Keywords: :
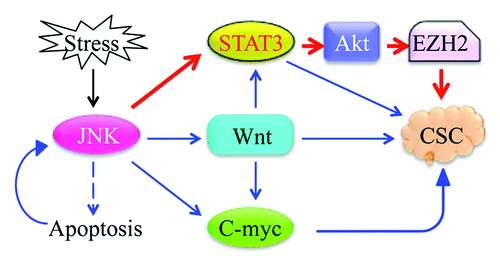
Activation of JNK signaling pathway has long been viewed as a one-way road to the destination where apoptotic or non-apoptotic cell death occurs in response to a plethora of extracellular stimuli. Paradoxically, JNK has also been implicated in the proliferation of transformed and cancer cells.Citation1 For example, several early studies have shown that activation of JNK kinase could increase the incidence of tumor formation or tumor size of hepatocellular carcinoma (HCC), gastric cancer and lung cancer induced by chemical carcinogens or tobacco smoke. However, it is still puzzling how JNK, which is a well-studied kinase regulating cell death, acts in the opposite direction to induce proliferation of cancer cells. In both compensatory growth model in Drosophila and cancer model in mice, JNK was viewed as an upstream kinase of Wnt/BMP signaling and JAK/STAT3 signaling that is important in providing growth advantages for the cells. Alternatively, JNK appears to be essential for the expression of c-myc, a tumor-promoting oncogene in human and mouse HCC.Citation2 In a recent issue of Cell Cycle, Chen and colleaguesCitation3 provide convincing evidence showing that activation of JNK by arsenic is an early event in the JNK-STAT3-Akt signaling axis that is linked to serine 21 (S21) phosphorylation of EZH2, an enzyme subunit of the PRC2 complex responsible for trimethylation of histone H3 lysine 27 (H3K27me3).
Previously, the Chen group demonstrated that JNK phosphorylates STAT3 at serine 727 (S727), which, in turn, activates Akt and induces VEGF expression and cell migration.Citation4 In this report, they validate the JNK regulation of STAT3 via miR-21 and link the JNK-STAT3-Akt signaling axis to the phosphorylation of EZH2. In addition, they found that STAT3 upregulates Akt through miR-21-mediated silencing of Spry2 rather than PTEN or PDCD4 as previously reported. Spry2 is a tumor suppressor that antagonizes multiple receptor tyrosine kinases associated with the activation of Ras/ERK and PI3K/Akt pathways. In prostate cancer and HCC, overactivation of PI3K/Akt is the major effect from loss of Spry2.Citation5,Citation6
Akt-dependent S21 phosphorylation of EZH2 has been reported in breast cancer cells treated with IGF-1 or estrogen.Citation7 It is believed that this phosphorylation weakens the association between EZH2 and other PRC2 subunits, which results in the decrease in H3K27me3 level in the genome. Unexpectedly, this report did not show a detectable effect of S21 phosphorylation of EZH2 on H3K27me3 level. The authors attribute this finding to the small fraction of EZH2 being phosphorylated by arsenic, which may not be sufficient to affect the methytransferase activity of EZH2. Interestingly, they observed that a substantial amount of S21-phosphorylated EZH2 is localized in the cytoplasm, which is unusual considering that EZH2 is predominantly a nuclear protein. The question to be answered is whether S21-phosphorylated EZH2 can partner with specific proteins in the cytoplasm to alter their function.
The significance of this paper is that it might reveal a novel mechanism of carcinogenesis induced by arsenic and other related metal carcinogens. It is worth noting that JNK is involved in the signaling of STAT3 and Akt that leads to EZH2 phosphorylation. The activation of JNK-STAT3-Akt signaling axis and EZH2 phosphorylation are potentially crucial steps in the generation of cancer stem cells (CSCs), since both STAT3 and EZH2 are known to be involved in the self-renewal, pluripotency and proliferation of CSCs ().Citation8 Interestingly, a recent report suggests that Oct4, one of the four Yamanaka factors for reprogramming, is a substrate of Akt.Citation9 Phosphorylation of Oct4 by Akt would enhance the transcriptional activity of Oct4 on other pluripotent or self-renewal genes, suggesting the potential new role of JNK-STAT3-Akt signaling and EZH2 phosphorylation in CSC growth and carcinogenesis.
References
- Chen F. Cancer Res 2012; 72:379 - 86; http://dx.doi.org/10.1158/0008-5472.CAN-11-1982; PMID: 22253282
- Hui L, et al. J Clin Invest 2008; 118:3943 - 53; http://dx.doi.org/10.1172/JCI37156; PMID: 19033664
- Chen B, et al. Cell Cycle 2012; 12:112 - 21; http://dx.doi.org/10.4161/cc.23030; PMID: 23255093
- Liu J, et al. Toxicol Sci 2012; 129:363 - 71; http://dx.doi.org/10.1093/toxsci/kfs199; PMID: 22696236
- Gao M, et al. EMBO Mol Med 2012; 4:776 - 90; http://dx.doi.org/10.1002/emmm.201100944; PMID: 22649008
- Wang C, et al. J Hepatol 2012; 57:577 - 83; http://dx.doi.org/10.1016/j.jhep.2012.04.026; PMID: 22617155
- Cha TL, et al. Science 2005; 310:306 - 10; http://dx.doi.org/10.1126/science.1118947; PMID: 16224021
- Chang CJ, et al. Br J Cancer 2012; 106:243 - 7; http://dx.doi.org/10.1038/bjc.2011.551; PMID: 22187039
- Lin Y, et al. Mol Cell 2012; 48:627 - 40; http://dx.doi.org/10.1016/j.molcel.2012.08.030; PMID: 23041284