Abstract
Periodontitis is an infection-induced inflammatory disease that causes loss of the tooth supporting tissues. Much focus has been put on comparison of the microbial biofilm in the healthy periodontium with the diseased one. The information arising from such studies is limited due to difficulties to compare the microbial composition in these two completely different ecological niches. A few longitudinal studies have contributed with information that makes it possible to predict which individuals who might have an increased risk of developing aggressive forms of periodontitis, and the predictors are either microbial or/and host-derived factors. The most conspicuous condition that is associated with disease risk is the presence of Aggregatibacter actinomycetemcomitans at the individual level. This Gram-negative bacterium has a great genetic variation with a number of virulence factors. In this review we focus in particular on the leukotoxin that, based on resent knowledge, might be one of the most important virulence factors of A. actinomycetemcomitans.
Periodontal Diseases
Inflammation is an essential immune response that enables survival during infection or injury and maintains tissue homeostasis under a variety of noxious conditions.Citation1 The type of pathway induced under a given condition depends on the nature of the inflammatory stimuli. Thus, bacterial pathogens are detected by receptors of the innate immune system on the tissue-resident macrophages, and those cells induce production, activation and secretion of inflammatory mediators.Citation1 These mediators induce vasodilation, neutrophil recruitment, and leakage of plasma into the infected tissue. The neutrophils recruited from the circulation, the tissue-resident macrophages, and the mast cells seek and destroy invading pathogens. Microbial pathogens located in the oral biofilm can contribute to the pathogenesis of periodontal diseases by releasing components that cause imbalance in the host inflammatory response.Citation2 Periodontitis is a bacteria-induced inflammatory disease that causes loss of the tooth-supporting tissues, alveolar bone and connective tissues.Citation3 The disease is highly prevalent and affects billions of people around the world. Inflammatory periodontal diseases generally progress slowly, but may at any stage undergo exacerbation resulting in additional loss of the tooth attachment and subsequently tooth loss. Various host genetic factors have been shown to interfere with the subgingival oral biofilm, a phenomenon named periodontal infectogenomics.Citation4 In addition, there is a significant association between periodontitis and an increased risk for systemic diseases, such as arteriosclerosis and endocarditis.Citation3,5,6
The periodontal infection is polymicrobial and has been shown to contain hundreds of different bacterial species in the same periodontal lesion.Citation7 Decades of investigations have demonstrated that the microbial communities associated with periodontitis differ from those in health.Citation8 Microbiome science continuously accumulates huge amounts of information about complex microbial communities, and that knowledge needs a healthy dose of skepticism.Citation9 The causality of the microbiome pattern in relation to disease initiation and progression is still not fully understood. The contributions of specific bacteria to disease may be unimportant according to the ecological plaque hypothesis.Citation10 Targeting one or more “pathogens” will not necessarily cure disease, since other organisms with similar activities might take their place.Citation2 Therefore, it may make more sense to focus on the specific virulence factors that contribute to disease, rather than on the microorganisms that produce those factors.Citation7 However, a subgroup of periodontitis includes the aggressive form that affects young individuals and has a high rate of periodontal disease progression. The aggressive forms of the disease are in most cases correlated to the presence of certain bacterial species in the subgingival biofilm with a predominance of Gram-negative anaerobic rods that colonize the periodontal crevice.Citation11 The contribution of specific pathogenic bacteria to disease implies an infection with a highly virulent microflora that releases factors, which can cause an imbalance in the host response.Citation2
The rapidly-progressing form of periodontal disease, named Aggressive periodontitis (AgP), affects young otherwise healthy individuals. The disease process is initiated by the microbial challenge that induces an aggressive inflammatory response, and the process results in a periodontal destruction in the tooth-supporting soft tissues and alveolar boneCitation12 (). If the inflammatory trigger is not eliminated by the acute inflammatory response or persists in the subgingival biofilm in the periodontal crevice, severe tissue damage may occur. The resulting tooth loss at an early age may have profound cosmetic, functional, and psychological effects.Citation13 It might be difficult to convince the observer that the bacterial burden in these cases can initiate an inflammatory response that may result in severe tooth loss, since the initial clinical presentation frequently shows little visible plaque accumulation.Citation14,15
Figure 1. Clinical presentation of localized aggressive periodontitis. A 16-year old female presenting with radiographic alveolar bone loss associated with bone defects (marked with arrows) and probing attachment loss at 2 permanent first molars, left upper premolars and lower incisors. Clinical photographs buccal view (A–C). Radiographs (D–F). The clinical presentation shows sparse plaque accumulation and localized gingival inflammation with 4–8 mm periodontal crevices with bleeding on probing in the affected regions. The results from the microbial sampling are presented in the inserted table and the sampled sites are indicated. Microbiological analysis, by cultivation technique, confirmed the presence of high levels of A. actinomycetemcomitans in the 3 lesions sampled. Diagnosis: localized aggressive periodontitis (LAgP).
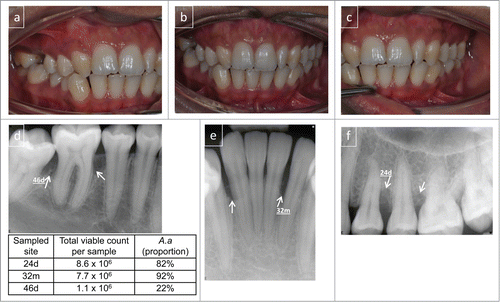
Two forms of AgP, the localized form (LAgP), and the generalized form (GAgP), have been distinguished based on the number of teeth affected and the distribution of the lesions within the dentition. For a long time, aggregation within families has been linked to aggressive forms of periodontitis and may be explained by a genetic predisposition related to susceptibility to the disease. Ethnicity and sociodemographic status have been shown to affect the carriage patterns of periodontal pathogens.Citation11,16,17 Childhood and adolescence are critical time points for the onset of AgP. The two main diagnostic criteria used for the detection of periodontitis are the loss of connective tissue attachment and loss of alveolar bone based on clinical and radiographic assessments. The fact that AgP, especially the localized form (LAgP), is associated with the presence of Aggregatibacter actinomycetemcomitans, is well known.Citation18 Data concerning this linkage are mainly based on association studies, while causality is difficult to determine due to the complexity of the disease.
Aggregatibacter actinomycetemcomitans
The Gram-negative A. actinomycetemcomitans is assumed to be the primary etiologic agent of LAgP and has also been implicated in chronic periodontitis and severe non-oral infections.Citation18 Currently seven serotypes of this bacterium (a-g) are recognized based on the immunodominant antigen, which is an O-polysaccharide of the lipopolysaccharide (LPS).Citation19,20
This bacterium has a complex lifecycle, acquired through transmission from saliva of infected individuals, and may initially colonize the oral mucosa possibly as a facultative intracellular pathogen.Citation11 The bacterium moves from the initial oral colonization site to the gingival crevices and competes with other bacteria in the niche. Successful establishment of persistent colonization in subgingival crevices by A. actinomycetemcomitans may lead to periodontal destruction and development of periodontitis in susceptible individuals.Citation21 Fine and co-workersCitation22 reported that soft tissue binding is mediated by the adhesins ApiA and Aae that bind specifically to the buccal epithelium. In vitro experiments show that the bacteria can be translocated from the epithelium to the hard surfaces (hydroxyapatite, enamel) but not in the opposite directions.Citation22 These data support the argument that A. actinomycetemcomitans may use buccal cells as a reservoir for initial attachment before the bacteria eventually move to the non-shedding tooth surfaces.
The virulence potential of A. actinomycetemcomitans appears to vary among strains, and specific serotypes/clonal types of the bacterium have been reported to be more prevalent in individuals with aggressive forms of the disease.Citation18 Prospective population-based studies have shown that individuals infected by strains of the serotype b JP2 genotype of A. actinomycetemcomitans have a significantly higher risk of developing AgP than individuals infected by strains of the non-JP2 genotype.Citation17,23 The JP2 genotype is defined by a 530-bp deletion in the promoter region of the leukotoxin operon, and this genotype is highly leukotoxic.Citation24 The within-species variable virulence of A. actinomycetemcomitans may be attributed to strain-to-strain variation in the genome content and in the regulation of virulence gene expressions.Citation20
The ability of the bacterium to express a leukotoxin (LtxA) is considered to be an important virulence property.Citation18 The toxin kills white blood cells in a variety of ways, and leukocyte destruction is essential for subsequent bacterial growth and stimulation of the host inflammatory response.Citation25 Leukotoxicity is substantially correlated with attachment loss in adolescents, indicating an important role of the toxin in the pathogenesis of AgP.Citation26 Among the non-oral infections, pneumonia caused by A. actinomycetemcomitans infections dominates of reports from adolescents.Citation27 There was an association to periodontitis in these A. actinomycetemcomitans infected individuals, but the virulence characteristic of these bacteria has not yet been examined. It has been demonstrated that members of the HACEK group (Haemophilus and Aggregatibacter spp., A. actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens and Kingella kingae), especially A. actinomycetemcomitans, are associated with systemic diseases far from the oral cavity.Citation28,29 The great genetic diversity within this species indicates that the highly virulent genotypes might be associated with the non-oral infections.
A. actinomycetemcomitans LtxA is a large pore-forming toxin that belongs to the RTX (Repeats-in-toxin) family of bacterial proteins.Citation30 There is great variation in LtxA expression in vitro, although all A. actinomycetemcomitans strains harbor a complete ltxA operon.Citation20 Expression of LtxA is not fully characterized, but it has been shown that the expression is regulated by both environmental and genetic factors.Citation25
Virulence Mechanisms of A. actinomycetemcomitans
The genetic diversity among different isolates of A. actinomycetemcomitans interferes with the ability of the bacterium to express and release virulence factors.Citation20 The different adhesins and fimbriae expressed by this bacterium have been shown to be important factors that promote colonization at the various ecological niches of the human oral cavity.Citation22 The periodontal infection by A. actinomycetemcomitans is accompanied by local and systemic immune responses, and this species may invade the gingival epithelium and release virulence factors such as endotoxins and exotoxins.Citation18 Endotoxin is expressed by all Gram-negative bacterial species and causes a general pro-inflammatory host response, while the 2 exotoxins, a cytolethal distending toxin (Cdt) and a leukotoxin (LtxA), are unique for A. actinomycetemcomitans within bacterial species colonizing the oral biofilm.Citation18 Cdt's are expressed by a number of Gram-negative bacteria and cause death of the host cells by blocking their proliferation and enhancing the expression of the Receptor activator of nuclear factor kappa-B ligand (RANKL), a key factor in osteoclastogenesis.Citation31,32 Despite the pathogenic potential of the Cdt in vitro, a recent longitudinal study performed on adolescents in Ghana could not demonstrate any significant differences in disease progression between individuals colonized with Cdt-expressing A. actinomycetemcomitans and individuals colonized with bacteria that lack Cdt expression.Citation33 The LtxA selectively affects human cells of haematopoietic origin by binding to the lymphocyte function-associated receptor 1 (LFA-1) and causes disruption of the membrane integrity.Citation34 Apart from causing death of the defense cells, LtxA also induces a massive pro-inflammatory response in human monocytes/macrophages.Citation35 There is a strong association between the presence of highly leukotoxic A. actinomycetemcomitans, both of the JP2-genotype and some highly leukotoxic variants of the non-JP2 genotype, and the disease progression in the infected individuals studied.Citation17,23,26 These findings attract enhanced attention concerning the importance of leukotoxicity acting toward the defense mechanisms in the host response.
Leukotoxin-Induced Pro-Inflammatory Cell Death
A. actinomycetemcomitans LtxA belongs to the RTX-toxin family and shares substantial molecular homology with other toxins of the RTX family such as Escherichia coli α-hemolysin and Mannheimia haemolytica leukotoxin.Citation30 RTX toxins are divided into 2 categories based on their target cell specificity. RTX hemolysins, such as E. coli α-hemolysin (HlyA) and Actinobacillus pleuropneumoniae ApxIA, are toxic to a wide range of cell types from many species. In contrast, leukotoxins of A. actinomycetemcomitans (LtxA) and M. haemolytica (LktA), are only toxic to specific types of cells in specific species.Citation34 It has long been known that LtxA selectively kills human leukocytes () and disturbs the local defense mechanisms.Citation25,36
Figure 2. (A) Interaction of A. actinomycetemcomitans leukotoxin (LtxA) with T-, B-lymphocytes, polymorphonuclear (PMN) leukocytes, and macrophages leads to cell lysis. (B) LtxA-induced cell death in macrophages involves several steps before the cell lysis occurs. LtxA binds to LFA-1 (1) and induces extracellular release of ATP (2). The released ATP binds to the P2X7-receptor (3) that subsequently causes efflux of potassium (4). The inflammasome complex is formed and activated (5), which promotes the cleavage and activation of a cysteine proteinase called caspase-1 (6). The cleaved caspase-1 is then responsible for activation (7) and release of abundant levels of active IL-1β and IL-18 (8).
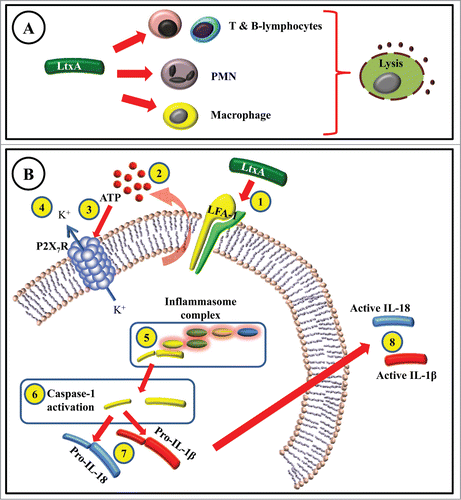
One of the most important pathogenic mechanisms involves the production of cytokines, which stimulate inflammatory events that induce other effector mechanisms. Regarding this aspect, macrophages are one of the major sources of pro-inflammatory cytokines such as interleukin-1 (IL-1) and tumor necrosis factor (TNF).Citation37 These cytokines improve the activation of cellular immunity and intensify the inflammatory cascade. However, when there is an imbalance in the host response, these cytokines can be harmful for the host tissues.Citation38,39 One such impaired immune response is the case of periodontitis, where osteoclast-differentiation is closely associated with the differentiation of macrophages.Citation40 IL-1 is involved in bone loss in various pathological conditions by promoting osteoclast survival and activation, although IL-1-mediated osteoclastogenesis requires the receptor activator of NF-kappaB ligand (RANKL).Citation41 Production of pro-inflammatory cytokines, including TNF-α, and IL-1β, by monocytes/macrophages in response to extracts and components of A. actinomycetemcomitans, has in fact been shown.Citation18 However, the involvement of LtxA in these cell activations has not been investigated in the 20th century, probably because LtxA has mainly been considered to disrupt the host defense system by killing the immune cells. With this in mind, we postulated that perhaps LtxA itself could not only be responsible for killing of the cells, but also play a major role in the induction of inflammatory responses of importance for the pathogenicity in periodontitis. Our in depth mechanistic studies of LtxA's effects on macrophages have revealed intriguing findings. We have shown for the first time that macrophages are the most sensitive cells to LtxA-induced cell lysis and that this process consequently causes a specific pro-inflammatory cell death. The LtxA-induced macrophage lysis also involves a specific activation of caspase-1 that subsequently leads to excessive secretion of IL-1β from the macrophages.Citation35,42 Since LtxA is primate-specific,Citation18 conducting experiments on animals is of little biological interest. Therefore, we used an alternative to test the already secreted IL-1β from LtxA-challenged human macrophages in an animal bone resorption model. The secreted IL-1β, from LtxA-exposed macrophages, was mainly the biologically active form and could act as the major activator of bone resorption in the tested bioactivity assay.Citation42 To investigate the contribution of LtxA in relation to other bacterial products from A. actinomycetemcomitans, macrophages were exposed to live strains of A. actinomycetemcomitans. In this study we were able to show that indeed the rapidly induced IL-1β secretion from the macrophages was mainly due to the LtxA.Citation43
There are major steps before the inactive form of this protein is activated and the active IL-1β is secreted. A crucial step is the activation of caspase-1 that occurs in specialized caspase-1 multi-protein complexes referred to as inflammasomes.Citation44 The investigation of mechanisms involved in the caspase-1 inflammasome-activation is now a “hot” field in cytokine research. There are numerous pathways for activation of the caspase-1 inflammasome by pathogen-associated molecular patterns (PAMPs) and danger associated molecular patterns (DAMPs). The inflammasones respond to various molecules such as extracellular adenosine triphosphate (ATP), different crystals (monosodium urate (MSU)), calcium pyrophosphate dihydrate (CPPD), silica, asbestos, aluminum salts, and ameloid-β), bacterial products (muramyl dipeptide (MDP) and flagellin), and toxins (Nigericin, Maitotoxin and aerolysin).Citation45,46 Furthermore, a newly discovered mechanism of cell death, designated pyroptosis, also leads to specific caspase-1 activation.Citation47 Pyroptosis has been accepted as a death mechanism, and macrophages undergoing pyroptosis have been shown to present features of both apoptosis and necrosis with a particular activation of caspase-148. Caspase-1, which was first described as the IL-1β-converting enzyme (ICE), is essential for the release of active IL-1β. In fact, caspase-1's “natural” substrates are certain members of the IL-1 family, which include pro-IL-1β, pro-IL-18, and pro-IL-33.Citation49,50 Cells dying by pyroptosis increase in size and show signs of plasma-membrane pores leading to increased osmotic pressure, water influx, cell swelling, and eventually osmotic lysis and specific abundant secretion of IL-1β and IL-18.Citation51 These features have also been shown in macrophages exposed to LtxA by our research group.Citation20,42 IL-1β secretion is induced robustly by extracellular ATP, which signals via the purinergic receptor, P2X7R. This causes K+ efflux from cells which activates pro-caspase-1 and consequently pro-IL-1β processing.Citation52 The efflux of potassium ions causes an influx of calcium ions, which in turn activate phospholipases.Citation53 Our research group was the first to show that the LtxA-induced pro-inflammatory cell death of macrophages involved ATP and P2X7RCitation54 ().
Several bacterial products, such as lipopolysaccharide (LPS), are known to increase expression of pro-IL-1β in macrophages.Citation55 In addition, the presence of A. actinomycetemcomitans has been shown to increase the expression of inflammasome components in co-cultures with macrophages.Citation56 However, a secondary stimulus is needed to induce a substantial secretion of the bioactive IL-1β. It has been shown that this activation can be induced by various bacterial species through activation of the inflammasome (intracellular signal-induced multiprotein complex), caspase-1 activation, and IL-1β secretion.Citation57 This secondary activation can be induced by direct interactions of bacteria or bacterial components with the inflammasome, such as proteins secreted by Salmonella typhimuriumCitation57 or Francisella tularensis invasion.Citation58 The other system for stimulating this secondary activation has been described through cell surface interactions such as with Listeria monocytogenes and Staphylococcus aureus.Citation57 Several studies have been able to show that LtxA can also cause this secondary activation in macrophages that consequently leads to secretion of bioactive IL-1β.Citation35,42,43,54
Macrophages play a significant role in periodontal tissue remodeling, and their ability to secrete large amounts of IL-1 indicates their involvement in the enhanced bone loss seen in periodontitis.Citation59,60 Moreover, IL-1 levels in gingival crevicular fluid from subjects with periodontitis are generally decreased after treatment. The levels of these cytokines in gingival crevicular fluid samples seem to play important roles for the bone resorption activity seen in periodontally diseased individuals.Citation43,61 IL-1 polymorphism has been suggested to be of importance for the severity of periodontitis,Citation62 which could enhance the pro-inflammatory response from macrophages exposed to LtxA.
It is tempting to speculate that in AgP cases infected with A. actinomycetemcomitans, where rapid tissue destruction is observed, the LtxA-induced interaction with the accumulated macrophages in the periodontal tissues plays a crucial role in the pathogenic potential of the A. actinomycetemcomitans infection.
A. actinomycetemcomitans in the Pre-Clinical Phase of Aggressive Periodontitis
The association between the presence of A. actinomycetemcomitans in subgingival plaque and the initiation and progression of AgP has been supported by observations in several population-based longitudinal studies.Citation17,21,23,63,64 Disease development in terms of progression of attachment loss has been evaluated prospectively among adolescentsCitation17,21,23 in relation to the carrier status of A. actinomycetemcomitans. In a recently followed West African cohort there was a significantly increased risk (OR=5 .126, 95% CI [2.994 - 8.779] P <0.001) for progression of attachment loss among the A. actinomycetemcomitans-positive young individuals as compared with the A. actinomycetemcomitans-negative reference individuals.Citation33 The carrier status of A. actinomycetemcomitans in this study is based on both cultivation of this bacterium and detection method for its DNA by polymerase chain reaction (PCR) analyzes. In both methods, pooled paper point samples from the gingival crevice of the examined individuals were analyzed. All serotypes of A. actinomycetemcomitans were related to the progression of attachment loss in this study, but the most pronounced risk (OR) for progression of attachment loss was associated with the presence of the serotype b (OR = 7.685; 95% CI [2.835 - 20.830] P <0.001).Citation23,33
The two exotoxins expressed by A. actinomyctemcomitans, the LtxA and the Cdt, have gained more attention since the pathogenic potential of this bacterium has been addressed.Citation18 The diversity of the LtxA expression by A. actinomycetemcomitans indicates a possible role of this toxin in the pathogenesis of periodontal disease, especially among young people. The importance of the leukotoxicity of A. actinomycetemcomitans and its contribution to the pathogenesis have recently been shown in a study where young individuals (mean age 15.0 years) harbouring highly leukotoxic A. actinomycetemcomitans (of the JP2 and of the non-JP2 genotypes) had a significantly increased risk for progression of localized periodontal attachment loss during a 2 y follow-up period.Citation23 A strong correlation between highly leukotoxic strains of A. actinomycetemcomitans and periodontitis has also been reported in Brazilian populations.Citation65 Different ethnic populations seem to show highly variable frequency of the JP2 clonal type of A. actinomycetemcomitans.Citation66,67 This specific genotype of A. actinomycetemcomitans significantly correlates to periodontal disease onset in infected individuals of African origin.Citation17,23,68 Subjects harboring highly leukotoxic A. actinomycetemcomitans are reported to be 18 times more likely to convert from a healthy status to localized aggressive periodontitis than those colonized by strains harboring the full-length leukotoxin promoter region.Citation17 However, there is also convincing evidence that phylogenetically diverse strains of A. actinomycetemcomitans of the non-JP2 genotype can carry pathogenic potential. Non-JP2 genotypes of A. actinomycetemcomitans of serotype b are strongly associated with disease onset and progression in African populations,Citation17,23,69 but also in other populations of non-African descent worldwide.Citation21,68,70
The fact that the low LtxA-producing strains of A. actinomycetemcomitans are present in patients with aggressive periodontal disease, as well as in healthy subjects,Citation18 suggests that genetic and environmental factors may interfere with the LtxA expression in individuals with various degrees of susceptibility to periodontal disease.
A. actinomycetemcomitans in the Complex Biofilm of the Periodontal Crevice
As discussed previously, the presence of A. actinomycetemcomitans in the oral cavity of young individuals is a strong prognostic marker for initiation and progression of AgP. The microbial composition of the biofilm of the pathogenic periodontal crevice is very diverse and complex, and it involves the presence of many different periodontal pathogens.Citation11 However, recent data indicate that once periodontitis has developed, the character of the periodontal infection may change over time from an infection primarily associated with A. actinomycetemcomitans into a more anaerobic, polymicrobial infection.Citation71 The subgingival environment provides a stable tooth surface and an unstable epithelial surface for the microbial colonization, the latter of which continuously desquamates.Citation7 Both surfaces are bathed with a continuous efflux of gingival crevicular fluid, derived from blood plasma and thus nutritionally rich in nitrogenous compounds such as amino acids, peptides and proteins. As the gingival crevice deepens, the subgingival microbial milieu becomes more anaerobic and under this condition, asaccharolytic and anaerobic and/or proteolytic bacteria such as Fusobacterium, Eubacterium, Campylobacter, Prevotella and Porphyromonas are found. It has previously been shown that the activity of LtxA is efficiently inhibited in the presence of black-pigmented periodontal pathogens, such as Porphyromonas gingivalis, Prevotella intermedia and Prevotella nigrescens.Citation72 In addition, P. gingivalis displays a competitive advantage over A. actinomycetemcomitans in co-cultured biofilms by inhibiting the growth and attachment of the A. actinomycetemcomitans cells.Citation73,74 These findings are supported by the negative association in subgingival samples between the presence of A. actinomycetemcomitans and P. gingivalis.Citation75 This indicates an important pre-clinical role of A. actinomycetemcomitans in AgP. However, it seems reasonable to assume that the role of this bacterium is limited in competition with the obligate anaerobic periodontal pathogens in the ecological niche of a periodontal crevice. These interactions may help to explain why several cross-sectional studies have also shown an association to other periodontal pathogens than A. actinomycetemcomitans in the diseased lesions of AgP individuals.Citation11,76
Moreover, an inflammatory host response has the capacity to modify leukotoxicity in the pathogenic periodontal crevice. McArthur and coworkersCitation77 reported that lipoproteins of human serum enhanced the activity of LtxA. In an attempt to repeat this finding it was shown that exclusion of the lipoproteins in human serum did not change its LtxA activating properties of the serum.Citation78 The serum protease inhibitors were shown to be the responsible components for the enhanced leukotoxicity by inhibiting LtxA degradation caused by PMN degranulation.Citation79,80 It has also been shown that systemic antibodies against LtxA efficiently neutralize the activity of the toxin, which might interfere with the pathogenicity of A. actinomycetemcomitans, both locally and systemically.Citation81 As described in detail above, LtxA induces a massive secretion of bioactive IL-1β from macrophages. Interestingly, a receptor protein for this cytokine with regulatory properties has been identified on A. actinomyctemcomitans, which indicates further interactions with the host inflammatory response.Citation82
Conclusion
Several different studies on various adolescent populations have shown that the presence of A. actinomycetemcomitans in the subgingival plaque significantly increases the risk for the colonized individuals for the initiation and progression of attachment loss. This correlation is further enhanced in the individuals that are carrying sub-types of the bacterium with an enhanced leukotoxicity. LtxA has been shown to be a virulence factor with capacity to cause imbalance in the host inflammatory response by activating and killing the immune cells in a variety of ways. The association between A. actinomycetemcomitans and leukotoxicity with aggressive forms of periodontitis is strong, while a possible causality is difficult to prove. The primate specificity of LtxA limits the ability to examine A. actinomycetemcomitans infections in animal models. In addition, the strong influence from hereditary and environmental factors in the pathogenesis of periodontitis makes causal conclusions difficult to draw. However, certain genetic host peculiarities, such as the IL-1β polymorphism, might act synergistically with the LtxA-induced pro-inflammatory response. Examination of this genetic host factor of the A. actinomycetemcomitans-infected individuals might help us in the future to take one step further in determination of causality and identification of risk individuals.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by the Research Fund (TUA), County of Västerbotten, Sweden and the Swedish Dental Association.
References
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell 2010; 140:771-6; PMID:20303867; http://dx.doi.org/10.1016/j.cell.2010.03.006
- Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends immunol 2014; 35:3-11; PMID:24269668; http://dx.doi.org/10.1016/j.it.2013.09.001
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005; 366:1809-20; PMID:16298220; http://dx.doi.org/10.1016/S0140-6736(05)67728-8
- Nibali L, Donos N, Henderson B. Periodontal infectogenomics. J Med Microbiol 2009; 58:1269-74; PMID:19528161; http://dx.doi.org/10.1099/jmm.0.012021-0
- Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation 2012; 125:2520-44; PMID:22514251; http://dx.doi.org/10.1161/CIR.0b013e31825719f3
- D'Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial. Am Heart J 2006; 151:977-84; PMID:16644317; http://dx.doi.org/10.1016/j.ahj.2005.06.018
- Teles R, Teles F, Frias-Lopez J, Paster B, Haffajee A. Lessons learned and unlearned in periodontal microbiology. Periodontology 2000 2013; 62:95-162; PMID:23574465; http://dx.doi.org/10.1111/prd.12010
- Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontology 2000 2005; 38:135-87; PMID:15853940; http://dx.doi.org/10.1111/j.1600-0757.2005.00107.x
- Hanage WP. Microbiology: Microbiome science needs a healthy dose of scepticism. Nature 2014; 512:247-8; PMID:25143098; http://dx.doi.org/10.1038/512247a
- Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiol 2003; 149:279-94; PMID:12624191; http://dx.doi.org/10.1099/mic.0.26082-0
- Kononen E, Muller HP. Microbiology of aggressive periodontitis. Periodontol 2000 2014; 65:46-78; PMID:24738586; http://dx.doi.org/10.1111/prd.12016
- Albandar JM. Aggressive and acute periodontal diseases. Periodontol 2000 2014; 65:7-12; PMID:24738583; http://dx.doi.org/10.1111/prd.12013
- Nibali L, Farias BC, Vajgel A, Tu YK, Donos N. Tooth loss in aggressive periodontitis: a systematic review. J Dent Res 2013; 92:868-75; PMID:23955159; http://dx.doi.org/10.1177/0022034513501878
- Westergaard J, Frandsen A, Slots J. Ultrastructure of the subgingival microflora in juvenile periodontitis. Scand J Dent Res 1978; 86:421-9; PMID:284566
- Listgarten MA. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol 1976; 47:1-18; PMID:1063849; http://dx.doi.org/10.1902/jop.1976.47.1.1
- Chung HJ, Chung CP, Son SH, Nisengard RJ. Actinobacillus actinomycetemcomitans serotypes and leukotoxicity in Korean localized juvenile periodontitis. J Periodontol 1989; 60:506-11; PMID:2677302; http://dx.doi.org/10.1902/jop.1989.60.9.506
- Haubek D, Ennibi OK, Poulsen K, Vaeth M, Poulsen S, Kilian M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet 2008; 371:237-42; PMID:18207019; http://dx.doi.org/10.1016/S0140-6736(08)60135-X
- Henderson B, Ward JM, Ready D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periodontopathogen? Periodontol 2000 2010; 54:78-105; PMID:20712635; http://dx.doi.org/10.1111/j.1600-0757.2009.00331.x
- Takada K, Saito M, Tsuzukibashi O, Kawashima Y, Ishida S, Hirasawa M. Characterization of a new serotype g isolate of Aggregatibacter actinomycetemcomitans. Mol Oral Microbiol 2010; 25:200-6; PMID:20536747; http://dx.doi.org/10.1111/j.2041-1014.2010.00572.x
- Kittichotirat W, Bumgarner RE, Asikainen S, Chen C. Identification of the pangenome and its components in 14 distinct Aggregatibacter actinomycetemcomitans strains by comparative genomic analysis. PloS one 2011; 6:e22420; PMID:21811606; http://dx.doi.org/10.1371/journal.pone.0022420
- Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, Nasri C, McKiernan M, Gunsolley J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: longitudinal cohort study of initially healthy adolescents. J Clin Microbiol 2007; 45:3859-69; PMID:17942658; http://dx.doi.org/10.1128/JCM.00653-07
- Fine DH, Markowitz K, Furgang D, Velliyagounder K. Aggregatibacter actinomycetemcomitans as an early colonizer of oral tissues: epithelium as a reservoir? J Clin Microbiol 2010; 48:4464-73; PMID:20881174; http://dx.doi.org/10.1128/JCM.00964-10
- Hoglund Aberg C, Kwamin F, Claesson R, Dahlen G, Johansson A, Haubek D. Progression of attachment loss is strongly associated with presence of the JP2 genotype of Aggregatibacter actinomycetemcomitans: a prospective cohort study of a young adolescent population. J Clin Periodontol 2014; 41:232-41; PMID:24304011; http://dx.doi.org/10.1111/jcpe.12209
- Brogan JM, Lally ET, Poulsen K, Kilian M, Demuth DR. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect Immun 1994; 62:501-8; PMID:8300209
- Johansson A. Aggregatibacter actinomycetemcomitans leukotoxin: a powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins 2011; 3:242-59; PMID:22069708; http://dx.doi.org/10.3390/toxins3030242
- Hoglund Aberg C, Haubek D, Kwamin F, Johansson A, Claesson R. Leukotoxic Activity of Aggregatibacter actinomycetemcomitans and Periodontal Attachment Loss. PloS one 2014; 9:e104095; PMID:25093857; http://dx.doi.org/10.1371/journal.pone.0104095
- Shilo S, Kassis I, Hakim F, Shachor-Meyouhas Y. Aggregatibacter actinomycemcomitans Pneumonia in Children: Two Case Reports and a Review of the Literature. Pediatr Infect Dis J 2014; PMID:25068288
- Das M, Badley AD, Cockerill FR, Steckelberg JM, Wilson WR. Infective endocarditis caused by HACEK microorganisms. Annu Rev Med 1997; 48:25-33; PMID:9046942; http://dx.doi.org/10.1146/annurev.med.48.1.25
- Norskov-Lauritsen N. Classification, identification, and clinical significance of haemophilus and aggregatibacter species with host specificity for humans. Clin Microbiol Rev 2014; 27:214-40; PMID:24696434; http://dx.doi.org/10.1128/CMR.00103-13
- Linhartova I, Bumba L, Masin J, Basler M, Osicka R, Kamanova J, Prochazkova K, Adkins I, Hejnova-Holubova J, Sadilkova L, et al. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol Rev 2010; 34:1076-112; PMID:20528947
- Belibasakis GN. Cellular and molecular responses of periodontal connective tissue cells to Actinobacillus actinomycetemcomitans cytolethal distending toxin. Odontological Dissertation, Faculty of Medicine: Umeå University, 2004:49
- Belibasakis GN, Johansson A, Wang Y, Chen C, Kalfas S, Lerner UH. The cytolethal distending toxin induces receptor activator of NF-kappaB ligand expression in human gingival fibroblasts and periodontal ligament cells. Infect Immun 2005; 73:342-51; PMID:15618171; http://dx.doi.org/10.1128/IAI.73.1.342-351.2005
- Hoglund Aberg C, Antonoglou G, Haubek D, Kwamin F, Claesson R, Johansson A. Cytolethal distending toxin in isolates of Aggregatibacter actinomycetemcomitans from Ghanaian adolescents and association with serotype and disease progression. PloS one 2013; 8:e65781; PMID:23922633; http://dx.doi.org/10.1371/journal.pone.0065781
- Lally ET, Hill RB, Kieba IR, Korostoff J. The interaction between RTX toxins and target cells. Trends Microbiol 1999; 7:356-61; PMID:10470043; http://dx.doi.org/10.1016/S0966-842X(99)01530-9
- Kelk P, Johansson A, Claesson R, Hanstrom L, Kalfas S. Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun 2003; 71:4448-55; PMID:12874324; http://dx.doi.org/10.1128/IAI.71.8.4448-4455.2003
- Claesson R, Johansson A, Belibasakis G, Hanstrom L, Kalfas S. Release and activation of matrix metalloproteinase 8 from human neutrophils triggered by the leukotoxin of Actinobacillus actinomycetemcomitans. J Periodontal Res 2002; 37:353-9; PMID:12366858; http://dx.doi.org/10.1034/j.1600-0765.2002.00365.x
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood 1996; 87:2095-147; PMID:8630372
- Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000 2003; 31:55-76; PMID:12656996; http://dx.doi.org/10.1034/j.1600-0757.2003.03105.x
- Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol 2008; 79:1585-91; PMID:18673014; http://dx.doi.org/10.1902/jop.2008.080183
- Lerner UH. Inflammation-induced bone remodeling in periodontal disease and the influence of post-menopausal osteoporosis. J Dent Res 2006; 85:596-607; PMID:16798858; http://dx.doi.org/10.1177/154405910608500704
- Jules J, Zhang P, Ashley JW, Wei S, Shi Z, Liu J, Michalek SM, Feng X. Molecular basis of requirement of receptor activator of nuclear factor kappaB signaling for interleukin 1-mediated osteoclastogenesis. J Biol Chem 2012; 287:15728-38; PMID:22416138; http://dx.doi.org/10.1074/jbc.M111.296228
- Kelk P, Claesson R, Hanstrom L, Lerner UH, Kalfas S, Johansson A. Abundant secretion of bioactive interleukin-1beta by human macrophages induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun 2005; 73:453-8; PMID:15618184; http://dx.doi.org/10.1128/IAI.73.1.453-458.2005
- Kelk P, Claesson R, Chen C, Sjostedt A, Johansson A. IL-1beta secretion induced by Aggregatibacter (Actinobacillus) actinomycetemcomitans is mainly caused by the leukotoxin. Int J Med Microbiol : IJMM 2008; 298:529-41; PMID:17888725; http://dx.doi.org/10.1016/j.ijmm.2007.06.005
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 2002; 10:417-26; PMID:12191486; http://dx.doi.org/10.1016/S1097-2765(02)00599-3
- McIntire CR, Yeretssian G, Saleh M. Inflammasomes in infection and inflammation. Apoptosis 2009; PMID:19156527
- Yu HB, Finlay BB. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe 2008; 4:198-208; PMID:18779046; http://dx.doi.org/10.1016/j.chom.2008.08.007
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 2009; 7:99-109; PMID:19148178; http://dx.doi.org/10.1038/nrmicro2070
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009; 16:3-11; PMID:18846107; http://dx.doi.org/10.1038/cdd.2008.150
- Dinarello CA. Interleukin-1 β, interleukin-18, and the interleukin-1 β converting enzyme. Ann N Y Acad Sci 1998; 856:1-11; PMID:9917859; http://dx.doi.org/10.1111/j.1749-6632.1998.tb08307.x
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005; 23:479-90; PMID:16286016; http://dx.doi.org/10.1016/j.immuni.2005.09.015
- Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infection and immunity 2005; 73:1907-16; PMID:15784530; http://dx.doi.org/10.1128/IAI.73.4.1907-1916.2005
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 2006; 176:3877-83; PMID:16547218; http://dx.doi.org/10.4049/jimmunol.176.7.3877
- Dinarello CA. Interleukin-1beta. Crit Care Med 2005; 33:S460-2; PMID:16340421; http://dx.doi.org/10.1097/01.CCM.0000185500.11080.91
- Kelk P, Abd H, Claesson R, Sandstrom G, Sjostedt A, Johansson A. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell death & disease 2011; 2:e126; PMID:21390060; http://dx.doi.org/10.1038/cddis.2011.6
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958-69; PMID:19029990; http://dx.doi.org/10.1038/nri2448
- Belibasakis GN, Johansson A. Aggregatibacter actinomycetemcomitans targets NLRP3 and NLRP6 inflammasome expression in human mononuclear leukocytes. Cytokine 2012; 59:124-30; PMID:22503597; http://dx.doi.org/10.1016/j.cyto.2012.03.016
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006; 440:228-32; PMID:16407890; http://dx.doi.org/10.1038/nature04515
- Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med 2005; 202:1043-9; PMID:16230474; http://dx.doi.org/10.1084/jem.20050977
- Boch JA, Wara-aswapati N, Auron PE. Interleukin 1 signal transduction–current concepts and relevance to periodontitis. J Dent Res 2001; 80:400-7; PMID:11332522; http://dx.doi.org/10.1177/00220345010800020101
- Gravallese EM, Goldring SR. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheum 2000; 43:2143-51; PMID:11037873; http://dx.doi.org/10.1002/1529-0131(200010)43:10<2143::AID-ANR1>3.0.CO;2-S
- Holmlund A, Hanstrom L, Lerner UH. Bone resorbing activity and cytokine levels in gingival crevicular fluid before and after treatment of periodontal disease. J Clin Periodontol 2004; 31:475-82; PMID:15142219; http://dx.doi.org/10.1111/j.1600-051X.2004.00504.x
- Kornman KS, di Giovine FS. Genetic variations in cytokine expression: a risk factor for severity of adult periodontitis. Ann Periodontol 1998; 3:327-38; PMID:9722717; http://dx.doi.org/10.1902/annals.1998.3.1.327
- Timmerman MF, Van der Weijden GA, Abbas F, Arief EM, Armand S, Winkel EG, Van Winkelhoff AJ, Van der Velden U. Untreated periodontal disease in Indonesian adolescents. Longitudinal clinical data and prospective clinical and microbiological risk assessment. J Clin Periodontol 2000; 27:932-42; PMID:11140561; http://dx.doi.org/10.1034/j.1600-051x.2000.027012932.x
- Van der Velden U, Abbas F, Armand S, Loos BG, Timmerman MF, Van der Weijden GA, Van Winkelhoff AJ, Winkel EG. Java project on periodontal diseases. The natural development of periodontitis: risk factors, risk predictors and risk determinants. J Clin Periodontol 2006; 33:540-8; PMID:16899096; http://dx.doi.org/10.1111/j.1600-051X.2006.00953.x
- Silveira VR, Nogueira MV, Nogueira NA, Lima V, Furlaneto FA, Rego RO. Leukotoxicity of Aggregatibacter actinomycetemcomitans in generalized aggressive periodontitis in Brazilians and their family members. J Appl Oral Sci 2013; 21:430-6; PMID:24212989; http://dx.doi.org/10.1002/app.38187
- Haubek D. The highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans: evolutionary aspects, epidemiology and etiological role in aggressive periodontitis. APMIS Suppl 2010:1-53; PMID:21214629; http://dx.doi.org/10.1111/j.1600-0463.2010.02665.x
- Haubek D, Johansson A. Pathogenicity of the highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans and its geographic dissemination and role in aggressive periodontitis. J Oral Microbiol 2014; 6; PMID:25206940; http://dx.doi.org/10.3402/jom.v6.23980
- Haraszthy VI, Hariharan G, Tinoco EM, Cortelli JR, Lally ET, Davis E, Zambon JJ. Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. J Periodontol 2000; 71:912-22; PMID:10914794; http://dx.doi.org/10.1902/jop.2000.71.6.912
- Elamin A, Albandar JM, Poulsen K, Ali RW, Bakken V. Prevalence of Aggregatibacter actinomycetemcomitans in Sudanese patients with aggressive periodontitis: a case-control study. J Periodontal Res 2011; 46:285-91; PMID:21332472; http://dx.doi.org/10.1111/j.1600-0765.2010.01337.x
- Zambon JJ. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol 1985; 12:1-20; PMID:3882766; http://dx.doi.org/10.1111/j.1600-051X.1985.tb01348.x
- Dahlen G, Claesson R, Aberg CH, Haubek D, Johansson A, Kwamin F. Subgingival bacteria in Ghanaian adolescents with or without progression of attachment loss. J Oral Microbiol 2014; 6; PMID:24834145; http://dx.doi.org/10.3402/jom.v6.23977
- Johansson A, Hanstrom L, Kalfas S. Inhibition of Actinobacillus actinomycetemcomitans leukotoxicity by bacteria from the subgingival flora. Oral Microbiol Immunol 2000; 15:218-25; PMID:11154406; http://dx.doi.org/10.1034/j.1399-302x.2000.150402.x
- Haraguchi A, Miura M, Fujise O, Hamachi T, Nishimura F. Porphyromonas gingivalis gingipain is involved in the detachment and aggregation of Aggregatibacter actinomycetemcomitans biofilm. Mol Oral Microbiol 2014; 29:131-43; PMID:24661327; http://dx.doi.org/10.1111/omi.12051
- Takasaki K, Fujise O, Miura M, Hamachi T, Maeda K. Porphyromonas gingivalis displays a competitive advantage over Aggregatibacter actinomycetemcomitans in co-cultured biofilm. J Periodontal Res 2013; 48:286-92; PMID:23033940; http://dx.doi.org/10.1111/jre.12006
- Chen C, Wang T, Chen W. Occurrence of Aggregatibacter actinomycetemcomitans serotypes in subgingival plaque from United States subjects. Molecular oral microbiology 2010; 25:207-14; PMID:20536748; http://dx.doi.org/10.1111/j.2041-1014.2010.00567.x
- Nibali L, D'Aiuto F, Ready D, Parkar M, Yahaya R, Donos N. No association between A actinomycetemcomitans or P gingivalis and chronic or aggressive periodontitis diagnosis. Quintessence Int 2012; 43:247-54; PMID:22299125
- McArthur WP, Tsai CC, Baehni PC, Genco RJ, Taichman NS. Leukotoxic effects of Actinobacillus actinomycetemcomitans. Modulation by serum components. J Periodontal Res 1981; 16:159-70; PMID:6453979; http://dx.doi.org/10.1111/j.1600-0765.1981.tb00962.x
- Johansson A, Claesson R, Belibasakis G, Makoveichuk E, Hanstrom L, Olivecrona G, Kalfas S. Lack of lipoprotein-dependent effects on the cytotoxic interactions of Actinobacillus actinomycetemcomitans leukotoxin with human neutrophils. APMIS 2002; 110:857-62; PMID:12645663; http://dx.doi.org/10.1034/j.1600-0463.2002.1101203.x
- Johansson A, Claesson R, Belibasakis G, Makoveichuk E, Hanstrom L, Olivecrona G, Sandstrom G, Kalfas S. Protease inhibitors, the responsible components for the serum-dependent enhancement of Actinobacillus actinomycetemcomitans leukotoxicity. Eur J Oral Sci 2001; 109:335-41; PMID:11695755; http://dx.doi.org/10.1034/j.1600-0722.2001.00055.x
- Johansson A, Claesson R, Hanstrom L, Sandstrom G, Kalfas S. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J Periodontal Res 2000; 35:85-92; PMID:10863962; http://dx.doi.org/10.1034/j.1600-0765.2000.035002085.x
- Brage M, Holmlund A, Johansson A. Humoral immune response to Aggregatibacter actinomycetemcomitans leukotoxin. J Periodontal Res 2011; 46:170-5; PMID:21118415; http://dx.doi.org/10.1111/j.1600-0765.2010.01325.x
- Paino A, Ahlstrand T, Nuutila J, Navickaite I, Lahti M, Tuominen H, Valimaa H, Lamminmaki U, Pollanen MT, Ihalin R. Identification of a novel bacterial outer membrane interleukin-1Beta-binding protein from Aggregatibacter actinomycetemcomitans. PloS one 2013; 8:e70509; PMID:23936223; http://dx.doi.org/10.1371/journal.pone.0070509
