Keywords: :
As one of the most intensively studied transcription factors, it is unsurprising that p53 is involved in many biological processes, including cell cycle arrest, DNA repair, senescence and apoptosis.Citation1 Its activities are mainly regulated by posttranslational modifications and interactions with other cellular components. Over the past few years, p53 has also been linked to redox homeostasis, the balance between cellular pro- and antioxidant levels.Citation2 On the one hand, reactive oxygen species (ROS) can modify the cysteine residues of p53, leading to a conformational change that affects its transcriptional activities.Citation2 On the other hand, p53 also acts as an upstream regulator of ROS production by activating or repressing several ROS-regulating genes, such as glutathine peroxidase (GPX), p53-induced genes (PIGs), nitric oxide synthase 2 (NOS2) and Mn superoxide dismutase (MnSOD).Citation2 Those genes have been shown to act either in a pro- or an antioxidant context. Hence, the role of p53 in overall ROS regulation is a complex network and still needs to be investigated in depth.
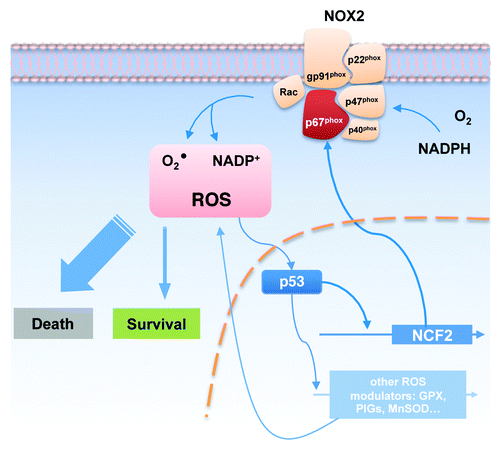
ROS are generated by both mitochondrial and non-mitochondrial pathways. In the latter pathway, the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) mainly contributes to ROS production. Among many family members, NOX2 is the one which has been most extensively studied (). NOX2 mainly localizes on the plasma membrane and generates superoxide utilizing NADPH and molecular oxygen upon activation, e.g., in the process of phagocytosis.Citation3 Recently, neutrophil cytosolic factor 2 (NCF2), the gene encoding p67phox, which is the cytosolic subunit and activator of NOX2, has been identified as a novel target of p53.Citation4 In this study, Italiano et al. have shown that p53 can bind to the promoter of NCF2 and activate gene expression, indicating that p53 is a transcription factor for NOX2. NCF2 is upregulated by doxorubicin treatment in p53-positive HCT 116 cells but not in p53-negative cells. Downregulation of NCF2 with siRNA-based approach results in reduced ROS production and increased cell death in HCT 116 and HaCat cells. This suggests that NOX2 expression is a sort of ROS-dependent cell survival factor owing to the influence of ROS on p53 (). It should be mentioned, however, that high ROS concentrations can also kill cells.Citation5 Taken together, these results provide a new insight into the network of p53 and ROS production. Since p53 can act both pro- and anti-apoptotically, the pro-oxidant NCF2/p67phox can thus support the anti-apoptotic role of p53 under certain conditions. In this case, p53 is probably required to maintain a sufficient level of NCF2/p67phox for cell survival. However, under which circumstances p53 activates NOX2 and how they modulate the cell fate are still questions of interest at the moment.
Figure 1. The network of ROS regulation by p53. Reactive oxygen species (ROS) can modulate the activity of p53, which, in turn, controls the expression of several ROS-regulating genes, such as GPX, PIGs and MnSOD. Those act as either pro- or antioxidant. NCF2, the gene encoding the intercellular subunit of NOX2 protein complex, is also transcriptional activated by p53. Upon activation, NOX2 generates ROS beneficial to cell survival. On the other hand, high ROS concentration may lead to cell death.
In summary, the identification of NCF2/p67phox as a novel target of p53 extends our understanding of the partnership between p53 and ROS production and further highlights the unknown role of p53 in inflammatory diseases, such as cardiovascular, autoimmune and allergic diseases as well as primary and secondary immunodeficiencies.
References
- Vousden KH, et al. Nat Rev Mol Cell Biol 2007; 8:275 - 83; http://dx.doi.org/10.1038/nrm2147; PMID: 17380161
- Maillet A, et al. Antioxid Redox Signal 2012; 16:1285 - 94; http://dx.doi.org/10.1089/ars.2011.4434; PMID: 22117613
- Bedard K, et al. Physiol Rev 2007; 87:245 - 313; http://dx.doi.org/10.1152/physrev.00044.2005; PMID: 17237347
- Italiano D, et al. Cell Cycle 2012; 11:4589 - 96; http://dx.doi.org/10.4161/cc.22853; PMID: 23187810
- Vurusaner B, et al. Free Radic Biol Med 2012; 52:7 - 18; http://dx.doi.org/10.1016/j.freeradbiomed.2011.09.035; PMID: 22019631