Abstract
Cryptococcus neoformans is an encapsulated opportunistic fungal pathogen that is found in multiple niches in the environment and that can cause fatal meningoencephalitis in susceptible patients, mainly HIV+ individuals. Cryptococcus also infects environmental hosts such as nematodes, insects and plants. In particular, C. neoformans can kill the lepidopteran Galleria mellonella, which offers a useful tool to study microbial virulence and drug efficacy. Galleria mellonella immunity relies on innate responses based on melanization, accumulation of antimicrobial peptides, and cellular responses as phagocytosis or multicellular encapsulation. In this work we have investigated the immune response of G. mellonella during cryptococcal infection. We found that G. mellonella infected with C. neoformans had a high lytic activity in their hemolymph. This response was temperature- and capsule-dependent. During interaction with phagocytic cells, C. neoformans behaved as an intracellular pathogen since it could replicate within hemocytes. Non-lytic events were also observed. In contrast to Candida species, C. neoformans did not induce melanization of G. mellonella after infection. Finally, passage of C. neoformans through G. mellonella resulted in changes in capsule structure as it has been also reported during infection in mammals. Our results highlight that G. mellonella is an optimal model to investigate innate immune responses against C. neoformans.
Introduction
Cryptococcus neoformans is an opportunistic fungal pathogen that is usually found in the environment, in particular soils and pigeon excreta. Infection begins by inhalation of infectious particles. In immunocompetent people the infection can remain in a latent state, or be cleared by the host. However, in immunosuppressed patients (mainly HIV infected patients and transplant recipients), the yeast can disseminate and invade the central nervous system causing a fatal meningoencephalitis.Citation1,2 Cryptococcus neoformans is responsible for over 600,000 deaths per year, which makes this pathogen a major global threat.Citation3 Defense against C. neoformans largely depends on innate response and Th1 polarization.Citation2
Cryptococcus neoformans has several virulence factors that have been associated with host damage, such as the polysaccharide capsule and melanin production.Citation4,8 Moreover, C. neoformans has developed mechanisms that prolong its survival in the host including the ability to form titan cells and to change the size of the capsule.Citation9,11 Cryptococcus neoformans behaves as an intracellular fungal pathogen, since it can survive and replicate inside phagocytic cells.Citation12,13
Cryptococcal infection is not human-specific, and this fungus can cause disease in a wide variety of hosts, including amoebas, insects, nematodes, koalas, birds, dolphins, and even plants.Citation14,20 These environmental interactions have important implications to understand how C. neoformans has acquired virulence traits required for host adaptation.Citation21 For this reason, the interaction between alternative models and pathogenic fungi is becoming a field of interest. The use of these hosts also offers bioethical advantages because it reinforces the application of the “3 Rs” rule to reduce animal suffering and pain. One of the main model host used to investigate fungal pathogenesis is the lepidopteran Galleria mellonella.Citation22,23 This insect offers important advantages to investigate microbial virulence,Citation24 so it has been successfully used to study the pathogenicity of some bacteria and fungi.Citation22,25,33 Cryptococcus neoformans can also infect and kill Galleria mellonella.Citation34 Virulence of this fungus in this model depends on the temperature and on the capsule. Cryptococcus neoformans can enlarge the capsule and produce titan cells in G. mellonella.Citation35 Recently, a multihost approach using Caenorhabditis elegans and G. mellonella has identified new genes required for cryptococcal virulence.Citation36 After challenge with microorganisms, G. mellonella induces melanization around the exogenous particles.Citation37 This insect contains hemocytes with phagocytic activity in the hemolymph, that also produce antimicrobial peptides.Citation38,44
Since some aspects of the G. mellonella innate immunity are similar to those elicited by mammals in response to C. neoformans, we have characterized the response of G. mellonella after challenge with this pathogenic yeast. We demonstrate that C. neoformans induce the accumulation of antimicrobial peptides in the hemolymph, but does not induce early melanization of the G. mellonella. We also show that this fungus behaves as an intracellular fungal pathogen in G. mellonella hemocytes. In conclusion, these findings support the use of non-mammalian models to investigate the immune response to C. neoformans.
Results
Lytic activity of the hemolymph
We first studied the accumulation of antimicrobial peptides in the hemolymph using a lytic assay based on agar plates containing the bacteria Micrococcus luteus. As shown in C. neoformans induced an increase in the lytic activity of the hemolymph. This induction was noticeable 6 hours after infection, and reached a maximal response after 24 h (). The increase was similar to the one observed after infection with the pathogenic yeast C. albicans (). We decided to characterize this phenomenon in detail and investigated if the capsule played a role in this induction. When we performed the same experiment with the acapsular mutant cap59, there was a slight increase in the lytic activity of the hemolymph compared to induction observed in G. mellonella infected with its parental strain B3501 (). This result suggested that the capsule played a role in the recognition of the yeast by G. mellonella, so we investigated the effect of capsular polysaccharide on the induction of antimicrobial peptides. For this purpose, we purified the main polysaccharide of the capsule (glucuronoxylomannan, GXM) and injected different doses in G. mellonella. We found that there was a dose-response effect, so that 50 µg induced a significant increase in the lytic activity of the hemolymph (). Interestingly, greater amounts of GXM did not induce the lytic activity. Next, we examined if cryptococcal cells with different capsule sizes induced different larval responses. However, we did not find any difference in the accumulation of lytic peptides when G. mellonella was challenged with cells of small or large capsule size ().
Figure 1. Effect of C. neoformans on the antimicrobial activity of the G. mellonella hemolymph. (A) Groups of 10 G. mellonella were infected with C. neoformans H99 strain (106 cells/G. mellonella) or with PBS (C), and then they were incubated at 37°C. The lytic activity of the hemolymph was evaluated as described in Material and Methods after different timepoints. Asterisks denote statistical difference between the sample and the corresponding control G. mellonella larvae treated with PBS. (B) G. mellonella larvae were infected with C. neoformans H99 or C. albicans SC5314 strain (105 cells per G. mellonella) and incubated at 37°C overnight. Then, the lytic of the hemolymph was evaluated. Asterisks denote statistical difference between the sample and the control G. mellonella treated with PBS.
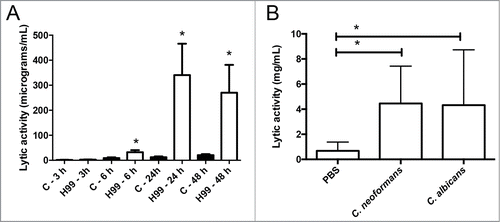
Figure 2. Role of the capsule on the accumulation of antimicrobial peptides in the hemolymph. (A) Galleria mellonella larvae were infected with approximately 105 cells from B3501 or cap59 strains as described in Material and Methods, and placed at 37°C for 24 h. Lytic activity of the hemolymph was assessed as described in M&M. Asterisks denote statistical difference. (B) Groups of 10 G. mellonella were infected with 106 H99 cells prepared in water or with different amounts of purified GXM. In parallel, G. mellonella were injected with sterile water. After 24 h of incubation at 37°C, the antimicrobial activity of the hemolymph was determined. Asterisks indicate statistical difference between the sample and the control treated with water. (C) Cells from H99 strain with different capsule size were obtained after growth in Sabouraud (Control, small capsule) or 10% Sabouraud, pH 7.3 (Induced, large capsule). Then, G. mellonella were infected with 105 cells from these cultures or with PBS, and lytic activity in the hemolymph was determined after 24 h of incubation at 37°C. Asterisks denote statistical difference between the sample and the larvae treated with PBS.
Cryptococcus neoformans is more virulent at 37°C than at 30°C, so we studied if the incubation temperature of infected G. mellonella played a role on the induction of lytic peptides. We observed that there was a trend to a higher induction of the lytic activity of the hemolymph when G. mellonella were placed at 37°C (data not shown). Induction of this larval response depended on living yeast, since dead cells did not show a significant effect on the lytic activity (data not shown).
Intracellular replication
Cryptococcus neoformas behaves as an intracellular fungal pathogen in murine and human macrophages. So we investigated if after phagocytosis by hemocytes, C. neoformans also replicated inside these cells. We infected G. mellonella larvae, and after 3 hours of incubation at 37°C, we isolated the hemocytes from the hemolymph and visualized internalized C. neoformans cells overnight at 28°C. As shown in and supplemental video 1, we found hemocytes in which C. neoformans replicated several times, indicating that this yeast behaves as a facultative intracellular pathogen. Moreover, we also found some cases in which internalized fungal cells were extruded from the hemocytes ( and supplemental video 2), which is another phenomenon also found after phagocytosis by mammalian macrophages and amoebas.
Figure 3. Behavior of C. neoformans inside hemocytes. Galleria mellonella were infected with 106 cryptococcal cells (H99 strain) and after 3 h of incubation at 37°C, the hemolymph was collected and hemocytes placed in a 96-well plate under the microscope. (A) Intracellular replication event; (B) Non lytic exocytosis of the cryptococcal cells from the hemocytes. In each case, the time difference between the pictures of the panels is 9 min.
Cryptococcus neoformans did not induce hemolymph melanization
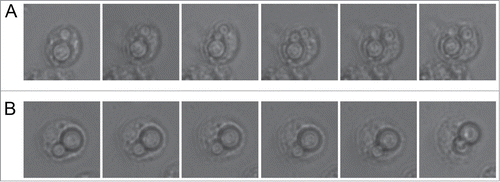
We then investigated how C. neoformans induced one of the early responses in G. mellonella after exposure to exogenous particles, which is melanization. This parameter can be monitored by measuring the OD of the hemolymph at 405 nm. We used as a positive control Candida albicans.Citation45 As shown in C. albicans induced melanization of the hemolymph as early as 1 hour after infection. In contrast, melanization was not observed when G. mellonella was infected with C. neoformans. This finding suggests that C. neoformans is differently recognized by G. mellonella.
Figure 4. Early melanization of G. mellonella after infection with C. neoformans and C. albicans. Groups of 10 G. mellonella larvae were injected with PBS (A), C. albicans SC5314 strain (106 cells/G. mellonella) or C. neoformans H99 (106 cells/G. mellonella). Quantification of hemolymph melanization as described in M&M. Asterisks denote statistical difference between the sample and the control G. mellonella treated with PBS.
Structural changes of the capsule during infection in G. mellonella
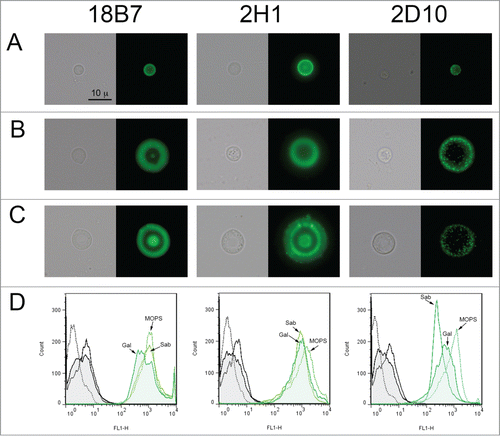
A typical feature of the capsule during infection is that it can change in size and structure. We have previously described that C. neoformans enlarges the capsule in G. mellonella.Citation35 In this work, we also investigated if the capsule suffered any structural change during infection. For this purpose, we tested if there was any change in the binding pattern of the GXM specific monoclonal antibodies to the capsule of yeast cells recovered from G. mellonella or grown in vitro in Sabouraud (small capsule) or under capsule enlargement conditions (10% Sabouradud, pH 7.3). We tested the binding of 2 IgG (18B7 and 2H1) and one IgM (2D10) mAbs. As shown in , mAb 18B7 gave a similar fluorescent pattern in all the cases, although cells obtained from G. mellonella presented lower fluorescent intensity, suggesting that the number of epitopes for this mAb were reduced after passage through G. mellonella. Monoclonal Ab 2H1 bound to cells grown in vitro in a homogenous way, showing an annular binding pattern. In contrast, we found that in cells isolated from G. mellonella, there was heterogeneity in the fluorescence binding pattern. While some cells also showed an annular pattern, in some others the fluorescence was not homogenously distributed throughout the capsule, and there was a trend to find higher fluorescence in the inner regions of the capsule (). Finally, mAb 2D10 showed a clear punctuate binding pattern to cells grown in vitro under capsule inducing conditions and isolated from G. mellonella. In contrast to the situation with mAb 18B7, cells obtained from G. mellonella gave higher fluorescence than cells grown in Sabouraud, but lower than in vitro cells with large capsule.
Figure 5. Structural features of the C. neoformans capsule in vitro and after passage through G. mellonella. Cells from H99 strain were obtained in vitro with different capsule sizes or from G. mellonella. (A) Cells with small capsule size after growth in vitro, (B) cells with enlarged capsule after incubation in 10% Sabouraud pH 7.3, (C) cells isolated from G. mellonella. These cells were incubated with different mAbs to the capsule (18B7, 2H1 and 2D10). mAb 18B7 was directly conjugated to Alexa488, and for the other 2, specific GAM-IgG (for 2H1) or GAM-IgM (for 2D10) were used. In each case, a representative cell is shown, including both light microscopy (left panel) and fluorescence (right panel). Scale bar in upper left panel applies to the rest of the pictures. (D) Quantification of the fluorescence of the cells shown in A, B and C by flow cytometry. Black histograms correspond to cells to which no primary anticryptococcal mAb has been added (negative controls), and green histograms indicate the fluorescence intensity of the cells to which both anti-cryptococcal and secondary Abs have been added. In all cases, straight line corresponds to cells grown in Sabouraud (Sab, small capsule in vitro), dotted line to cells incubated in 10% Sabouraud pH 7.3 (MOPS, large capsule in vitro), and filled histogram to yeast cells isolated from G. mellonella (Gal).
Discussion
Cryptococcus neoformans is a pathogenic yeast that can cause disease in susceptible patients. But it can also infect a wide variety of environmental hosts, such as amoebas, insects, nematodes, mammals and plants. Interestingly, the virulence mechanisms seem to be conserved independently of the host. For this reason, the use of alternative models to mammals has raised the interest of the scientific community. In particular, the lepidopteran G. mellonella is being largely used to investigate microbial pathogenesis. In this work, we have studied different aspects of the immune response of G. mellonella after infection with C. neoformans. Some of these responses, such as the induction of antimicrobial peptides and phagocytic cells, are also present in mammals.
Cryptococcus neoformans induced the production of lytic activity in the hemolymph, which is also another well-known response of G. mellonella. In this case, the capsule played a role, since acapsular mutants did not induce this process as encapsulated strains. However, cap59 mutants are avirulent, and presumably, they replicate inside G. mellonella at a slower rate than encapsulated strains. Induction of antimicrobial peptides in the hemolymph depended on live yeasts and on the pathogen dose. So it is possible that the decrease response elicited by cap59 mutant is due to the presence of a lower number of viable yeast cells during infection compared to wild type strains. The capsular polysaccharide has many effects on the immune response of mammals, and they produce immunomodulation, complement depletion, decreased Ab production and apoptosis.Citation46,51 So it could be argued that a similar situation might be found during cryptococcal infection in G. mellonella. We found that purified GXM produced a dose response effect, so intermediate amounts of polysaccharide induced the accumulation of antimicrobial peptides, but higher doses did not have any effect. This result suggests that this polysaccharide can also induce “immunological paralysis” in G. mellonella, as it has been also described in mammalian models (see reviews inCitation2,6). Moreover, it cannot be discarded that higher GXM amounts are toxic. So, this lepidopteran provides an alternative model to investigate some aspects of the effect of the main cryptococcal virulence factor on the immune response. The induction of lytic activity is of interest because in mammals, some antimicrobial peptides also play a role in immune defense, such as defensins and histatins, which show anticryptococcal activity. An interesting aspect is that the induction of antimicrobial peptides did not protect G. mellonella from death, indicating that the natural role of this response might be to eliminate other type of pathogens, such as bacteria.
We noticed that the induction of antimicrobial activity is very variable between larvae, and even injection with PBS already induced accumulation of these peptides. This indicates that this response is very sensitive and tightly regulated, so minor changes in the hemolymph homeostasis are sensed by G. mellonella, resulting in an enhancement of innate responses. In agreement, physical stress is enough to prime the immune response of G. mellonella and the expression of some genes encoding lytic peptides.Citation44
One of the most important aspects of the cryptococcal virulence is its interaction with phagocytic cells.Citation52,53 Cryptococcus neoformans is phagocytosed by hemocytes, but it was unknown whether it could behave as a facultative intracellular pathogen. We report that after phagocytosis, C. neoformans undergoes 2 of the outcomes described in mammalian phagocytic cells, which are intracellular replication and non-lytic exocytosis.Citation12,13,54,56 Similar phenomena have been described also in other non-mammalian hosts, such as amoebas and hemocytes from Drosophila melanogaster.Citation20,57,58 These results enforce the hypothesis that the intracellular pathogenesis is a virulence trait that C. neoformans has acquired after environmental interactions,Citation21 and allows the yeast to escape from death by macrophages in more complex hosts. Some entomopathogenic fungi, such as Metarhizium anisopliae or Beauveria bassiana, can also affect the phagocytic activity of hemocytes,Citation59 most probably due to the secretion of secondary metabolites that impair the recognition of the fungal cells. Our findings confirm that this lepidopteran can be used to investigate aspects of the fungal intracellular pathogenesis.
We have found that C. neoformans is recognized differently compared to other pathogenic yeast. After injection with Candida species, G. mellonella induces melanization of the hemolymph that is noticeable after 30 minutes postinfection. This response has been associated to β-glucans on the surface.Citation45 This early response was not observed after challenge with C. neoformans, although G. mellonella did melanize when they died (4–6 d after infection). The capsule could in fact inhibit the recognition of the yeast by G. mellonella, but preliminary results show that the melanization of the hemolymph measured after infection with acapsular mutants is barely above the control (data not shown). Since the cryptococcal cell wall has very low levels of β-glucans, this result suggests that the structural differences at the yeast surface determine the type of response elicited by G. mellonella. At the moment, it cannot be discarded that other factors play a role in this phenomenon. For example, C. neoformans can melanize when incubated in G. mellonella homogenates,Citation60 so maybe the fungus has the ability to act as a scavenger of melanin precursors from the hemolymph. When G. mellonella is infected by Beauveria bassiana, some strains also fail to induce larval melanization and in this case, this effect correlates with the production of toxins by the fungus.Citation37
Capsule enlargement is a process that has a great impact on phagocytosis. In the case of mammalian macrophages, this transition inhibits complement-mediated phagocytosis and protects against free radicals.Citation61,62 Capsule growth also occurs during infection in G. mellonella and amoebas,Citation35,63 and this process impairs phagocytosis by hemocytes.Citation35 In this work, we have also shown that after passage through G. mellonella, the capsule of C. neoformans suffers rearrangements of its structure. This has been described both in vitro and in mice,Citation64, 65 and it seems to be a process associated with brain invasion.Citation65 Since the capsule plays an important role during phagocytosisCitation35 and in the induction of antimicrobial peptides in G. mellonella, we argue that capsular rearrangements might influence the recognition of the pathogen by the host and contribute to immune evasion.
Galleria mellonella is a versatile model host to study fungal pathogenesis. In this work, we have demonstrated that this lepidopteran can be used to investigate mechanisms of fungal immune evasion and intracellular pathogenesis. These new aspects open new perspectives in the fungal field for different reasons. First, we confirm that some of the cryptococcal virulence mechanisms used in mammals also occur in this insect, which highlights the importance of environmental interactions in the acquisition of virulence traits. Moreover, using G. mellonella as model host, it has been possible to show that microbial virulence is a complex phenotype that cannot be explained by single virulence factors,Citation66 and that virulence must be considered from a holistic point of view. For this reason, the characterization of the immune response of G. mellonella during cryptococcal infection described in this work will contribute to the understanding of the pathogenesis of this microorganism. Second, our findings expand the use of G. mellonella to study fungal virulence. Classically, this model host has been used to investigate antifungal efficacy and differences in virulence between strains and species. Finally, our data expands the use of G. mellonella for more “sophisticated” studies, as it has been already shown in the case of some pathogenic bacteria, which demonstrated that this model host can be used to investigate brain infections and maternal transfer of immunityCitation25,67 (see comment inCitation68).
Material and Methods
Yeast strains and growth conditions. In this work we used the following C. neoformans strains: H99 (variety grubii,Citation69), a derivative from this strain which expresses the green fluorescent protein,Citation70 B3501 (variety neoformans,Citation71) and the acapsular strain C536 (cap59,Citation72 derived from B3501). In addition, C. albicans SC5314 strainCitation73 was used in some experiments. The cells were routinely grown in Sabouraud solid (Oxoid, Reference CM0041) or liquid media (Oxoid, Reference CM0147). In the case of liquid media, the cultures were incubated at 30°C with moderate shaking (150 r.p.m.). In some experiments, the size of the capsule was induced by placing the cells overnight in 10% Sabouraud at pH 7.3 buffered with 50 mM MOPS (Sigma Aldrich, Reference M1254) buffer.Citation74 When indicated, the yeast cells were killed by incubation at 65°C for 30 min.
Insects and incubation conditions media. Galleria mellonella was obtained from Alcotán (Valencia, Spain). The larvae were selected by weight (0.2–0.3 g) and by the absence of dark spots on the cuticle. The larvae were maintained at room temperature, and the day before the experiment, they were transferred to the temperature at which the experiment was going to be performed (30 or 37°C, as indicated).
Injection
Galleria mellonella larvae were infected with different pathogen doses (ranging from 104 to 106 cells/larva) as describedCitation34 (a visual demonstration can be found in Youtube (https://www.youtube.com/watch?v=2XEu_5aF1qk)). The larva abdomen was cleaned with 70% ethanol and the yeast suspensions prepared in PBS (137 mM NaCl, 2,7 mM KCl, 10 mM Na2HPO4, 1,8 mM KH2PO4, pH 7,3) were injected in the hemocele through the last right pro-leg of the larvae using a 10 µL Hamilton syringe (Hamilton, USA, Reference 80366, distributed by Fisher Scientific, Reference W0166E).
Lytic activity of the hemolymph
The presence of antimicrobial peptides in the hemolymph was performed as describedCitation67 with some modifications. This assay is based on the degradation of the bacteria M. luteus by lytic peptides and enzymes from the hemolymph (mainly lysozyme). Briefly, plates containing M. luteus (1.7 mg/mL, Sigma Aldrich, Reference M3770–5G), phosphate buffer (Na2HPO4, Panreac, Reference 121512, 67 mM, pH 6.4) and 1 % agar (BD, Reference 214010) were prepared, and 2 mm holes were punched in the agar. Then, 3 microliters of recently isolated hemolymph were placed in these wells. In parallel, the same volume of lysozyme prepared at different concentrations (0.25; 0.03 and 0.0039 mg/L, Sigma Aldrich, Reference L6876) was placed as internal standard in each plate. The plates were incubated for 4–5 hours at 37°C, and then, pictures were taken using a Nikon D60 digital camera. The width of the halos was measured with Adobe Photoshop (Adobe, San Jose, CA), and lytic activity was calculated for each sample using the internal controls of lysozyme present in each plate. To test the effect of capsular polysaccharide on the accumulation of antimicrobial peptides, GXM was purified as described.Citation75 After the final lyophilization, the polysaccharide was dissolved in sterile distilled water at 15 mg/mL. Serial dilutions were prepared (10, 5 and 2.5 mg/mL), and 10 µL were injected in groups of 10 larvae (corresponding to 150, 100, 50 and 25 µg/larva). For this particular experiment, the control larvae were injected with 10 µL of sterile distilled water.
In vivo microscopy of infected hemocytes
Galleria mellonella larvae were infected with 106 C. neoformans cells, and after 3 hours of incubation at 37°C, the hemolymph was collected and centrifuged in a bench centrifuge at 12,000 r.p.m. (13,800 g). The pellet was suspended in Schneider´s medium (Sigma Aldrich, Reference S0146) supplemented with 10% of fetal bovine serum (HyClone-Perbio Thermo Fisher Scientific, Reference SV30160). Two hundred microliters of this suspension were placed in a 96-well plate (Corning Inc., Reference 3599), and videos were taken as described.Citation76 Briefly, the plate was placed under a Leica DMI 4000B microscope and observed with a 20x lens objective. This microscope had a temperature-regulated chamber, which was adjusted at 28°C. Pictures were taken every 3 min overnight. Pictures were exported as .avi documents and processed with ImageJ (http://rsb.info.nih.gov/ij).
Hemolymph melanization
Hemolymph melanization assay was performed as described.Citation29 Briefly, 10 larvae were infected with 106cells/larva of C. neoformans H99 or C. albicans SC5314 and incubated at 37°C for 1 and 3 hours. After the incubation period the hemolymph were collected and diluted 1:10 in cold PBS and centrifuged at 12,000 rpm (13,800 g) in a Heraus Fresco 21 bench centrifuge (Thermo Electron Corporation). The supernatants were placed in a 96 well microdilution plate (Corning Inc., Reference 3599), and optical density determined at 405 nm in a iEMS Reader MS (LabSystems, Thermo Scientific). PBS-injected larvae were used as control.
Binding of mAbs to the C. neoformans capsule after passage through G. mellonella
Galleria mellonella larvae were infected with 106 C. neoformans cells as described above and incubated at 37°C for 24 hours. After this time, the hemolymph was collected and centrifuged. The pellet, containing C. neoformans cells, was washed with PBS and suspended in PBS with 1% of bovine serum albumin (BSA, Sigma Aldrich, Reference A4503–50G). After 30 min of incubation at 37°C, mAbs to the capsule 18B7, 2H1 (both mouse IgG1) and 2D10 (mouse IgM)Citation77 were added at 1 μg/mL. Monoclonal antibody 18B7 was directly conjugated to Alexa 488 using the Alexa Fluor 488 Protein Labeling Kit (Molecular Probes, Life Technologies, Reference A10235) following the manufacturer´s recommendations. The cells were incubated for 30 min at 37°C, and washed with PBS/BSA. In the case of samples incubated mAb 2H1 and 2D10, a goat anti-mouse (GAM) IgG-Alexa 488 (Life Technologies, Reference A11001) or GAM IgM secondary Ab conjugated to Alexa 488 (Life Technologies, Reference A21042) was added at 1 μg/mL, respectively. The cells were washed with PBS/BSA and suspended in 50 μL of same buffer. Cells were placed on a glass slide and mounted with Fluoromount G (Southern Biotech, Reference 0100–01). Fluorescence binding pattern was visualized in a Leica DMI3000B fluorescence microscope coupled to a Leica DFC300 digital camera and pictures were taken using the LAS 3.3.1 software (Leica Microsystems). To quantify the fluorescence intensity, parallel samples without the primary antibodies, but with the secondary antibodies, were prepared. Fluorescence of the cells was analyzed in a FACS Calibur flow cytometer (BD) in the FL1 channel. Data was processed with FlowJo software (FlowJo, LLC). In addition, and as controls, Ab binding was evaluated in parallel in cells grown in Sabouraud and in capsule growth inducing conditions (10% Sabouraud, pH 7.3, see above).
Statistics
Data was analyzed with GraphPad 5.0 software (GraphPad Software, La Jolla California USA). Normality of the samples was assessed with the Kolgomorov-Smirnov (KS) test. In case of normality, data was analyzed with ANOVA and T-test. When samples were not normally distributed (p < 0.05 in the KS test), statistical differences were evaluated with the non-parametric tests Kruskal-Wallis and Mann-Whitney. In all cases, statistical differences were considered when p < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
986412_Supplementary_Materials.zip
Download Zip (464.4 KB)Acknowledgments
We are indebted to Dr. Krishnendu Mukherjee and Dr. Andreas Vilcinskas (Giessen University, Germany) for training our group in some of the techniques used in the work. We thank Dr. Robin May and Dr. Kerstin Voelz (Birmingham University, UK) for the gift of the H99-GFP strain, Dr. June Kwon-Chung (NIAID, NIH, Bethesda, Washington DC) for the gift of the cap59 mutant, Dr. Adela González de la Campa (National Center for Microbiology, ISCIII, Spain) for the gift of lysozyme and Dr. Arturo Casadevall (Albert Einstein College of Medicine, Bronx, NY) for the gift of Abs. We also thank Julie Wolf and Rafael Prados-Rosales (Albert Einstein College of Medicine, Bronx, New York) for reading and editing the manuscript.
Funding
O.Z. is funded by grant SAF2011–25140 from the Spanish Ministry for Economics and Competitivity. N.T-C. is supported by a FPI fellowship (reference BES-2012–051837). C.R. has a Sara Borrell contract from the Fondo de Investigaciones Sanitarias from Instituto de Salud Carlos III (reference number CD11/00110). I.H-F and I.G-B participated in this work in the frame of the Master “Parasitology and Microbiology: Research and Development” from the Pharmacy and Biology Faculties from the Complutense University of Madrid. Liliana Scorzoni was supported by Fundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP) 2013/10917–9. S.A.R was supported by Programa PDSE-CAPES – 1909–13–4.
Supplemental Materials
Supplemental data for this article can be accessed on the publisher's website at http://dx.doi.org/10.4161/21505594.2014.986412
References
- Casadevall A, Perfect J. Cryptococcus neoformans. Washington DC: ASM, 1998.
- Heitman J, Kozel TR, Kwon-Chung KJ, Perferct JR, Casadevall A. Cryptococcus. From Human pathogen to model yeast. Washington DC: ASM Press, 2011.
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23: 525-30; PMID:19182676; http://dx.doi.org/10.1097/QAD.0b013e328322ffac
- Vecchiarelli A, Pietrella D, Dottorini M, Monari C, Retini C, Todisco T, Bistoni F. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin Exp Immunol 1994; 98: 217-23; PMID:7955525; http://dx.doi.org/10.1111/j.1365-2249.1994.tb06128.x
- Chang YC, Kwon-Chung KJ. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol 1994; 14: 4912-9; PMID:8007987
- Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol 2009; 68: 133-216; PMID:19426855; http://dx.doi.org/10.1016/S0065-2164(09)01204-0
- Nosanchuk JD, Valadon P, Feldmesser M, Casadevall A. Melanization of Cryptococcus neoformans in murine infection. Mol Cell Biol 1999; 19: 745-50; PMID:9858597
- Williamson PR. Laccase and melanin in the pathogenesis of Cryptococcus neoformans. Front Biosci 1997; 2: e99-107; PMID:9342305
- Feldmesser M, Kress Y, Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 2001; 147: 2355-65; PMID:11496012
- Zaragoza O, Garcia-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. Fungal cell gigantism during mammalian infection. PLOS Pathog 2010; 6: e1000945; PMID:20585557; http://dx.doi.org/10.1371/journal.ppat.1000945
- Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chretien F, Heitman J, Dromer F, Nielsen K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLOS Pathog 2010; 6: e1000953; PMID:20585559; http://dx.doi.org/10.1371/journal.ppat.1000953
- Diamond RD, Bennett JE. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun 1973; 7: 231-6; PMID:4697791
- Tucker SC, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci U S A 2002; 99: 3165-70; PMID:11880650; http://dx.doi.org/10.1073/pnas.052702799
- Apidianakis Y, Rahme LG, Heitman J, Ausubel FM, Calderwood SB, Mylonakis E. Challenge of Drosophila melanogaster with Cryptococcus neoformans and role of the innate immune response. Eukaryot Cell 2004; 3: 413-9; PMID:15075271; http://dx.doi.org/10.1128/EC.3.2.413-419.2004
- Eshar D, Mayer J, Parry NM, Williams-Fritze MJ, Bradway DS. Disseminated, histologically confirmed Cryptococcus spp infection in a domestic ferret. J Am Vet Med Assoc 2010; 236: 770-4; PMID:20367045; http://dx.doi.org/10.2460/javma.236.7.770
- Kido N, Makimura K, Kamegaya C, Shindo I, Shibata E, Omiya T, Yamamoto Y. Long-term surveillance and treatment of subclinical cryptococcosis and nasal colonization by Cryptococcus neoformans and C. gattii species complex in captive koalas (Phascolarctes cinereus). Med Mycol 2012; 50: 291-8; PMID:21859391; http://dx.doi.org/10.3109/13693786.2011.594967
- McGill S, Malik R, Saul N, Beetson S, Secombe C, Robertson I, Irwin P. Cryptococcosis in domestic animals in Western Australia: a retrospective study from 1995–2006. Med Mycol 2009; 47: 625-39; PMID:19306217; http://dx.doi.org/10.1080/13693780802512519
- Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc Natl Acad Sci U S A 2002; 99: 15675-80; PMID:12438649; http://dx.doi.org/10.1073/pnas.232568599
- Warpeha KM, Park YD, Williamson PR. Susceptibility of intact germinating Arabidopsis thaliana to human fungal pathogens Cryptococcus neoformans and C. gattii. Appl Environ Microbiol 2013; 79: 2979-88; PMID:23435895; http://dx.doi.org/10.1128/AEM.03697-12
- Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A 2001; 98: 15245-50; PMID:11742090; http://dx.doi.org/10.1073/pnas.261418798
- Steenbergen JN, Casadevall A. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microbes Infect 2003; 5: 667-75; PMID:12787743; http://dx.doi.org/10.1016/S1286-4579(03)00092-3
- Cotter G, Doyle S, Kavanagh K. Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol 2000; 27: 163-9; PMID:10640612; http://dx.doi.org/10.1111/j.1574-695X.2000.tb01427.x
- Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol 2002; 34: 153-7; PMID:12381467; http://dx.doi.org/10.1111/j.1574-695X.2002.tb00617.x
- Fuchs BB, O'Brien E, Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 2010; 1: 475-82; PMID:21178491; http://dx.doi.org/10.4161/viru.1.6.12985
- Mukherjee K, Raju R, Fischer R, Vilcinskas A. Galleria mellonella as a model host to study gut microbe homeostasis and brain infection by the human pathogen Listeria monocytogenes. Adv Biochem Eng Biotechnol 2013; 135: 27-39; PMID:23708825
- Mesa-Arango AC, Forastiero A, Bernal-Martinez L, Cuenca-Estrella M, Mellado E, Zaragoza O. The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med Mycol 2013; 51:461-72; PMID:23170962
- Morton DB, Barnett RI, Chadwick JS. Structural alterations to Proteus mirabilis as a result of exposure to haemolymph from the larvae of Galleria mellonella. Microbios 1984; 39: 177-85; PMID:6374385
- Reeves EP, Messina CG, Doyle S, Kavanagh K. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia 2004; 158: 73-9; PMID:15487324; http://dx.doi.org/10.1023/B:MYCO.0000038434.55764.16
- Scorzoni L, de Lucas MP, Mesa-Arango AC, Fusco-Almeida AM, Lozano E, Cuenca-Estrella M, Mendes-Giannini MJ, Zaragoza O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS One 2013; 8: e60047; PMID:23555877; http://dx.doi.org/10.1371/journal.pone.0060047
- St Leger RJ, Screen SE, Shams-Pirzadeh B. Lack of host specialization in Aspergillus flavus. Appl Environ Microbiol 2000; 66: 320-4; PMID:10618242; http://dx.doi.org/10.1128/AEM.66.1.320-324.2000
- Thomaz L, Garcia-Rodas R, Guimaraes AJ, Taborda CP, Zaragoza O, Nosanchuk JD. Galleria mellonella as a model host to study Paracoccidioides lutzii and Histoplasma capsulatum. Virulence 2013; 4: 139-46; PMID:23302787; http://dx.doi.org/10.4161/viru.23047
- Borghi E, Romagnoli S, Fuchs BB, Cirasola D, Perdoni F, Tosi D, Braidotti P, Bulfamante G, Morace G, Mylonakis E. Correlation between Candida albicans biofilm formation and invasion of the invertebrate host Galleria mellonella. Future Microbiol 2014; 9: 163-73; PMID:24571071; http://dx.doi.org/10.2217/fmb.13.159
- Navarro-Velasco GY, Prados-Rosales RC, Ortiz-Urquiza A, Quesada-Moraga E, Di Pietro A. Galleria mellonella as model host for the trans-kingdom pathogen Fusarium oxysporum. Fungal Genet Biol 2011; 48: 1124-9; PMID:21907298; http://dx.doi.org/10.1016/j.fgb.2011.08.004
- Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, Calderwood SB, Ausubel FM, Diener A. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 2005; 73: 3842-50; PMID:15972469; http://dx.doi.org/10.1128/IAI.73.7.3842-3850.2005
- Garcia-Rodas R, Casadevall A, Rodriguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLOS One 2011; 6: e24485; PMID:21915338; http://dx.doi.org/10.1371/journal.pone.0024485
- Desalermos A, Tan X, Muthiah R, Arvanitis M, Wang Y, Li D, Kourkoumpetis TK, Fuchs BB, Mylonakis E. A Multi-Host Approach for the Systematic Analysis of Virulence Factors in Cryptococcus neoformans. J Infect Dis 2015; 211:298-305; PMID:25114160
- Fuguet R, Vey A. Comparative analysis of the production of insecticidal and melanizing macromolecules by strains of Beauveria spp.: in vivo studies. J Invertebr Pathol 2004; 85: 152-67; PMID:15109898; http://dx.doi.org/10.1016/j.jip.2004.03.001
- Tojo S, Naganuma F, Arakawa K, Yokoo S. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J Insect Physiol 2000; 46: 1129-35; PMID:10817839; http://dx.doi.org/10.1016/S0022-1910(99)00223-1
- Mak P, Chmiel D, Gacek GJ. Antibacterial peptides of the moth Galleria mellonella. Acta Biochim Pol 2001; 48: 1191-5; PMID:11995991
- Hu K, Li J, Li B, Webster JM, Chen G. A novel antimicrobial epoxide isolated from larval Galleria mellonella infected by the nematode symbiont, Photorhabdus luminescens (Enterobacteriaceae). Bioorg Med Chem 2006; 14: 4677-81; PMID:16644226; http://dx.doi.org/10.1016/j.bmc.2006.01.025
- Bergin D, Reeves EP, Renwick J, Wientjes FB, Kavanagh K. Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun 2005; 73: 4161-70; PMID:15972506; http://dx.doi.org/10.1128/IAI.73.7.4161-4170.2005
- Schuhmann B, Seitz V, Vilcinskas A, Podsiadlowski L. Cloning and expression of gallerimycin, an antifungal peptide expressed in immune response of greater wax moth larvae, Galleria mellonella. Arch Insect Biochem Physiol 2003; 53: 125-33; PMID:12811766; http://dx.doi.org/10.1002/arch.10091
- Garcia-Hermoso D, Dromer F, Janbon G. Cryptococcus neoformans capsule structure evolution in vitro and during murine infection. Infect Immun 2004; 72: 3359-65; PMID:15155641; http://dx.doi.org/10.1128/IAI.72.6.3359-3365.2004
- Mowlds P, Barron A, Kavanagh K. Physical stress primes the immune response of Galleria mellonella larvae to infection by Candida albicans. Microbes Infect 2008; 10: 628-34; PMID:18457977; http://dx.doi.org/10.1016/j.micinf.2008.02.011
- Rueda C, Cuenca-Estrella M, Zaragoza O. Paradoxical growth of Candida albicans in the presence of caspofungin is associated with multiple cell wall rearrangements and decreased virulence. Antimicrob Agents Chemother 2014; 58: 1071-83; PMID:24295973; http://dx.doi.org/10.1128/AAC.00946-13
- Mody CH, Syme RM. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun 1993; 61: 464-9; PMID:8423074
- Blackstock R, Hall NK. Non-specific immunosuppression by Cryptococcus neoformans infection. Mycopathologia 1984; 86: 35-43; PMID:6234468; http://dx.doi.org/10.1007/BF00437227
- Gadebusch HH. Active immunization against Cryptococcus neoformans. J Infect Dis 1958; 102: 219-26; PMID:13549767; http://dx.doi.org/10.1093/infdis/102.3.219
- Murphy JW, Cozad GC. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun 1972; 5: 896-901; PMID:4564405
- Blackstock R. Cryptococcal capsular polysaccharide utilizes an antigen-presenting cell to induce a T-suppressor cell to secrete TsF. J Med Vet Mycol 1996; 34: 19-30; PMID:8786467; http://dx.doi.org/10.1080/02681219680000041
- Chiapello LS, Aoki MP, Rubinstein HR, Masih DT. Apoptosis induction by glucuronoxylomannan of Cryptococcus neoformans. Med Mycol 2003; 41: 347-53; PMID:12964728; http://dx.doi.org/10.1080/1369378031000137260
- Garcia-Rodas R, Zaragoza O. Catch me if you can: phagocytosis and killing avoidance by Cryptococcus neoformans. FEMS Immunol Med Microbiol 2012; 64: 147-61; PMID:22029633; http://dx.doi.org/10.1111/j.1574-695X.2011.00871.x
- Mcquiston T, del Poeta M. The interaction of Cryptococcus neoformans with host macrophages and neutrofils. Cryptococcus. From human pathogen to model host. In: Heitman J, Kozel TR, Kwon-Chung KJ, Perfect JR and Casadevall A., ed. Washington: ASM, 2011: 373-87.
- Ma H, Croudace JE, Lammas DA, May RC. Expulsion of live pathogenic yeast by macrophages. Curr Biol 2006; 16: 2156-60; PMID:17084701; http://dx.doi.org/10.1016/j.cub.2006.09.032
- Alvarez M, Burn T, Luo Y, Pirofski LA, Casadevall A. The outcome of Cryptococcus neoformans intracellular pathogenesis in human monocytes. BMC Microbiol 2009; 9: 51; PMID:19265539; http://dx.doi.org/10.1186/1471-2180-9-51
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol 2006; 16: 2161-5; PMID:17084702; http://dx.doi.org/10.1016/j.cub.2006.09.061
- Chrisman CJ, Alvarez M, Casadevall A. Phagocytosis of Cryptococcus neoformans by, and nonlytic exocytosis from, Acanthamoeba castellanii. Appl Environ Microbiol 2010; 76: 6056-62; PMID:20675457; http://dx.doi.org/10.1128/AEM.00812-10
- Qin QM, Luo J, Lin X, Pei J, Li L, Ficht TA, de Figueiredo P. Functional analysis of host factors that mediate the intracellular lifestyle of Cryptococcus neoformans. PLoS Pathog 2011; 7: e1002078; PMID:21698225; http://dx.doi.org/10.1371/journal.ppat.1002078
- Vilcinskas A, Matha V, Götz P. Inhibition of phagocytic activity of plasmatocytes isolated from Galleria mellonella by entomogenous fungi and their secondary metabolites. J. Insect Physiol. 1997; 43: 475-83; http://dx.doi.org/10.1016/S0022-1910(96)00120-5
- Eisenman HC, Duong R, Chan H, Tsue R, McClelland EE. Reduced virulence of melanized Cryptococcus neoformans in Galleria mellonella. Virulence 2014; 5: 611-8; PMID:24846144; http://dx.doi.org/10.4161/viru.29234
- Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol 2008; 10: 2043-57; PMID:18554313; http://dx.doi.org/10.1111/j.1462-5822.2008.01186.x
- Zaragoza O, Taborda CP, Casadevall A. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur J Immunol 2003; 33: 1957-67; PMID:12884862; http://dx.doi.org/10.1002/eji.200323848
- Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLOS Pathog 2011; 7: e1002047; PMID:21637814; http://dx.doi.org/10.1371/journal.ppat.1002047
- McFadden DC, Fries BC, Wang F, Casadevall A. Capsule structural heterogeneity and antigenic variation in Cryptococcus neoformans. Eukaryot Cell 2007; 6: 1464-73; PMID:17601878; http://dx.doi.org/10.1128/EC.00162-07
- Charlier C, Chretien F, Baudrimont M, Mordelet E, Lortholary O, Dromer F. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am J Pathol 2005; 166: 421-32; PMID:15681826; http://dx.doi.org/10.1016/S0002-9440(10)62265-1
- Garcia-Solache MA, Izquierdo-Garcia D, Smith C, Bergman A, Casadevall A. Fungal virulence in a lepidopteran model is an emergent property with deterministic features. MBio 2013; 4: e00100-13; PMID:23631914; http://dx.doi.org/10.1128/mBio.00100-13
- Freitak D, Schmidtberg H, Dickel F, Lochnit G, Vogel H, Vilcinskas A. The maternal transfer of bacteria can mediate trans-generational immune priming in insects. Virulence 2014; 5: 547-54; PMID:24603099; http://dx.doi.org/10.4161/viru.28367
- Trevijano-Contador N, Zaragoza O. Expanding the use of alternative models to investigate novel aspects of immunity to microbial pathogens. Virulence 2014; 5: 454-6; PMID:24717215; http://dx.doi.org/10.4161/viru.28775
- Perfect JR, Lang SD, Durack DT. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol 1980; 101: 177-94; PMID:7004196
- Byrnes EJ, 3rd, Li W, Lewit Y, Ma H, Voelz K, Ren P, Carter DA, Chaturvedi V, Bildfell RJ, May RC, Heitman J. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLOS Pathog 2010; 6: e1000850; PMID:20421942; http://dx.doi.org/10.1371/journal.ppat.1000850
- Kwon-Chung KJ. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 1976; 68: 821-33; PMID:790172; http://dx.doi.org/10.2307/3758800
- Chang YC, Wickes BL, Kwon-Chung KJ. Further analysis of the CAP59 locus of Cryptococcus neoformans: structure defined by forced expression and description of a new ribosomal protein-encoding gene. Gene 1995; 167: 179-83; PMID:8566774; http://dx.doi.org/10.1016/0378-1119(95)00640-0
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 1984; 198: 179-82; PMID:6394964; http://dx.doi.org/10.1007/BF00328721
- Zaragoza O, Casadevall A. Experimental modulation of capsule size in Cryptococcus neoformans. Biol Proced Online 2004; 6: 10-5; PMID:15103395; http://dx.doi.org/10.1251/bpo68
- Cherniak R, Valafar H, Morris LC, Valafar F. Cryptococcus neoformans chemotyping by quantitative analysis of 1H nuclear magnetic resonance spectra of glucuronoxylomannans with a computer-simulated artificial neural network. Clin Diagn Lab Immunol 1998; 5: 146-59; PMID:9521136
- Garcia-Rodas R, Gonzalez-Camacho F, Rodriguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. The interaction between Candida krusei and murine macrophages results in multiple outcomes, including intracellular survival and escape from killing. Infect Immun 2011; 79: 2136-44; PMID:21422181; http://dx.doi.org/10.1128/IAI.00044-11
- Casadevall A, Mukherjee J, Scharff MD. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Methods 1992; 154: 27-35; PMID:1401941; http://dx.doi.org/10.1016/0022-1759(92)90209-C
