology.
Perhaps the field of normal biology in which STAT3 is most well studied is that of inflammation and the immune response. The actions of many cytokines and chemokines that led to the discovery of the STAT family, especially IL-6 and interferon (IFN), are mediated principally by STAT3 and are critical for proper immune function. STAT3 activation is triggered in epithelial tissue and associated macrophages in response to immunoglobulin G complex deposition or injury.Citation23 In dendritic cells, the proinflammatory activity of IL-6 is mediated by transient activation of STAT3, whereas the antiinflammatory effects of IL-10 result from more sustained STAT3 activation.Citation24 Interestingly, artificial early termination of IL-10 signaling leads to an IL-6-like cellular response.Citation24 This rapid termination of IL-6 signaling appears to be mediated by SOCS3, which is encoded by a STAT3 target gene upregulated by both IL-6 and IL-10 that can inhibit signaling through the IL-6 receptor, but not the IL-10 receptor.Citation24 These findings suggest that the divergent consequences of various signals upstream of STAT3 may in turn be determined by the contribution of STAT3 inactivators that ultimately determine the duration of STAT3 signaling.
Increased STAT3 activity is also associated with wound healing. As many of the genes involved in wound healing are also involved in oncogenesis, it is not surprising that STAT3 regulates many of the same genes in both of these processes.Citation25 After cutaneous wounding in mice, IL-6 is upregulated in the epidermis primarily at the leading edge of the wound.Citation26 Genetic knockout of IL-6 in mice leads to deficient cutaneous wound healing, with knockout mice requiring up to three-fold longer to heal than wild-type mice.Citation26 Keratinocyte-specific STAT3 knockout in mice leads to impaired skin remodeling that results from impaired epidermal cell regeneration, confirming a central role for STAT3 in normal wound healing.Citation27 In the gut, STAT3 activation in intestinal epithelial cells regulates immune homeostasis.Citation28 Colonic CD11c+ cells secrete IL-22 in response to Toll-like receptor activation, leading to STAT3 activation in intestinal epithelial cells and promoting wound healing, demonstrating that STAT3 is essential for the wound healing process in a variety of tissues.Citation28
STAT3 plays additional roles in several other normal cellular processes. For example, STAT3 functions as the downstream effector of important hormones such as insulin and leptin in both the brain and peripheral tissues, allowing for regulation of energy and metabolite homeostasis.Citation29–Citation32 STAT3 is also involved in autophagy, embryogenesis, proper thymic function, mammary development, and other processes.Citation33–Citation36 The importance of STAT3 activity in normal biology is demonstrated in part by the ubiquity of its tissue distribution. STAT3 activation across these tissues is a transient event, and STAT3 is quickly downregulated. When aberrations occur in the strict regulation of STAT3, malignancies can develop.
Role of STAT3 in cancer
Genomic and epigenomic deregulation of STAT3 in cancer
The STAT3 protein is overexpressed and/or hyperactivated in the majority of human cancers.Citation37 The prevalence of STAT3 overactivation in cancer cannot be explained by mutational activation of STAT3 because somatic mutation of the STAT3 gene in cancer is rare (1.08%; 54/4980 tumors analyzed to date by whole exome sequencing by The Cancer Genome Atlas).Citation38 Instead, STAT3 is the common effector of activating events affecting oncoproteins and deactivating events affecting tumor-suppressive proteins that ultimately lead to constitutive STAT3 activation. Dysregulation of diverse pathways that converge on STAT3 allows escape from the strict regulation that maintains transient STAT3 signaling in normal cell biology, leading to tumor-promoting cell proliferation, survival, motility, invasion, and angiogenesis. In addition, activation of STAT3 is associated with emergent resistance to targeted therapies and decreased patient survival.Citation39,Citation40
Among the first observations that indicated the importance of STAT3 in cancer was the phosphorylation of STAT3 by v-Src – a known oncoprotein at that time – as well as constitutive STAT3 tyrosine phosphorylation and DNA-binding in several v-Src-transformed cell lines.Citation41 Further study revealed that STAT3 activation and specific gene regulation is required for Src-mediated transformation of NIH-3T3 cells, leading to the conclusion that activation of STAT3 signaling is a critical component of malignant transformation.Citation42,Citation43 Additional studies generated similar findings in diverse systems, providing a strong case for the central role of STAT3 in a wide array of cancers.Citation39,Citation44–Citation49 Years of continued research have convinced physicians and scientists of the significance of STAT3 in cancer and have elucidated many, though certainly not all, of the mechanisms by which aberrant STAT3 signaling contributes to malignancy.
In addition to Src kinase, many kinases upstream of STAT3 activation are frequently found to be altered in cancer cells, leading to constitutive kinase and STAT3 signaling. In neuroblastoma, frequent point mutation of the RTK anaplastic lymphoma kinase (ALK) in the kinase domain (F1174L) leads to constitutive activation of STAT3.Citation50 Forced expression of this mutant, but not wild-type ALK, is sufficient to transform Ba/F3 cells, enables cytokine-independent growth, and confers sensitivity to the small molecule ALK inhibitor TAE684 in neuroblastoma cell line models.Citation50 Further, in ALK-positive anaplastic large-cell lymphoma cells that overexpress STAT3, inhibition of ALK leads to downregulation of total and active STAT3.Citation51 Similar results have been found for other kinase domain mutations, including the well-studied JAK2 mutation V617F, which is primarily found in myeloproliferative disorders.Citation52,Citation53 Activation of JAK2 caused by this mutation leads to constitutive activation of STAT3 and is associated with reduced survival in idiopathic myelofibrosis.Citation54,Citation55 Another mechanism of kinase-driven STAT3 activation in cancer is genomic amplification of kinase genes or RTK ligands with subsequent protein overexpression, leading to enhanced activation of wild-type kinases. For example, gene amplification of PDGFRα or EGFR in distinct subsets of glial tumors leads to enhanced expression of those proteins and downstream signaling events, including activation of STAT3.Citation56 Overexpression of RTK ligands, such as IL-6 or transforming growth factor-α, can also lead to persistent STAT3 activation via autocrine signaling through their receptors.Citation57,Citation58 Other genomic events and rearrangements can also lead to kinase and STAT3 activation, such as that observed for the EGFRvIII protein, a constitutively active EGFR variant that is missing a large portion of the extracellular domain and exhibits impaired EGF binding. EGFRvIII expression is sufficient to transform NR6 cells (murine fibroblasts) and is associated with STAT3 activation and target gene expression.Citation59,Citation60
Conversely, activation of STAT3 in human cancers can result from genomic or epigenomic inactivation of proteins that normally downregulate STAT3 activity. In contrast to the frequent activation of kinases by point mutation, deactivation of tumor-suppressive proteins by point mutation is relatively rare because of the necessity for such mutations to function in a dominant negative manner. These events do occur, however, as evinced by recently reported mutations in GRIM-19 that ablate its STAT3 inhibitory activity and promote tumor growth.Citation16 Many investigators have recently begun to focus on epigenomic silencing of tumor-suppressive proteins that normally downregulate STAT3, especially by promoter hypermethylation. In lung cancer, for example, SOCS3 is frequently downregulated by promoter hypermethylation, and restoration of SOCS3 expression in cells where it was previously silenced leads to downregulation of active STAT3, induction of apoptosis, and suppression of cell growth.Citation61 As SOCS proteins have not been demonstrated to inhibit kinases other than JAKs, inactivation of the SOCS family is unlikely to contribute substantially to aberrant STAT3 signaling across cancer types. Indeed, SOCS1 is unable to inhibit STAT3-mediated transformation of NIH-3T3 cells by v-Src and does not reduce STAT3 target gene expression in this system.Citation62 Abnormal epigenomic alteration of other proteins that normally cause direct inactivation of STAT3, especially PTPs, remains incompletely understood and warrants further study. For instance, frequent methylation in the promoter region of PTPN6 is strongly correlated with decreased PTPN6 messenger ribonucleic acid (mRNA) expression and increased pSTAT3 expression in immunodeficiency-related non-Hodgkin lymphoma, demonstrating that epigenetic silencing of a phosphatase targeting pSTAT3 can lead to STAT3 activation.Citation63 Other phosphatases that act upon pSTAT3, including PTPRD and PTPRT, have recently been reported to have tumor-suppressive functions and are frequently found to be altered in cancer cells, including, by promoter methylation.Citation18,Citation64 Additionally, the glutathione S-transferase family member GSTP1, which downregulates EGF-mediated STAT3 signaling and expression of STAT3 target genes via a direct interaction with STAT3, is promoter-hypermethylated in hepatitis B virus-associated hepatocellular carcinoma and prostate cancer, and is subsequently downregulated.Citation65–Citation67
The diversity of genomic and epigenomic alterations in both activators and deactivators of STAT3 signaling is, in part, responsible for the high degree of difficulty in developing therapeutics that are applicable to a wide array of cancers; this suggests that targeting STAT3 directly may prove more efficacious. The further understanding of the many mechanisms contributing to aberrant STAT3 pathway activation may lead to the identification of biomarkers that can be used to establish subsets of patients who will most likely to respond to STAT3 inhibition.
STAT3 in cell growth and proliferation
STAT3 is a critical driver of cell growth in cancer, but not in normal cells.Citation68 Constitutive STAT3 signaling has been implicated in aberrant cell growth and proliferation in many cancers, including head and neck squamous cell carcinoma, colorectal carcinoma, melanoma, glioblastoma multiforme, multiple myeloma, non-small cell lung cancer, and others.Citation58,Citation69–Citation73 A critical mediator of cell growth downstream of STAT3 is its target CCND1, encoding gene cyclin D1, which is upregulated transcriptionally by active STAT3 and is required for STAT3-mediated transformation.Citation74 Cyclin D1, in turn, acts through cyclin-dependent kinase (cdk)-dependent and cdk-independent mechanisms to allow passage through the G1 checkpoint of the cell cycle, ultimately leading to continuous and unregulated cell growth and proliferation.Citation75 In addition, the STAT3 target gene MYC, which itself encodes a transcription factor, is also a potent promoter of cell growth and is required for Src-mediated cellular transformation via STAT3.Citation45
Other target genes of STAT3 that contribute to cell growth and proliferation include cytokines and growth factors that often act in an autocrine manner to further increase STAT3 signaling and/or other mitogenic pathways. It has recently come to be appreciated that nontraditional gene products, including micro-RNA molecules that downregulate specific genes by binding to specific mRNA transcripts, are also mediators of STAT3 mitogenic function. For example, STAT3 is persistently active in Wilms’ tumor, a genetically heterogeneous childhood kidney cancer, where it transcriptionally upregulates the microRNA miR-370, which in turn regulates cell proliferation and tumorigenicity in mice.Citation76 Cells transfected with miR-370 exhibit downregulation of the tumor suppressor WTX via direct binding to the 3′-untranslated region of WTX mRNA, leading to its degradation.Citation76 These cells also exhibit downregulation of the proteins p21Cip1 and p27Cip1 (which inhibit progress through the cell cycle) and upregulation of cyclin D1, illustrating novel mechanisms downstream of STAT3 that contribute to its proliferative capacity.Citation76
STAT3 in apoptosis and cell survival
Constitutive STAT3 activation leads to evasion of apoptosis and a subsequent increase in cell survival. STAT3 transcriptionally regulates several Bcl-2 family members, including the antiapoptotic proteins Bcl-xL, Bcl-2, and Mcl-1.Citation77 The Bcl-2 family regulates apoptosis via homodimerization/heterodimerization (the dynamics of which are determined stoichiometrically) and translocation to the mitochondrial membrane, where they ultimately regulate cytochrome c release and the initiation of apoptosis. STAT3-mediated upregulation of Bcl-xL, Bcl-2, and Mcl-1 contributes to apoptosis evasion in several cancers.Citation77–Citation79 STAT3-mediated Bcl-2 expression in metastatic subclones of the parental cell line MDA-MB435 (estrogen receptor-negative breast cancer) correlates with increased pSTAT3, but not with other transcription factors that regulate Bcl-2, and contributes to chemoresistance in this cell line, suggesting that the antiapoptotic effects of STAT3 contribute to treatment sensitivity.Citation80 Furthermore, a small peptide, ST3-H2A2, that inhibits the function of the N-terminal PPID of STAT3 induces the expression of multiple proapoptotic genes (and others) in prostate cancer cells, suggesting that STAT3 inhibition may restore normal apoptosis.Citation81
STAT3 target genes that are not themselves in the Bcl-2 family can also contribute to evasion of apoptosis. Octamer transcription factor-1 (Oct-1) has been reported to be a target gene of STAT3 in esophageal squamous carcinoma cells (Eca-109), where STAT3 and Oct-1 coordinately regulate apoptosis.Citation39 In these cells, activation of STAT3 by IL-6 treatment suppresses apoptosis as assessed by TUNEL staining, and knockdown of either STAT3 or Oct-1 by RNA interference enhances apoptosis.Citation39 Conversely, forced overexpression of Oct-1 – even in the presence of STAT3 knockdown – is sufficient to reduce apoptosis to similar levels as IL-6 treatment, suggesting that STAT3-driven Oct-1 expression may be sufficient to reduce apoptosis to minimal levels in these cells.Citation39STAT3 and Oct-1 knockdown leads to increased expression of proapoptotic Bax and Bad proteins, cytochrome c release from mitochondria, subsequent cleavage of caspases 3 and 9, and decreased expression of antiapoptotic Bcl-2 and Bcl-xL proteins.Citation39 These findings provide a mechanism by which STAT3 overactivation leads to positive feedback in the suppression of apoptosis in conjunction with its target gene Oct-1.
STAT3 in migration and invasion
Constitutively active STAT3 further contributes to the cancer phenotype by promoting motility and invasion, including in human melanoma where increased activation of STAT3 promotes metastasis to the brain.Citation82 The metastatic action of STAT3 is, in part, mediated by matrix metalloproteinases, a family of zinc-dependent endopeptidases that are secreted into the extracellular matrix. There the matrix metalloproteinases degrade extracellular matrix proteins, leading to facilitated cell migration, invasion through the basement membrane, and ultimately to the establishment of metastatic secondary tumors. The STAT3 target genes MMP-2 and MMP-9 are upregulated in esophageal squamous carcinoma cells (Eca-109) that express high pSTAT3, and STAT3 knockdown by RNA interference in these cells leads to downregulation of MMP-2 and MMP-9, dysregulation of cell migration directionality, decreased migration speed, and disorganization of F-actin formation; this demonstrates a central role for STAT3 in MMP-2/9-mediated cell motility.Citation39 In addition, activation of STAT3 is required for maximal MMP-1 and MMP-10 induction in response to EGF in T24 bladder cancer cells, where STAT3 is a critical mediator of malignant characteristics.Citation83
Other mechanisms that contribute to STAT3-mediated cell migration have been elucidated. For instance, EGFR activation via autocrine signaling in near-confluent, but not sparse, squamous cell carcinoma cells leads to activation of STAT3 and subsequent overexpression of the transmembrane glycoprotein podoplanin (PDPN).Citation84 This cell density-regulated PDPN expression leads to increased cell migration and invasion, and these effects are reversed by shRNA knockdown of PDPN.Citation84 Importantly, the observation of increased PDPN extends to clinical samples, in which PDPN is overexpressed in basal cell layers at the invading front of in situ SCC lesions, providing an additional clinically relevant mechanism by which STAT3 contributes to motility and invasion.Citation84 Similarly, STAT3 is necessary for EGFR-mediated migration and invasion in prostate carcinoma cells.Citation85 In addition, shRNA knockdown of STAT3 in Tu-2449 glioma cells leads to decreased PDPN expression and microvilli formation relative to vector-infected cells.Citation86 Thus, inhibition of STAT3 may be an effective strategy for preventing malignant transformation and metastasis in several human cancers.
STAT3 in the tumor microenvironment
STAT3 is a critical regulator of the tumor microenvironment. For example, STAT3 is the downstream effector of several cytokine receptors that are involved in promoting angiogenesis, including those for vascular endothelial growth factor (VEGF), basic fibroblast growth factor, leptin, IL-6, and granulocyte-macrophage colony-stimulating factor.Citation87–Citation90 In addition, STAT3 can promote transcription of proangiogenic factors, including vascular endothelial growth factor and IL-6, leading to paracrine and/or autocrine feedback.Citation91–Citation93 Cytokine excretion from tumor cells also acts upon neighboring endothelial cells to promote proliferation, migration, and microvascular tube formation, leading to the development of mature blood vessels. The contribution of STAT3 activation to tumor angiogenesis, both in tumor cells and in endothelial cells, suggests that the inhibition of STAT3 may be an efficient method to block angiogenesis and tumor progression.
STAT3 is also involved in inflammation-associated carcinogenesis, suppression of the antitumor immune response, and maintenance of cancer stem cells. For example, nuclear factor-kappa B-mediated expression of IL-6 and subsequent activation of STAT3 is required for survival and evasion of apoptosis in intestinal epithelial cells during the development of colitis-associated cancer, a serious morbidity of irritable bowel disease.Citation94,Citation95 Furthermore, STAT3 activity is associated with reduced T-cell infiltration in isogenic murine melanomas, suggesting a role for STAT3 in suppressing tumor immunity.Citation96 Inhibition of STAT3 in these tumor cells and also in glioblastoma cell models stimulates secretion of soluble factors, including TNF-α and IFN-β, that ultimately lead to increased infiltration of lymphocytes, natural killer cells, neutrophils, and macrophages, and also activates nitric oxide production from macrophages in vivo and in vitro.Citation96,Citation97 In addition, genetic or pharmacologic inhibition of STAT3 in glioblastoma stem cells, even transiently, leads to a loss of multipotency and an irreversible growth arrest, suggesting that STAT3 is required for maintenance and proliferation of cancer stem cells in this system.Citation98 Thus, several mechanisms exist by which STAT3 inhibition may lead to tumor microenvironment disruption and subsequent regression.
Overview of the current STAT3 inhibitors in clinical development
summarizes the STAT3 inhibitors that are currently in clinical development according to www.clinicaltrials.gov. Of the four trials listed, one is complete and three are recruiting or ongoing. Both the Isis (Isis Pharmaceuticals, Inc, Carlsbad, CA, USA) and AstraZeneca (London, UK) compounds are antisense oligonucleotide inhibitors of STAT3, whereas the Otsuka (Otsuka Pharmaceutical Development and Commercialization, Inc., Rockville, MD, USA) compound is a small molecule that downregulates STAT3 through an unknown mechanism of action. No results have yet been published from any of these three studies. Preclinical data recently reported in a poster abstract at the 2013 Annual Meeting of the American Association for Cancer Research demonstrate potent and selective downregulation of STAT3 mRNA and protein following AZD9150 treatment in several murine models, including human tumor xenografts. Downregulation resulted in strong antitumor activity and the study suggests that AZD9150 may be effective clinically.Citation99
Table 1 STAT3 inhibitors currently in clinical development according to www.clinicaltrials.gov
A full report of the completed STAT3 decoy Phase 0 trial and further development has been published.Citation100 The decoy, which was designed to bind to the STAT3 DNA-binding domain and prevent STAT3 binding to chromatin, consists of a 15-mer duplex oligonucleotide with phosphorothioate caps at the 5′ and 3′ ends to enhance stability in vivo. Intratumoral injection of this molecule prior to surgical resection in head and neck squamous cell carcinoma (HNSCC) patients led to decreased expression of STAT3 target genes relative to saline-injected tumors in a Phase 0 trial, confirming the ability of the decoy to downregulate STAT3 signaling in human tumors. Systemic administration of the decoy in a murine xenograft model failed to demonstrate any effect on tumor growth or STAT3 signaling as a result of low stability of the decoy in serum. To overcome this difficulty, modified decoys were designed and tested. A circularized decoy consisting of the original decoy with two hexaethylene glycol linkages demonstrated enhanced stability in serum, with detectable levels observed for up to 12 hours. Importantly, systemic administration by intravenous injection of the cyclic decoy in murine xenograft models inhibited tumor growth and expression of STAT3 target genes, demonstrating a successful strategy to inhibit intratumoral STAT3 signaling via systemic, rather than intratumoral, administration. The cyclic decoy has not yet been tested in humans; efforts are underway to further improve its preclinical pharmacodynamic and pharmacokinetic parameters.
In addition to targeting STAT3 via its DNA-binding domain with an oligonucleotide decoy, STAT3 may be targeted via its SH2 domain by small molecules, peptides, or peptidomimetic compounds.Citation101–Citation104 Such molecules are designed to disrupt STAT3 dimerization, thus preventing its translocation to the nucleus and transcription. Other inhibition strategies include the introduction of antisense oligonucleotides, as in the case of the Isis Pharmaceuticals, Inc/AstraZeneca drug in clinical development, designed to cause degradation of STAT3 mRNA or prevent its translation via complementary base pairing, thereby reducing total STAT3 protein levels.Citation105 Antisense strategies in particular will require exquisite tissue specificity, as they may lead to underexpression of STAT3 in normal tissues where its function is required. Recent high throughput and in silico screens also have the potential to identify novel strategies for targeting STAT3.Citation101,Citation106
Critical analysis of the potential for the use of STAT3 inhibitors in the management of human malignancy
For any protein to be the optimal target of inhibition for cancer treatment, it must exhibit several characteristics. Inhibition of the target protein must lead to downregulation of cell growth/proliferation, motility/invasion, and angiogenesis, as well as upregulation of apoptosis, cell death, and the antitumor immune response. The ideal target would also be applicable across a wide variety of cancer types. The inhibition of STAT3 in preclinical models has demonstrated all these characteristics across a wide variety of cancers, most likely via reversal of the many mechanisms discussed previously in this review. This suggests that STAT3 may be the ultimate target for inhibition in human malignancy. Importantly, a Phase 0 trial has demonstrated that STAT3 can be effectively targeted in human tumors, and further preclinical studies have suggested that systemic delivery of STAT3 inhibitors is likely to be effective.Citation100 In addition, because STAT3 signaling is transient in normal tissues and cells, the potential for adverse events following systemic administration of a STAT3 inhibitor is minimal. Indeed, toxicology studies in nonhuman primates demonstrate a lack of toxicity of a STAT3 decoy oligonucleotide.Citation107
Many of the recently approved cancer therapies target tyrosine kinases that are upstream of STAT3 activation, among other pathways. One hypothesis is that mutations in these kinases would signify constitutive activation and serve as a biomarker for patients who will most likely respond to these therapies. Unfortunately, clinical success with these agents has been limited, although in some cases they do prove extremely effective. Studies in preclinical models demonstrate that non-small cell cancer cell lines with mutations in select tyrosine kinases do not exhibit decreased STAT3 activation upon treatment with the respective targeted small molecules erlotinib (EGFR), U0126 (MEK1/2), sunitinib (PDGFRA), or crizotinib (MET), though other downstream effects of these inhibitors, including downregulation of phosphatidylinositol 3-kinase signaling, do occur.Citation108 These findings support the notion that directly targeting STAT3, rather than any large number of its upstream activators, may be more efficacious in reversing the effects of constitutive STAT3 signaling.
Though some patients initially respond to targeted therapies, many develop chemoresistance and secondary cancers that are associated with increased STAT3 signaling. For example, overactivation of STAT3 is associated with resistance to EGFR-targeted therapies in several cancers, including HNSCC, bladder cancer, and others.Citation40,Citation80 Biopsies of recurrent HNSCC following treatment with cetuximab, a Food and Drug Administration-approved monoclonal antibody targeting EGFR, exhibit elevated pSTAT3 relative to pretreatment samples. These results suggest that STAT3 inhibition may be effective at overcoming acquired resistance or as adjuvant therapy to prevent recurrence.Citation40 Treatment of bladder cancer cell lines that are resistant to cetuximab and exhibit elevated levels of activated STAT3 relative to cetuximab-sensitive cell lines leads to reduced cell viability and downregulation of STAT3 target genes.Citation40 Importantly, a combination of STAT3 inhibition with EGFR blockade significantly enhances antitumor effects in vivo relative to EGFR blockade alone, suggesting that the efficacy of already existing (and approved) drugs may be significantly increased by concomitant treatment with STAT3 inhibitors.Citation40 An additional mechanism of acquired resistance to targeted therapy is the activation of IL-6 following treatment. For example, acquired resistance to trastuzumab (a monoclonal antibody targeting the HER2/neu receptor) in HER2-positive breast cancer is associated with the activation of an IL-6 inflammatory feedback loop in which downstream STAT3 signaling contributes to cancer stem cell proliferation, providing additional rationale for cotargeting with a STAT3 inhibitor.Citation109 Likewise, resistance to the tyrosine kinase inhibitor imatinib mesylate, which targets the BCR-ABL oncoprotein, in chronic myeloid leukemia cell models is also associated with increased STAT3 activation and target gene expression.Citation110 Knockdown of STAT3 by small interfering RNA in this context resensitizes the cells to imatinib mesylate-induced cell death, suggesting that STAT3 inhibition may also be effective at overcoming targeted therapy resistance in hematological malignancies.Citation110
Challenges in the development of STAT3 inhibitors to date have largely been overcome. Firstly, STAT3 and other transcription factors were widely regarded as untreatable with drugs mainly because they are not exposed to the extracellular surface and do not have clear ligand-binding domains that can be targeted for competitive inhibition. These perceived difficulties have proven surmountable in the case of STAT3 in both preclinical and clinical models, with STAT3-targeting agents effectively downregulating the pathway and reversing its oncogenic effects. A second obstacle in targeting STAT3 was its structural homology with STAT1, a family member with tumor-suppressive properties in many systems. This challenge has been overcome both with oligonucleotide inhibitors, which exploit the exquisite specificity of the DNA-binding domain, and with recent high-throughput screens of compound libraries to identify candidates that specifically inhibit STAT3 and not STAT1. Both of these strategies may lead in the near future to novel clinical therapeutics targeting STAT3.
Conclusion
Recent advances in the understanding of STAT3 signaling and its role in cancer have led to the establishment of STAT3 as a potential target for a wide variety of human malignancies. Whereas some clinical success has been found in the treatment of cancer with nonspecific chemotherapeutics and some targeted agents, there remains an urgent need for new classes of inhibitors of novel targets that will be widely applicable, well tolerated, and highly effective. The sum of preclinical and clinical data to date supports a unique role for STAT3 as one such target. Indeed, many therapeutic clinical successes to date have been associated with decreased STAT3 signaling, but because of the diversity of signaling components upstream of STAT3, the high cost of developing inhibitors for each one, the potential for emergent compensatory mechanisms, and the development of resistance to a given therapy, directly targeting STAT3 will likely be a preferred strategy.
Disclosure
The authors report no conflicts of interest in this work.
References
- VinkemeierUCohenSLMoarefiIChaitBTKuriyanJDarnellJEJrDNA binding of in vitro activated Stat1 alpha, Stat1 beta and truncated Stat1: interaction between NH2-terminal domains stabilizes binding of two dimers to tandem DNA sitesEMBO J19961520561656268896455
- WojciakJMMartinez-YamoutMADysonHJWrightPEStructural basis for recruitment of CBP/p300 coactivators by STAT1 and STAT2 transactivation domainsEMBO J200928794895819214187
- ZhongZWenZDarnellJEJrStat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6Science1994264515595988140422
- AkiraSNishioYInoueMMolecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathwayCell199477163717512451
- HartKCRobertsonSCKanemitsuMYMeyerANTynanJADonoghueDJTransformation and Stat activation by derivatives of FGFR1, FGFR3, and FGFR4Oncogene200019293309332010918587
- CirriPChiarugiPMarraFc-Src activates both STAT1 and STAT3 in PDGF-stimulated NIH3T3 cellsBiochem Biophys Res Commun199723924934979344858
- EhretGBReichenbachPSchindlerUDNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sitesJ Biol Chem200127696675668811053426
- XuXSunYLHoeyTCooperative DNA binding and sequence-selective recognition conferred by the STAT amino-terminal domainScience199627352767947978670419
- AndrésRMHaldAJohansenCKragballeKIversenLStudies of Jak/STAT3 expression and signalling in psoriasis identifies STAT3-Ser727 phosphorylation as a modulator of transcriptional activityExp Dermatol201322532332823614738
- WakaharaRKunimotoHTaninoKPhospho-Ser727 of STAT3 regulates STAT3 activity by enhancing dephosphorylation of phospho-Tyr705 largely through TC45Genes Cells201217213214522233524
- WangRCherukuriPLuoJActivation of Stat3 sequence-specific DNA binding and transcription by p300/CREB-binding protein-mediated acetylationJ Biol Chem200528012115281153415649887
- YuanZLGuanYJChatterjeeDChinYEStat3 dimerization regulated by reversible acetylation of a single lysine residueScience2005307570726927315653507
- SasakiAYasukawaHSuzukiACytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domainGenes Cells19994633935110421843
- YasukawaHMisawaHSakamotoHThe JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loopEMBO J19991851309132010064597
- ChungCDLiaoJLiuBSpecific inhibition of Stat3 signal transduction by PIAS3Science19972785344180318059388184
- NallarSCKalakondaSLindnerDJTumor-derived mutations in the gene associated with retinoid interferon-induced mortality (GRIM-19) disrupt its anti-signal transducer and activator of transcription 3 (STAT3) activity and promote oncogenesisJ Biol Chem2013288117930794123386605
- ZhangXGuoAYuJIdentification of STAT3 as a substrate of receptor protein tyrosine phosphatase TProc Natl Acad Sci USA2007104104060406417360477
- VeeriahSBrennanCMengSThe tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancersProc Natl Acad Sci USA2009106239435944019478061
- YamamotoTSekineYKashimaKThe nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylationBiochem Biophys Res Commun2002297481181712359225
- ZhangWChanRJChenHNegative regulation of Stat3 by activating PTPN11 mutants contributes to the pathogenesis of Noonan syndrome and juvenile myelomonocytic leukemiaJ Biol Chem200928433223532236319509418
- NieYErionDMYuanZSTAT3 inhibition of gluconeogenesis is downregulated by SirT1Nat Cell Biol200911449250019295512
- BernierMPaulRKMartin-MontalvoANegative regulation of STAT3 protein-mediated cellular respiration by SIRT1 proteinJ Biol Chem201128622192701927921467030
- GaoHGuoR-FSpeyerCStat3 activation in acute lung injuryJ Immunol2004172127703771215187153
- BraunDAFribourgMSealfonSCCytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activationJ Biol Chem201328852986299323166328
- DauerDJFerraroBSongLStat3 regulates genes common to both wound healing and cancerOncogene200524213397340815735721
- GallucciRMSimeonovaPPMathesonJMImpaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed miceFASEB J200014152525253111099471
- SanoSItamiSTakedaKKeratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesisEMBO J199918174657466810469645
- PickertGNeufertCLeppkesMSTAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healingJ Exp Med200920671465147219564350
- CofferPJvan PuijenbroekABurgeringBMInsulin activates Stat3 independently of p21ras-ERK and PI-3 K signal transductionOncogene19971521252925399399641
- CarvalheiraJBSilotoRMIgnacchittiIInsulin modulates leptin-induced STAT3 activation in rat hypothalamusFEBS Lett2001500311912411445068
- InoueHOgawaWAsakawaARole of hepatic STAT3 in brain-insulin action on hepatic glucose productionCell Metab20063426727516581004
- ZongCSChanJLevyDEHorvathCSadowskiHBWangLHMechanism of STAT3 activation by insulin-like growth factor I receptorJ Biol Chem200027520150991510510747872
- Niso-SantanoMShenSAdjemianSDirect interaction between STAT3 and EIF2AK2 controls fatty acid-induced autophagyAutophagy20139341541723221979
- TakedaKNoguchiKShiWTargeted disruption of the mouse Stat3 gene leads to early embryonic lethalityProc Natl Acad Sci USA1997948380138049108058
- SanoSTakahamaYSugawaraTStat3 in thymic epithelial cells is essential for postnatal maintenance of thymic architecture and thymocyte survivalImmunity200115226127311520461
- ChapmanRSLourencoPCTonnerESuppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3Genes Dev199913192604261610521404
- YuHJoveRThe STATs of cancer – new molecular targets come of ageNat Rev Cancer2004429710514964307
- CeramiEGaoJDogrusozUThe cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics dataCancer Discov20122540140422588877
- WangZZhuSShenMSTAT3 is involved in esophageal carcinogenesis through regulation of Oct-1Carcinogenesis201334367868823172665
- SenMJoyceSPanahandehMTargeting Stat3 abrogates EGFR inhibitor resistance in cancerClin Cancer Res201218184986499622825581
- CaoXTayAGuyGRTanYHActivation and association of Stat3 with Src in v-Src-transformed cell linesMol Cell Biol1996164159516038657134
- BrombergJFHorvathCMBesserDLathemWWDarnellJEStat3 activation is required for cellular transformation by v-srcMol Cell Biol1998185255325589566875
- TurksonJBowmanTGarciaRCaldenhovenEDe GrootRPJoveRStat3 activation by Src induces specific gene regulation and is required for cell transformationMol Cell Biol1998185254525529566874
- PedranziniLLeitchABrombergJStat3 is required for the development of skin cancerJ Clin Invest2004114561962215343379
- BowmanTBroomeMASinibaldiDStat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesisProc Natl Acad Sci USA200198137319732411404481
- YuCYWangLKhaletskiyASTAT3 activation is required for interleukin-6 induced transformation in tumor-promotion sensitive mouse skin epithelial cellsOncogene200221253949396012037677
- ChiarleRSimmonsWJCaiHStat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic targetNat Med200511662362915895073
- VulturAArulanandamRTurksonJNiuGJoveRRaptisLStat3 is required for full neoplastic transformation by the Simian Virus 40 large tumor antigenMol Biol Cell20051683832384615917293
- DasguptaARaychaudhuriBHaqqiTStat3 activation is required for the growth of U87 cell-derived tumours in miceEur J Cancer200945467768419121577
- GeorgeRESandaTHannaMActivating mutations in ALK provide a therapeutic target in neuroblastomaNature2008455721597597818923525
- AnandMLaiRGelebartPβ-catenin is constitutively active and increases STAT3 expression/activation in anaplastic lymphoma kinase-positive anaplastic large cell lymphomaHaematologica201196225326120971814
- JonesAVKreilSZoiKWidespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disordersBlood200510662162216815920007
- ScottLMCampbellPJBaxterEJThe V617F JAK2 mutation is uncommon in cancers and in myeloid malignancies other than the classic myeloproliferative disordersBlood200510682920292116204151
- CampbellPJGriesshammerMDöhnerKV617F mutation in JAK2 is associated with poorer survival in idiopathic myelofibrosisBlood200610752098210016293597
- OkuSTakenakaKKuriyamaTJAK2 V617F uses distinct signalling pathways to induce cell proliferation and neutrophil activationBr J Haematol2010150333434420553273
- FlemingTPSaxenaAClarkWCAmplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumorsCancer Res19925216455045531322795
- YehHHLaiWWChenHHLiuHSSuWCAutocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusionOncogene200625314300430916518408
- GrandisJRDrenningSDChakrabortyARequirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitroJ Clin Invest19981027138513929769331
- BatraSKCastelino-PrabhuSWikstrandCJEpidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII geneCell Growth Differ1995610125112598845302
- LoHWCaoXZhuHAli-OsmanFCyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axesMol Cancer Res20108223224520145033
- HeBYouLUematsuKSOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancerProc Natl Acad Sci USA200310024141331413814617776
- IwamotoTSengaTNaitoYThe JAK-inhibitor, JAB/SOCS-1 selectively inhibits cytokine-induced, but not v-Src induced JAK-STAT activationOncogene200019414795480111032030
- CapelloDGloghiniABaldanziGAlterations of negative regulators of cytokine signalling in immunodeficiency-related non-Hodgkin lymphomaHematol Oncol2013311222822488585
- LaczmanskaIKarpinskiPBebenekMProtein tyrosine phosphatase receptor-like genes are frequently hypermethylated in sporadic colorectal cancerJ Hum Genet2013581111523096495
- KouXChenNFengZLuoLYinZGSTP1 negatively regulates Stat3 activation in epidermal growth factor signalingOncol Lett2013531053105723426146
- ZhongSTangMWYeoWLiuCLoYMJohnsonPJSilencing of GSTP1 gene by CpG island DNA hypermethylation in HBV-associated hepatocellular carcinomasClin Cancer Res2002841087109211948118
- LinXAsgariKPutziMJReversal of GSTP1 CpG island hypermethylation and reactivation of pi-class glutathione S-transferase (GSTP1) expression in human prostate cancer cells by treatment with procainamideCancer Res200161248611861611751372
- SchlessingerKLevyDEMalignant transformation but not normal cell growth depends on signal transducer and activator of transcription 3Cancer Res200565135828583415994959
- CorvinusFMOrthCMorigglRPersistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growthNeoplasia20057654555516036105
- NiuGBowmanTHuangMRoles of activated Src and Stat3 signaling in melanoma tumor cell growthOncogene200221467001701012370822
- RahamanSOHarborPCChernovaOBarnettGHVogelbaumMAHaqueSJInhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cellsOncogene200221558404841312466961
- BhardwajASethiGVadhan-RajSResveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cellsBlood200710962293230217164350
- WeerasinghePGarciaGEZhuQInhibition of Stat3 activation and tumor growth suppression of non-small cell lung cancer by G-quartet oligonucleotidesInt J Oncol200731112913617549413
- LeslieKLangCDevganGCyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3Cancer Res20066652544255216510571
- EwenMELambJThe activities of cyclin D1 that drive tumorigenesisTrends Mol Med200410415816215059606
- CaoXLiuDYanXStat3 inhibits WTX expression through up-regulation of microRNA-370 in Wilms tumorFEBS Lett2013587663964423333300
- ZhuangLLeeCSScolyerRAMcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanomaMod Pathol200720441642617384650
- Catlett-FalconeRLandowskiTHOshiroMMConstitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cellsImmunity199910110511510023775
- ZushiSShinomuraYKiyoharaTSTAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cellsInt J Cancer19987833263309766567
- RealPJSierraADe JuanASegoviaJCLopez-VegaJMFernandez-LunaJLResistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cellsOncogene200221507611761812400004
- TimofeevaOATarasovaNIZhangXSTAT3 suppresses transcription of proapoptotic genes in cancer cells with the involvement of its N-terminal domainProc Natl Acad Sci USA201311041267127223288901
- XieTXHuangFJAldapeKDActivation of stat3 in human melanoma promotes brain metastasisCancer Res20066663188319616540670
- ItohMMurataTSuzukiTRequirement of STAT3 activation for maximal collagenase-1 (MMP-1) induction by epidermal growth factor and malignant characteristics in T24 bladder cancer cellsOncogene20062581195120416205632
- FujiiMHonmaMTakahashiHIshida-YamamotoAIizukaHIntercellular contact augments epidermal growth factor receptor (EGFR) and signal transducer and activator of transcription 3 (STAT3)-activation which increases podoplanin-expression in order to promote squamous cell carcinoma motilityCell Signal201325476076523266472
- ZhouWGrandisJRWellsASTAT3 is required but not sufficient for EGF receptor-mediated migration and invasion of human prostate carcinoma cell linesBr J Cancer200695216417116804520
- PriesterMCopanakiEVafaizadehVSTAT3 silencing inhibits glioma single cell infiltration and tumor growthNeuro-oncology201315784085223486688
- CascioSFerlaRD’AndreaAExpression of angiogenic regulators, VEGF and leptin, is regulated by the EGF/PI3K/STAT3 pathway in colorectal cancer cellsJ Cell Physiol2009221118919419492417
- WeiLHKuoMLChenCAInterleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathwayOncogene200322101517152712629515
- ValdembriDSeriniGVaccaARibattiDBussolinoFIn vivo activation of JAK2/STAT-3 pathway during angiogenesis induced by GM-CSFFASEB J200216222522711744626
- ZhaoMGaoFHWangJYJAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancerLung Cancer201173336637421333372
- SriuranpongVParkJIAmornphimolthamPPatelVNelkinBDGutkindJSEpidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine systemCancer Res200363112948295612782602
- NiuGWrightKLHuangMConstitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesisOncogene200221132000200811960372
- MasudaMRuanHYItoASignal transducers and activators of transcription 3 up-regulates vascular endothelial growth factor production and tumor angiogenesis in head and neck squamous cell carcinomaOral Oncol200743878579017169602
- GrivennikovSKarinETerzicJIL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancerCancer Cell200915210311319185845
- LiangJNagahashiMKimEYSphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancerCancer Cell201323110712023273921
- BurdelyaLKujawskiMNiuGStat3 activity in melanoma cells affects migration of immune effector cells and nitric oxide-mediated antitumor effectsJ Immunol200517473925393115778348
- SeeAPHanJEPhallenJThe role of STAT3 activation in modulating the immune microenvironment of GBMJ Neurooncol2012110335936823096132
- SherryMMReevesAWuJKCochranBHSTAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cellsStem Cells200927102383239219658181
- KimYHsuJZhouTPotent in vivo pharmacology of AZD9150, a next-generation, constrained ethyl-modified antisense oligonucleotide targeting STAT3 in multiple preclinical cancer models [abstract]Proceedings of the 104th Annual Meeting of the American Association for Cancer Research2013 Apr 6–10Washington, DC. Philadelphia (PA) AACR; 2013. Abstract nr LB-317
- SenMThomasSMKimSFirst-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapyCancer Discov20122869470522719020
- SchustJSperlBHollisAMayerTUBergTStattic: a small-molecule inhibitor of STAT3 activation and dimerizationChem Biol200613111235124217114005
- RenZCabellLASchaeferTSMcMurrayJSIdentification of a high-affinity phosphopeptide inhibitor of Stat3Bioorg Med Chem Lett200313463363612639546
- ZhangXYuePFletcherSZhaoWGunningPTTurksonJA novel small-molecule disrupts Stat3 SH2 domain-phosphotyrosine interactions and Stat3-dependent tumor processesBiochem Pharmacol201079101398140920067773
- GomezCBaiLZhangJDesign, synthesis, and evaluation of peptidomimetics containing Freidinger lactams as STAT3 inhibitorsBioorg Med Chem Lett20091961733173619243938
- Epling-BurnettePKLiuJHCatlett-FalconeRInhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expressionJ Clin Invest2001107335136211160159
- SongHWangRWangSLinJA low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cellsProc Natl Acad Sci USA2005102134700470515781862
- SenMToscaPJZwayerCLack of toxicity of a STAT3 decoy oligonucleotideCancer Chemother Pharmacol200963698399518766340
- LooyengaBDHutchingsDCherniIKingsleyCWeissGJMackeiganJSTAT3 is activated by JAK2 independent of key oncogenic driver mutations in non-small cell lung carcinomaPLoS ONE201272e3082022319590
- KorkayaHKimGIDavisAActivation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell populationMol Cell201247457058422819326
- BewryNNNairRREmmonsMFBoulwareDPinilla-IbarzJHazlehurstLAStat3 contributes to resistance toward BCR-ABL inhibitors in a bone marrow microenvironment model of drug resistanceMol Cancer Ther20087103169317518852120
- Marcher-BauerALuSAndersonJBCDD: a Conserved Domain Database for the functional annotation of proteinsNuclieic Acids Res201139225229