Abstract
Four series of forty-five nitrogen-containing polyhydroxylated aromatics based on caffeic acid phenethyl ester were designed and synthesized as HIV-1 integrase (IN) inhibitors. Most of these compounds inhibited IN catalytic activities in low micromolar range. Among these new analogues, compounds 9e and 9f were the most potent IN inhibitors with IC50 value of 0.7 μM against strand transfer reaction. Their key structure-activity relationships were also discussed.
Introduction
Human immunodeficiency virus type-1 (HIV-1) is the etiological agent of acquired immunodeficiency syndrome (AIDS) that is a challenging pandemic of the 21st century. The highly active anti-retroviral therapy (HAART), based on the use of a combination of reverse transcriptase (RT) and protease (PR) inhibitors, effectively inhibits the replication cycle of HIV-1Citation1,Citation2. However, prolonged therapy is associated with drug-related toxicities, drug–drug/food interactions, and the emergence of multidrug resistant viral strains. Therefore, it is necessary to develope novel inhibitors, which target other stages of the viral life cycleCitation3.
As one of the essential viral enzymes, integrase (IN) catalyzes the insertion of proviral cDNA into the host genome through two biochemical steps: (i) cleavage of a dinucleotide from each 3′-end of viral cDNA (termed “3′-processing”, 3′-P) and (ii) insertion of the two newly processed 3′-viral DNA ends into the host-cell chromosome (termed “strand transfer”, STCitation2,Citation4). Due to the absence of any known human homologCitation5, therefore, in principle, selective inhibitors with few or no side effects can be designed, which makes HIV-1 IN an attractive and validated target for the development of novel anti-HIV-1 drugs.
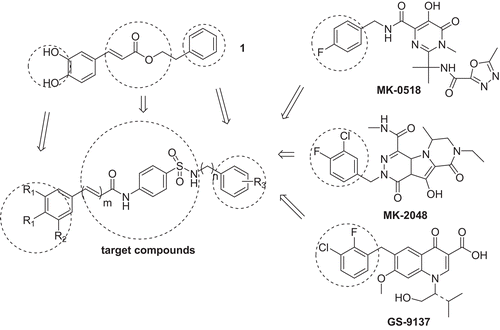
Caffeic acid phenylethyl ester (CAPE 1, ), an antioxidant component of the propolis, is one of the first HIV-1 IN inhibitors reported. It also exhibits significant anti-viral activity with little cytotoxicity in primary human peripheral blood mononuclear cells (PBMC) cultureCitation6,Citation7. Therefore, CAPE is a potential lead for further structural optimization as potent IN inhibitors. The phenolic hydroxyl group, as a pharmacophore of CAPE coordinates with metal ions, like Mg2+ or Mn2+, blocking the 3′-P and STCitation8,Citation9. However, the linker and the phenyl ring are supposed to be accommodated at the hydrophobic pocket around the active site domain to reinforce their affinity and selectivity to INCitation10.
In our lead-optimization work based on CAPE, the galloyl group which was reported to have higher affinity to metal ions had been introduced instead of the catechol group as a necessary pharmacophoreCitation11. A sulfanilamide was also been introduced replacing the ester as the linker, because it was suggested that compounds containing sulfanilamide groups show higher anti-retroviral activitiesCitation12. It has been reported that halogen substitution on the aryl group showed significant potency and selectivity against IN and viral replication in cell cultureCitation13. Several IN inhibitors containing different halogen substitution on the aryl group had been tested in the clinic, including MK-2048, GS-9137 and MK-0518 ()Citation14,Citation15. Especially MK-0518, also known as raltegravir, was the first IN inhibitor drug approved by the United States Food and Drug Administration (US FDA). Therefore, we introduced diversity of halogen substitution on the aryl group to investigate the roles of substitutions on the phenyl portion. So a set of nitrogen-containing trihydroxylated aromatics had been synthesized. This set of compounds exhibited potent IN inhibitory activities at low micromolar concentrations and compound 9e and 9f were the most potent ST inhibitors with IC50 value of 0.7 μM among these new analogues. However, when tested for the ability to inhibit HIV replication in cell cultures, they didn’t exhibit anti-viral activity at a concentration of 25 μM. Recently, it had been reported that the compounds with the catechol group displayed strong anti-viral activity at nanomolar concentrationsCitation16. So another set of nitrogen-containing dihydroxylated aromatics have also been synthesized in order to increase their anti-viral activity. This set of compounds showed higher cytotoxicity although they exhibited improved anti-viral activity.
Materials and methods
Chemistry
Unless otherwise noted, all reagents were obtained from commercial suppliers and used without further purification. Melting points were determined on a Büchi capillary melting point apparatus. Infrared (IR) spectra were measured on KBr pellets, using a Nicolet Nexus 470FT-IR and were expressed in cm−1. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded with a Bruker Avance DRX600 spectrometer with DMSO-d6 as the solvent and tetramethyl-silane (TMS) as the internal standard. The chemical shifts were reported in δ (ppm). High resolution mass spectra (HRMS) data were obtained using an Accela UPLC-LTQ Orbitrap Mass Spectrometer. Petroleum ether used for column chromatography had a boiling range of 60–90°C.
General methods for preparing 4-amino-N-substituted-benzenesulfonamides 3
To a stirred solution of substituted amine 2 (20 mmol) in pyridine (129 mmol) 4-acetamidobenzene-1-sulfonyl chloride (16 mmol) was added in portion with external cooling. The reaction mixture was stirred at room temperature for 4 h. Then water (100 mL) was added and the resulting solid was collected by filtration. The solid was then dissolved in 10% NaOH and the precipitate was removed by filtration. The solution was acidified with hydrochloric acid solution (5 M) to pH 3–4. The precipitate was collected and dissolved in 20 mL of NaOH (5 M) and 12 mL of methanol. The solution was stirred at 70°C for 3 h and then was acidified with hydrochloric acid solution (2 M) to pH 6. The resulting precipitate was collected by filtration, and then was recrystallized from ethanol/water to afford compound 3.
4-Amino-N-(4-fluorophenyl)benzenesulfonamide (3a)
White powder, yield 76.9%, mp 130.9–132.4°C. 1H-NMR (DMSO-d6) δ: 10.02 (s, 1H), 7.36 (d, J = 9.0 Hz, 2H), 7.15 (m, 2H), 6.65 (d, J = 7.2 Hz, 2H), 6.52 (d, J = 9.0 Hz, 2H), 5.94 (s, 2H).
4-Amino-N-(3,4-difluorophenyl)benzenesulfonamide (3b)
White powder, yield 75.9%, mp 178.5–180.0°C. 1H-NMR (DMSO-d6) δ: 10.06 (s, 1H), 7.37 (d, J = 9.0 Hz, 2H), 7.30 (m, 1H), 7.03 (m, 1H), 6.84 (t, J = 4.8 Hz, 1H), 6.53 (d, J = 9.0 Hz, 2H), 6.03 (s, 2H).
4-Amino-N-(2,3,4-trifluorophenyl)benzenesulfonamide (3c)
White powder, yield 82.6%, mp 169.5–171.1°C. 1H-NMR (DMSO-d6) δ: 10.04 (s, 1H), 7.41 (d, J = 9.0 Hz, 2H), 7.32 (m, 1H), 7.09 (m, 1H), 6.51 (d, J = 9.0 Hz, 2H), 6.03 (s, 2H).
4-Amino-N-(4-bromophenyl)benzenesulfonamide (3d)
White powder, yield 80.9%, mp 216.3–217.7°C. 1H-NMR (DMSO-d6) δ: 10.03 (s, 1H), 7.30 (d, J = 9.6 Hz, 2H), 7.21 (d, J = 9.6 Hz, 2H), 6.98 (d, J = 9.6 Hz, 2H), 6.42 (d, J = 9.6 Hz, 2H), 5.96 (s, 2H).
4-Amino-N-(3-bromophenyl)benzenesulfonamide (3e)
White powder, yield 66.2%, mp 142.8–144.0°C. 1H-NMR (DMSO-d6) δ: 10.14 (s, 1H), 7.40 (d, J = 8.4 Hz, 2H), 7.21 (s, 1H), 7.16 (m, 2H), 7.07 (d, J = 7.8 Hz, 1H), 6.54 (d, J = 8.4 Hz, 2H), 6.04 (s, 2H).
4-Amino-N-(2-bromophenyl)benzenesulfonamide (3f)
White powder, yield 68.2%, mp 170.8–171.5°C. 1H-NMR (DMSO-d6) δ: 10.06 (s, 1H), 7.37 (d, J = 8.4 Hz, 2H), 7.29 (t, J = 9.0 Hz, 1H), 7.17 (dd, J = 6.0 Hz, J = 2.4 Hz, 1H), 7.03 (m, 1H), 6.55 (d, J = 8.4 Hz, 2H), 6.04 (s, 2H).
4-Amino-N-(3,4-dichlorophenyl)benzenesulfonamide (3g)
White powder, yield 70.0%, mp 169.5–171.1°C. 1H-NMR (DMSO-d6) δ: 10.28 (s, 1H), 7.48 (d, J = 9.0 Hz, 1H), 7.41 (d, J = 8.4 Hz, 2H), 7.23 (d, J = 2.4 Hz, 1H), 7.05 (dd, J = 9.0 Hz, J = 2.4 Hz, 1H), 6.55 (d, J = 8.4 Hz, 2H), 6.07 (s, 2H).
4-Amino-N-(4-butylphenyl)benzenesulfonamide (3h)
White powder, yield 78.5%, mp 163.7–164.9°C. 1H-NMR (DMSO-d6) δ: 9.68 (s, 1H), 7.35 (d, J = 9.6 Hz, 2H), 7.01 (d, J = 8.4 Hz, 2H), 6.94 (d, J = 9.6 Hz, 2H), 6.51 (d, J = 8.4 Hz, 2H), 5.94 (s, 2H), 2.44 (t, J = 7.8 Hz, 2H), 1.45 (quintet, J = 7.8 Hz, 2H), 1.24 (sextet, J = 7.2 Hz, 2H), 0.86 (t, J = 7.2 Hz, 3H).
4-Amino-N-(4-ethoxylphenyl)benzenesulfonamide (3i)
Orange yellow powder, yield 62.8%, mp 205.9–207.8°C. 1H-NMR (DMSO-d6) δ: 9.65 (s, 1H), 7.32 (dd, J = 9.0 Hz, J = 1.8 Hz, 2H), 6.98 (dd, J = 7.2 Hz, J = 1.8 Hz, 2H), 6.80 (dd, J = 7.2 Hz, J = 1.8 Hz, 2H), 6.52 (dd, J = 9.0 Hz, J = 1.8 Hz, 2H), 5.92 (s, 2H), 4.04 (q, J = 7.8 Hz, 2H), 1.52 (t, J = 7.8 Hz, 3H).
4-Amino-N-(3,5-dimethylphenyl)benzenesulfonamide (3j)
Orange yellow powder, yield 54.0%, mp 151.8–153.0°C. 1H-NMR (DMSO-d6) δ: 9.71 (s, 1H), 7.39 (d, J = 9.0 Hz, 2H), 6.68 (s, 2H), 6.59 (s, 1H), 6.52 (d, J = 9.0 Hz, 2H), 5.95 (s, 2H), 2.14 (s, 6H).
4-Amino-N-(3-chloro-2-fluorophenyl)benzenesulfonamide (3k)
White powder, yield 67.2%, mp 142.2–143.4°C. 1H-NMR (DMSO-d6) δ: 9.95 (s, 1H), 7.36 (d, J = 8.4 Hz, 2H), 7.30 (t, J = 7.2 Hz, 1H), 7.21 (t, J = 7.8 Hz, 1H), 7.12 (t, J = 7.8 Hz, 1H), 6.55 (d, J = 9.0 Hz, 2H), 6.04 (s, 2H).
4-Amino-N-phenylbenzenesulfonamide (3l)
Yellowish powder, yield 79.1%, mp 197.2–198.8°C. 1H-NMR (DMSO-d6) δ: 9.85 (s, 1H), 7.38 (d, J = 9.0 Hz, 2H), 7.20 (t, J = 7.8 Hz, 2H), 7.05 (d, J = 8.4 Hz, 2H), 6.96 (t, J = 7.2 Hz, 1H), 6.53 (d, J = 9.0 Hz, 2H), 5.96 (s, 2H).
4-Amino-N-(4-chlorophenyl)benzenesulfonamide (3m)
White powder, yield 59.6%, mp 198.1–201.1°C. 1H-NMR (DMSO-d6) δ: 10.01 (s, 1H), 7.38 (d, J = 9.6 Hz, 2H), 7.26 (d, J = 9.6 Hz, 2H), 7.07 (d, J = 9.6 Hz, 2H), 6.53 (d, J = 9.6 Hz, 2H), 6.01 (s, 2H).
4-Amino-N-(3,4-dimethylphenyl)benzenesulfonamide (3n)
Yellowish powder, yield 72.1%, mp 169.5–171.1°C. 1H-NMR (DMSO-d6) δ: 9.61 (s, 1H), 7.35 (d, J = 9.6 Hz, 2H), 6.93 (d, J = 8.4 Hz, 1H), 6.83 (s, 1H), 6.78 (dd, J = 7.8 Hz, J = 1.8 Hz, 1H), 6.51 (d, J = 8.4Hz, 2H), 5.93 (s, 2H), 2.08 (s, 6H).
4-Amino-N-(4-methoxyphenyl)benzenesulfonamide (3o)
White powder, yield 75.3%, mp 202.1–204.3°C. 1H-NMR (DMSO-d6) δ: 9.45 (s, 1H), 7.29 (dd, J = 9.0 Hz, J = 1.8 Hz, 2H), 6.94 (dd, J = 7.2 Hz, J = 1.8 Hz, 2H), 6.77 (dd, J = 7.2 Hz, J = 1.8 Hz, 2H), 6.50 (dd, J = 9.0 Hz, J = 1.8 Hz, 2H), 5.92 (s, 2H), 3.66 (s, 3H).
4-Amino-N-(4-nitrophenyl)benzenesulfonamide (3p)
Orange yellow powder, yield 55.0%, mp 167.2–168.0°C. 1H-NMR (DMSO-d6) δ: 10.04 (s, 1H), 8.14 (d, J = 9.0 Hz, 2H), 7.60 (d, J = 9.0 Hz, 2H), 6.72 (d, J = 9.0 Hz, 2H), 6.59 (d, J = 9.0 Hz, 2H), 5.96 (s, 2H).
4-Amino-N-(2-methylphenyl)benzenesulfonamide (3q)
White powder, yield 75.0%, mp 153.0–155.0°C. 1H-NMR (DMSO-d6) δ: 10.03 (s, 1H), 7.57 (d, J = 9.0 Hz, 2H), 6.82 (d, J = 9.0 Hz, 2H), 6.58 (dd, J = 6.0 Hz, J = 3.0 Hz, 1H), 6.45 (m, 2H), 6.32 (dd, J = 6.0 Hz, J = 2.4 Hz, 1H,), 5.91 (s, 2H), 2.18 (s, 3H).
4-Amino-N-(3-bromo-4-fluorophenyl)benzenesulfonamide (3r)
Pink powder, yield 63.5%, mp 169.1–169.9°C. 1H-NMR (DMSO-d6) δ: 10.04 (s, 1H), 7.37 (d, J = 7.8 Hz, 2H), 7.29 (d, J = 4.8 Hz, 1H), 7.25 (t, J = 9.0 Hz, 1H), 7.08 (t, J = 4.8 Hz, 1H), 6.55 (d, J = 8.4 Hz, 2H), 6.04 (s, 2H).
4-Amino-N-(3-chloro-4-fluorophenyl)benzenesulfonamide (3s)
White powder, yield 72.5%, mp 175.5–176.4°C. 1H-NMR (DMSO-d6) δ: 10.06 (s, 1H), 7.37 (d, J = 8.4 Hz, 2H), 7.29 (t, J = 9.0 Hz, 1H), 7.17 (dd, J = 6.6 Hz, J = 1.8 Hz, 1H), 7.04 (m, 1H), 6.55 (d, J = 9.0 Hz, 2H), 6.04 (s, 2H).
4-Amino-N-(4-fluorobenzyl)benzenesulfonamide (3t)
Yellowish powder, yield 53.6%, mp 163.4–165.0°C. 1H-NMR (DMSO-d6) δ: 7.65 (t, J = 6.6 Hz, 1H), 7.41 (d, J = 10.4 Hz, 2H), 7.27 (dd, J = 8.4 Hz, J = 5.4 Hz, 2H), 7.10 (m, 2H), 6.59 (d, J = 9.6 Hz, 2H), 5.93 (s, 2H), 3.85 (d, J = 6.0 Hz, 2H).
4-Amino-N-(4-chlorobenzyl)benzenesulfonamide (3u)
White powder, yield 62.7%, mp 122.9–123.6°C. 1H-NMR (DMSO-d6) δ: 7.62 (t, J = 6.6 Hz, 1H), 7.44 (d, J = 8.4 Hz, 2H), 7.28 (t, J = 7.2 Hz, 2H), 7.23 (m, 2H), 6.61 (d, J = 8.4 Hz, 2H), 5.93 (s, 2H), 3.87 (d, J = 6.6 Hz, 2H).
4-Amino-N-(4-fluorophenethyl)benzenesulfonamide (3v)
Pink powder, yield 64.5%, mp 155.4–156.7°C. 1H-NMR (DMSO-d6) δ: 7.40 (d, J = 8.4 Hz, 2H), 7.18 (m, 3H), 7.08 (t, J = 8.4 Hz, 2H), 6.59 (d, J = 8.4 Hz, 2H), 5.92 (s, 2H), 2.85 (m, 2H), 2.64 (t, J = 7.2 Hz, 2H).
General methods for preparing acyl chlorides 6 and 717,18
To a stirred solution of acetic anhydride (212.1 mmol) and pyridine (61.9 mmol) compound 4 or 5 (20 mmol) was added. The reaction mixture was stirred for 24 h at room temperature, and then water (200 mL) was added to the mixture. The precipitate was collected by filtration and was then recrystallized from acetone/cyclohexane to afford white solid. The solid was dissolved in 20 mL of thionyl chloride and the solution was stirred at 80°C for 5 h. The solvent was concentrated under reduced pressure at 50°C to obtain acyl chloride 6 or 7, which was diluted with 20 mL of acetone and was used for the next step without further purification.
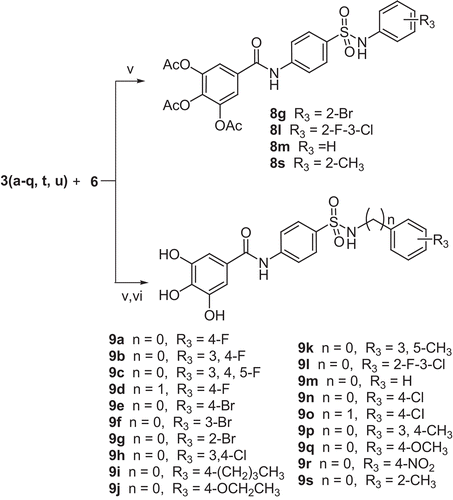
General methods for preparing target compounds 8
To a stirred solution of 4-amino-N-substituted-benzenesulfonamide 3 (2.8 mmol) and pyridine (2.6 mmol) in acetone (10 mL) acyl chloride 6 (2.2 mmol) was added in portion at 0°C. The reaction mixture was stirred at room temperature for 20 h and the precipitate was removed by filtration. The filtrate was concentrated under reduced pressure and the residue was purified by column chromatography using ethyl acetate/petroleum ether (1:1) as eluent to yield compound 8.
N-(4-(2-Bromophenylsulfamoyl)phenyl)-3,4,5-triacetoxybenzamide (8g)
White powder, yield 76.1%, mp 187.1–189.3°C. 1H-NMR (DMSO-d6) δ: 10.69 (s, 1H), 9.83 (s, 1H), 7.89 (d, J = 7.8 Hz, 2H), 7.80 (s, 2H), 7.69 (d, J = 7.8 Hz, 2H), 7.56 (d, J = 8.4 Hz, 1H), 7.31 (s, 1H), 7.18 (d, J = 8.4 Hz, 1H), 7.12 (s, 1H), 2.32 (s, 3H), 2.31 (s, 6H). IR (KBr, cm−1): υNH: 3313.66, 3278.59, 3244.09; υ=CH: 3111.59; υCH: 2937.43; υC=O: 1777.62, 16869.87; υC=C: 1590.18, 1533.08, 1497.91; υS=O: 1328.26, 1198.19; υC-O: 1157.57; γ=C-H: 844.88, 755.64. HRMS (ESI) m/z for C25H25N3O9BrS [M+NH4]+: calcd 622.0489, found 622.0504.
N-(4-(3-chloro-2-fluorophenylsulfamoyl)phenyl)-3,4,5-triacetoxybenzamide (8l)
White powder, yield 73.2%, mp 228.3–229.1°C. 1H-NMR (DMSO-d6) δ: 10.71 (s, 1H), 10.39 (s, 1H), 7.91 (d, J = 9.0 Hz, 2H), 7.81 (s, 2H), 7.73 (d, J = 9.0 Hz, 2H), 7.35 (t, J = 7.2 Hz, 1H), 7.21 (t, J = 7.2 Hz, 1H), 7.14 (d, J = 8.4 Hz, 1H), 2.33 (s, 3H), 2.30 (s, 6H). IR (KBr, cm−1): υNH: 3376.37, 3191.70; υ=CH: 3070.52; υCH: 2936.09; υC=O: 1780.50, 1694.29; υC=C: 1591.55, 1532.28, 1486.11; υS=O: 1333.73, 1191.54; υC-O: 1226.26, 1160.30; γ=C-H: 842.75, 729.80. HRMS (ESI) m/z for C25H24N3O9FClS [M+H]+: calcd 596.0900, found 596.0911.
N-(4-Phenylsulfamoylphenyl)-3,4,5-triacetoxybenzamide (8m)
White powder, yield 79.1%, mp 215.1–217.3°C. 1H-NMR (DMSO-d6) δ: 10.65 (s, 1H), 10.22 (s, 1H), 7.88 (d, J = 8.4 Hz, 2H), 7.80 (s, 2H), 7.75 (d, J = 9.0 Hz, 2H), 7.23 (t, J = 7.8 Hz, 2H), 7.09 (d, J = 7.8 Hz, 2H), 7.02 (t, J = 7.2 Hz, 1H), 2.34 (s, 3H), 2.32 (s, 6H). IR (KBr, cm−1): υNH: 3341.36, 3294.47, 3112.12; υC=O: 1778.68, 1762.49, 1689.88; υC=C: 1589.19, 1531.64, 1495.69; υS=O: 1326.81, 1157.95; υC-O: 1201.45; γ=C-H: 844.82, 758.59. HRMS (ESI) m/z for C25H23N2O9S [M+H]+: calcd 527.1119, found 527.1129.
N-(4-(2-Methylphenylsulfamoyl)phenyl)-3,4,5-triacetoxybenzamide (8s)
White powder, yield 84.0%, mp 201.4–203.0°C. 1H-NMR (DMSO-d6) δ: 10.68 (s, 1H), 9.50 (s, 1H), 7.90 (d, J = 9.0 Hz, 2H), 7.82 (s, 2H), 7.65 (d, J = 9.0 Hz, 2H), 7.12 (m, 3H), 6.98 (dd, J = 6.0 Hz, J = 3.0 Hz, 1H), 2.35 (s, 3H), 2.33 (s, 6H), 2.02 (s, 3H). IR (KBr, cm−1): υNH: 3337.80; υCH: 2936.46; υC=O: 1776.04, 1687.54; υC=C: 1588.61, 1531.71, 1496.08; υS=O: 1328.19, 1196.03; υC-O: 1235.57; γ=C-H: 845.53, 754.37. HRMS (ESI) m/z for C26H25N2O9S [M+H]+: calcd 541.1275, found 541.1287.
General methods for preparing target compounds 9
Following the aforementioned procedure, amidation between compound 3 and compound 6 was carried out. The product was added to a solution of tetrahydrofuran (4 mL), methanol (4 mL) and concentrated hydrochloric acid (37%, 2 mL). The reaction mixture was stirred for 1h at 60°C. Evaporation of the solvent gave a residue, which was recrystallized from ethanol/water to obtain compound 9.
N-(4-(4-Fluorophenylsulfamoyl)phenyl)galloylamide (9a)
Yellowish powder, yield 61.7%, mp 274.7–275.8°C. 1H-NMR (DMSO-d6) δ: 10.24 (s, 1H), 10.13 (s, 1H), 9.22 (s, 2H), 8.92 (s, 1H), 7.89 (d, J = 9.0 Hz, 2H), 7.65 (d, J = 8.4 Hz, 2H), 7.09 (d, J = 7.2 Hz, 4H), 6.94 (s, 2H). IR (KBr, cm−1): υOH: 3388.90; υC=O: 1652.96; υC=C: 1610.39, 1590.27, 1507.16, 1444.29; υS=O: 1317.34, 1153.40; υC-O: 1209.12; γ=C-H: 833.72, 757.35. HRMS (ESI) m/z for C19H15N2O6FS [M+H]+: calcd 419.0708, found 419.0718.
N-(4-(3,4-Difluorophenylsulfamoyl)phenyl)galloylamide (9b)
Yellowish powder, yield 58.6%, mp 265.1–266.7°C.Citation1H-NMR (DMSO-d6) δ: 10.41 (s, 1H), 10.26 (s, 1H), 9.22 (s, 2H), 8.91 (s, 1H), 7.91 (d, J = 8.4 Hz, 2H), 7.70 (d, J = 9.0 Hz, 2H), 7.33 (m, 1H), 7.10 (m, 1H), 6.94 (s, 2H), 6.89 (d, J = 8.4 Hz, 1H). IR (KBr, cm−1): υOH: 3384.58; υCH: 2975.49; υC=O: 1652.52; υC=C: 1614.13, 1590.56, 1519.04, 1445.08; υS=O: 1320.77, 1162.37; υC-O: 1252.83, 1210.51; γ=C-H: 837.12, 757.97. HRMS (ESI) m/z for C19H15N2O6F2S [M+H]+: calcd 437.0613, found 437.0623.
N-(4-(2,3,4-Trifluorophenylsulfamoyl)phenyl)galloylamide (9c)
White powder, yield 80.8%, mp 254.1–255.5°C. 1H-NMR (DMSO-d6) δ: 10.29 (s, 1H), 10.27 (s, 1H), 9.23 (s, 2H), 8.92 (s, 1H), 7.93 (d, J = 9.0 Hz, 2H), 7.65 (d, J = 9.0 Hz, 2H), 7.28 (m, 1H), 7.01 (m, 1H), 6.97 (s, 2H). IR (KBr, cm−1): υOH: 3388.04; υC=O: 1661.31; υC=C: 1617.05, 1590.26, 1510.49; υS=O: 1335.16, 1160.41; υC-O: 1255.26, 1231.65, 1208.82; γ=C-H: 842.2, 757.07. HRMS (ESI) m/z for C19H14N2O6F3S [M+H]+: calcd 455.0519, found 455.0527.
N-(4-(4-Fluorobenzylsulfamoyl)phenyl)galloylamide (9d)
Yellowish powder, yield 58.3%, mp 225.4–227.1°C. 1H-NMR (DMSO-d6) δ: 10.59 (s, 1H), 9.20-9.80 (br, 3H), 8.03 (s, 1H), 7.87 (d, J = 8.4 Hz, 2H), 7.77 (d, J = 9.0 Hz, 2H), 7.26 (m, 6H), 3.95 (s, 2H). IR (KBr, cm−1): υNH: 3269.41; υCH: 2976.42; υC=O: 1608.98; υC=C: 1591.15, 1512.59, 1445.86; υS=O: 1325.20, 1154.31; υC-O: 1231.19; γ=C-H: 824.18, 757.05. HRMS (ESI) m/z for C20H18N2O6FS [M+H]+: calcd 433.0864, found 433.0873.
N-(4-(4-Bromophenylsulfamoyl)phenyl)galloylamide (9e)
White powder, yield 56.7%, mp 271.1–273.3°C. 1H-NMR (DMSO-d6) δ: 10.25 (s, 1H), 9.21 (br, 3H), 7.90 (d, J = 9.0 Hz, 2H), 7.70 (d, J = 9.0 Hz, 2H), 7.42 (d, J = 9.0 Hz, 2H), 7.05(d, J = 9.0 Hz, 2H), 6.94 (s, 2H). IR (KBr, cm−1): υOH: 3397.52; υNH: 3157.91; υC=O: 1676.87; υC=C: 1589.25, 1504.69, 1445.40; υS=O: 1327.51, 1143.64; υC-O: 1306.20, 1246.77, 1210.30; γ=C-H: 825.04, 757.39, 708.40. HRMS (ESI) m/z for C19H16N2O6BrS [M+H]+: calcd 478.9907, found 478.9916.
N-(4-(3-Bromophenylsulfamoyl)phenyl)galloylamide (9f)
White powder, yield 44.2%, mp 260.6–261.3°C. 1H-NMR (DMSO-d6) δ: 10.45 (s, 1H), 10.26 (s, 1H), 9.22 (s, 2H), 8.91 (s, 1H), 7.91 (d, J = 9.0 Hz, 2H), 7.72 (d, J = 9.0 Hz, 2H), 7.25 (s, 1H), 7.20 (d, J = 4.8 Hz, 2H), 7.12 (m, 1H), 6.94 (s, 2H). IR (KBr, cm−1): υOH: 3376.08; υC=O: 1653.00; υC=C: 1609.83, 1590.78, 1521.10, 1473.20; υS=O: 1329.27, 1153.64; υC-O: 1257.32, 1212.64; γ=C-H: 838.01, 755.03. HRMS (ESI) m/z for C19H16N2O6BrS [M+H]+: calcd 478.9907, found 478.9920.
N-(4-(2-Bromophenylsulfamoyl)phenyl)galloylamide (9g)
White powder, yield 49.3%, mp 241.6–243.4°C. 1H-NMR (DMSO-d6) δ: 10.27 (s, 1H), 9.77 (s, 1H), 9.23 (s, 2H), 8.92 (s, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.66 (d, J = 8.4 Hz, 2H), 7.25 (dd, J = 7.8 Hz, J = 1.8 Hz, 1H), 7.32 (td, J = 7.8 Hz, J = 1.2 Hz, 1H), 7.19 (dd, J = 7.8 Hz, J = 1.8 Hz, 1H), 7.13 (td, J = 7.8 Hz, J = 1.2 Hz, 1H), 6.96 (s, 2H). IR (KBr, cm−1): υOH: 3454.11, 3301.65; υNH: 3212.44; υCH: 2924.26, 2853.12; υC=O: 1658.80; υC=C: 1603.47, 1588.64, 1524.84, 1475.51; υS=O: 1311.38, 1156.59; υC-O: 1254.71, 1218.76; γ=C-H: 841.34, 755.98. HRMS (ESI) m/z for C19H16N2O6BrS [M+H]+: calcd 478.9907, found 478.9913.
N-(4-(3,4-Dichlorophenylsulfamoyl)phenyl)galloylamide (9h)
Yellowish powder, yield 60.4%, mp 259.4–261.2°C. 1H-NMR (DMSO-d6) δ: 10.61 (s, 1H), 10.27 (s, 1H), 9.22 (s, 2H), 8.91 (s, 1H), 7.93 (d, J = 9.0 Hz, 2H), 7.73 (d, J = 9.0 Hz, 2H), 7.51 (d, J = 9.0 Hz, 1H), 7.28 (d, J = 3.0 Hz, 1H), 7.28 (dd, J = 9.0 Hz, J = 2.4 Hz, 1H), 6.94 (s, 2H). IR (KBr, cm−1): υOH: 3403.21; υC=O: 1662.34; υC=C: 1590.43, 1518.80, 1472.34; υS=O: 1321.73, 1159.18; υC-O: 1222.24; γ=C-H: 829.85, 756.69. HRMS (ESI) m/z for C19H15N2O6Cl2S [M+H]+: calcd 469.0022, found 469.0033.
N-(4-(4-Butylphenylsulfamoyl)phenyl)galloylamide (9i)
White powder, yield 73.4%, mp 260.5–261.4°C. 1H-NMR (DMSO-d6) δ: 10.22 (s, 1H), 10.02 (s, 1H), 9.22 (s, 2H), 8.91 (s, 1H), 7.88 (d, J = 9.0 Hz, 2H), 7.66 (d, J = 9.0 Hz, 2H), 7.04 (d, J = 7.8 Hz, 2H), 6.98 (d, J = 8.4 Hz, 2H), 6.94 (s, 2H), 2.45 (t, J = 7.8 Hz, 2H), 1.46 (quintet, 2H), 1.24 (sextet, 2H), 0.85 (t, J = 7.2 Hz, 3H). IR (KBr, cm−1): υOH: 3433.45; υNH: 3333.27, 3223.55; υCH: 2954.15, 2927.03, 2858.20; υC=O: 1667.19; υC=C: 1592.20, 1529.40, 1512.43, 1442.07; υS=O: 1341.85, 1159.04; υC-O: 1311.75, 1263.80, 1219.59; γ=C-H: 841.51, 760.55, 704.06. HRMS (ESI) m/z for C23H25N2O6S [M+H]+: calcd 457.1428, found 457.1436.
N-(4-(4-Ethoxylphenylsulfamoyl)phenyl)galloylamide (9j)
White powder, yield 55.8%, mp 271.4–272.9°C.Citation1H-NMR (DMSO-d6) δ: 10.22 (s, 1H), 9.68 (s, 1H), 9.18 (br, 3H), 7.87 (d, J = 9.0 Hz, 2H), 7.60 (d, J = 9.0 Hz, 2H), 6.95 (m, 4H), 6.78 (d, J = 9.0 Hz, 2H), 3.91 (q, J = 7.2 Hz, 2H), 1.26 (t, J = 7.2 Hz, 3H). IR (KBr, cm−1): υOH: 3400.03; υNH: 3186.03; υCH3: 2983.01, 2937.89; υC=O: 1676.82; υC=C: 1589.77, 1509.67, 1445.41; υS=O: 1326.67, 1144.63; υC-O: 1253.28, 1212.47; γ=C-H: 823.77, 756.19. HRMS (ESI) m/z for C21H21N2O7S [M+H]+: calcd 445.1064, found 445.1074.
N-(4-(3,5-Dimethylphenylsulfamoyl)phenyl)galloylamide (9k)
Yellowish powder, yield 78.3%, mp 266.8–267.7°C. 1H-NMR (DMSO-d6) δ: 10.23 (s, 1H), 10.05 (s, 1H), 9.21 (s, 2H), 8.90 (s, 1H), 7.89 (d, J = 8.4 Hz, 2H), 7.70 (d, J = 9.0 Hz, 2H), 6.94 (s, 2H), 6.72 (s, 2H), 6.64 (s, 1H), 2.09 (s, 6H). IR (KBr, cm−1): υOH: 3339.34; υCH: 2919.14; υC=O: 1661.65; υC=C: 1590.17, 1519.36, 1440.46; υS=O: 1314.22, 1146.64; υC-O: 1253.37, 1226.94; γ=C-H: 837.99, 755.38. HRMS (ESI) m/z for C21H21N2O6S [M+H]+: calcd 429.1115, found 429.1123.
N-(4-(3-chloro-2-fluorophenylsulfamoyl)phenyl)galloylamide (9l)
White powder, yield 67.4%, mp 247.5–248.6°C. 1H-NMR (DMSO-d6) δ: 10.32 (s, 1H), 10.28 (s, 1H), 9.27 (s, 2H), 8.94 (s, 1H), 7.92 (d, J = 9.0 Hz, 2H), 7.69 (d, J = 9.0 Hz, 2H), 7.36 (t, J = 7.8 Hz, 1H), 7.22 (d, J = 7.2 Hz, 1H), 7.15 (t, J = 7.8 Hz, 1H), 6.96 (s, 2H). IR (KBr, cm−1): υOH: 3350.58; υCH: 2918.51, 2848.49; υC=O: 1779.88, 1662.78; υC=C: 1608.49, 1589.24, 1518.57; υS=O: 1325.02, 1159.47; υC-O: 1252.94, 1232.28, 1206.56; γ=C-H: 839.31, 818.89, 756.97, 728.04. HRMS (ESI) m/z for C19H15N2O6FClS [M+H]+: calcd 453.0318, found 453.0325.
N-(4-Phenylsulfamoylphenyl)galloylamide (9m)
Yellowish powder, yield 68.0%, mp 257.3–259.1°C. 1H-NMR (DMSO-d6) δ: 10.22 (s, 1H), 10.17 (s, 1H), 9.21 (s, 2H), 8.90 (s, 1H), 7.88 (d, J = 9.0 Hz, 2H), 7.69 (d, J = 9.0 Hz, 2H), 7.23 (t, J = 8.4 Hz, 2H), 7.09 (d, J = 9.0 Hz, 2H), 7.02 (t, J = 7.20 Hz, 1H), 6.93 (s, 2H). IR (KBr, cm−1): υOH: 3444.79; υNH: 3311.73, 3222.61; υC=O: 1667.96; υC=C: 1591.21, 1518.26, 1442.69; υS=O: 1342.43, 1159.67; υC-O: 1313.09, 1260.81, 1214.68; γ=C-H: 838.09, 757.59, 707.72. HRMS (ESI) m/z for C19H17N2O6S [M+H]+: calcd 401.0802, found 401.0812.
N-(4-(4-Chlorophenylsulfamoyl)phenyl)galloylamide (9n)
White powder, yield 58.9%, mp 273.5–275.9°C. 1H-NMR (DMSO-d6) δ: 10.28 (s, 1H), 10.24 (s, 1H), 9.21 (br, 2H), 8.85 (s, 1H), 7.90 (d, J = 9.0 Hz, 2H), 7.69 (d, J = 9.0 Hz, 2H), 7.30 (d, J = 9.0 Hz, 2H), 7.10 (d, J = 9.0 Hz, 2H), 6.94 (s, 2H). IR (KBr, cm−1): υOH: 3397.19; υNH: 3156.01; υC=O: 1678.21; υC=C: 1589.50, 1537.67, 1505.70, 1446.78; υS=O: 1326.60, 1143.40; υC-O: 1306.79, 1246.77, 1209.73; γ=C-H: 812.75, 757.20, 721.44. HRMS (ESI) m/z for C19H16N2O6ClS [M+H]+: calcd 435.0412, found 435.0422.
N-(4-(4-Chlorobenzylsulfamoyl)phenyl)galloylamide (9o)
White powder, yield 52.1%, mp 217.2–217.8°C. 1H-NMR (DMSO-d6) δ: 10.26(s, 1H), 8.80-9.35 (br, 3H), 8.04 (s, 1H), 7.95 (d, J = 9.0 Hz, 2H), 7.74 (d, J = 8.4 Hz, 2H), 7.29 (dd, J = 7.8 Hz, J = 6.0 Hz, 2H), 7.12 (t, J = 9.0 Hz, 2H), 6.98 (s, 2H), 3.95 (s, 2H). IR (KBr, cm−1): υOH: 3553.90, 3519.08; υNH: 3318.34; υC=O: 1671.22; υC=C: 1591.76, 1533.63, 1510.29, 1455.98; υS=O: 1321.46, 1142.46; υC-O: 1299.01, 1256.86, 1207.48; γ=C-H: 840.42, 773.10, 701.21. HRMS (ESI) m/z for C20H18N2O6ClS [M+H]+: calcd 449.0569, found 449.0578.
N-(4-(3,4-Dimethylphenylsulfamoyl)phenyl)galloylamide (9p)
Yellowish powder, yield 50.5%, mp 273.4–275.1°C. 1H-NMR (DMSO-d6) δ: 10.21 (s, 1H), 9.94 (s, 1H), 9.21 (s, 2H), 8.90 (s, 1H), 7.86 (d, J = 9.0 Hz, 2H), 7.67 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 8.4 Hz, 1H), 6.94 (s, 2H), 6.87 (s, 1H), 6.81 (d, J = 7.8 Hz, 1H), 2.10(s, 6H). IR (KBr, cm−1): υOH: 3442.41; υNH: 3254.22; υC=O: 1659.33; υC=C: 1590.35, 1522.84, 1443.73; υS=O: 1309.64, 1152.17; υC-O: 1254.57, 1216.87; γ=C-H: 864.39, 757.37. HRMS (ESI) m/z for C21H21N2O6S [M+H]+: calcd 429.1115, found 429.1125.
N-(4-(4-Methoxylphenylsulfamoyl)phenyl)galloylamide (9q)
White powder, yield 73.9%, mp 269.8–271.7°C. 1H-NMR (DMSO-d6) δ: 10.22 (s, 1H), 9.80 (s, 1H), 9.21 (s, 2H), 8.90 (s, 1H), 7.87 (d, J = 9.0 Hz, 2H), 7.62 (d, J = 9.0 Hz, 2H), 6.96 (m, 4H), 6.80 (d, J = 8.4Hz, 2H), 3.67(s, 3H). IR (KBr, cm−1): υOH: 3512.95, 3365.67; υNH: 3269.09, 3149.97; υC=O: 1656.12; υC=C: 1624.31, 1591.57, 1524.28, 1444.13; υS=O: 1317.11, 1143.35; υC-O: 1267.10, 1228.40; γ=C-H: 833.09, 793.31, 750.04. HRMS (ESI) m/z for C20H19N2O6S [M+H]+: calcd 431.0907, found 431.0915.
N-(4-(4-Nitrophenylsulfamoyl)phenyl)galloylamide (9r)
White powder, yield 52.3%, mp 256.1–257.5°C. 1H-NMR (DMSO-d6) δ: 11.20 (s, 1H), 10.28 (s, 1H), 9.22 (s, 2H), 8.91 (s, 1H), 8.14 (d, J = 9.0 Hz, 2H), 7.94 (d, J = 9.0 Hz, 2H), 7.82 (d, J = 8.40 Hz, 2H), 7.31 (d, J = 9.0 Hz, 2H), 6.93 (s, 2H). IR (KBr, cm−1): υOH: 3431.20; υNH: 3193.81; υC=O: 1656.40; υC=C: 1597.41, 1586.22, 1496.84; υNO2: 1524.80; υS=O: 1343.40, 1149.71; υC-O: 1240.82, 1195.61, 1182.13; γ=C-H: 850.10, 756.31. HRMS (ESI) m/z for C19H16N3O8S [M+H]+: calcd 446.0653, found 446.0665.
N-(4-(2-Methylphenylsulfamoyl)phenyl)galloylamide (9s)
White powder, yield 69.7%, mp 263.9–264.8°C. 1H-NMR (DMSO-d6) δ: 10.25 (s, 1H), 9.44 (s, 1H), 9.22 (s, 2H), 8.91 (s, 1H), 7.90 (d, J = 9.0 Hz, 2H), 7.59 (d, J = 8.4 Hz, 2H), 7.13 (dd, J = 6.0 Hz, J = 3.0 Hz, 1H), 7.10 (m, 2H), 7.0 (d, J = 2.4 Hz, 1H,), 6.96 (s, 2H), 2.02 (s, 3H). IR (KBr, cm−1): υOH: 3449.51; υNH: 3329.29, 3231.72; υC=O: 1665.62; υC=C: 1591.26, 1522.43, 1442.05; υS=O: 1309.67, 1152.55; υC-O: 1257.98, 1210.03; γ=C-H: 839.62, 754.00. HRMS (ESI) m/z for C20H19N2O6S [M+H]+: calcd 415.0958, found 415.0968.
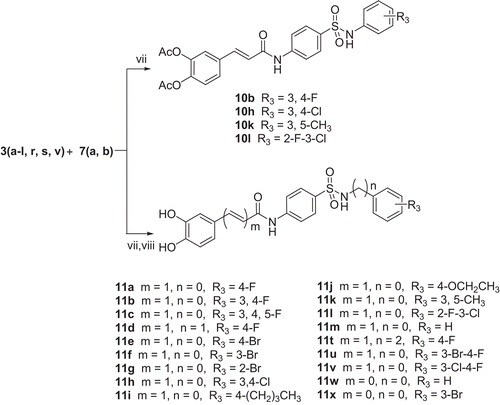
General methods for preparing target compounds 10
To a stirred solution of 4-amino-N-substituted-benzenesulfonamide 3 (2.2 mmol) and pyridine (2.2 mmol) in acetone (8 mL) acyl chloride 7 (1.8 mmol) was added in portion at -5°C. The reaction mixture was stirred at room temperature for 16 h and the precipitate was removed by filtration. The filtrate was concentrated under reduced pressure and the residue was purified by column chromatography using acetone/petroleum ether (1:1) as eluent to yield compound 10.
(E)-3-(3,4-Diacetoxyphenyl)-N-(4-(3,4-Difluorophenylsulfamoyl)phenyl)acrylamide (10b)
White powder, yield 74.0%, mp 219.1–221.2°C. 1H-NMR (DMSO-d6) δ: 10.63 (s, 1H), 10.43 (s, 1H), 7.84 (d, J = 9.0 Hz, 2H), 7.73 (d, J = 9.0 Hz, 2H), 7.61(d, J = 16.2 Hz, 1H), 7.58 (m, 2H), 7.34 (m, 2H), 7.10 (m, 1H), 6.89 (d, J = 8.4 Hz, 2H), 6.80 (d, J = 15.6 Hz, 1H), 2.31(s, 3H), 2.30(s, 3H). IR (KBr, cm−1): υNH: 3350.16; υ=CH: 3137.78; υC=O: 1774.84; υC=C: 1677.03, 1631.36, 1590.99, 1518.04; υS=O: 1341.07, 1163.01; υC-O: 1256.67, 1209.85; γ=C-H: 836.15. HRMS (ESI) m/z for C25H21N2O7F2S [M+H]+: calcd 531.1032, found 531.1035.
(E)-3-(3,4-Diacetoxyphenyl)-N-(4-(3,4-Dichlorophenylsulfamoyl)phenyl)acrylamide(10h)
White powder, yield 69.8%, mp 246.7–247.8°C. 1H-NMR (DMSO-d6) δ: 10.64 (s, 1H), 10.64 (s, 1H), 7.86 (d, J = 8.4 Hz, 2H), 7.76 (d, J = 8.4 Hz, 2H), 7.61(d, J = 16.2 Hz, 1H), 7.59 (d, J = 1.8 Hz, 1H), 7.57 (dd, J = 6.6 Hz, J = 1.8 Hz, 1H), 7.51 (d, J = 9.0 Hz, 1H), 7.36 (d, J = 8.4 Hz, 1H), 7.28(d, J = 8.4 Hz, 1H), 7.10 (dd, J = 9.0 Hz, J = 2.4 Hz, 1H), 7.80(d, J = 16.2 Hz, 1H), 2.31(s, 3H), 2.30(s, 3H). IR (KBr, cm−1): υNH: 3529.13; υ=CH: 3180.00, 3117.79; υC=O: 1780.80; υC=C: 1678.00, 1630.52, 1590.02, 1525.71, 1505.44; υS=O: 1320.85, 1152.62; υC-O: 1255.30, 1210.20; γ=C-H: 838.31. HRMS (ESI) m/z for C25H21N2O7Cl2S [M+H]+: calcd 563.0441, found 563.0444.
(E)-3-(3,4-Diacetoxyphenyl)-N-(4-(3,5-Dimethylphenylsulfamoyl)phenyl)acrylamide(10k)
White powder, yield 75.4%, mp 188.5–189.3°C. 1H-NMR (DMSO-d6) δ: 10.59 (s, 1H), 10.08 (s, 1H), 7.82 (d, J = 9.0 Hz, 2H), 7.73 (d, J = 9.0 Hz, 2H), 7.61(d, J = 15.6 Hz, 1H), 7.57 (d, J = 4.8 Hz, 2H), 7.35 (d, J = 7.8 Hz, 1H), 6.80(d, J = 15.6 Hz, 1H), 6.72 (s, 2H), 6.64 (s, 1H), 2.31(s, 3H), 2.30(s, 3H). IR (KBr, cm−1): υNH: 3352.62; υ=CH: 3124.55, 3038.96; υCH: 2937.62, 2814.11; υC=O: 1766.03; υC=C: 1669.22, 1631.74, 1591.10, 1530.77, 1476.82; υS=O: 1333.40, 1162.66; υC-O: 1255.80, 1214.97; γ=C-H: 840.23. HRMS (ESI) m/z for C27H27N2O7S [M+H]+: calcd 523.1533, found 523.1536.
(E)-3-(3,4-Diacetoxyphenyl)-N-(4-(3-chloro-2-fluorophenylsulfamoyl)phenyl)acrylamide (10l)
White powder, yield 61.7%, mp 240.5–241.8°C. 1H-NMR (DMSO-d6) δ: 10.64 (s, 1H), 10.35 (s, 1H), 7.85 (d, J = 9.0 Hz, 2H), 7.71 (d, J = 9.0 Hz, 2H), 7.62(d, J = 15.6 Hz, 1H), 7.58 (m, 2H), 7.36 (d, J = 8.4 Hz, 2H), 7.21 (t, J = 7.2 Hz, 1H), 7.15 (t, J = 8.4 Hz, 1H), 6.81(d, J = 15.6 Hz, 1H), 2.31(s, 3H), 2.30(s, 3H). IR (KBr, cm−1): υNH: 3373.57, 3126.20; υCH: 2894.37, 2839.76; υC=O: 1780.48, 1756.71; υC=C: 1675.56, 1628.51, 1588.90, 1523.21; υS=O: 1369.15, 1343.53, 1165.62; υC-O: 1258.58, 1215.36; γ=C-H: 839.78. HRMS (ESI) m/z for C25H21N2O7FClS [M+H]+: calcd 547.0737, found 547.0738.
General methods for preparing target compounds 11
Following the aforementioned procedure, amidation between compound 3 and compound 7 was carried out. The product was added to a solution of tetrahydrofuran (3 mL), ethanol (3 mL) and concentrated hydrochloric acid (37%, 1.5 mL). The reaction mixture was stirred for 0.5 h at 65°C. Evaporation of the solvent gave a residue, which was recrystallized from ethanol/water to obtain compound 11.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(4-Fluorophenylsulfamoyl)phenyl)acrylamide(11a)
Yellowish powder, yield 68.2%, mp 183.5–184.6°C. 1H-NMR (DMSO-d6) δ: 10.44 (s, 1H), 10.13 (s, 1H), 9.54 (s, 1H), 9.24 (s, 1H), 7.81 (d, J = 8.4 Hz, 2H), 7.67 (d, J = 9.0 Hz, 2H), 7.44(d, J = 15.6 Hz, 1H), 7.09 (d, J = 6.6 Hz, 4H), 7.98 (s, 1H), 6.93 (d, J = 8.4 Hz, 1H), 6.79 (d, J = 8.4 Hz, 1H), 6.53(d, J = 15.6 Hz, 1H). IR (KBr, cm−1): υOH: 3363.42; υNH: 3258.97; υ=CH: 2975.16; υC=O: 1659.57; υC=C: 1610.17, 1591.22, 1524.05, 1506.90; υS=O: 1329.78, 1155.88; υC-O: 1278.26, 1256.35; γ=C-H: 833.42. HRMS (ESI) m/z for C21H18N2O5FS [M+H]+: calcd 429.0915, found 429.0919.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(3,4-Difluorophenylsulfamoyl)phenyl)acrylamide(11b)
Yellowish powder, yield 79.1%, mp 179.1–180.3°C. 1H-NMR (DMSO-d6) δ: 10.46 (s, 1H), 10.41 (s, 1H), 9.54 (s, 1H), 9.23 (s, 1H), 7.83 (d, J = 9.0 Hz, 2H), 7.71(d, J = 9.0 Hz, 2H), 7.44(d, J = 16.2 Hz, 1H,), 7.33 (m, 1H), 7.10 (m, 1H), 7.01 (d, J = 1.8 Hz, 1H), 6.93 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H), 6.88 (dd, J = 9.0 Hz, J = 5.4 Hz, 1H), 6.78 (d, J = 7.8 Hz, 1H), 6.53(d, J = 15.6 Hz, 1H). IR (KBr, cm−1): υOH: 3330.64; υ=CH: 2975.38; υC=O: 1671.56; υC=C: 1591.98, 1519.39, 1447.75; υS=O: 1333.14, 1162.93; υC-O: 1285.61, 1256.85; γ=C-H: 879.14. HRMS (ESI) m/z for C21H17N2O5F2S [M+H]+: calcd 447.0821, found 447.0826.
(E)-3-(3,4-Dihydroxyphenyl)-N-(2,3,4-Trifluorophenylsulfamoyl)phenyl)acrylamide(11c)
Yellowish powder, yield 71.2%, mp 165.7–167.2°C. 1H-NMR (DMSO-d6) δ: 10.48 (s, 1H), 10.30 (s, 1H), 9.54 (s, 1H), 9.24 (s, 1H), 7.84 (d, J = 8.4 Hz, 2H), 7.66 (d, J = 8.4 Hz, 2H), 7.45(d, J = 15.6 Hz, 1H), 7.26 (d, J = 9.0 Hz, 1H), 7.02 (s, 1H), 7.0 (d, J = 3.0 Hz, 1H), 6.94 (d, J = 8.4 Hz, 1H), 6.79 (d, J = 8.4 Hz, 1H), 6.54(d, J = 15.6 Hz, 1H). IR (KBr, cm−1): υNH: 3256.63; υ=CH: 2954.98, 2924.60; υC=O: 1669.82; υC=C: 1591.16, 1511.81; υS=O: 1339.11, 1157.60; υC-O: 1284.13, 1255.86; γ=C-H: 812.00. HRMS (ESI) m/z for C21H16N2O5F3S [M+H]+: calcd 465.0727, found 465.0732.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(4-Fluorobenzylsulfamoyl)phenyl)acrylamide(11d)
White powder, yield 61.7%, mp 232.6–233.4°C. 1H-NMR (DMSO-d6) δ: 10.45 (s, 1H), 9.54 (s, 1H), 9.24 (s, 1H), 8.05 (t, J = 6.0 Hz, 1H), 7.85 (d, J = 8.4 Hz, 2H), 7.74 (d, J = 9.0 Hz, 2H), 7.46(d, J = 15.6 Hz, 1H), 7.28 (dd, J = 6.6 Hz, J = 2.4 Hz, 2H), 7.11 (t, J = 2.4 Hz, 2H), 7.03 (d, J = 1.8 Hz, 1H), 6.94 (dd, J = 8.4 Hz, J = 1.8 Hz, 1H), 6.79(d, J = 8.4 Hz, 1H), 6.56(d, J = 15.6 Hz, 1H), 3.96(d, J = 6.0 Hz, 2H). IR (KBr, cm−1): υOH: 3554.10; υNH: 3417.04, 3343.80, 3142.88; υ=CH: 2923.71; υC=O: 1673.54; υC=C: 1630.45, 1591.89, 1534.26, 1510.76; υS=O: 1316.31, 1300.72, 1150.78; υC-O: 1263.14, 1226.07; γ=C-H: 834.15. HRMS (ESI) m/z for C22H20N2O5FS [M+H]+: calcd 443.1071, found 443.1074.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(4-Bromophenylsulfamoyl)phenyl)acrylamide(11e)
Yellowish powder, yield 59.7%, mp 192.4–193.8°C. 1H-NMR (DMSO-d6) δ: 10.45 (s, 1H), 10.37 (s, 1H), 9.54 (s, 1H), 9.24 (s, 1H), 7.82 (d, J = 8.4 Hz, 2H), 7.71 (d, J = 8.4 Hz, 2H), 7.43(d, J = 15.6 Hz, 1H), 7.42 (d, J = 6.6 Hz, 2H), 7.09 (d, J = 9.0 Hz, 2H), 7.02 (s, 1H), 6.93 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H), 6.78 (d, J = 7.8 Hz, 1H), 6.53(d, J = 15.6 Hz, 1H). IR (KBr, cm−1): υNH: 3252.48; υ=CH: 2973.66, 2923.03; υC=O: 1658.84; υC=C: 1609.69, 1590.86, 1523.07; υS=O: 1329.76, 1155.90; υC-O: 1278.82, 1256.06; γ=C-H: 832.77. HRMS (ESI) m/z for C21H18N2O5BrS [M+H]+: calcd 489.0114, found 489.0113.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(3-Bromophenylsulfamoyl)phenyl)acrylamide(11f)
White powder, yield 66.4%, mp 197.8–198.8°C. 1H-NMR (DMSO-d6) δ: 10.48 (s, 1H), 10.46 (s, 1H), 9.53 (s, 1H), 9.23 (s, 1H), 7.83 (d, J = 8.4 Hz, 2H), 7.73 (d, J = 9.0 Hz, 2H), 7.43(d, J = 15.6 Hz, 1H), 7.24 (s, 1H), 7.20 (d, J = 4.8 Hz, 2H), 7.10 (dd, J = 8.4 Hz, J = 4.8 Hz, 1H), 7.01 (d, J = 1.8 Hz, 1H), 6.92 (d, J = 8.4 Hz, 1H), 6.52(d, J = 15.6 Hz, 1H). IR (KBr, cm−1): υOH: 3373.22; υ=CH: 2974.84; υC=O: 1672.16; υC=C: 1592.69, 1533.27, 1497.67, 1474.69; υS=O: 1335.92, 1159.47; υC-O: 1286.96, 1257.41; γ=C-H: 879.69. HRMS (ESI) m/z for C21H18N2O5BrS [M+H]+: calcd 489.0114, found 489.0118.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(2-Bromophenylsulfamoyl)phenyl)acrylamide(11g)
White powder, yield 68.9%, mp 208.7–209.3°C. 1H-NMR (DMSO-d6) δ: 10.46 (s, 1H), 9.77 (s, 1H), 9.54 (s, 1H), 9.24 (s, 1H), 7.83 (d, J = 8.4 Hz, 2H), 7.66 (d, J = 8.4 Hz, 2H), 7.58(dd, J = 8.4 Hz, J = 1.8 Hz, 1H), 7.45(d, J = 15.6 Hz, 1H), 7.32 (m, 1H), 7.19 (dd, J = 8.4 Hz, J = 1.8 Hz, 1H), 7.14(dt, J = 7.8 Hz, J = 1.8 Hz, 1H), 7.02 (d, J = 2.4 Hz, 1H), 6.93 (dd, J = 7.8 Hz, J = 1.8 Hz, 1H), 6.78(d, J = 7.8 Hz, 1H), 6.54(d, J = 15.6 Hz, 1H). IR (KBr, cm−1): υOH:3354.20; υC=O:1670.63; υC=C:1589.37, 1514.80, 1476.41; υS=O:1332.90, 1153.28; υC-O:1282.67, 1253.48; γ=C-H: 817.01. HRMS (ESI) m/z for C21H18N2O5BrS [M+H]+: calcd 489.0114, found 489.0120.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(3,4-Dichlorophenylsulfamoyl)phenyl)acrylamide(11h)
White powder, yield 77.4%, mp 226.8–228.1°C. 1H-NMR (DMSO-d6) δ: 10.62 (s, 1H), 10.47 (s, 1H), 9.54 (s, 1H), 9.23 (s, 1H), 7.84 (d, J = 8.4 Hz, 2H), 7.74 (d, J = 8.4 Hz, 2H), 7.51 (d, 1H, J = 9.0 Hz), 7.44(d, J = 16.2 Hz, 1H), 7.27 (d, J = 2.4Hz, 1H), 7.09 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H), 7.02 (s, 1H), 6.93(d, J = 7.8 Hz, 1H), 6.78 (d, J = 7.8 Hz, 1H), 6.52(d, J = 16.2 Hz, 1H). IR (KBr, cm−1): υOH: 3350.13; υ=C-H: 2975.28; υC=O: 1671.24; υC=C: 1590.80, 1528.13, 1473.75; υS=O: 1303.19, 1158.09; υC-O: 1254.24, 1182.35; γ=C-H: 812.90. HRMS (ESI) m/z for C21H17N2O5Cl2S [M+H]+: calcd 479.0230, found 479.0240.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(4-Butylphenylsulfamoyl)phenyl)acrylamide(11i)
Yellowish powder, yield 61.7%, mp 197.5–198.8°C. 1H-NMR (DMSO-d6) δ: 10.43 (s, 1H), 10.03 (s, 1H), 9.54 (s, 1H), 9.23 (s, 1H), 7.79 (d, J = 9.0 Hz, 2H), 7.68 (d, J = 9.0 Hz, 2H), 7.44(d, J = 15.6 Hz, 1H), 7.03 (t, J = 7.8 Hz, 3H), 7.98 (d, J = 8.4 Hz, 2H), 6.93 (d, J = 8.4 Hz, 1H), 6.78 (d, J = 8.4 Hz, 1H), 6.53(d, J = 15.6 Hz, 1H), 2.45(t, 2H), 1.46(qui, 2H), 1.22(sxt, 2H), 0.85(t, 3H). IR (KBr, cm−1): υNH: 3254.37; υ=CH: 2955.51, 2927.02; υCH: 2856.14; υC=O: 1661.42; υC=C: 1591.22, 1523.20, 1446.16; υS=O: 1332.40, 1154.78; υC-O: 1280.48, 1254.34; γ=C-H: 833.60. HRMS (ESI) m/z for C25H27N2O5S [M+H]+: calcd 467.1635, found 467.1641.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(4-Ethoxylphenylsulfamoyl)phenyl)acrylamide(11j)
Yellowish powder, yield 51.7%, mp 184.7–185.6°C. 1H-NMR (DMSO-d6) δ: 10.40 (s, 1H), 10.40 (s, 1H), 9.74 (s, 1H), 9.37 (s, 1H), 7.78 (d, J = 9.0 Hz, 2H), 7.62 (d, J = 8.4 Hz, 2H), 7.43(d, J = 15.6 Hz, 1H), 7.01 (s, 1H), 6.95 (d, J = 8.4 Hz, 1H), 6.92 (d, J = 7.2 Hz, 1H), 6.78 (d, J = 8.4 Hz, 3H), 6.52(d, J = 15.6 Hz, 1H). IR (KBr, cm−1): υNH: 3256.27; υ=C-H: 2979.28, 2929.24; υC=O: 1667.04; υC=C: 1591.04, 1509.65; υS=O: 1332.67, 1154.38; υC-O: 1282.50, 1252.17; γ=C-H: 836.69. HRMS (ESI) m/z for C23H23N2O6S [M+H]+: calcd 455.1271, found 455.1271.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(3,5-Dimethylphenylsulfamoyl)phenyl)acrylamide (11k)
Yellowish powder, yield 72.7%, mp 222.4–223.8°C. 1H-NMR (DMSO-d6) δ: 10.42 (s, 1H), 10.05 (s, 1H), 9.52 (s, 1H), 9.22 (s, 1H), 7.80 (d, J = 8.4 Hz, 2H), 7.71 (d, J = 8.4 Hz, 2H), 7.43(d, J = 15.6 Hz, 1H), 7.01 (d, J = 1.8 Hz, 1H), 6.92 (dd, J = 8.4 Hz, J = 2.4 Hz, 1H), 6.78 (d, J = 8.4 Hz, 1H), 6.71 (s, 2H), 6.64 (s, 1H), 6.52(d, J = 15.6 Hz, 1H), 2.15 (s, 6H). IR (KBr, cm−1): υOH: 3349.14; υNH: 3245.78; υCH: 2919.73; υC=O: 1670.03; υC=C: 1591.23, 1520.88; υS=O: 1357.42, 1150.45; υC-O: 1285.02, 1253.35; γ=C-H: 839.76. HRMS (ESI) m/z for C23H23N2O5S [M+H]+: calcd 439.1322, found 439.1327.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(3-chloro-2-fluorophenylsulfamoyl)phenyl)acrylamide (11l)
Yellowish powder, yield 76.7%, mp 229.5–230.8°C. 1H-NMR (DMSO-d6) δ: 10.48 (s, 1H), 10.34 (s, 1H), 9.54 (s, 1H), 9.24 (s, 1H), 7.83 (d, J = 9.0 Hz, 2H), 7.69(d, J = 9.0 Hz, 2H), 7.45(d, J = 15.6 Hz, 1H), 7.37 (t, J = 6.6 Hz, 1H), 7.21 (t, J = 7.8 Hz, 1H), 7.15 (t, J = 8.4 Hz, 1H), 7.02 (d, J = 1.8 Hz, 1H), 6.93 (dd, J = 8.4 Hz, J = 1.8 Hz, 1H), 6.78 (d, J = 8.4 Hz, 1H), 6.54(d, J = 16.2 Hz, 1H). IR (KBr, cm−1): υOH: 3353.47; υNH: 3251.51; υ=CH: 2923.67; υC=O: 1668.04; υC=C: 1591.11, 1522.04, 1498.29; υS=O: 1329.46, 1158.03; υC-O: 1281.46, 1253.84; γ=C-H: 816.97. HRMS (ESI) m/z for C21H17N2O5FClS [M+H]+: calcd 463.0525, found 463.0532.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-phenylsulfamoylphenyl)acrylamide (11m)
Yellowish powder, yield 62.2%, mp 190.3–192.2°C. 1H-NMR (DMSO-d6) δ: 10.42 (s, 1H), 10.18 (s, 1H), 9.52 (s, 1H), 9.22 (s, 1H), 7.80 (d, J = 8.40 Hz, 2H), 7.70 (d, J = 9.0 Hz, 2H), 7.43 (d, J = 15.60 Hz, 1H), 7.22 (t, J = 7.80 Hz, 2H), 7.09 (d, J = 7.80 Hz, 2H), 7.02 (t, J = 7.20 Hz, 2H), 6.92 (d, J = 8.40 Hz, 1H), 6.78 (d, J = 7.80 Hz, 1H), 6.52 (d, J = 15.60 Hz, 1H). IR (KBr, cm−1): υOH: 3452.60; υNH: 3348.49; υC=O: 1672.72; υC=C: 1607.40, 1590.00, 1524.01, 1494.62; υS=O: 1296.63, 1151.19; υC-O: 1250.85, 1182.95, γ=C-H: 810.24, 754.32, 690.90. HRMS (ESI) m/z for C21H19N2O5S [M+H]+: calcd 411.1009, found 411.1019.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(4-Fluorophenethylsulfamoyl)phenyl)acrylamide(11t)
White powder, yield 60.9%, mp 219.7–220.8°C. 1H-NMR (DMSO-d6) δ: 10.46 (s, 1H), 9.54 (s, 1H), 9.24 (s, 1H), 7.86 (d, J = 9.0 Hz, 2H), 7.72 (d, J = 9.0 Hz, 2H), 7.58(t, J = 6.0 Hz, 1H), 7.46(d, J = 15.6 Hz, 1H), 7.19 (m, 2H), 7.08 (m, 2H), 7.03 (d, J = 1.8 Hz, 1H), 6.94 (dd, J = 8.4 Hz, J = 1.8 Hz 1H), 6.79(d, J = 7.8 Hz, 1H), 6.56(d, J = 15.6 Hz, 1H), 2.94(m, 2H), 2.67(t, J = 7.2 Hz, 2H). IR (KBr, cm−1): υOH: 3531.21; υNH: 3343.43, 3193.45; υ=CH: 2924.33; υC=O: 1676.65; υC=C: 1601.44, 1587.46, 1526.17, 1507.89; υS=O: 1311.10, 1298.07, 1147.08; υC-O: 1255.07, 1228.00; γ=C-H: 824.78. HRMS (ESI) m/z for C23H22N2O5FS [M+H]+: calcd 457.1228, found 457.1231.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(3-bromo-4-fluorophenylsulfamoyl)phenyl)acrylamide (11u)
White powder, yield 73.2%, mp 227.4–228.3°C. 1H-NMR (DMSO-d6) δ: 10.47 (s, 1H), 10.38 (s, 1H), 9.54 (s, 1H), 9.24 (s, 1H), 7.83 (d, J = 8.4 Hz, 2H), 7.69(d, J = 8.4 Hz, 2H), 7.44(d, J = 15.6 Hz, 1H), 7.32 (dd, J = 6.0 Hz, J = 2.4 Hz, 1H), 7.28 (t, J = 9.0 Hz, 1H), 7.10 (m, 1H), 7.01 (d, J = 1.8 Hz, 1H), 6.93 (dd, J = 8.4 Hz, J = 1.8 Hz, 1H), 6.78 (d, J = 7.8 Hz, 1H), 6.53(d, J = 15.6 Hz, 1H). IR (KBr, cm−1): υOH: 3347.33; υ=CH: 2923.74; υC=O: 1668.79; υC=C: 1590.82, 1525.03, 1492.73; υS=O: 1302.36, 1155.37; υC-O: 1282.35, 1255.38; γ=C-H: 815.04. HRMS (ESI) m/z for C21H17N2O5FBrS [M+H]+: calcd 509.0000, found 508.9988.
(E)-3-(3,4-Dihydroxyphenyl)-N-(4-(3-chloro-4-fluorophenylsulfamoyl)phenyl)acrylamide (11v)
Yellowish powder, yield 79.1%, mp 220.3–221.3°C. 1H-NMR (DMSO-d6) δ: 10.46 (s, 1H), 10.40 (s, 1H), 9.53 (s, 1H), 9.23 (s, 1H), 7.82 (d, J = 8.4 Hz, 2H), 7.69(d, J = 9.0 Hz, 2H), 7.44(d, J = 15.6 Hz, 1H), 7.32 (t, J = 9.0 Hz, 1H), 7.22 (dd, J = 6.6 Hz, J = 2.4 Hz, 1H), 7.06 (m, 1H), 7.01 (d, J = 1.8 Hz, 1H), 6.93 (dd, J = 8.4 Hz, J = 1.8 Hz, 1H), 6.78 (d, J = 8.4 Hz, 1H), 6.52(d, J = 15.6 Hz, 1H). IR (KBr, cm−1): υOH: 3348.49; υNH: 3262.00; υC=O: 1667.98; υC=C: 1590.60, 1526.70, 1497.30; υS=O: 1325.69, 1302.62, 1155.45; υC-O: 1256.54; γ=C-H: 814.94. HRMS (ESI) m/z for C21H17N2O5FClS [M+H]+: calcd 463.0525, found 463.0527.
N-(4-phenylsulfamoylphenyl)3,4-dihydroxybenzamidebenzamide (11w)
White powder, yield 77.6%, mp 257.9–258.6°C. 1H-NMR (DMSO-d6) δ: 10.25 (s, 1H), 10.17 (s, 1H), 9.69 (br, 1H), 8.28 (br, 1H), 7.89 (d, J = 9.0 Hz, 2H), 7.70 (d, J = 8.4 Hz, 2H), 7.36 (d, J = 2.4 Hz, 1H), 7.33 (dd, J = 8.4 Hz, J = 1.8 Hz, 1H), 7.22 (t, J = 8.4 Hz, 2H), 7.09 (d, J = 7.8 Hz, 2H), 7.01 (t, J = 7.8 Hz, 1H), 6.82 (d, J = 7.8 Hz, 1H). IR (KBr, cm−1): υOH: 3477.24, 3395.34; υNH: 3304.26, 3225.24; υC=O: 1652.66; υC=C: 1589.62, 1521.36, 1440.46; υS=O: 1316.36, 1153.08; υC-O: 1252.05, 1228.84; γ=C-H: 835.70, 755.85, 720.74. HRMS (ESI) m/z for C19H17N2O5S [M+H]+: calcd 385.0853, found 385.0869.
N-(4-(3-Bromophenylsulfamoyl)phenyl)3,4-dihydroxybenzamide (11x)
White powder, yield 59.7%, mp 237.8–238.9°C. 1H-NMR (DMSO-d6) δ: 10.49 (s, 1H), 10.29 (s, 1H), 9.72 (s, 1H), 9.30 (s, 1H), 7.92 (d, J = 8.4 Hz, 2H), 7.73 (d, J = 9.0 Hz, 2H), 7.37(d, J = 2.4 Hz, 1H), 7.33 (dd, J = 7.8 Hz, J = 2.4 Hz, 1H), 7.25 (s, 1H), 7.20 (d, J = 3.6 Hz, 2H), 7.12 (m, 1H), 6.83(d, J = 7.8 Hz, 1H). IR (KBr, cm−1): υOH: 3366.32; υNH: 3259.89; υC=O: 1655.16; υC=C: 1590.39, 1506.42, 1474.57; υS=O: 1321.75, 1151.66; υC-O: 1290.01, 1251.33; γ=C-H: 841.39. HRMS (ESI) m/z for C19H16N2O5BrS [M+H]+: calcd 462.9958, found 462.9962.
Biological assays
All compounds were dissolved in DMSO, and the stock solutions were stored at−20°C. The γ[32P]ATP was purchased from MP Biomedical. The expression systems for the wild-type IN were generous gifts of Dr. Robert Craigie, Laboratory of Molecular Biology, NIDDK, NIH, Bethesda, MD.
Preparation of oligonucleotide substrates
The oligonucleotides 21 top, 5′-GTGTGGAAAATCTCTAGCAGT-3′ and 21 bot, 5′-ACTGCTAGAG- ATTTTCCACAC-3′ were purchased from the Norris Cancer Center Core Facility (University of Southern California), and purified by UV shadowing on a polyacrylamide gel. To analyze the extent of 3′-processing and strand transfer using 5′-end-labeled substrates, 21 top was 5′-end-labeled using T4 polynucleotide kinase (Epicenter, Madison, WI) and γ[32P]ATP (MP Biomedical). The kinase was heat-inactivated, and 21 bot was added in 1.5-molar excess. The mixture was heated to 95°C, allowed to cool slowly to room temperature, and run through a spin 25 mini-column (USA Scientific) to separate the annealed double-stranded oligonucleotide from unincorporated material.
All biological assays were performed essentially as previously describedCitation19.
Results and discussion
Chemistry
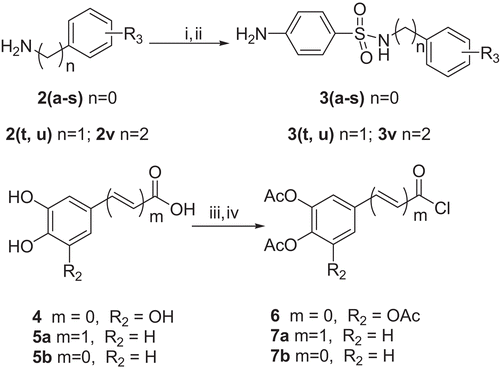
The synthesis of compound 3, 6 and 7 series were shown in . Using compound 2 series as raw material, compound 3 series were synthesized by direct mono-sulfonylation with 4-acetamidobenzene-1-sulfonyl followed by the acetyl removal under alkaline conditions in methanol at room temperature. Compound 4 and 5 series were protected with ethanoyl in the presence of pyridine, and then reacted with thionyl chloride under reflux at 80°C to give compound 6 and 7 series. describes the synthesis of compounds 8 and 9 series. Compounds 8 series were synthesized through amidation between 3 and 6 in acetone. Compound 9 series were finally synthesized by esterolysis using tetrahydrofuran-methanol-concentrated hydrochloric acid (2:2:1, v/v/v) as a solvent avoided the hydrolysis of the amide linkage. The synthesis of compound 10 and 11 were similar with compound 9 and 10 series with 3 and 7 as raw material as shown in .
Scheme 1. Reagents and conditions: (i) 4-acetamidobenzene-1-sulfonyl chloride, pyridine, 0°C− rt, 4 h; (ii) 5 M NaOH, MeOH, 70°C, 4 h; (iii) Ac2O, pyridine, rt, 24 h; (iv) SOCl2, 80°C, 5 h.
Structure-activity relationships in HIV-1 IN inhibition
All compounds were initially tested in an in vitro assay specific for IN. As shown in , and , these inhibitors demonstrated activities against IN-mediated 3′-P and ST at micromolar concentrations.
Table 1. Inhibition of HIV-1 integrase catalytic activities of compounds 9a-s.
Table 2. Inhibition of HIV-1 integrase catalytic activities of compounds 11a-m, t-x.
Table 3. Inhibition of HIV-1 integrase catalytic activities of compounds 8g, l, m, s and 10b, h, k, l.
In series 9, installing weak electron-donating substituents on the terminal aromatic ring caused a drop of activity, while introduction of electron-withdrawing substituents resulted in similar or improved potency. However, introduction of both electron-withdrawing and electron-donating substituents on the terminal aromatic in series 11 gave slightly higher ST inhibitory potency while such introduction resulted in irregular diversifications in 3′-P inhibitory potency. Either extending the chain length between the terminal aromatic ring and the sulfonamide group (9a v 9d and 11a v 11d v 11t) or increasing the number of fluoro substituents (9a v 9b v 9c and 11a v 11b v 11c) caused a loss of ST inhibitory activity in both series 9 and 11. When the 4-fluoro substituent was replaced by a 4-bromo group (9a v 9e and 11a v 11e), intrinsic ST inhibition potency was maintained for both series 9 and 11, while 3′-P inhibitory activity increased by 2-fold for series 9 and decreased by 4-fold for series 11. Migration of the bromo substituent from the 4- to the 3-position or 2-position resulted in diversity of results of inhibitory potencies for series 9 and 11. In series 9, bromo substituent at the 4- and 3-position exhibited higher 3′-P and ST inhibitory activity relative to 2-position analogue (9e v 9f v 9g). In series 11, the 4- and 2-position Br analogues showed higher ST inhibitory activity relative to 3-position analogue, while the 2-position Br analogue displayed stronger 3′-P inhibitory activity relative to 3- and 4-position analogues (11e v 11f v 11g).
A noteworthy feature is that these halogens could be incorporated in different combinations of substituted positions to further improve potency, such as compounds 9l, 11u and 11v. Compounds 9l is one of the best inhibitors with IC50 values of 1.3 and 0.8 μM for 3′-P and ST in series 9 and compounds 11u and 11v had the best efficacy against ST in series 11 with an IC50 value of 4 μM. These observations suggested that halogen substitution on the terminal aromatic ring showed improved potency, consistent with the previous reportCitation13.
In series 11, compound 11m exhibited higher inhibitory potencies relative to compound 11w, while compound 11f exhibited a comparable inhibitory profile to that of compound 11x, which suggested that a double bond between the caffeoyl group and the amide group had irregular effect on IN inhibitory potency.
Intrigued by the blockage from the phenolic hydroxyl group, several acetylated derivatives have been synthesized and studied. Masking of the phenolic hydroxyl groups with acetylate group caused various IN inhibitory activities diversity. Compounds 8g, 8l, 10b and 10k showed slight weaker inhibitory activities against ST and 3′-P compared to 9g, 9l, 11b and 11k, while compounds 8m, 8s, 10h and 10l kept the important ST inhibitory activity compared to 9m, 9s, 11h and 11l. We consider that these acetylated compounds may be pro-inhibitors which are deacetylated by the enzyme in the assay; or/and the carbonyl, instead of the phenolic hydroxyl group, bond to Mg2+ or Mn2+ to carry out inhibitory activities for IN, which needs to be further investigated.
Inhibition of HIV replication in culture
To assess the potential utility of the new compounds as anti-HIV agents, we tested some of synthesized compounds for the ability to inhibit HIV replication in cell cultures. The toxicity of the compounds was also evaluated using MT-4 cells ().
Table 4. Anti-HIV activity and cytotoxicity of selected compounds in HIV-1 infected MT-4 cells.
At first, some of compounds 9 series with a galloyl group (such as 9g and 9l) had been tested for their anti-HIV activity and cytotoxicity; however, all of them didn’t exhibit anti-viral activity at a concentration of 25 μM. Recently, it was reported that the compounds with the catechol group displayed strong anti-viral activity at nanomole concentrationsCitation16. So compounds series 11 had also been synthesized in order to increase their anti-viral activity. This set of compounds showed higher cytotoxicity although they exhibited improved anti-viral activity. Compounds 11b and 11l showed similar anti-viral activity and cytotoxicity at a concentration of around 20 μM. Compound 11g exhibited a moderate anti-viral activity with a therapeutic index of ~4. However, compounds 11b, 11l and 11g were less potent IN inhibitors compared with compound 9l which was one of the best inhibitors but didn’t exhibit anti-viral activity at a concentration of 25 μM. We believed that the disparity between IN inhibitory activities and anti-viral activities was resulted from off-target effects and cell penetrance issues.
Conclusion
In conclusion, four series of novel nitrogen-containing polyhydroxylated aromatics with sulfanilamide linkers have been identified as IN inhibitors. Most of the compounds inhibited the catalytic activities of IN in micromolar concentrations and compounds 9e and 9f were the most potent ST inhibitors with IC50 value of 0.7 μM. The biological experimental results indicated that (1) the role of the halogen substitution on the terminal aromatic ring is important for potency; (2) the length of the linker between the terminal aromatic ring and the sulfonamide group has a weak deleterious effect on potency; (3) masking of catechol or galloyl functionality with acetyl group causes no substantial loss of inhibitory activities against ST. These results prompted us to carry out further research mainly focusing on designing novel IN inhibitors with the aim to further increase the IN inhibitory activity and anti-viral activity and decrease the toxicities.
Declaration of interest
This work was financially supported by the National Natural Science Foundation of China (No20872082).
References
- De Clercq E. New approaches toward anti-HIV chemotherapy. j Med Chem 2005;48:1297–1313.
- Geffen N. Beyond HAART: scientists and activists need to work together. Lancet 2009;374:860–861.
- Dayam R, Gundla R, Al-Mawsawi LQ, Neamati N. HIV-1 integrase inhibitors: 2005-2006 update. Med Res Rev 2008;28:118–154.
- Zhao G, Wang C, Liu C, Lou H. New developments in diketo-containing inhibitors of HIV-1 integrase. Mini Rev Med Chem 2007;7:707–725.
- Johnson AA, Marchand C, Pommier Y. HIV-1 integrase inhibitors: a decade of research and two drugs in clinical trial. Curr Top Med Chem 2004;4:1059–1077.
- Fesen MR, Kohn KW, Leteurtre F, Pommier Y. Inhibitors of human immunodeficiency virus integrase. Proc Natl Acad Sci usa 1993;90:2399–2403.
- Ho CC, Lin SS, Chou MY, Chen FL, Hu CC, Chen CS et al. Effects of CAPE-like compounds on HIV replication in vitro and modulation of cytokines in vivo. j Antimicrob Chemother 2005;56:372–379.
- Neamati N, Lin Z, Karki RG, Orr A, Cowansage K, Strumberg D et al. Metal-dependent inhibition of HIV-1 integrase. j Med Chem 2002;45:5661–5670.
- Tchertanov L, Mouscadet JF. Target recognition by catechols and beta-ketoenols: potential contribution of hydrogen bonding and Mn/Mg chelation to HIV-1 integrase inhibition. j Med Chem 2007;50:1133–1145.
- Zhao H, Neamati N, Mazumder A, Sunder S, Pommier Y, Burke TR Jr. Arylamide inhibitors of HIV-1 integrase. j Med Chem 1997;40:1186–1194.
- King PJ, Ma G, Miao W, Jia Q, McDougall BR, Reinecke MG et al. Structure-activity relationships: analogues of the dicaffeoylquinic and dicaffeoyltartaric acids as potent inhibitors of human immunodeficiency virus type 1 integrase and replication. j Med Chem 1999;42:497–509.
- Supuran CT, Innocenti A, Mastrolorenzo A, Scozzafava A. Antiviral sulfonamide derivatives. Mini Rev Med Chem 2004;4:189–200.
- Fisher TE, Kim B, Staas DD, Lyle TA, Young SD, Vacca JP et al. 8-Hydroxy-3,4-dihydropyrrolo[1,2-a]pyrazine-1(2H)-one HIV-1 integrase inhibitors. Bioorg Med Chem Lett 2007;17:6511–6515.
- Marchand C, Maddali K, Métifiot M, Pommier Y. HIV-1 IN inhibitors: 2010 update and perspectives. Curr Top Med Chem 2009;9:1016–1037.
- Serrao E, Odde S, Ramkumar K, Neamati N. Raltegravir, elvitegravir, and metoogravir: the birth of “me-too” HIV-1 integrase inhibitors. Retrovirology 2009;6:25.
- Lee-Huang S, Huang PL, Zhang D, Lee JW, Bao J, Sun Y et al. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol: part II. integrase inhibition. Biochem Biophys Res Commun 2007;354:879–884.
- Wu B, Zhou L, Cai HH. Synthesis and neuroprotective properties of novel cinnamide derivatives. Chinese Chem Lett 2008;19:1163–1166.
- Andrus MB, Liu J, Meredith EL, Nartey E. Synthesis of resveratrol using a direct decarbonylative Heck approach from resorcylic acid. Tetrahedron Lett 2003;44:4819–4822.
- Dayam R, Al-Mawsawi LQ, Zawahir Z, Witvrouw M, Debyser Z, Neamati N. Quinolone 3-carboxylic acid pharmacophore: design of second generation HIV-1 integrase inhibitors. J Med Chem 2008;51:1136–1144.